Abstract
Despite significant advances in our understanding of HIV, a cure has not been realized for the more than 34 million infected with this virus. HIV is incurable because infected individuals harbor cells where the HIV provirus is integrated into the host’s DNA but is not actively replicating and thus is not inhibited by antiviral drugs. Similarly, these latent viruses are not detected by the immune system. In this review, we discuss HIV-1 latency and the mechanisms that allow this pathogenic retrovirus to hide and persist by exploiting the cellular vehicles of immunological memory.
Introduction
AIDS was first recognized in the summer of 1981 (Friedman-Kien, 1981; Gottlieb et al., 1981; Siegal et al., 1981) and HIV was first isolated two years later (Barre-Sinoussi et al., 1983) and proven to be the cause of AIDS in 1984 (Gallo et al., 1984; Popovic et al., 1984; Sarngadharan et al., 1984; Schupbach et al., 1984). More than 35 million people have died of AIDS. The virus continues to hit the hardest in Sub-Saharan Africa, where 1 in every 20 adults is infected. Although there is no cure for HIV, more than 30 different anti-HIV drugs have now been approved for clinical use targeting different steps in the viral life cycle. While not eradicating HIV, combinations of these agents can routinely drive viral loads down to undetectable levels. Indeed, HIV is now managed as a chronic rather than acute disease. Nevertheless, for every 10 people started on antiretroviral therapy in the developing world, 16 people are newly infected. We clearly do not yet have a winning HIV/AIDS strategy plus the escalating cost for treatment will become increasingly difficult for developed countries to meet. Strategies for either eradicating the virus from infected individuals or boosting their immune response so that antiviral drugs can be discontinued—a functional cure—are urgently needed.
HIV-1 Latency in CD4+ T cells
Post-integration HIV latency refers to the rare but extremely stable proviral reservoir formed within resting memory CD4+ T cells. Latency is established early during acute infection, likely within days of initial infection (Chun et al., 1998a). Although transcriptionally silent, this reservoir is fully capable of producing infectious virus when the host cell is reactivated by recall antigen or various cytokines or when ART is discontinued.
Naïve CD4+ T cells exist in a resting state until they encounter an antigen, after which they undergo activation and proliferation to generate effector cells that clear the associated pathogen from the body. The majority of these activated cells die within a few weeks. However, some of these cells revert back to a resting state and persist as memory T cells that are capable of responding to the same antigen in the future. It is precisely these cells that form a primary reservoir for latent HIV proviruses. It is possible that latent infection reflects infection just as these cells retreat to a resting state. Because these cells can persist in a quiescent state for long periods of time, they represent an ideal cellular reservoir for the maintenance of latent virus. Antigen or cytokine activation of these cells leads to the induction of transcription factors, like NF-κ B and NFAT, that, in turn, promote reactivation of the latent HIV proviruses. Following activation, cytopathic effects or immune responses cause the rapid death of most HIV-infected cells (Figure 1). Importantly, it was recently shown that antigen-specific stimulation of patient cytolytic T lymphocytes (CTLs) prior to virus reactivation from latently infected cells is essential for effective killing of HIV-1 infected cells in vitro (Shan et al., 2012). This suggests that boosting the CTL response in infected patients may be necessary to deplete the HIV reservoir.
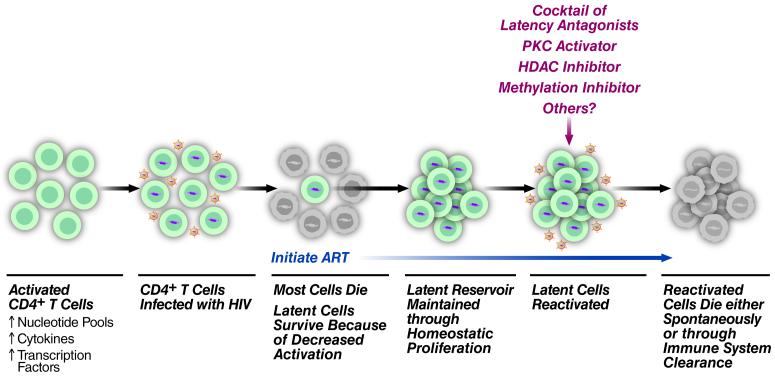
Figure 1. HIV-1 Infection and Reactivation of CD4+ T cells.
HIV-1 infects activated CD4+ T cells, which have increased nucleotide pools, cytokines, and transcription factors compared to non-activated cells. Most of these infected cells die due to cytopathic effects of the virus or lysis by HIV-specific CTLs. Cells that revert back to a resting memory T cell survive and may undergo homeostatic proliferation. Upon initiation of ART these latently infected cells persist. However, treatment of these cells with reactivating agents causes the cells to actively produce virus and ultimately leads to either spontaneous cell death or death through immune system clearance.
Cellular Reservoirs
The introduction of combination antiretroviral therapy (ART) in 1996 was a major advance that revolutionized the care of HIV-infected individuals. These drugs also provided new insights into the dynamics of HIV-1 replication in vivo. Specifically, a four-phase decline in virus was detected following ART administration. The initial rapid phase of decay occurs within the first two weeks of treatment and is accompanied by a 99% drop in HIV levels in the blood. This is largely due to the rapid decline of free virus (t1/2≤6 hours) and productively infected cells (t1/2≤1.6 days) (Ho et al., 1995; Wei et al., 1995).
The second phase of decay proceeds more slowly and results mainly from the death of infected macrophages, partially activated CD4+ T cells and dendritic cells. The half-life of macrophages can be several weeks long, depending on the type of tissue in which they reside. Macrophages are susceptible to infection with HIV, but are more resistant to the cytopathic effects of the virus compared to CD4+ T cells (Ho et al., 1986). Partially activated CD4+ T cells are also described as having a longer turnover rate than fully activated CD4+ T cells, and are therefore likely to contribute to the slower decay of the second phase. In general, the dendritic cell lifespan is highly dependent on anatomical location and cellular subtype (Shortman and Naik, 2007). Following HIV infection in vitro, myeloid dendritic cells are capable of surviving for over 45 days (Popov et al., 2005). However, in vivo it has been shown that peripheral blood myeloid dendritic cells do not contain detectable HIV DNA following a 6-week ART regimen (Otero et al., 2003). Langerhans cells have been shown to resist HIV infection unless stressed by skin abrasion or co-exposed to other sexually transmitted organisms (reviewed by (Coleman and Wu, 2009)), and when infected have a half-life of about 15 days. For these reasons, HIV-infected Langerhans cells are not thought to contribute significantly to the latent reservoir. In addition, follicular dendritic cells, found in the lymphoid tissues, are capable of trapping and retaining HIV virions on their surface for several months following infection (Smith et al., 2001). However, it is unclear whether these cell-associated virions meaningfully contribute to the latent reservoir that is able to persist for years in infected subjects on ART.
Taking into account this second phase of decay, it was initially predicted that 2.3–3.1 years of treatment would be sufficient to eliminate HIV-1-infected cells, including latently infected lymphocytes (Perelson et al., 1997). However, this calculation was made with the acknowledgement that additional longitudinal studies were necessary to exclude the presence of very long-lived infected cells.
In 1995, the first in vivo evidence of the presence of a latent HIV reservoir was revealed. This was demonstrated by isolating a highly purified population of resting CD4+ T cells from HIV-1-infected patients and performing inverse PCR to detect integrated HIV-1 DNA (Chun et al., 1995). At the time, a critical area of investigation was to determine the frequency of latently infected cells as well as the replication competency of the virus in patients on ART. Because resting memory CD4+ T cells can persist for months to years, these cells could represent a long-term viral reservoir in patients on ART. Indeed, several laboratories demonstrated that replication-competent virus could persist in the resting CD4+ T cells of patients on ART regimens and that these infected cells were present in about 1 in every 106 resting CD4+ T cells (Chun et al., 1997; Finzi et al., 1997; Wong et al., 1997). Using direct longitudinal analysis of the decay rate of the latent reservoir, it was demonstrated that latent cells capable of producing replication-competent virus had a very long half-life (43.9 months). Based on this turnover rate, it was estimated to take 73 years of therapy to eradicate the latent reservoir (Siliciano et al., 2003). Recently, the Siliciano lab identified and characterized proviruses present in viral outgrowth assays that were not induced by maximal PHA stimulation, and showed that a portion of these proviruses are intact and replication-competent. Taking these non-induced cells into account, statistical modeling indicated that the number of latently infected cells is ~60 fold larger than originally estimated (this issue of Cell), proving that eradication of the viral reservoir will be even more challenging than previously predicted.
The exact cellular subsets responsible for the third and fourth phases of decline are not yet clear, but are likely to include the well-defined latent reservoir of resting memory CD4+ T cells. In support of this idea, Chomont et al. identified two viral reservoirs within the memory CD4+ T-cell pool. Known as central memory (TCM) and transitional memory (TTM) CD4+ T cells, these reservoirs are characterized by differing decay rates in ART-treated patients and persist by two distinct mechanisms. HIV-infected patients who have normal CD4 counts and who have begun an early treatment regimen carry a viral reservoir of limited size that consists mainly of TCM cells. TCM cells proliferate at extremely low levels and are able to survive for decades, although this reservoir may be partially depleted at a slow rate due to CTL killing and the virus’ intrinsic cytopathic effects. In contrast, HIV-infected patients with low CD4 counts have a viral reservoir consisting mainly of TTM cells. This is accompanied by continuous immune activation in the majority of these patients, including increased levels of IL-7. IL-7 has been shown to induce proliferation of memory CD4+ T cells, and may induce the homeostatic proliferation and survival of TTM cells in ART-treated individuals with low CD4 counts (Chomont et al., 2009). This could be an additional mechanism by which this latently infected reservoir persists for long periods of time. This study also raises the possibility that earlier therapeutic intervention could more effectively limit the size of this proliferating reservoir.
Although ART is capable of decreasing the viral load to levels below the limit of detection, the persistence of a latent reservoir of replication-competent provirus remains a major obstacle to achieving a cure. It is well known that latently infected CD4+ T cells can be activated to produce infectious virus in vitro as well as in vivo. Therefore, stimulation of this latent reservoir and forcing virus to enter into a productive viral lifecycle may be an essential step in eradicating HIV-1 in infected patients.
Viral Persistence
The mechanism by which virus persists in the presence of ART is uncertain and may actually involve multiple mechanisms. The most widely accepted school of thought is that the long-lived reservoirs of memory CD4+ T cells establish latency early on and persist for an extremely long time. Another school suggests that ongoing rounds of replication are occurring perhaps in tissues where drug penetration is decreased (for example the CNS and GALT) and that latent infection is established within these tissues ((Chun et al., 2008; Gras and Kaul, 2010). In support of this latter model, recent studies demonstrated that intensification of a suppressive ART regimen by the addition of raltegravir (an HIV integration inhibitor) results in a temporary increase in episomal DNA in some HIV-infected patients consistent with active viral replication despite ART (Buzon et al., 2010). Conversely, therapy intensification does not appear to affect reservoir size (Dinoso et al., 2009; Gandhi et al., 2010). Furthermore, longitudinal clonal genotypic analyses demonstrate that ART-suppressed patients do not develop drug resistance (Kieffer et al., 2004), suggesting that the latent reservoir is not evolving during treatment. Importantly, it has also been shown that there is a lack of HIV-1 evolution in the GALT in patients that initiated ART during primary infection (Evering et al., 2012). It should be noted that blips in viral load do not represent replication of drug-resistant virus, but instead simply reflect biological and statistical variations in levels of the prevailing virus (Nettles et al., 2005). Even in patients that respond well to ART (e.g. patients exhibiting undetectable plasma viral loads below 40-50 copies of HIV-1 RNA in standard assays), a low level of free virus can be detected by sensitive assays (Dornadula et al., 1999). When considered together, the balance of evidence favors the conclusion that residual viremia most likely reflects the intermittent production of virus from stable long-lived reservoirs rather than ongoing, low-level productive infection. However, this remains a hotly debated issue.
Mechanisms of Post-integration Latency
A better understanding of the mechanisms of post-integration latency will be necessary to uncover novel targets and methods for attacking and eradicating the latent reservoir. In particular, it will be important to find new ways to reactivate the latent provirus within each and every cell in the reservoir where it resides. It is also essential that the cellular host be induced to die either as a result of viral reactivation or a subsequent immune response against the virus. In the next sections, we discuss the various mechanisms that likely underlie HIV latency. However, it is important to point out that latency is likely a multifactorial process. This fact could complicate development of an effective anti-latency therapy.
Site and Orientation of Integration
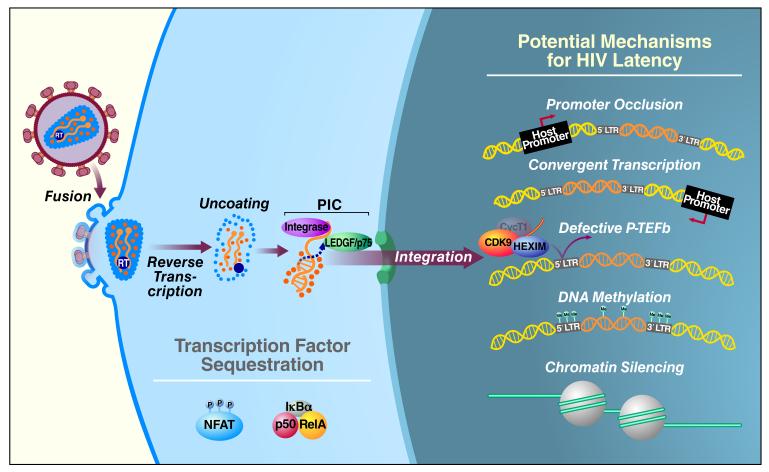
In most instances, HIV-1 proviral cDNA integrates into regions of the host genome that are actively transcribed (Schroder et al., 2002). Following integration, the provirus is replicated and transcribed along with the cellular DNA that surrounds it. Some of these viral RNAs are then translated into viral proteins, while two copies of the full-length viral RNA are incorporated as the genetic material for new infectious virions. Along with viral RNA, a number of viral proteins are incorporated into these virions including the Gag, Pol and Env structural proteins. Additionally, the viral integrase protein, which associates with viral DNA and the preintegration complex (PIC) is incorporated as is Vpr. Various host proteins are included as well. For example, the cellular LEDGF/p75 is incorporated due to its tightly binding to integrase (Cherepanov et al., 2003). LEDGF/p75 is believed to guide the protein complex and viral DNA to intronic regions of actively transcribed genes (Lewinski et al., 2006). Most latent proviruses from virally suppressed patients on ART reside in host genes that are actively transcribed (Han et al., 2004). Although it may seem contradictory that latent provirus exists in actively transcribed regions, this phenomenon points to the potential involvement of transcriptional interference as a mechanism for suppressing expression of the integrated provirus.
One mechanism of transcriptional interference involves promoter occlusion, where the provirus integrates downstream of the host gene in the same transcriptional orientation or polarity (Figure 2). This may result in ―read-though‖ by the host RNA Pol II, which displaces constitutively expressed transcription factors like Sp1 that bind to the HIV-1 LTR and are essential for viral gene expression (Greger et al., 1998). Furthermore, it has been shown in a Jurkat CD4+ T-cell model (J-Lat cells) that transcriptional interference can be reversed by inhibiting transcription of the upstream gene or by cooperatively activating viral transcription initiation and elongation through viral Tat or TNFα-mediated NF-κB activation (Lenasi et al., 2008). Specifically, NF-κB can bind the HIV LTR with exceptionally high affinity and block the upstream elongating RNA Pol II, thus overcoming transcriptional interference leading to HIV reactivation. Another mechanism of transcriptional interference, referred to as convergent transcription, occurs when the provirus integrates in the opposite orientation or polarity relative to the host gene (Figure 2). This leads to collision of the RNA Pol II complexes from the host and viral promoters and early arrest of transcription from both promoters or the weaker of the two (Lewinski et al., 2005). It is also possible that convergent transcription results in double-stranded RNA when both strands of viral DNA are elongated. This may lead to silencing of viral transcription or translation through RNA interference (Hu et al., 2004), RNA-directed DNA methylation (Morris et al., 2004), or the generation of antisense RNA (Scherer and Rossi, 2003).
Figure 2. Major Mechanisms of HIV-1 Latency in CD4+ T cells.
Sequestration of essential transcription factors, like NFAT and NF-κB, in the cytoplasm leads to silencing of viral gene expression. Two major mechanisms of transcriptional interference occur in latently infected cells. In promoter occlusion, the host promoter is positioned upstream of the provirus and the host RNA Pol II reads through the HIV-1 LTR, causing displacement of necessary transcription factors. In convergent transcription, provirus and the host gene are in the opposite orientation, leading to collision of the RNA Pol II complexes. Levels of CyclinT1, which forms P-TEFb and is important for HIV-1 transcription and Tat transactivation, are low in resting CD4+ T cells. DNA methylation and restrictive chromatin structures contribute to transcriptional silencing leading to HIV-1 latency.
Initiation of Transcription
The 5′LTR of HIV contains multiple sites for the binding of cellular transcription factors, including NF-κB, NFAT, Sp1, and AP1 (Figure 3). These key cellular factors may be activated by external stimuli in order to enhance HIV-1 transcription. In resting cells, NF-κB and NFAT are sequestered in the cytoplasm (and are unable to promote HIV-1 transcription in the nucleus), but undergo nuclear translocation following appropriate cellular activation. Both NFAT and NF-κB are able to bind to κB sites in the HIV-1 LTR (Kinoshita et al., 1998). However, it is likely that NF-κB plays a larger role in HIV transcription (Kim et al., 2011). It has been shown that binding of NF-κB is not sufficient to induce HIV gene expression, while Sp1 is necessary (Perkins et al., 1993).
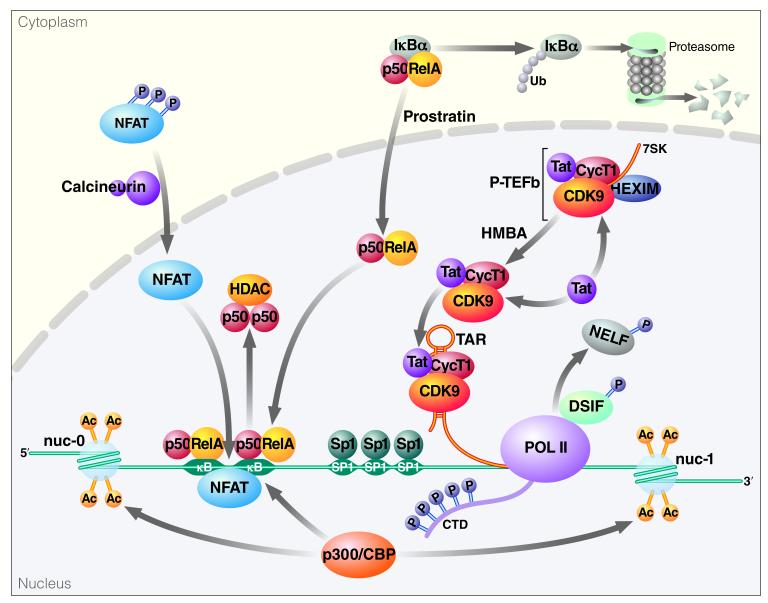
Figure 3. Initiation of Transcription and Translational Elongation at the HIV-1 LTR.
Following cellular activation or drug treatment NFAT and NF-κB translocate to the nucleus and bind sites at the HIV-1 LTR. NFAT and NF-κB recruit p300/CBP to the LTR, resulting in acetylation of histone tails and transcriptional activation. In the case of NF-κB, proteosomal degradation of IκBα permits NF-κB translocation and displacement of the p50 homodimers. This is followed by Tat-dependent elongation, in which Tat recruits the P-TEFb complex to TAR. Cdk9 phosphorylates the CTD of RNA Pol II, resulting in increased processivity. P-TEFb phosphorylates DSIF and NELF, resulting in removal of NELF from Pol II, converting DSIF into a positive elongation factor, thereby promoting productive elongation.
In the cytoplasm of resting cells, NF-κB exists as a p50/RelA heterodimer bound in an inactive form through interactions with Iκ Bβ. In the nucleus of HIV-infected resting cells, NF-κB p50/p50 homodimers are bound to the 5′LTR. The p50/p50 homodimer lacks the essential transactivation domain present in the p50/RelA heterodimer (the prototypical NF-κB complex). In addition, p50 homodimers can actively inhibit transcription by recruiting HDAC-1 to the LTR and promoting latency through histone deacetylation and chromatin condensation (Williams et al., 2006). The NF-κB heterodimer is activated by IKK-mediated phosphorylation of serine-32 and -36 on Iκ Bα followed by βTrCP-mediated ubiquitylation and degradation of IκBα by the 26S proteasome. These events enable NF-κB translocation to the nucleus and displacement of p50/p50 NF-κB homodimers. The p50/RelA heterodimers recruit histone acetyltranserases (HATs), like p300/CBP, resulting in acetylation of histone tails and transcriptional activation (Zhong et al., 2002). To initiate transcription, RelA interacts with the Cdk7 kinase subunit of TFIIH to stimulate phosphorylation of serine-5 within the CTD of RNA Pol II (Kim et al., 2006). Similarly, interaction of RelA with P-TEFb results in phosphorylation of the RNA Pol II CTD at serine-2 and subsequent elongation (Barboric et al., 2001). P300 also mediates acetylation of RelA at lysines 218, 221 and 310. Acetylation at lysine-310 enhances the transcriptional activity of RelA while acetylation at lysine-218 and −221 enhance DNA binding and make RelA resistant to IκBα binding.
Following Ca2+ release via the PKC pathway, calcineurin dephosphorylates and induces nuclear localization of the cytoplasmic components of NFAT. NFAT binds to the 5′LTR at two sets of NFAT binding sites in addition to the κB sites. Furthermore, it has been shown that CsA, which is a potent inhibitor of NFAT, is capable of inhibiting HIV LTR promoter activity in primary cells (Cron et al., 2000). Like NF-κB, NFAT can also bind p300/CBP and recruit HATs from nuclear extracts of cell lines (Garcia-Rodriguez and Rao, 1998).
Our understanding of the key signaling pathways that promote transcription in active versus latent cells has helped to identify points of intervention that could be used to overcome latency and purge the latent reservoir. The non-tumor-promoting phorbol ester, prostratin, promotes HIV transcription by PKC-mediated activation of NF-κB, which thereby induces RelA to bind the latent HIV-1 promoter (Williams et al., 2004). Activation of the PKC pathway by prostratin also results in down-regulation of the CD4 receptor and the CXCR4 and CCR5 coreceptors on the host cell, decreasing the likelihood of new infections (Hezareh et al., 2004). Although NF-κB-inducing agents are strong activators of HIV-1 transcription, they also cause global cellular activation and may induce a toxic inflammatory response in patients in the form of a cytokine storm.
In addition to PKC activation, there have been attempts to purge the latent reservoir through global cellular activation using cytokines. For example, an array of cytokines, including IL-6, TNFα, and IL-2, are capable of being potent inducers of viral replication in the resting CD4+ T cells of ART-treated patients (Chun et al., 1998b). In clinical trials of ART-suppressed patients, researchers observed no difference in the amount of proviral DNA and determined that IL-2 had little effect on viral latency (Dybul et al., 2002; Stellbrink et al., 2002). In another study, HIV-infected patients were co-treated with ART and a combination of IL-2 and IFN-γ. At the end of the trial patients rebounded with high plasma HIV-1 RNA levels when ART was stopped (Lafeuillade et al., 2001). In patients with near-complete suppression of plasma viremia (below 5 copies/mL), treatment with anti-CD3 antibodies and IL-2 triggered activation and proliferation of T cells along with stimulation of HIV replication (up to 1500 copies/mL). However, the anti-CD3/IL-2 therapy was toxic, and resulted in long-lasting depletion of CD4+ T cells in the peripheral blood and lymph nodes (van Praag et al., 2001). IL-7 is another cytokine that is capable of activating viral gene expression. For example, in latently infected cells from SCID-hu (Thy/Liv) mice, ex vivo treatment with IL-7 was sufficient to reactivate latent virus while causing minimal cell cycle progression (Brooks et al., 2003). However, Chomont et al. showed that IL-7 mediates homeostatic proliferation and subsequent viral persistence in patients with low CD4+ T cell levels through effects on transitional memory T cells (Chomont et al., 2009). Although IL-7, and possibly other cytokines, can influence the stability of the HIV reservoir, these findings suggest that global immune activation strategies may do more harm than good, and that limiting immune activation in combination with agents like IL-7 might be more effective.
Transcriptional Elongation
The HIV transactivator Tat plays a major role in the elongation phase of transcription (Figure 3). During the earlier (Tat-independent) initiation phase, cellular transcription factors (like NF-κB) are recruited to the HIV LTR promoter region. This is followed by the Tat-dependent elongation phase, which promotes much higher levels of HIV-1 transcription compared to the initiation phase. When Tat is present, 99% of the transcripts are transcribed to their full length. However, in the absence of Tat, 87% of the initiated transcripts terminate prematurely at positions +55 to +59 (Kao et al., 1987). This block is due to the activity of negative transcription elongation factor (N-TEF), which is composed of the negative elongation factor (NELF) and DRB-sensitive inducing factor (DSIF). These factors cooperatively restrict the transcription of other cellular genes (Yamaguchi et al., 2013). Although these short transcripts predominate in the absence of Tat, a small amount of full-length transcript is produced, including Tat, thereafter promoting full-length HIV transcription by positive feedback. To overcome the transcription block caused by N-TEF, Tat binds to the transactivation-responsive element (TAR), a short nucleotide stem-loop located at the 5′end of all HIV transcripts. Following TAR binding, Tat recruits the P-TEFb complex, composed of CyclinT1 and Cdk9 (Wei et al., 1998). As part of the P-TEFb complex, Cdk9 phosphorylates the CTD of RNA Pol II, primarily at serine-2, promoting enhanced processivity of RNA Pol II (Kim et al., 2002). In addition, P-TEFb mediates phosphorylation of DSIF and NELF, which results in removal of NELF from the LTR and allows productive elongation to begin (Fujinaga et al., 2004; Ivanov et al., 2000).
Transcriptional activation also depends on the acetylation state of Tat. Tat can be acetylated at lysines 50 and 51 by CBP/p300 and GCN5, respectively, within the TAR-binding domain. This leads to the dissociation of Tat from TAR, thus promoting the switch between the early and late phases in HIV transcriptional elongation (Ott et al., 2004). Also, the bromodomain of p300/CBP-associated factor (PCAF) can bind to acetylated Tat at lysine-50, thereby competing with the Tat/TAR interaction and promoting the dissociation of Tat from TAR (Mujtaba et al., 2002). Disruption of the Tat/CyclinT1/TAR complex via p300-mediated Tat acetylation facilitates the transfer of Tat to the elongating RNA Pol II complex (Kaehlcke et al., 2003). Furthermore, both in vitro and in vivo, Tat is deacetylated by human sirtuin 1 (SIRT1), a NAD+-dependent class-II protein deacetylase. SIRT1-mediated deacetylation allows Tat to return to its unacetylated form, thereby enabling the initiation of a new transcription cycle (Pagans et al., 2005). In addition, experimental and computational studies performed by Weinberger et al. (2005) demonstrate how stochastic fluctuations (noise) in the activity of the LTR governed by Tat can influence the viral latency decision (Weinberger et al., 2005). This provides further evidence for the importance of Tat in regulating HIV-1 latency.
Levels of active P-TEFb are key in promoting the switch to productive elongation, and therefore play a large role in controlling the expression levels of viral genes. P-TEFb is inactive when its components CyclinT1 and Cdk9 are bound to the 7SK small nuclear ribonucleoprotein complex containing the inhibitory molecule HEXIM1(Yik et al., 2003). The release of P-TEFb from HEXIM1 is mediated through stress-inducing agents, including the small molecule hexamethylene biacetamide (HMBA) (Contreras et al., 2007). Choudhary et al. showed that HMBA-treated resting memory CD4+ T cells from ART-suppressed patients were reactivated through phosphorylation of the CTD of RNA Pol II in a Cdk9-dependent manner. HMBA increases the nuclear expression level of Cdk9, which is then recruited to the LTR by Sp1 (Choudhary et al., 2008).
There has been great interest in a novel group of transcriptional regulators shown to control viral gene expression. Recent studies demonstrated the effectiveness of JQ1 and other bromodomain (BET family) inhibitors in inducing latent reactivation in cell lines and primary cell models of HIV latency (Banerjee et al., 2012; Bartholomeeusen et al., 2012; Li et al., 2013) Specifically, it was shown that bromodomain-containing protein 4 (Brd4) could compete with HEXIM1 for P-TEFb binding. Therefore, inhibiting the binding of Brd4 to P-TEFb could promote increased binding of Tat to P-TEFb. In contrast, Boehm et al. recently showed that BET inhibitors, like JQ1, are dependent on P-TEFb, but independent from Tat. In addition, Brd2 was identified as a new Tat-independent suppressor of HIV transcription (Boehm et al., 2013). This mechanism of action has not yet been fully worked out. Interestingly, it is also known that HMBA increases the level of active P-TEFb-Brd4 complexes (He et al., 2006). The BET family therefore represents a promising new target for therapies aimed at overcoming HIV-1 latency.
Chromatin Modification
The expression of genes, including those of integrated HIV-1, is dependent on chromatin structure, which can be altered by epigenetic modifications. The main structural units of chromatin are nucleosomes, which consist of pairs of four core histones (H2A, H2B, H3, and H4) that form an octamer and become enwrapped by 1.7 turns of DNA (147 base pairs). When formed around promoters, these structures can epigenetically determine whether the associated genes are expressed or not. For example, a genome in a compact structure bound tightly by nucleosomes is transcriptionally repressed due to blockade of the promoter region from the transcriptional machinery; DNA in this state is called heterochromatin. In contrast, euchromatin refers to the relaxed and transiently open state of chromatin, which encourages transcription. It is known that changes in chromatin condensation status can be mediated through chromatin remodeling complexes that use ATP to disrupt nucleosome-DNA contacts, move nucleosomes along DNA, and remove or exchange nucleosomes. However, changes in chromatin structure are also brought about by various posttranslational modifications, including acetylation and methylation.
Histone acetyl transferases (HATs) catalyze the addition of acetyl groups to target histones, whereas histone deacetylases (HDACs) remove these acetyl groups. These opposing functions of HATs and HDACs allow for switching between different acetylation states, and determine whether genes are transcriptionally activated or repressed, respectively. In terms of HIV-1 therapy, there has been great interest in using HDAC inhibitors to block HDAC deacetylase activity, which would create a less repressive chromatin state and allow for HIV transcription. Early experiments showed that, independent of the viral integration site, the 5′LTR of HIV contains two nucleosomes, nuc-0 and nuc-1 (Verdin et al., 1993). Nuc-1 is rapidly and specifically disrupted following treatment with HDAC inhibitors, thus allowing for increased HIV-1 transcription via chromatin modification (Van Lint et al., 1996). More recent studies revealed that multiple DNA-binding complexes could be responsible for the recruitment of HDACs to initiator and enhancer regions of the HIV-1 promoter, thereby inducing repressive effects on chromatin structure. HDAC1 is recruited by a number of transcription factors to the 5′LTR, including (but not limited to) Late SV40 Factor (LSF) (He and Margolis, 2002), Activating Protein-4 (AP-4) (He and Margolis, 2002), NF-κB p50/p50 homodimers (Williams, Chen et al. 2006), C-promoter Binding Factor-1 (CBF-1) (Tyagi and Karn, 2007), and Sp1 (Jiang et al., 2007). Although four different classes of HDACs exist, class-I HDACs (HDAC1, 2, and 3) dominate at the HIV LTR in CD4+ cell line models (Keedy et al., 2009).
As previously noted, increasing HIV-1 transcription represents a possible strategy for eradicating latent provirus from HIV-1 infected patients. Initially, HDAC inhibitors held promise as good candidates for viral purging because they failed to promote global T cell activation yet could activate the virus. These cells could also act on a broad range of cellular types, not just T cells. However, a prominent concern is their ability to affect multiple biological processes, as reflected by cancer studies showing that HDAC inhibitors affect transcription of about 10% of cellular genes (Glaser et al., 2003).
Initial studies in patients using valproic acid (VPA), an HDAC1 inhibitor, were encouraging. When given to patients on intensified ART, VPA was reported to accelerate the reduction of HIV-infected resting T cells (Lehrman et al., 2005). Unfortunately, later reports did not demonstrate the same decay of infected resting T cells following VPA treatment (Sagot-Lerolle et al., 2008; Siliciano et al., 2007). Another more potent class-I HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA), can induce HIV-1 transcription in resting CD4+ T cells from patients on ART (Archin et al., 2009). In other studies, treatment with either VPA or SAHA results in synergistic activation of latent HIV in patient PBMCs when combined with the PKC agonist, prostratin (Reuse et al., 2009). In addition to prostratin, another PKC agonist, bryostatin, also had synergistic effects when used in combination with HDAC inhibitors (Perez et al., 2010). Combinatorial strategies, in which multiple pathways are targeted, are likely to be a more effective strategy for clearing the latent reservoir to levels that are necessary for achieving a cure.
DNA Methylation
Transcriptional regulation by DNA methylation is catalyzed by DNA methyltransferases and occurs largely at CpG dinucleotides. DNA methylation is associated with gene silencing and is present at high levels within heterochromatic regions of mammalian DNA. CpG methylation occurs at the 5′LTR of HIV-1, and likely acts by preventing the binding of essential transcription factors such as NF-κB and Sp1 (Bednarik et al., 1991). In a study by Kauder et al., it was demonstrated that during latency the HIV-1 promoter is hypermethylated at two CpG islands surrounding the transcriptional start site in both J-Lat cell lines and in primary CD4+ T cells. One of the two methylated CpG islands is occupied by methyl-CpG binding protein 2 (MBD2) and HDAC2, thereby promoting a transcriptionally repressive state. Inhibition of DNA methylation with 5-aza-2′deoxycytidine (aza-CdR) prevents the recruitment of MBD2 and HDAC2 and synergizes with NF-κB activators to promote a dramatic increase in viral gene expression (Kauder et al., 2009). Furthermore, in another study by Blazkova et al., CpG methylation of the HIV-1 5′LTR was shown to prevent reactivation in an in vitro model of latency and in memory CD4+ T cells from HIV-infected patients. This methylation pattern was responsible for the maintenance but not the establishment of HIV-1 latency (Blazkova et al., 2009). In addition, the combination of 5-aza-CdR and prostratin had a synergistic effect on HIV-1 reactivation. In contrast, recent studies have demonstrated that only 2.4% of HIV 5′LTRs contained methylated CpG dinucleotides in infected individuals receiving ART (Blazkova et al., 2012). Therefore, it is likely that DNA methylation at the HIV 5′LTR plays a less prominent role in maintenance of the latent reservoir than other mechanisms described in this review.
Examples of HIV Cures
Thirty years ago, the term “HIV cure” was equated to complete eradication of virus from an HIV-infected patient. Now, there is mounting evidence supporting the concept of a “functional cure” represented by the persistence of a low and stable viral load in the absence of ART. Timothy Ray Brown, also known as “the Berlin patient”, appears to represent an HIV cure involving complete eradication of the virus since recent detection of low level residual virus may be artifactual (Yukl et al., 2013). Brown was an HIV-positive patient who developed acute myeloid leukemia, and was treated for his cancer by stem cell transplantation from an individual homozygous for the CCR5 gene variant Δ32 (Allers et al., 2011). Individuals with this mutation express a nonfunctional truncated variant of the CCR5 coreceptor, and are therefore resistant to infection with CCR5-tropic viral strains. This treatment resulted in eradication of virus from his body without the need for any further ART following transplant.
Functional cures have also been described likely reflecting an enhanced immune response against the virus. For example, recent studies have identified 14 HIV patients who maintained long-lasting control of viremia for several years following the interruption of ART (Saez-Cirion et al., 2013). These 14 patients were distinct from elite controllers in that they lacked protective HLA alleles and did not develop strong HIV-specific T-cell responses. Of note, these patients initiated ART during the very early primary stage of HIV infection. In contrast, most patients begin treatment regimens during the later chronic stage of HIV infection. Follow-up studies showed that following the interruption of therapy in these individuals, long-lived resting CD4+ T cells represented only a minor population within the total viral reservoir compared to patients who initiated therapy later. These individuals provide additional evidence that a functional cure is possible, and that treatment during the early stage of infection may significantly decrease the formation of viral reservoirs in longer-lived memory cells.
Further support for the concept of very early treatment may be found in a recent report of a baby girl from Mississippi infected with HIV either in utero or during birth. She received three antiretroviral drugs within 31 hours of birth and then continued therapy for the first 15 months of her life, until her mother stopped administering drugs for several months. Surprisingly, since terminating the treatments, the child has not exhibited a viral rebound nor any detectable virus in her blood. This case again raises the possibility that early and aggressive treatment might lead to a cure, although whether this situation represents full eradication or a functional cure remains unclear. Although this case is less relevant in developed countries where expectant mothers with HIV routinely receive treatment to prevent transmission, it is highly relevant for developing regions like Sub-Saharan Africa where it is more common for untreated HIV-positive mothers to give birth. In sum, these studies suggest that a “cure” may not require a complete viral purge, but instead may be achieved functionally by minimizing the size of the latent reservoir via early and aggressive antiretroviral treatment. In addition, it is possible that a functional cure can be attained through pharmacological agents that cause the reactivation of a significant proportion of the latent proviruses. Since it may not be necessary to reactivate every cell in the viral reservoir, it is possible that we are in fact closer to a functional cure than previously thought.
Concluding Remarks
A key goal in the field of HIV research is to identify a safe, effective, and scalable cure for the roughly 34 million people currently infected with HIV around the world. The ability to cure infected individuals would yield both a cost-effective prevention strategy for curbing the spread of HIV and a solution to the unsustainable global cost of treatment by eliminating the need for lifelong antiviral therapy. Currently, we are only reaching approximately one third of those infected who need treatment based on new WHO criteria. Further, for every 10 individuals placed on treatment 16 more are newly infected. Clearly, we do not yet have a winning strategy, particularly in sub-Saharan Africa where the virus is hitting the hardest. The development of a cure for HIV infection would be game changing. Our progress toward a cure will be enhanced by a better understanding of the molecular underpinnings of HIV latency. We also need a more systematic effort to identify viral activating agents that can be combined to form a highly effective cocktail for purging virus from the latent reservoir. Similarly, more work is required to identify the full range of cells that comprise the latent reservoir and the nature of the immune factors that drive its maintenance. We also need to prepare for the possibility that rousing virus from its transcriptional slumber in latently infected cells will not lead to clearance of the cellular host. If this is the case, additional technologies will be needed to kill these cells thereby depleting the reservoir. Complete viral eradication is a desirable but lofty goal. Alternatively, it may be possible to enhance the intrinsic immune response against the virus in a manner that allows antiviral drug discontinuation even though the virus remains—a functional cure. Such a functional cure may in fact be easier to achieve than complete viral eradication although the latter remains the ultimate goal. Certainly the recent individual cases where at least a functional cure has been achieved provides hope for the field. However, it will be important to ensure that new anti-latency therapies are not only deployable in developed countries, but instead can be used throughout the world.
References
- Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS research and human retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, Sebastiani P, Margolis DM, Montano M. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. Journal of leukocyte biology. 2012;92:1147–1154. doi: 10.1189/jlb.0312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboric M, Nissen RM, Kanazawa S, Jabrane-Ferrat N, Peterlin BM. NF-kappaB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Molecular cell. 2001;8:327–337. doi: 10.1016/s1097-2765(01)00314-8. [DOI] [PubMed] [Google Scholar]
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. The Journal of biological chemistry. 2012;287:36609–36616. doi: 10.1074/jbc.M112.410746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik DP, Duckett C, Kim SU, Perez VL, Griffis K, Guenthner PC, Folks TM. DNA CpG methylation inhibits binding of NF-kappa B proteins to the HIV-1 long terminal repeat cognate DNA motifs. The New biologist. 1991;3:969–976. [PubMed] [Google Scholar]
- Blazkova J, Murray D, Justement JS, Funk EK, Nelson AK, Moir S, Chun TW, Fauci AS. Paucity of HIV DNA methylation in latently infected, resting CD4+ T cells from infected individuals receiving antiretroviral therapy. Journal of virology. 2012;86:5390–5392. doi: 10.1128/JVI.00040-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, Guiguen A, Verdin E, Olive D, Van Lint C, Hejnar J, et al. CpG methylation controls reactivation of HIV from latency. PLoS pathogens. 2009;5:e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D, Calvanese V, Dar RD, Xing S, Schroeder S, Martins L, Aull K, Li PC, Planelles V, Bradner JE, et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle. 2013;12:452–462. doi: 10.4161/cc.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Hamer DH, Arlen PA, Gao L, Bristol G, Kitchen CM, Berger EA, Zack JA. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19:413–423. doi: 10.1016/s1074-7613(03)00236-x. [DOI] [PubMed] [Google Scholar]
- Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nature medicine. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. The Journal of biological chemistry. 2003;278:372–381. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature medicine. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary SK, Archin NM, Margolis DM. Hexamethylbisacetamide and disruption of human immunodeficiency virus type 1 latency in CD4(+) T cells. The Journal of infectious diseases. 2008;197:1162–1170. doi: 10.1086/529525. [DOI] [PubMed] [Google Scholar]
- Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 1998a;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. The Journal of experimental medicine. 1998b;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nature medicine. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. The Journal of infectious diseases. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Barboric M, Lenasi T, Peterlin BM. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS pathogens. 2007;3:1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cron RQ, Bartz SR, Clausell A, Bort SJ, Klebanoff SJ, Lewis DB. NFAT1 enhances HIV-1 gene expression in primary human CD4 T cells. Clin Immunol. 2000;94:179–191. doi: 10.1006/clim.1999.4831. [DOI] [PubMed] [Google Scholar]
- Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, O’Shea A, Callender M, Spivak A, Brennan T, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr., Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA: the journal of the American Medical Association. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- Dybul M, Hidalgo B, Chun TW, Belson M, Migueles SA, Justement JS, Herpin B, Perry C, Hallahan CW, Davey RT, et al. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)-specific immune responses in patients treated during recently acquired HIV infection. The Journal of infectious diseases. 2002;185:61–68. doi: 10.1086/338123. [DOI] [PubMed] [Google Scholar]
- Evering TH, Mehandru S, Racz P, Tenner-Racz K, Poles MA, Figueroa A, Mohri H, Markowitz M. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS pathogens. 2012;8:e1002506. doi: 10.1371/journal.ppat.1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Friedman-Kien AE. Disseminated Kaposi’s sarcoma syndrome in young homosexual men. Journal of the American Academy of Dermatology. 1981;5:468–471. doi: 10.1016/s0190-9622(81)80010-2. [DOI] [PubMed] [Google Scholar]
- Fujinaga K, Irwin D, Huang Y, Taube R, Kurosu T, Peterlin BM. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Molecular and cellular biology. 2004;24:787–795. doi: 10.1128/MCB.24.2.787-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, Kallungal B, Palmer S, Medvik K, Lederman MM, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS medicine. 2010;7 doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodriguez C, Rao A. Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP) The Journal of experimental medicine. 1998;187:2031–2036. doi: 10.1084/jem.187.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Molecular cancer therapeutics. 2003;2:151–163. [PubMed] [Google Scholar]
- Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. The New England journal of medicine. 1981;305:1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Demarchi F, Giacca M, Proudfoot NJ. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic acids research. 1998;26:1294–1301. doi: 10.1093/nar/26.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Lassen K, Monie D, Sedaghat AR, Shimoji S, Liu X, Pierson TC, Margolick JB, Siliciano RF, Siliciano JD. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. Journal of virology. 2004;78:6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Margolis DM. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Molecular and cellular biology. 2002;22:2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Pezda AC, Zhou Q. Modulation of a P-TEFb functional equilibrium for the global control of cell growth and differentiation. Molecular and cellular biology. 2006;26:7068–7076. doi: 10.1128/MCB.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezareh M, Moukil MA, Szanto I, Pondarzewski M, Mouche S, Cherix N, Brown SJ, Carpentier JL, Foti M. Mechanisms of HIV receptor and co-receptor down-regulation by prostratin: role of conventional and novel PKC isoforms. Antiviral chemistry & chemotherapy. 2004;15:207–222. doi: 10.1177/095632020401500404. [DOI] [PubMed] [Google Scholar]
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Ho DD, Rota TR, Hirsch MS. Infection of monocyte/macrophages by human T lymphotropic virus type III. The Journal of clinical investigation. 1986;77:1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WY, Bushman FD, Siva AC. RNA interference against retroviruses. Virus research. 2004;102:59–64. doi: 10.1016/j.virusres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Ivanov D, Kwak YT, Guo J, Gaynor RB. Domains in the SPT5 protein that modulate its transcriptional regulatory properties. Molecular and cellular biology. 2000;20:2970–2983. doi: 10.1128/mcb.20.9.2970-2983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Espeseth A, Hazuda DJ, Margolis DM. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. Journal of virology. 2007;81:10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehlcke K, Dorr A, Hetzer-Egger C, Kiermer V, Henklein P, Schnoelzer M, Loret E, Cole PA, Verdin E, Ott M. Acetylation of Tat defines a cyclinT1-independent step in HIV transactivation. Molecular cell. 2003;12:167–176. doi: 10.1016/s1097-2765(03)00245-4. [DOI] [PubMed] [Google Scholar]
- Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS pathogens. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedy KS, Archin NM, Gates AT, Espeseth A, Hazuda DJ, Margolis DM. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. Journal of virology. 2009;83:4749–4756. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TL, Finucane MM, Nettles RE, Quinn TC, Broman KW, Ray SC, Persaud D, Siliciano RF. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. The Journal of infectious diseases. 2004;189:1452–1465. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- Kim YK, Bourgeois CF, Isel C, Churcher MJ, Karn J. Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation. Molecular and cellular biology. 2002;22:4622–4637. doi: 10.1128/MCB.22.13.4622-4637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Bourgeois CF, Pearson R, Tyagi M, West MJ, Wong J, Wu SY, Chiang CM, Karn J. Recruitment of TFIIH to the HIV LTR is a rate-limiting step in the emergence of HIV from latency. The EMBO journal. 2006;25:3596–3604. doi: 10.1038/sj.emboj.7601248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Mbonye U, Hokello J, Karn J. T-cell receptor signaling enhances transcriptional elongation from latent HIV proviruses by activating P-TEFb through an ERK-dependent pathway. Journal of molecular biology. 2011;410:896–916. doi: 10.1016/j.jmb.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Chen BK, Kaneshima H, Nolan GP. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Chouraqui M, Pisapia M, Delbeke E. Pilot study of a combination of highly active antiretroviral therapy and cytokines to induce HIV-1 remission. J Acquir Immune Defic Syndr. 2001;26:44–55. doi: 10.1097/00126334-200101010-00006. [DOI] [PubMed] [Google Scholar]
- Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenasi T, Contreras X, Peterlin BM. Transcriptional interference antagonizes proviral gene expression to promote HIV latency. Cell host & microbe. 2008;4:123–133. doi: 10.1016/j.chom.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski MK, Bisgrove D, Shinn P, Chen H, Hoffmann C, Hannenhalli S, Verdin E, Berry CC, Ecker JR, Bushman FD. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. Journal of virology. 2005;79:6610–6619. doi: 10.1128/JVI.79.11.6610-6619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski MK, Yamashita M, Emerman M, Ciuffi A, Marshall H, Crawford G, Collins F, Shinn P, Leipzig J, Hannenhalli S, et al. Retroviral DNA integration: viral and cellular determinants of target-site selection. PLoS pathogens. 2006;2:e60. doi: 10.1371/journal.ppat.0020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Guo J, Wu Y, Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic acids research. 2013;41:277–287. doi: 10.1093/nar/gks976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- Mujtaba S, He Y, Zeng L, Farooq A, Carlson JE, Ott M, Verdin E, Zhou MM. Structural basis of lysine-acetylated HIV-1 Tat recognition by PCAF bromodomain. Molecular cell. 2002;9:575–586. doi: 10.1016/s1097-2765(02)00483-5. [DOI] [PubMed] [Google Scholar]
- Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, Cofrancesco J, Jr., Gallant JE, Quinn TC, Jackson B, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA: the journal of the American Medical Association. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- Otero M, Nunnari G, Leto D, Sullivan J, Wang FX, Frank I, Xu Y, Patel C, Dornadula G, Kulkosky J, et al. Peripheral blood Dendritic cells are not a major reservoir for HIV type 1 in infected individuals on virally suppressive HAART. AIDS research and human retroviruses. 2003;19:1097–1103. doi: 10.1089/088922203771881194. [DOI] [PubMed] [Google Scholar]
- Ott M, Dorr A, Hetzer-Egger C, Kaehlcke K, Schnolzer M, Henklein P, Cole P, Zhou MM, Verdin E. Tat acetylation: a regulatory switch between early and late phases in HIV transcription elongation. Novartis Foundation symposium. 2004;259:182–193. discussion 193-186, 223-185. [PubMed] [Google Scholar]
- Pagans S, Pedal A, North BJ, Kaehlcke K, Marshall BL, Dorr A, Hetzer-Egger C, Henklein P, Frye R, McBurney MW, et al. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS biology. 2005;3:e41. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- Perez M, de Vinuesa AG, Sanchez-Duffhues G, Marquez N, Bellido ML, Munoz-Fernandez MA, Moreno S, Castor TP, Calzado MA, Munoz E. Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Current HIV research. 2010;8:418–429. doi: 10.2174/157016210793499312. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Edwards NL, Duckett CS, Agranoff AB, Schmid RM, Nabel GJ. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. The EMBO journal. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov S, Chenine AL, Gruber A, Li PL, Ruprecht RM. Long-term productive human immunodeficiency virus infection of CD1a-sorted myeloid dendritic cells. Journal of virology. 2005;79:602–608. doi: 10.1128/JVI.79.1.602-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Reuse S, Calao M, Kabeya K, Guiguen A, Gatot JS, Quivy V, Vanhulle C, Lamine A, Vaira D, Demonte D, et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PloS one. 2009;4:e6093. doi: 10.1371/journal.pone.0006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS pathogens. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot-Lerolle N, Lamine A, Chaix ML, Boufassa F, Aboulker JP, Costagliola D, Goujard C, Pallier C, Delfraissy JF, Lambotte O. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008;22:1125–1129. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- Sarngadharan MG, Popovic M, Bruch L, Schupbach J, Gallo RC. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984;224:506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Scherer LJ, Rossi JJ. Approaches for the sequence-specific knockdown of mRNA. Nature biotechnology. 2003;21:1457–1465. doi: 10.1038/nbt915. [DOI] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Schupbach J, Popovic M, Gilden RV, Gonda MA, Sarngadharan MG, Gallo RC. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science. 1984;224:503–505. doi: 10.1126/science.6200937. [DOI] [PubMed] [Google Scholar]
- Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nature reviews Immunology. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Lopez C, Hammer GS, Brown AE, Kornfeld SJ, Gold J, Hassett J, Hirschman SZ, Cunningham-Rundles C, Adelsberg BR, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. The New England journal of medicine. 1981;305:1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Lai J, Callender M, Pitt E, Zhang H, Margolick JB, Gallant JE, Cofrancesco J, Jr., Moore RD, Gange SJ, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. The Journal of infectious diseases. 2007;195:833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, Kerkering TM, Ferreira-Gonzalez A, Szakal AK, Tew JG, et al. Persistence of infectious HIV on follicular dendritic cells. J Immunol. 2001;166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- Stellbrink HJ, van Lunzen J, Westby M, O’Sullivan E, Schneider C, Adam A, Weitner L, Kuhlmann B, Hoffmann C, Fenske S, et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial) AIDS. 2002;16:1479–1487. doi: 10.1097/00002030-200207260-00004. [DOI] [PubMed] [Google Scholar]
- Tyagi M, Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. The EMBO journal. 2007;26:4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. The EMBO journal. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- van Praag RM, Prins JM, Roos MT, Schellekens PT, Ten Berge IJ, Yong SL, Schuitemaker H, Eerenberg AJ, Jurriaans S, de Wolf F, et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. Journal of clinical immunology. 2001;21:218–226. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- Verdin E, Paras P, Jr., Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. The EMBO journal. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell. 2005;122:169–182. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, Verdin E, Greene WC. Prostratin antagonizes HIV latency by activating NF-kappaB. The Journal of biological chemistry. 2004;279:42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. The EMBO journal. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Shibata H, Handa H. Transcription elongation factors DSIF and NELF: promoter-proximal pausing and beyond. Biochimica et biophysica acta. 2013;1829:98–104. doi: 10.1016/j.bbagrm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Molecular cell. 2003;12:971–982. doi: 10.1016/s1097-2765(03)00388-5. [DOI] [PubMed] [Google Scholar]
- Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, Eisele E, Haase A, Ho YC, Hutter G, et al. Challenges in Detecting HIV Persistence during Potentially Curative Interventions: A Study of the Berlin Patient. PLoS pathogens. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Molecular cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]