Abstract
Anxiety disorders increase risk for the early development of several diseases of aging. Elevated inflammation, a common risk factor across diseases of aging, may play a key role in the relationship between anxiety and physical disease. However, the neurobiological mechanisms linking anxiety with elevated inflammation remain unclear. In this review, we present a neurobiological model of the mechanisms by which anxiety promotes inflammation. Specifically we propose that exaggerated neurobiological sensitivity to threat in anxious individuals may lead to sustained threat perception, which is accompanied by prolonged activation of threat-related neural circuitry and threat-responsive biological systems including the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system (ANS), and inflammatory response. Over time, this pattern of responding can promote chronic inflammation through structural and functional brain changes, altered sensitivity of immune cell receptors, dysregulation of the HPA axis and ANS, and accelerated cellular aging. Chronic inflammation, in turn, increases risk for diseases of aging. Exaggerated neurobiological sensitivity to threat may thus be a treatment target for reducing disease risk in anxious individuals.
Keywords: Anxiety, Attentional bias, Cellular aging, Diseases of aging, Hypothalamic-pituitary-adrenal axis, Inflammation, Information processing, Neurobiological, Parasympathetic nervous system, Psychoneuroimmunology, Sympathetic nervous system, Threat
Still, thou art blest, compar’d wi’ me!
The present only toucheth thee:
But Och! I backward cast my e’e,
On prospects drear!
An’ forward, tho’ I canna see,
I guess an’ fear!
“To A Mouse” by Robert Burns
Being anxious throughout life has implications not just for subjective wellbeing, but also for physical health and longevity. This is because individuals who experience chronically high levels of anxiety are at increased risk for several diseases of aging, including cardiovascular, autoimmune, and neurodegenerative diseases, as well as for early mortality (Benninghoven et al., 2006; Carroll et al., 2009; Eaker et al., 2005; Kubzansky and Kawachi, 2000; Li et al., 2008; Martens et al., 2010; Roy-Byrne et al., 2008; Spitzer et al., 2009). Given that anxiety disorders have the highest lifetime prevalence of any psychiatric disorder, affecting up to 30% of the population over the lifespan, these findings highlight a highly prevalent and modifiable risk factor for physical disease (Demyttenaere et al., 2004; Kessler et al., 2005). Nonetheless, when compared with other major psychiatric disorders like depression, relatively little attention has been paid to examining the role anxiety plays in promoting and exacerbating physical disease. Moreover, little clinical consideration is given to addressing the physical health consequences of anxiety. This lack of attention is striking given that anxiety may be an even stronger risk factor for physical illness than depression (Kubzansky and Kawachi, 2000).
Despite strong evidence that anxiety negatively impacts physical health, the mechanisms that underlie these effects remain poorly understood. Previous research confirms that various forms of anxiety – including trait anxiety, state anxiety, and clinical anxiety disorders – are associated with elevated inflammation (Carroll et al., 2011; Hoge et al., 2009; O’Donovan et al., 2010; Pitsavos et al., 2006). In turn, elevated inflammation is a strong and robust risk factor for several diseases of aging including cardiovascular, autoimmune, and neurodegenerative disorders (Akiyama et al., 2000; Bruunsgaard et al., 2001; Freund et al., 2010; O’Donovan et al., 2011b; Ridker et al., 2000). However, an integrative model of the cognitive-behavioral and neurobiological mechanisms linking anxiety and inflammation has been lacking.
In the present paper, we address this gap in the literature by proposing a neurobiological model of the mechanisms by which anxiety may increase risk for diseases of aging. In this model, exaggerated neurobiological sensitivity to threat is proposed as a common feature across multiple anxiety disorders that plays a key role in the relationship between anxiety and inflammation. To introduce this model, we first outline differences and commonalities among the anxiety disorders. Then, we introduce evidence that diverse anxiety disorders are characterized by exaggerated neurobiological sensitivity to threat, as indexed by cognitive biases in threat-related information processing, and abnormalities in central and peripheral neurobiological systems involved in threat perception. We then explore the consequences of such neurobiological responding for inflammation, and present evidence for the role of chronic inflammation in promoting the development and progression of diseases of aging that have earlier onset and greater prevalence in anxious individuals. Finally, we bring these ideas together in a single integrative model, and discuss the clinical and public health implications of this work, as well as several avenues for future research.
1. Anxiety disorders and diseases of aging
Anxiety disorders, the most prevalent neuropsychiatric disorders worldwide, include generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), social anxiety disorder, panic disorder, obsessive-compulsive disorder (OCD), agoraphobia, and specific phobia (Kessler et al., 2005). Although these disorders are phenotypically diverse with symptoms ranging from enduring worry in GAD, to hypervigilance in PTSD, to compulsive hand washing in OCD, they also have common genetic, cognitive-behavioral, and biological features (Enoch et al., 2008; Lara et al., 2006; Zhou et al., 2008). One shared biological feature is elevated inflammation (Brennan et al., 2009; Gill et al., 2009; Hoge et al., 2009; O’Donovan et al., 2010; Pace and Heim, 2011; Von Kanel et al., 2010), which in turn is associated with accelerated biological aging and increased risk for the development of a variety of chronic diseases (Akiyama et al., 2000; Freund et al., 2010; Koenig et al., 2008; O’Donovan et al., 2011b; Ridker and Morrow, 2003; Ridker et al., 2002). Although inflammation may contribute to elevated disease risk in anxious individuals, it is not clear how having an anxiety disorder confers increased risk for elevated inflammation.
One possibility is that anxious individuals have elevated inflammation because of a greater tendency to smoke, eat poorly, be physically inactive, and abuse substances such as alcohol and drugs (Azevedo Da Silva et al., 2012; Schneider et al., 2010; Strine et al., 2005; Wolitzky-Taylor et al., 2012). However, not all anxious individuals exhibit poor health behaviors (Eifert et al., 1996), and most (Hoge et al., 2009; Pitsavos et al., 2006; von Kanel et al., 2007) but not all (Copeland et al., 2012) studies of the relationship between anxiety disorders and inflammation indicate that the association is independent of such factors. Another possibility is that neurobiological abnormalities associated with anxiety disorders promote inflammation. Exaggerated neurobiological sensitivity to threat, a common abnormality across diverse anxiety disorders, may increase risk for repeated and prolonged activation of biological stress systems, including inflammatory systems. When sustained, as in the case of a chronically anxious individual, such activation could drive functional and structural biological changes that promote chronic inflammation. Thus, exaggerated neurobiological sensitivity to threat may play a key role in the relationship between anxiety and inflammation.
2. Neurobiological sensitivity to threat
The ability to perceive and respond to environmental threats is fundamental to survival; without it, our ancestors would have died young and failed to pass on their genes. Given this strong selective pressure, it is not surprising that human threat perception and response systems comprise an exquisitely coordinated network that extends across central and peripheral bodily systems and is programmed to respond proactively to protect against injury and infection (Stein and Nesse, 2011; Woody and Szechtman, 2011). To facilitate survival, humans maintain vigilance for threatening information and are able to quickly mount appropriate biological and behavioral responses to threat. This vigilance and preparedness is costly, though, and can interfere with the pursuit of other goals such as reward seeking and bodily repair. Thus, threat perception and response systems must be tightly regulated and appropriately calibrated to the environment (Blanchard et al., 2011). Negative emotions may play a key role in the calibration of this system, insofar as they promote vigilance for threatening information and readiness to confront or avoid threats (Dolan, 2002). Although emotional enhancement of threat-related information processing confers obvious advantages in dangerous environments, it is biologically costly and needs to be switched off when no longer appropriate. That is, threat-related vigilance and preparedness must be up-regulated when physical or social threats are likely (e.g., in a warzone) and down-regulated when such threats are unlikely (e.g., in one’s own home).
2.1. Anxiety and exaggerated neurobiological sensitivity to threat
Anxiety disorders represent one context in which emotional enhancement of threat-related information processing is maladaptive, leading to exaggerated threat sensitivity. Unlike fear, which is a generally adaptive short-lived state of apprehension related to a proximal threat, anxiety is a sustained emotion aroused by distal and diffuse threats (Davis et al., 2009; Grillon, 2008). Thus, anxiety-related enhancement of threat-related information processing can persist across time and have chronic effects. A large body of literature and numerous excellent reviews document the complexities of enhanced threat-related information processing in anxious individuals (Britton et al., 2011; Cisler and Koster, 2010; Stein and Nesse, 2011), and here we provide a broad overview.
In the earliest stages of threat-related information processing, anxious individuals detect threatening stimuli (e.g., angry faces or predatory animals) more quickly than non-anxious individuals (Bar-Haim et al., 2007; El Khoury-Malhame et al., 2011; MacLeod et al., 1986). In the subsequent appraisal stage, anxious individuals are likely to regard ambiguous and threatening stimuli (e.g., mild electric shocks in the laboratory and motor vehicle accidents in real life) as more threatening than they actually are (Boddez et al., 2012; Britton et al., 2011; Dash and Davey, 2012; Lazarus and Folkman, 1984; Meiser-Stedman et al., 2009). This may contribute to the tendency for anxious individuals to show greater neurobiological reactivity to standardized threatening stimuli such as a virtual audience for a public speaking task or angry and fearful human faces (Cornwell et al., 2011; Eldar et al., 2011; Robinson et al., 2012a). Finally, following such reactions, anxious individuals engage in cognitive-behavioral avoidance of perceived threats, which limits their ability to challenge inappropriate threat perception, confront and resolve threatening situations, and reshape expectations for the future (Cisler and Koster, 2010; Koster et al., 2006).
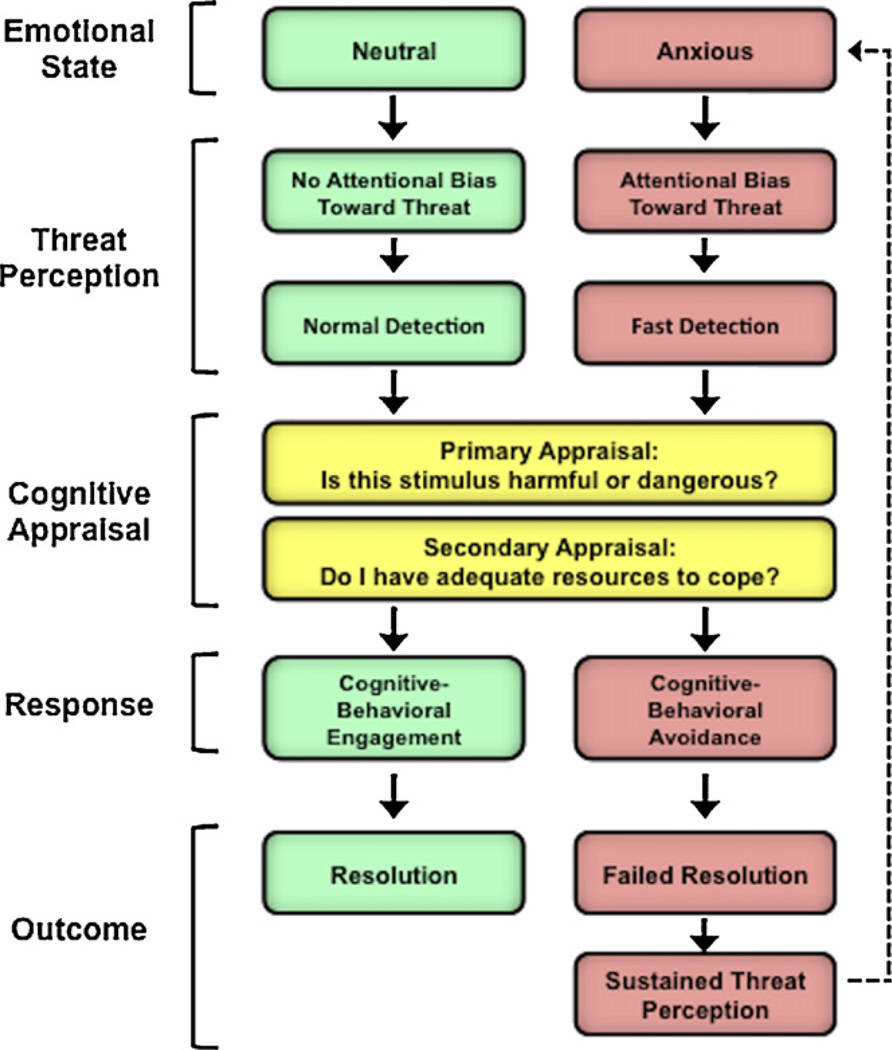
Even worry, a symptom of anxiety that may superficially resemble an attempt to engage with perceived threats, has been conceptualized as a form of cognitive avoidance that facilitates evasion of threatening imagery and inhibition of emotion processing (Borkovec and Hu, 1990; Borkovec and Inz, 1990). For example, worry can facilitate avoidance of a distressing mental image (e.g., of oneself being humiliated in front of a large audience) by allowing the attention to be directed away from the image and towards abstract thoughts related to the situation (e.g., “I am a failure”). Evidence for this avoidance function of worry is found in studies demonstrating that worry is associated with enhanced distraction from other emotionally distressing images and reduced physiological reactivity to subsequently encountered threats (Borkovec et al., 1993; Borkovec and Roemer, 1995; Llera and Newman, 2010). Ultimately, however, the avoidance function of worry is only partially fulfilled, because worry in itself leads to sustained emotional distress and prolonged physiological arousal (e.g., Newman and Llera, 2011). The ultimate result of this process is failure to achieve resolution of perceived threats, resulting in sustained threat perception. We illustrate these dynamics in Fig. 1, which depicts a model of threat-related information processing in anxious and non-anxious individuals.
Fig. 1.
A broad overview of cognitive-behavioral responses to perceived threats in anxious and non-anxious individuals. Anxious individuals show cognitive biases toward threatening information, which leads them to detect threatening stimuli (e.g., angry faces or predatory animals) more quickly than non-anxious individuals, and to appraise both ambiguous and threatening stimuli as more threatening. Anxious individuals also show a tendency to engage in cognitive-behavioral avoidance, which limits their ability to challenge inappropriate threat perception, confront and resolve threatening situations, and reshape expectations for the future. The ultimate result of this process is failure to achieve resolution of perceived threats, resulting in sustained threat perception.
Support for the existence of biases in threat-related information processing in anxious individuals is particularly strong in the context of clinical anxiety disorders. In fact, several theoretical models implicate threat-related attentional biases in both the development and maintenance of anxiety disorders including GAD, PTSD, social anxiety disorder, panic disorder, OCD, and simple phobia (Beck, 1985; Beck et al., 1985; Dalgleish et al., 2003; MacLeod and McLaughlin, 1995; Mathews et al., 1990, 1989; Taghavi et al., 2003). Despite the diverse clinical presentations of individuals with different anxiety disorders, a meta-analytic review indicated that the magnitude or severity of the threat-related attentional bias is not significantly different between these disorders (Bar-Haim et al., 2007) and cognitive-behavioral avoidance is also a core feature of all anxiety disorders (APA, 2000). In fact, the most successful psychotherapeutic treatments for anxiety disorders are cognitive-behavior therapy and prolonged exposure, both of which involve progressively exposing anxious individuals to stimuli perceived as increasingly threatening in order to break the cycle of vigilance and avoidance (Beck, 1976; Beck et al., 1985; Foa and Kozak, 1986). When such treatments are ineffective, or when they are not applied, anxious individuals tend to experience sustained threat perception, which leads to emotional distress and impaired quality of life (Beck et al., 1985; Cisler and Koster, 2010; Stein and Nesse, 2011). Moreover, such biases in threat-related information processing may promote neurobiological processes that increase disease risk. Below, we outline the neural underpinnings of threat-related information processing and the potentially deleterious consequences of threat perception for neuroendocrine, autonomic, and inflammatory systems.
3. Neurobiology of threat sensitivity
3.1. Neural processing of threatening information
The neural substrates that subserve threat-related information processing have been studied extensively in animal models and in humans. In humans, this has been done by exposing individuals to a wide range of stimuli including angry and fearful faces, dangerous scenes, threatening words, distressing memories, and phylogenetic threats such as snakes and spiders (Bishop, 2007, 2008; Woody and Szechtman, 2011). Although the specific sites of neural activation differ somewhat across these stimuli, several brain regions have emerged as being engaged in response to stimuli perceived as threatening. These regions include the amygdala, hippocampus, medial prefrontal cortex (mPFC), bed nucleus of the stria terminalus (BNST), and periaqueductal gray (PAG) (Davis et al., 2009). Coordinated engagement of these regions is critical for detecting threats, and for regulating behavioral and biological responses to threat. Importantly, however, activity in this threat-related neural network is potentiated for individuals with anxiety disorders, as well as for persons exhibiting high levels of trait anxiety (Bishop, 2007, 2008).
One particularly important brain region for threat-related information processing is the amygdala (Bishop, 2008; Davis and Whalen, 2001; LeDoux, 2000). The amygdala is a limbic brain structure that has been described as a “neural watchdog” insofar as it responds quickly – even before conscious awareness – to possible threats in the environment (Anderson et al., 2003; Whalen et al., 2004). The amygdala responds to both positive and negative stimuli (Hennenlotter et al., 2005; Somerville et al., 2004), and thus plays a key role in determining the extent to which environments are perceived as safe versus dangerous (Tottenham and Sheridan, 2010). The amygdala is particularly responsive to cues representing danger or threat, such as emotional faces and masked fearful whites of human eyes (Cunningham et al., 2008; Davis and Whalen, 2001; Whalen et al., 2004). Amygdalar responses are tightly calibrated under normal circumstances, but are exaggerated in the context of anxiety disorders (Rauch et al., 2000; Stein and Nesse, 2011). In fact, greater amygdala responses to threat, as measured by functional imaging paradigms involving exposure to threatening stimuli such as emotional faces, is positively correlated with the severity of anxiety symptoms (Armony et al., 2005; Fredrikson and Furmark,2003; Phan et al., 2006; Protopopescu et al., 2005). Neural activity in the amygdala has also been found to mediate symptoms related to anxiety, such as PTSD-related hyperarousal (Rauch et al., 2003).
The amygdala does not function in isolation but rather in concert with other brain regions that regulate its activity, such as the hippocampus and mPFC, which have extensive connections to the amygdala (Bishop, 2007, 2008). The hippocampus is involved in learning and episodic memory, and is critical for explicit encoding of threat-related contextual cues (Bishop, 2007; Phelps, 2004; Shin et al., 2006). Patients with hippocampal damage, for example, are physiologically aroused by neutral stimuli that have been paired with shock but are unable to explicitly recollect that the stimuli and shock were ever associated with each another (Bechara et al., 1995). Moreover, the hippocampus stores information that gives the amygdala the ability to respond to environmental threats that a person has been told about, but never directly experienced (e.g., a dangerous predator or neighborhood), enabling individuals to experience anticipatory anxiety for symbolic or imagined situations, a key process in anxiety disorders (Phelps et al., 2001).
The mPFC, on the other hand, is involved in fear extinction learning, or the acquired down-regulation of threat-related responses after threats have passed (Milad and Quirk, 2002; Milad et al., 2006). This mechanism for down-regulating cued and contextual fear is impaired in anxious individuals, resulting in sustained physiological symptoms of anxiety (Indovina et al., 2011). It has been proposed, therefore, that the maintenance of anxiety stems from exaggerated amygdala responsivity to threat resulting from a lack of adequate regulation of amygdala activity by the mPFC (Bishop, 2007; Indovina et al., 2011).
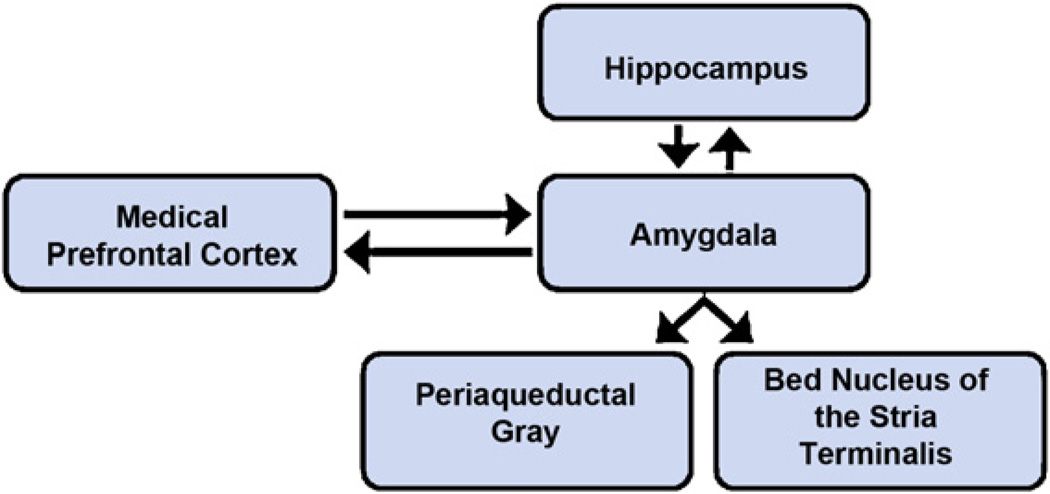
The amygdala, hippocampus, and mPFC function as components of an integrated network involved in detecting and monitoring potential threats, and regulating behavioral and biological responses to such threats (Bishop, 2007; Indovina et al., 2011; Rauch et al., 2003; Shin et al., 2006). Other brain structures in this network include the BNST and the PAG. The BNST is involved in monitoring the proximity of threat and in coordinating autonomic and motor responses to threat (Davis et al., 2009; Davis and Whalen, 2001; Mobbs et al., 2007; Somerville et al., 2010; Walker et al., 2003), and the PAG is involved in activating stereotyped defensive reactions to threat, such as immobility and panic, which are engaged during periods of intense fear or imminent threat (Mobbs et al., 2007; Nashold et al., 1969). Finally, a large number of neuroimaging studies have examined the brain regions that are engaged during exposure to social threats, such as social exclusion or rejection, which signal an increased likelihood of possible physical threat. Brain regions engaged during these experiences include the dorsal anterior cingulate cortex (dACC) and bilateral anterior insula, which are key nodes in the neural pain network (Eisenberger et al., 2003; Kross et al., 2011; Slavich et al., 2010b). Fig. 2 illustrates some of the key reciprocal connections within the neural network involved in threat-related information processing.
Fig. 2.
Hypothetical model of the neural systems involved in detecting threats, and regulating behavioral and biological responses to threat. Central to this network is the amygdala, which responds quickly to potential threats in the environment and plays a key role in determining whether environments are perceived as safe or dangerous. The amygdala functions in concert with other brain regions including the hippocampus and medial prefrontal cortex, which can up-regulate or down-regulate amygdalar responses to threat. Moreover, behavioral and biological responses to threat depend on activation of other brain areas, including the bed nucleus of the stria terminalis, which coordinates autonomic and motor responses to threat, and the periaqueductal gray, which coordinates stereotyped defensive reactions to threat, such as immobility and panic. Activity in this threat-related neural network is potentiated for individuals with anxiety disorders, as well as for persons exhibiting high levels of trait anxiety.
3.2. Neurobiological responses to threat perception
The brain regions involved in processing threatening information can activate biological stress-response systems, including the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) (Dickerson and Kemeny, 2004; Mendes et al., 2007; Seery, 2011; Thayer et al., 2012). In the case of the HPA axis, cortical and subcortical brain regions involved in threat-related information processing have direct projections to neurons in the paraventricular nucleus of the hypothalamus, which signals downstream via the pituitary release of ACTH to promote the secretion of the glucocorticoid hormone cortisol from the adrenal cortex (An et al., 1998; Ongür et al., 1998). Numerous experimental studies have shown increases in cortisol in response to social-evaluative threat (Epel et al., 2000; Gruenewald et al., 2006; Taylor et al., 2008). Moreover, in a meta-analytic review of 208 studies, threat emerged as one of the key features of psychological stressors that elicit increases in cortisol (Dickerson and Kemeny, 2004).
The brain regions involved in threat-related information processing also regulate the ANS, and activity in the amygdala strongly correlates with activity in both the sympathetic and parasympathetic branches of the ANS (An et al., 1998; Critchley, 2005, 2009; Ongür et al., 1998; Salomé et al., 2007; Thayer et al., 2012; Tsukiyama et al., 2011). The mPFC can influence the ANS indirectly through inhibitory effects on the amygdala and directly through projections to both the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) (Thayer and Lane, 2009). Within the ANS, threat-related brain activity up-regulates SNS activity as indexed by peripheral vasoconstriction, increased blood pressure and greater release of the catecholamines norepinephrine and epinephrine from the adrenal medulla and norepinephrine from sympathetic nerves throughout the body (Blascovich and Mendes, 2010; Mendes et al., 2008). At the same time, threat perception downregulates the PNS, as indexed indirectly by decreases in respiratory sinus arrhythmia (Thayer et al., 2012; Thayer and Sternberg, 2009).
3.3. Threat perception and inflammation
Following these changes in the HPA axis and ANS, increased cortisol and catecholamines circulating in the blood can bind to receptors on immune cells and initiate intracellular signaling cascades that regulate immune cell gene expression and the release of inflammatory cytokines (Black, 2002; Padgett and Glaser, 2003; Sternberg, 2006; Thayer et al., 2011). In response to acute social-evaluative threat, activation of the HPA axis and ANS is accompanied by increased inflammation (Carroll et al., 2011; Dickerson et al., 2009a,b; Moons et al., 2010; Murphy et al., in press). Chronic threat perception appears to have similar effects; people living in a state of fear about specific threats (e.g., fear of terrorist acts), and individuals with a dispositional bias toward threat-related information (i.e., highly trait anxious and highly pessimistic individuals), have both been found to have elevated resting levels of inflammation (Melamed et al., 2004; O’Donovan et al., 2009; Pitsavos et al., 2006). Overall, this evidence suggests that threat-related activation of central and peripheral systems is accompanied by increased inflammation.
However, the mechanisms by which activation of the HPA axis and ANS permit or promote elevated inflammation are complex and incompletely understood. One complexity is that high doses of endogenous and synthetic glucocorticoids have well-documented anti-inflammatory effects (Auphan et al., 1995). Thus, the cortisol release following threat-related HPA axis activation should theoretically be associated with less, not more, inflammation. One explanation for this apparent paradox is that threat simultaneously up-regulates the HPA axis and increases cortisol production, while down-regulating the sensitivity of receptors for glucocorticoids on immune cells, thus reducing the extent to which cortisol can inhibit inflammation (Sheridan et al., 2000; Stark et al., 2002). Support for this hypothesis is provided by a human laboratory-based study that involved exposing individuals to an acute episode of social-evaluative threat. In this study, immune cells taken from participants who completed a stressful public speaking task in front of a socially rejecting panel of raters exhibited decreased sensitivity to the anti-inflammatory effects of glucocorticoids (Dickerson et al., 2009a). Mounting evidence also indicates that glucocorticoids can have pro- as well as anti-inflammatory effects, and that low levels of glucocorticoids are actually required for activation of the inflammatory response (Sapolsky et al., 2000; Sorrells and Sapolsky, 2007).
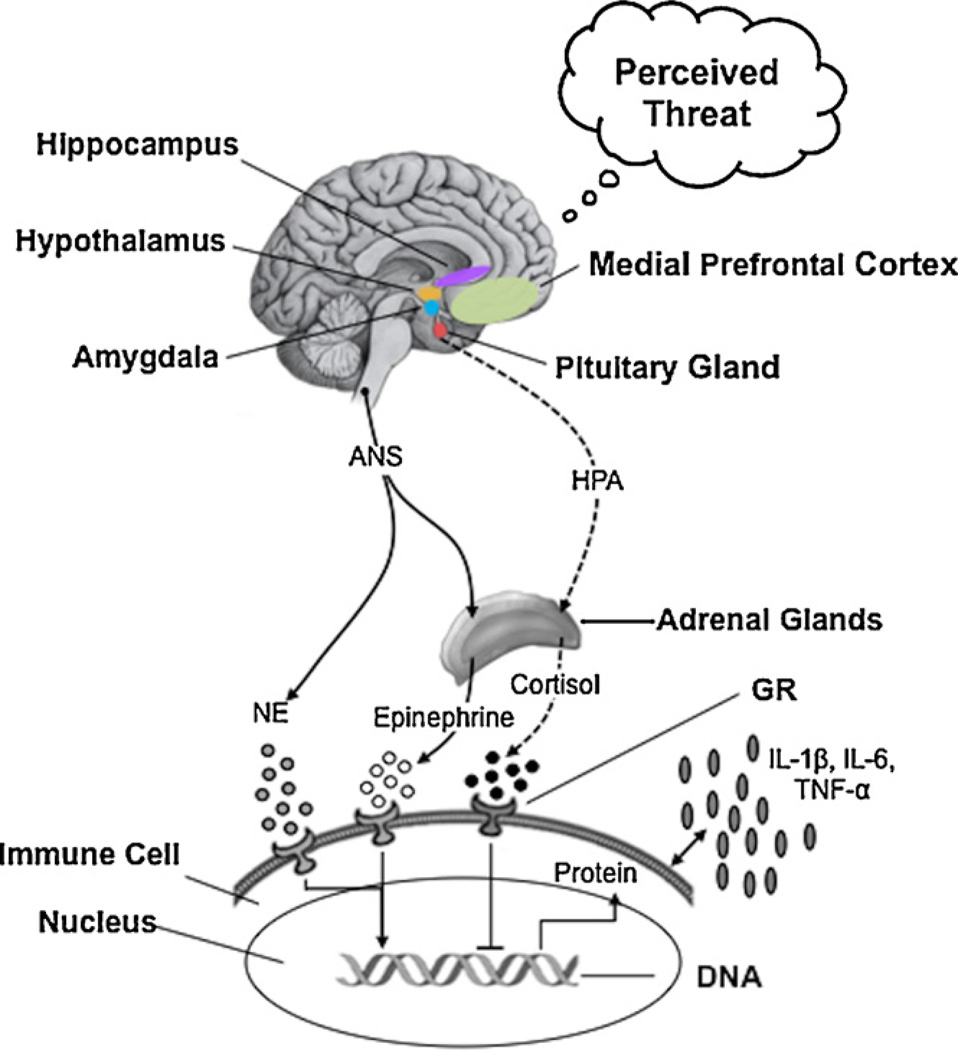
Activation of the ANS can also have both pro- and anti-inflammatory effects via the release of catecholamines and direct innervation of immune organs including the spleen, thymus, and lymph nodes (Bierhaus et al., 2003; Borovikova et al., 2000; Flierl et al., 2008; Röntgen et al., 2004; Sternberg, 2006; Thayer et al., 2011; Thayer and Sternberg, 2010; Tracey, 2002). The sympathetic arm of the ANS (SNS) can both inhibit and promote inflammation (Elenkov and Chrousos, 2006; Thayer and Sternberg, 2010). However, up-regulation of the SNS in anxious individuals is typically accompanied by down-regulation of the parasympathetic arm of the ANS (PNS), and reduced PNS activity has been associated with increased inflammation (Haensel et al., 2008). Moreover, lower PNS activity has been associated with elevated inflammation even when adjusting for the contributions of SNS activity (Thayer and Fischer, 2009). Thus, decreased PNS activity in threatened individuals may play a key role in permitting the elevated systemic inflammation observed in anxious individuals. Fig. 3 illustrates some of the pathways that link threat-related neural activity with elevated inflammation.
Fig. 3.
Illustration of the pathways linking threat-related neural activity in the amygdala, medial prefrontal cortex and hippocampus with elevated inflammation. Threat perception leads to activation of the hypothalamic-pituitary-adrenal (HPA) axis leading to increased release of the glucocorticoid hormone cortisol from the adrenal glands (broken lines). Threat perception also activates the sympathetic arm and deactivates the parasympathetic arm of the autonomic nervous system (ANS), leading to increased release of the catecholamines epinephrine and norepinephrine (solid lines). This pattern of activation and deactivation is accompanied by increased synthesis and release of pro-inflammatory cytokines including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Binding of these factors to receptors on immune cells regulates gene expression, including expression of genes for pro-inflammatory cytokines. Thus, the effects of the HPA axis and ANS on the immune system depend on the expression of immune cell receptors for cortisol and catecholamines, as well as on the release of these hormones. The glucocorticoid receptor (GR) appears to be down-regulated in response to threat, limiting the anti-inflammatory effects of cortisol. Although there are complex bidirectional relationships between the various factors in this model, threat perception ultimately leads to elevated inflammation.
4. Chronic anxiety and inflammation
Thus far, we have described threat-related changes in central and peripheral systems that have the ability to increase systemic levels of inflammation. It is important to note that these changes provide short-term protection against the potential physical harm associated with threatening events. Specifically, acute inflammation prevents infection, elicits pain to encourage avoidance of further injury, and up-regulates cellular (e.g., neutrophils, monocytes) and humoral (e.g., antibody, complement) immune processes targeted at removing pathogens and healing damaged or infected sites (Suffredini et al., 1999). Systemic inflammation also promotes behavioral changes, collectively known as sickness behavior, which resemble some symptoms of major depression (e.g., fatigue and anhedonia) and limit social-behavioral activity to reduce risk for further infection or injury (Dantzer et al., 2008). However, inflammatory activity must be down-regulated when no longer necessary. If sustained, elevated inflammation can have deleterious effects, increasing risk for clinical depression as well as for diseases of aging and early mortality (Cohen et al., 1997; Dantzer et al., 2008; Morrow et al., 1998; Pradhan et al., 2001; Strandberg and Tilvis, 2000; Volpato et al., 2001; Yin et al., 2004).
Anxiety-related increases in inflammation may be sustained across years or decades because the onset of many anxiety disorders occurs during sensitive periods in early life when biological systems develop, and because symptoms tend to persist over long periods of time (Danese et al., 2011; Hertzman, 1999; Prenoveau et al., 2011). There are several pathways by which anxiety-related exaggerated neurobiological sensitivity to threat could promote chronic inflammation. However, we will confine our discussion to four key neurobiological pathways: structural and functional brain changes; changes in receptor sensitivity; changes in basal HPA axis and ANS activity; and accelerated cellular aging.
4.1. Structural and functional brain changes
One pathway by which exaggerated threat sensitivity may lead to chronic inflammation is through changes in the brain regions involved in detecting and processing subsequently encountered threats (McEwen, 2007; Yirmiya and Goshen, 2011). Much of the evidence for these changes comes from studies showing changes in the structure and functional connectivity of the brain in the aftermath of adverse early life experiences that increase neurobiological sensitivity to threat (Tottenham and Sheridan, 2010). These neurobiological changes can be generated through neural plasticity and can involve alterations in the physical structure of the brain, changes in synapse turnover, dendritic remodeling or neuronal replacement, and alterations in functional activity or connectivity between target brain areas (McEwen et al., 2012). Importantly, accumulating evidence confirms that such alterations occur throughout the lifespan and not only in early life (Li et al., 2006).
The brain areas involved in detecting, processing, and remembering threatening information have dense catecholamine and glucocorticoid receptors, making them highly sensitive to the effects of repeated and prolonged activation of the HPA axis and ANS (Buffalari and Grace, 2007; Jöels, 2006; McEwen, 2010). As evidence for the fact that sustained activation of biological stress systems has neurotoxic effects, exposure to traumatic stress involving threat to life or physical integrity results in smaller hippocampal and mPFC volumes (Apfel et al., 2011; Rao et al., 2010; Shin et al., 2006), although possibly only in vulnerable individuals (Gilbertson et al., 2002; Gross and Hen, 2004). Connectivity in various brain circuits can change due to experience as well (Saibeni et al., 2005). For example, exposure to early life stress is associated with impaired connectivity between the amygdala and the right ventrolateral PFC (Robinson et al., 2012b). Such structural and functional brain changes are relevant for health because they can impair the regulation of central and peripheral responses to threat, promoting the sustained threat perception that may drive chronic inflammation.
4.2. Changes in receptor sensitivity
Sustained threat perception also changes how immune cells respond to signals from threat-related neuroendocrine and inflammatory factors. These changes can come about through altered sensitivity of immune cell receptors. For example, several studies have indicated that exposure to chronic stress down-regulates glucocorticoid receptors on immune cells, making them less sensitive to the anti-inflammatory effects of cortisol (Miller et al., 2002; Rohleder et al., 2009, 2010). In addition, bioinformatics and other approaches have revealed less signaling to immune cells via the glucocorticoid receptor in individuals who have a greater tendency to perceive ambiguous situations as threatening, specifically those who are socioeconomically disadvantaged during early life (Chen et al., 2004) or who develop PTSD in the aftermath of trauma (O’Donovan et al., 2011c). Thus, sustained threat perception in anxious individuals may down-regulate glucocorticoid receptors on immune cells, leading to a failure of the negative feedback effects of cortisol on threat-related inflammation.
4.3. Changes in HPA and ANS activity
In addition to influencing immune cell receptors for stress hormones, sustained threat perception may alter resting and reactivity levels of HPA axis and ANS activity. Many studies have documented elevated resting and reactivity levels of cortisol in patients with clinical anxiety disorders (Maes et al., 1998; Thayer, 2006; Thayer et al., 1995). However, there is also evidence that some anxiety disorders may be associated with lower resting levels of cortisol (O’Donovan et al., 2010; Thayer et al., 2009; Yehuda, 2009; Yehuda et al., 1990). Potential reasons for this lack of convergence in findings include differences across anxiety disorders and the absence of controls for circadian factors, comorbid psychiatric symptoms (such as depression), duration of anxious symptoms, or the inherent complexity of the HPA axis, which can become dysregulated at many levels. The relationship between anxiety and the SNS appears dependent on the timescale of particular analyses. A meta-analysis of relevant studies of acute stress indicates that anxious individuals exhibit hypoactive sympathetic responses to acute threat, but prolonged activation of the SNS when threats have passed (Chida and Hamer, 2008). Patients with anxiety disorders have shown lower PNS activity in most studies (Blechert et al., 2007; Sharma et al., 2011; Watkins et al., 1998), although not all (Davis et al., 2002). Large-scale studies that take timescale, circadian rhythms and comorbidities into account are needed to clarify the nature of dysregulation in the HPA axis and ANS in chronically anxious individuals.
4.4. Accelerated cellular aging
Sustained threat perception can also lead to elevated inflammation through an accelerated rate of cellular aging, as indexed by telomere shortening. Telomeres are DNA–protein complexes that cap the ends of chromosomes and protect against damage to the DNA that encodes our genes. Telomeres shorten with each cycle of cell division and with age, and immune cell telomere length is an emerging marker and mechanism of cellular aging (Blackburn, 2000; Sahin et al., 2011). Moreover, short telomere length confers increased risk for several major diseases of aging including cardiovascular, autoimmune, and neurodegenerative diseases, as well as for early mortality (Cawthon et al., 2003; Grodstein et al., 2008; Willeit et al., 2010). Individuals with high levels of threat sensitivity – as indexed by pessimism, childhood trauma exposure, phobic anxiety or post-traumatic stress disorder – have shorter telomere length (Kananen et al., 2010; O’Donovan et al., 2011a, 2009; Okereke et al., 2012; Surtees et al., 2011; Tyrka et al., 2010). Individuals experiencing various forms of chronic psychological stress also have shorter telomere length (Cherkas et al., 2006; Damjanovic et al., 2007; Epel et al., 2004), which appears to be mediated at least in part by increased threat perception (O’Donovan et al., 2012). Although accelerated cellular aging may be driven by elevated inflammation (Jaiswal et al., 2000), the relationship between inflammation and cellular aging is likely to be bidirectional, given that cells with short telomeres release higher quantities of proinflammatory factors such as IL-6 and TNF-α (Coppé et al., 2010; O’Donovan et al., 2011b). Thus, accelerated cellular aging may be a potential contributor to increased disease risk in anxiety, largely through threat-related elevated inflammation.
5. A neurobiological model of anxiety-related risk for diseases of aging
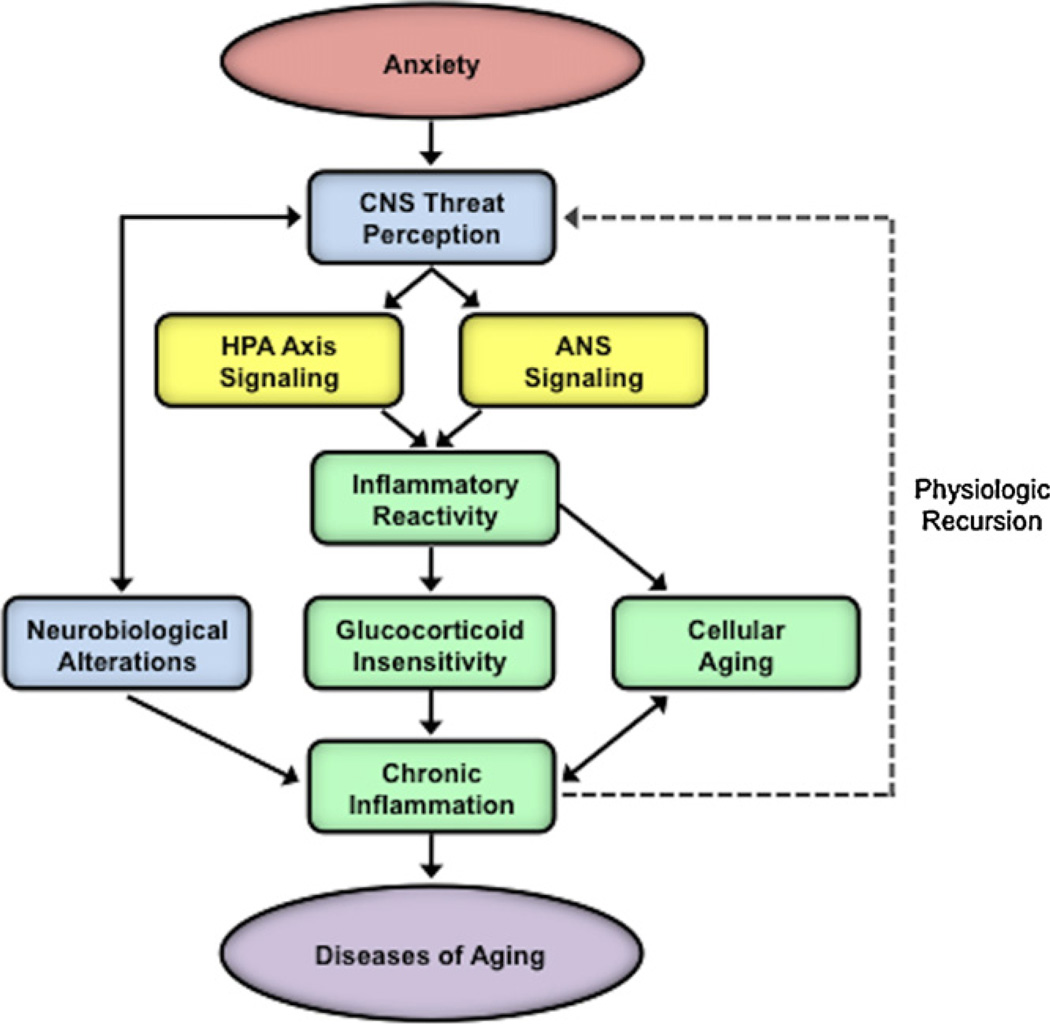
Based on the findings presented above, we propose a neurobiological model showing how anxiety disorders may promote the development of chronic diseases such as cardiovascular, autoimmune, and neurodegenerative diseases, and increase risk for early mortality (Fig. 4). In this model, exaggerated neurobiological sensitivity to threat in anxious individuals leads to cognitive-behavioral responses characterized by a pattern of vigilance-avoidance, which ultimately results in sustained threat perception. Accompanying this sustained threat perception is prolonged activation of threat-related neural circuitry and threat-responsive biological systems including the HPA axis, ANS, and inflammatory response. Over time, these effects on central and peripheral systems may become chronic through structural changes in the CNS, altered sensitivity of receptors on immune cells, and accelerated cellular aging, as well as through other pathways. As a consequence, chronically elevated inflammation can have toxic effects throughout the body and increase risk for early onset and accelerated progression of diseases of aging.
Fig. 4.
Integrative neurobiological model showing the pathways mediating anxiety-related increased risk for diseases of aging. The model depicts how exaggerated neurobiological sensitivity to threat in anxious individuals leads to cognitive-behavioral threat responses characterized by a pattern of vigilance-avoidance, which ultimately results in sustained threat perception. Such sustained threat perception is accompanied by prolonged activation of threat-related neural circuitry and threat-responsive biological systems including the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system (ANS), and inflammatory response, ultimately leading to elevated inflammation. Over time, the effects on central and peripheral systems may become chronic through structural changes in the central nervous system (CNS), altered sensitivity of receptors on immune cells, and accelerated cellular aging. Finally, such chronic elevations in inflammation can increase risk for, and accelerate the progression of, diseases of aging.
5.1. Implications for understanding stress and health
The proposed model has implications for understanding trends in public health such as upswings in disease during certain stressful periods, as well as in specific geographic regions and in individuals exposed to various forms of psychological stress (Cohen et al., 2009; Maunder et al., 1999; Seal et al., 2007, 2012). Although there are documented links between stress exposure and both physical and mental disease outcomes (Cohen et al., 2007; Monroe et al., 2009), these associations are not easily deconstructed and remain understood only at the level of the global stress response. However, the apparently complex relationship between stressor exposure and disease might be understood in terms of threat sensitivity. Specifically, in stressful contexts, increased neurobiological sensitivity to threat at individual, community, or societal levels might increase risk for the early development of diseases of aging by increasing inflammation. Moreover, following such experiences, individual differences leave those predisposed to anxiety with persistently exaggerated threat sensitivity and systemic inflammation. Thus, even someone with an initially stable emotional temperament may develop an anxious style of emotional processing, driven by the neural changes described above (Bar-Haim et al., 2011a; Britton et al., 2011; Melamed et al., 2004; Pine et al., 2005). Subsequently, the tendency toward negative mood reactivity to daily life stress may promote biological stress responses and increase risk for the development of chronic physical diseases (Piazza et al., 2012). Variation in the degree of increase in threat sensitivity following exposure to psychological stress may explain why some people do and other people do not show increased risk for disease in the aftermath of stressful experiences.
5.2. Implications for treatment
The proposed model highlights several potential targets for pharmacological and psychological intervention. Although single-dose administration of selective serotonin reuptake inhibitors (SSRIs) may increase threat sensitivity (Browning et al., 2007; Grillon et al., 2007), longer term administration of commonly used psychopharmacological agents including SSRIs and selective noradrenaline reuptake inhibitors may reduce threat sensitivity (Harmer et al., 2006; Mogg et al., 2004; Murphy et al., 2009; Rawlings et al., 2010). Pharmacological interventions that target inflammation or HPA and ANS mechanisms involved in inflammation may be warranted as an adjunct or replacement for psychological interventions. However, the effectiveness of anti-inflammatory treatments for individuals with psychiatric disorders remains unclear (Miller et al., 2009; Warner-Schmidt et al., 2011), and both psychopharmacological and anti-inflammatory medications have adverse side effects. Thus, there are several potential pharmacological treatments that may target causal elements in our proposed model, but additional research is needed to assess their effects on disease risk in anxious individuals.
In the meantime, there is evidence that a number of existing and emerging cognitive-behavioral therapies (CBT) may be helpful. Different forms of CBT have been found to reduce threat-related cognitive-behavioral biases as well as pro-inflammatory signaling (Antoni et al., 2011; Beck, 1976; Smits et al., 2012). Prolonged exposure treatments that involve carefully titrated exposure to stimuli perceived as threatening may break the cycle of vigilance-avoidance in anxious individuals and thereby reduce inflammation (Foa, 2011). In recent years, computerized threat-related cognitive bias modification has emerged as an effective treatment that reduces threat-related attentional biases and anxiety (Bar-Haim et al., 2011b; Browning et al., 2010). Because such treatments may be effectively administered online, they may prove to be a targeted and cost-effective method for reducing anxiety-related disease risk (MacLeod et al., 2007). Lastly, extrapolating from a cross-sectional study, therapies targeted at increasing positive personal resources may dampen threat-related neural activity through prefrontal cortex enhancement and decreased threat perception (Taylor et al., 2008).
5.3. Future research
Our review highlights that insufficient attention has been paid to the mechanisms of anxiety-related increased risk for chronic physical diseases. Although a large literature now exists linking depression and inflammation (Dantzer et al., 2008; Raison et al., 2006; Slavich and Irwin, submitted for publication; Slavich et al., 2010a), the relationship between anxiety and inflammation has received very little attention. In the present review, we emphasize common threat-related cognitive-behavioral biases across anxiety disorders and postulate that these biases have important effects on central and peripheral systems that regulate inflammation.
Although it is clear that different anxiety disorders confer increased risk for diseases of aging, threat-related biases take different forms across the anxiety disorders (Krusemark and Li, 2011), and further research is needed to clarify common and distinct patterns of responding to threat across diagnostic groups. In addition, there are unanswered questions regarding the association between threat and inflammation in anxious individuals. Some of the most pressing questions relate to specificity in the relationship between threat perception and inflammation (Kemeny, 2009). Previous research on threat-related activation of inflammation in humans has relied on manipulating the social environment to evoke threat-related responses (Carroll et al., 2011; Dickerson et al., 2009a; Moons et al., 2010). However, the inflammatory response may be activated by many different types of threat, ranging from phylogenetic threats (e.g., spiders, snakes), to contamination threats (e.g., open infected wounds, sneezing), to conspecific violence (e.g., angry aggressive humans). Although ethical concerns preclude research on some important categories of threat, the use of virtual reality technology permits the exposure of humans to a wide range of stimuli (e.g., war zones, deadly predators, infected wounds). Research examining common and specific responses to different forms of threat in anxious and non-anxious individuals will shed light on the applicability of our model across groups and situations. Moreover, it would be helpful to assess additional neurobiological and psychosocial aspects of anxiety disorders that may increase risk for physical disease across diagnostic groups or in specific anxiety disorders (e.g., social isolation, sleep disturbance, and health behaviors).
Our review also highlights a pressing need to identify the specific biological processes mediating inflammatory responses to threat. Although in vitro and non-human research may provide important information on these mechanistic processes, research with humans is also necessary because some types of threat – such as symbolic, imagined, or anticipated threats – appear unique to humans (Gilbert and Wilson, 2007). Although mechanistic research is limited in humans because genetic and pharmacological manipulations of biological pathways are seldom possible, developments in bioinformatics provide a partial solution to this problem as they permit researchers to probe large amounts of genomic, proteomic, and metabolomic data for underlying causal mechanisms. For example, the Transcription Element Listening System (TELiS) permits analysis of the intracellular signaling pathways underlying gene expression patterns of specific cell types (Cole et al., 2005). Of relevance to the present review, research conducted with TELiS suggests that decreased anti-inflammatory signaling through the glucocorticoid receptor and increased pro-inflammatory signaling by nuclear factor-κB may underlie chronic stress and PTSD-related elevations in inflammation (Chen et al., 2011; O’Donovan et al., 2011c; van Vollenhoven, 2009).Thus, technological advances in the field of bioinformatics and increases in computing power may permit more detailed mechanistic understanding of the relationship between threat sensitivity and inflammation.
In addition, it is important to elucidate factors that moderate the effects of anxiety on inflammation and chronic disease risk. These moderating factors include social–environmental factors like childhood adversity that promote epigenetic changes and increase risk for anxiety (McEwen, 2010); genetic polymorphisms, such as the serotonin transporter polymorphism (5HTTLPR) and polymorphisms in the promoter of the pro-inflammatory interleukin-6 gene (rs1800795), which influence stress responding and inflammation (Cole et al., 2010; Hariri et al., 2005, 2002); and health behaviors that affect inflammation-related disease risk, such as voluntary sleep curtailment and physical inactivity (Puterman et al., 2011; Taylor et al., 2011). Thus, integrative multidisciplinary studies will be needed to uncover more specific treatment targets for the reduction of inflammation in sub-populations of anxious individuals.
We have drawn on several related lines of research in proposing our integrative neurobiological model and have presented evidence for each of the individual pathways in the model. To date, however, no studies have examined all of the pathways concurrently. As a result, there is no existing research evaluating our model as a whole. Such research would involve exposing individuals to different types of threat while monitoring their cognitive-behavioral, neural, and inflammatory responses. To our knowledge, only one study has examined neural processes underlying inflammatory responses to social threat. In this study, individuals who exhibited greater activation of the dACC and bilateral anterior insula in response to social rejection showed greater inflammatory responses to a subsequent episode of acute social stress (Slavich et al., 2010b). A complementary strategy for identifying causal mechanisms of anxiety-related inflammation involves manipulating specific factors in the model and analyzing the downstream consequences of such manipulations. One study that employed this approach used a cognitive-behavior stress management intervention to target anxiety-related affective and behavioral processes in a sample of patients with breast cancer. Results indicated that the intervention led to down-regulation of pro-inflammatory signaling to immune cells through NF-κB and reduced expression of genes that regulate pro-inflammatory factors (Antoni et al., 2011). Similar intervention studies that include analysis across cognitive-behavioral, neural, and inflammatory systems are necessary for advancing research on anxiety and health.
6. Conclusions
Drawing on research from cognitive psychology, neuroimaging, neuroendocrinology, and psychoneuroimmunology, we propose that exaggerated neurobiological sensitivity to threat is a key mediator of anxiety-related increases in inflammation, and that inflammation, in turn, promotes the development and accelerated progression of a variety of major diseases of aging. This model identifies several psychological and biological processes that can become the target of interventions designed to reduce risk for disease in anxious individuals. The model also generates several testable hypotheses for future research. Coordinated biobehavioral responses to perceived threat are critical for ensuring fitness within dangerous environments. When threat perception is sustained as in the case of highly threat-sensitive anxious individuals, however, chronically elevated inflammation may promote the development and accelerated progression of diseases of aging and, shorten the lifespan. Given the high lifetime prevalence of anxiety disorders, and the substantial social and economic costs associated with anxiety-related physical disease, addressing anxiety-related health problems should be of paramount public concern.
Acknowledgments
Aoife O’Donovan and George M. Slavich were supported by Society in Science – The Branco Weiss Fellowship during the preparation of this review. We thank Joshua Woolley, Keely Muscatell, Kristen Nishimi, and Annika Rose for their helpful feedback on a previous version of this manuscript.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole G, Cooper N, Eikelen-boom P, Emmerling M, Fiebich B, Finch C, Frautschy S, Griffin W, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie I, McGeer P, O’Banion M, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel F, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. The Journal of Comparative Neurology. 1998;401:455–479. [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JM, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biological Psychiatry. 2011;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: 2000. Text Revision. [Google Scholar]
- Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Weiner MW, Schuff N, Neylan TC. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biological Psychiatry. 2011;69:541–548. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. The American Journal of Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Azevedo Da Silva M, Singh-Manoux A, Brunner EJ, Kaffashian S, Shipley MJ, Kivimäki M, Nabi H. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. European Journal of Epidemiology. 2012;27:537–546. doi: 10.1007/s10654-012-9692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel TI, Muller D, Charney DS, Pine DS, Fox NA, Wald I. Life-threatening danger and suppression of attention bias to threat. The American Journal of Psychiatry. 2011a;167:694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Morag I, Glickman S. Training anxious children to disengage attention from threat: a randomized controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011b;52:861–869. doi: 10.1111/j.1469-7610.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive Therapy and the Emotional Disorders. New York: International Universities Press; 1976. [Google Scholar]
- Beck AT. Theoretical perspectives on clinical anxiety. In: Tuma AH, Maser J, editors. Anxiety and the Anxiety Disorders. Hillsdale, NJ: Lawrence Erlbaum Associates; 1985. pp. 183–196. [Google Scholar]
- Beck AT, Emery G, Greenberg RL. Anxiety Disorders and Phobias: a Cognitive Perspective. New York: Basic Books; 1985. [Google Scholar]
- Benninghoven D, Kaduk A, Wiegand U, Specht T, Kunzendorf S, Jantschek G. Influence of anxiety on the course of heart disease after acute myocardial infarction – risk factor or protective function? Psychotherapy and Psychosomatics. 2006;75:56–61. doi: 10.1159/000089227. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain, Behavior, and Immunity. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ. Risk assessment as an evolved threat detection and analysis process. Neuroscience and Biobehavioral Reviews. 2011;35:991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, editors. The Handbook of Social Psychology. 5th ed. New York: Wiley; 2010. [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosomatic Medicine. 2007;69:935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- Boddez Y, Vervliet B, Baeyens F, Lauwers S, Hermans D, Beckers T. Expectancy bias in a selective conditioning procedure: trait anxiety increases the threat value of a blocked stimulus. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43:832–837. doi: 10.1016/j.jbtep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Hu S. The effect of worry on cardiovascular response to phobic imagery. Behaviour Research and Therapy. 1990;28:69–73. doi: 10.1016/0005-7967(90)90056-o. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Inz J. The nature of worry in generalized anxiety disorder: a predominance of thought activity. Behaviour Research and Therapy. 1990;28:153–158. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Lyonfields JD, Wiser SL, Deihl L. The role of worrisome thinking in the suppression of cardiovascular response to phobic imagery. Behaviour Research and Therapy. 1993;31:321–324. doi: 10.1016/0005-7967(93)90031-o. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Roemer L. Perceived functions of worry among generalized anxiety disorder subjects: distraction from more emotionally distressing topics? Journal of Behavior Therapy and Experimental Psychiatry. 1995;26:25–30. doi: 10.1016/0005-7916(94)00064-s. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Fargnoli JL, Williams CJ, Li T, Willett W, Kawachi I, Qi L, Hu FB, Mantzoros CS. Phobic anxiety is associated with higher serum concentrations of adipokines and cytokines in women with diabetes. Diabetes Care. 2009;32:926–931. doi: 10.2337/dc08-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depression and Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Harmer CJ. The modification of attentional bias to emotional information: a review of the techniques, mechanisms, and relevance to emotional disorders. Cognitive, Affective & Behavioral Neuroscience. 2010;10:8–20. doi: 10.3758/CABN.10.1.8. [DOI] [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. Journal of Psychopharmacology. 2007;21:684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Current Opinion in Hematology. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Thomas GN, Gale CR, Deary I, Batty GD. Generalized anxiety disorder is associated with metabolic syndrome in the Vietnam experience study. Biological Psychiatry. 2009;66:91–93. doi: 10.1016/j.biopsych.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity. 2011;25:232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Development. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA: the Journal of the American Medical Association. 2009;302:489–492. doi: 10.1001/jama.2009.1084. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KMK, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1997;52:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA: the Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21:803–810. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychological Medicine. 2012;42:2641–2650. doi: 10.1017/S0033291712000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual Review of Pathology. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Heller R, Biggs A, Pine DS, Grillon C. Becoming the center of attention in social anxiety disorder: startle reactivity to a virtual audience during speech anticipation. The Journal of Clinical Psychiatry. 2011;72:942–948. doi: 10.4088/JCP.09m05731blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychological Science: A Journal of the American Psychological Society. 2008;19:152–160. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32:10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng N. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s Disease patients. Journal of Immunology. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Werts H, Freeman J, Pariante CM, Moffitt TE, Arseneault L. Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash SR, Davey GC. An experimental investigation of the role of negative mood in worry: the role of appraisals that facilitate systematic information processing. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43:823–831. doi: 10.1016/j.jbtep.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Davis M, Montgomery I, Wilson G. Worry and heart rate variables: autonomic rigidity under challenge. Journal of Anxiety Disorders. 2002;16:639–659. doi: 10.1016/s0887-6185(02)00132-9. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2009;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, Bernert S, de Girolamo G, Morosini P, Polidori G, Kikkawa T, Kawakami N, Ono Y, Takeshima T, Uda H, Karam EG, Fayyad JA, Karam AN, Mneimneh ZN, Medina-Mora ME, Borges G, Lara C, de Graaf R, Ormel J, Gureje O, Shen Y, Huang Y, Zhang M, Alonso J, Haro JM, Vilagut G, Bromet EJ, Gluzman S, Webb C, Kessler RC, Merikangas KR, Anthony JC, Von Korff MR, Wang PS, Brugha TS, Aguilar-Gaxiola S, Lee S, Heeringa S, Pennell BE, Zaslavsky AM, Ustun TB, Chatterji S. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA: the Journal of the American Medical Association. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychological Science: A Journal of the American Psychological Society. 2009a;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. Psychobiological responses to social self threat: functional or detrimental? Self Identity. 2009b;8:270–285. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB, Benjamin EJ. Tension and anxiety and the prediction of the 10-year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosomatic Medicine. 2005;67:692–696. doi: 10.1097/01.psy.0000174050.87193.96. [DOI] [PubMed] [Google Scholar]
- Eifert GH, Hodson SE, Tracey DR, Seville JL, Gunawardane K. Heart-focused anxiety, illness beliefs, and behavioral impairment: comparing healthy heart-anxious patients with cardiac and surgical inpatients. Journal of Behavioral Medicine. 1996;19:385–399. doi: 10.1007/BF01904764. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- El Khoury-Malhame M, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, Gellato C, Eric F, Lefebvre MN, Rouby F, Samuelian JC, Anton JL, Blin O, Khalfa S. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology. 2011;85:252–257. doi: 10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress system – organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–267. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Waheed J, Goldman D. Neurophysiological and genetic distinctions between pure and comorbid anxiety disorders. Depression and Anxiety. 2008;25:383–392. doi: 10.1002/da.20378. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Molecular Medicine. 2008;14:195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB. Prolonged exposure therapy: past, present, and future. Depression and Anxiety. 2011;28:1043–1047. doi: 10.1002/da.20907. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Fredrikson M, Furmark T. Amygdaloid regional cerebral blood flow and subjective fear during symptom provocation in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:341–347. doi: 10.1111/j.1749-6632.2003.tb07092.x. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends in Molecular Medicine. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Prospection: experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspectives in Psychiatric Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, De Vivo I. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses’ health study. PloS ONE. 2008;3:e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Hen R. Genetic and environmental factors interact to influence anxiety. Neurotoxicity Research. 2004;6:493–501. doi: 10.1007/BF03033286. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N. Subjective social status moderates cortisol responses to social threat. Brain, Behavior, and Immunity. 2006;20:410–419. doi: 10.1016/j.bbi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. 2008;33:1305–1312. doi: 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, Lange KW, Ceballos-Baumann AO. A common neural basis for receptive and expressive communication of pleasant facial affect. NeuroImage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depression and Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Research. 2000;60:184–190. [PubMed] [Google Scholar]
- Jöels M. Corticosteroid effects in the brain: U-shape it. Trends in Pharmacological Sciences. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, Ripatti S, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PloS ONE. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME. Psychobiological responses to social threat: evolution of a psychological model in psychoneuroimmunology. Brain, Behavior, and Immunity. 2009;23:1–9. doi: 10.1016/j.bbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984–1998. Clinical Chemistry. 2008;54:335–342. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Verschuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behaviour Research and Therapy. 2006;44:1757–1771. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemark EA, Li W. Do all threats work the same way? Divergent effects of fear and disgust on sensory perception and attention. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31:3429–3434. doi: 10.1523/JNEUROSCI.4394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I. Going to the heart of the matter: do negative emotions cause coronary heart disease? Journal of Psychosomatic Research. 2000;48:323–337. doi: 10.1016/s0022-3999(99)00091-4. [DOI] [PubMed] [Google Scholar]
- Lara DR, Pinto O, Akiskal K, Akiskal HS. Toward an integrative model of the spectrum of mood, behavioral and personality disorders based on fear and anger traits: I. Clinical implications. Journal of Affective Disorders. 2006;94:67–87. doi: 10.1016/j.jad.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal and Coping. New York: Springer-Verlag; 1984. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li C, Barker L, Ford ES, Zhang X, Strine TW, Mokdad AH. Diabetes and anxiety in US adults: findings from the 2006 Behavioral Risk Factor Surveillance System. Diabetic Medicine: A Journal of the British Diabetic Association. 2008;25:878–881. doi: 10.1111/j.1464-5491.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- Li SC, Brehmer Y, Shing YL, Werkle-Bergner M, Lindenberger U. Neuromodulation of associative and organizational plasticity across the life span: empirical evidence and neurocomputational modeling. Neuroscience and Biobehavioral Reviews. 2006;30:775–790. doi: 10.1016/j.neubiorev.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Llera SJ, Newman MG. Effects of worry on physiological and subjective reactivity to emotional stimuli in generalized anxiety disorder and nonanxious control participants. Emotion. 2010;10:640–650. doi: 10.1037/a0019351. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, McLaughlin K. Implicit and explicit memory bias in anxiety: a conceptual replication. Behaviour Research and Therapy. 1995;33:1–14. doi: 10.1016/0005-7967(94)e0004-3. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Soong LY, Rutherford EM, Campbell LW. Internet-delivered assessment and manipulation of anxiety-linked attentional bias: validation of a free-access attentional probe software package. Behavior Research Methods. 2007;39:533–538. doi: 10.3758/bf03193023. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, Biondi M, Bosmans E, Kenis G, Sharpé S. Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatrica Scandinavica. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Martens EJ, de Jonge P, Na B, Cohen BE, Lett H, Whooley MA. Scared to death? Generalized anxiety disorder and cardiovascular events in patients with stable coronary heart disease: The Heart and Soul Study. Archives of General Psychiatry. 2010;67:750–758. doi: 10.1001/archgenpsychiatry.2010.74. [DOI] [PubMed] [Google Scholar]
- Mathews A, May J, Mogg K, Eysenck M. Attentional bias in anxiety: selective search or defective filtering. Journal of Abnormal Psychology. 1990;99:166–173. doi: 10.1037//0021-843x.99.2.166. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mogg K, May J, Eysenck M. Implicit and explicit memory bias in anxiety. Journal of Abnormal Psychology. 1989;98:236–240. doi: 10.1037//0021-843x.98.3.236. [DOI] [PubMed] [Google Scholar]
- Maunder R, Toner B, de Rooy E, Moskovitz D. Influence of sex and disease on illness-related concerns in inflammatory bowel disease. Canadian Journal of Gastroenterology. 1999;13:728–732. doi: 10.1155/1999/701645. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Annals of the New York Academy of Sciences. 2010;1204(Suppl.):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser-Stedman R, Dalgleish T, Glucksman E, Yule W, Smith P. Maladaptive cognitive appraisals mediate the evolution of posttraumatic stress reactions: a 6-month follow-up of child and adolescent assault and motor vehicle accident survivors. Journal of Abnormal Psychology. 2009;118:778–787. doi: 10.1037/a0016945. [DOI] [PubMed] [Google Scholar]
- Melamed S, Shirom A, Toker S, Berliner S, Shapira I. Association of fear of terror with low-grade inflammation among apparently healthy employed adults. Psychosomatic Medicine. 2004;66:484–491. doi: 10.1097/01.psy.0000130963.52755.b9. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Hunter SB, Lickel B, Jost JT. Threatened by the unexpected: physiological responses during social interactions with expectancy-violating partners. Journal of Personality and Social Psychology. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of Personality and Social Psychology. 2008;94:278–291. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: aglucocorticoid-resistance model. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Baldwin DS, Brodrick P, Bradley BP. Effect of short-term SSRI treatment on cognitive bias in generalised anxiety disorder. Psychopharmacology. 2004;176:466–470. doi: 10.1007/s00213-004-1902-y. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Georgiades K. The social environment and life stress in depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. second ed. New York: Guilford Press; 2009. pp. 340–360. [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, Braunwald E. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin Tin acute coronary syndromes: a T1M1A substudy. Journal of the American College of Cardiology. 1998;31:1460–1465. doi: 10.1016/s0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- Murphy MLM, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clinical Psychological Science. doi: 10.1177/2167702612455743. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. The British Journal of Psychiatry: the Journal of Mental Science. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold BS, Jr., Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. Journal of Neurosurgery. 1969;30:14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- Newman MG, Llera SJ. A novel theory of experiential avoidance in generalized anxiety disorder: a review and synthesis of research supporting a contrast avoidance model of worry. Clinical Psychology Review. 2011;31:371–382. doi: 10.1016/j.cpr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biological Psychiatry. 2011a;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain, Behavior, and Immunity. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, Epel E. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain, Behavior, and Immunity. 2009;23:446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayon-ayon HN, Rubin SM, Harris TB, Epel ES. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS ONE. 2011b;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Disease Markers. 2011c;30:123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Wolkowitz OM, Blackburn EH, Epel ES. Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity. 2012;26:573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke OI, Prescott J, Wong JYY, Han J, Rexrode KM, De Vivo I. High phobic anxiety is related to lower leukocyte telomere length in women. PLoS ONE. 2012;7:e40516. doi: 10.1371/journal.pone.0040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. The Journal of Comparative Neurology. 1998;401:480–505. [PubMed] [Google Scholar]
- Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain, Behavior, and Immunity. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Glaser R. How stress influences the immune response. Trends in Immunology. 2003;24:444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Piazza JR, Charles ST, Sliwinski MJ, Mogle J, Almeida DM. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Annals of Behavioral Medicine. 2012 doi: 10.1007/s12160-012-9423-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E, Guyer AE, Ernst M, Charney DS, Kaufman J. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. The American Journal of Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA Study. Atherosclerosis. 2006;185:320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type-2 diabetes mellitus. JAMA: the Journal of the American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Prenoveau JM, Craske MG, Zinbarg RE, Mineka S, Rose RD, Griffith JW. Are anxiety and depression just as stable as personality during late adolescence? Results from a three-year longitudinal latent variable study. Journal of Abnormal Psychology. 2011;120:832–843. doi: 10.1037/a0023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Puterman E, O’Donovan A, Adler NE, Tomiyama AJ, Kemeny M, Wolkowitz OM, Epel E. Physical activity moderates effects of stressor-induced rumination on cortisol reactivity. Psychosomatic Medicine. 2011;73:604–611. doi: 10.1097/PSY.0b013e318229e1e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rawlings NB, Norbury R, Cowen PJ, Harmer CJ. A single dose of mirtazapine modulates neural responses to emotional faces in healthy people. Psychopharmacology. 2010;212:625–634. doi: 10.1007/s00213-010-1983-8. [DOI] [PubMed] [Google Scholar]
- Ridker P, Hennekens C, Buring J, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England Journal of Medicine. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker P, Morrow D. C-reactive protein, inflammation, and coronary risk. Cardiology Clinics. 2003;21:315–325. doi: 10.1016/s0733-8651(03)00079-1. [DOI] [PubMed] [Google Scholar]
- Ridker P, Rifai N, Rose L, Buring J, Cook N. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. The New England Journal of Medicine. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. NeuroImage. 2012a;60:523–529. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Allen PS, Pine DS, Grillon C. Acute tryptophan depletion increases translational indices of anxiety but not fear: serotonergic modulation of the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2012b;37:1963–1971. doi: 10.1038/npp.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2009;27:2909–2915. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Wolf OT. Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews. 2010;35:104–114. doi: 10.1016/j.neubiorev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Röntgen P, Sablotzki A, Simm A, Silber RE, Czeslick E. Effect of catecholamines on intracellular cytokine synthesis in human monocytes. European Cytokine Network. 2004;15:14–23. [PubMed] [Google Scholar]
- Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB. Anxiety disorders and comorbid medical illness. General Hospital Psychiatry. 2008;30:208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibeni S, Cortinovis I, Beretta L, Tatarella M, Ferraris L, Rondonotti E, Corbellini A, Bortoli A, Colombo E, Alvisi C, Imperiali G, de Franchis R. Gender and disease activity influence health-related quality of life in inflammatory bowel diseases. Hepato-Gastroenterology. 2005;52:509–515. [PubMed] [Google Scholar]
- Salomé N, Ngampramuan S, Nalivaiko E. Intra-amygdala injection of GABAA agonist, muscimol, reduces tachycardia and modifies cardiac sympatho-vagal balance during restraint stress in rats. Neuroscience. 2007;148:335–341. doi: 10.1016/j.neuroscience.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schneider KL, Appelhans BM, Whited MC, Oleski J, Pagoto SL. Trait anxiety, but not trait anger, predisposes obese individuals to emotional eating. Appetite. 2010;55:701–706. doi: 10.1016/j.appet.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Archives of Internal Medicine. 2007;167:476–482. doi: 10.1001/archinte.167.5.476. [DOI] [PubMed] [Google Scholar]
- Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA: the Journal of the American Medical Association. 2012;307:940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- Seery MD. Challenge or threat? Cardiovascular indexes of resilience and vulnerability to potential stress in humans. Neuroscience and Biobehavioral Reviews. 2011;35:1603–1610. doi: 10.1016/j.neubiorev.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Balhara YP, Sagar R, Deepak KK, Mehta M. Heart rate variability study of childhood anxiety disorders. Journal of Cardiovascular Disease Research. 2011;2:115–122. doi: 10.4103/0975-3583.83040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Annals of the New York Academy of Sciences. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. Stress, inflammation, and depression: a review of empirical evidence and underlying mechanisms. submitted for publication. [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: a psychobiological model of social rejection and depression. Neuroscience and Biobehavioral Reviews. 2010a;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Julian K, Rosenfield D, Powers MB. Threat reappraisal as a mediator of symptom change in cognitive-behavioral treatment of anxiety disorders: a systematic review. Journal of Consulting and Clinical Psychology. 2012;80:624–635. doi: 10.1037/a0028957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain, Behavior, and Immunity. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Völzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosomatic Medicine. 2009;71:1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. Journal of Neuroimmunology. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Nesse RM. Threat detection, precautionary responses, and anxiety disorders. Neuroscience and Biobehavioral Reviews. 2011;35:1075–1079. doi: 10.1016/j.neubiorev.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Reviews. Immunology. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg TE, Tilvis RS. C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1057–1060. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- Strine TW, Chapman DP, Kobau R, Balluz L. Associations of self-reported anxiety symptoms with health-related quality of life and health behaviors. Social Psychiatry and Psychiatric Epidemiology. 2005;40:432–438. doi: 10.1007/s00127-005-0914-1. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NP. New insights into the biology of the acute phase response. Journal of Clinical Immunology. 1999;19:203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC) – Norfolk population study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2011;66:1152–1162. doi: 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- Taghavi MR, Dalgleish T, Moradi AR, Neshat-Doost HT, Yule W. Selective processing of negative emotional information in children and adolescents with Generalized Anxiety Disorder. The British Journal of Clinical Psychology. 2003;42:221–230. doi: 10.1348/01446650360703348. [DOI] [PubMed] [Google Scholar]
- Taylor L, Loerbroks A, Herr RM, Lane RD, Fischer JE, Thayer JF. Depression and smoking: mediating role of vagal tone and inflammation. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2011;42:334–340. doi: 10.1007/s12160-011-9288-7. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Thayer JF. On the importance of inhibition: central and peripheral manifestations of nonlinear inhibitory processes in neural systems. Dose Response. 2006;4:2–21. doi: 10.2203/dose-response.004.01.002.Thayer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic antiinflammatory pathway in healthy human adults. Journal of Internal Medicine. 2009;265:439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Loerbroks A, Sternberg EM. Inflammation and cardiorespiratory control: the role of the vagus nerve. Respiratory Physiology & Neurobiology. 2011;178:387–394. doi: 10.1016/j.resp.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Neural concomitants of immunity–focus on the vagus nerve. NeuroImage. 2009;47:908–910. doi: 10.1016/j.neuroimage.2009.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain, Behavior, and Immunity. 2010;24:1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, von Eye A, Rovine MJ. Assessment of neural network models using prediction analysis. Biomedical Sciences Instrumentation. 1995;31:25–28. [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3 doi: 10.3389/neuro.09.068.2009. 1477 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, Tanida M, Tajiri M, Hazama K, Ogata K, Hashimoto H, Baba A. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress. 2011;14:368–375. doi: 10.3109/10253890.2010.544345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vollenhoven RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Medicine. 2009;7:12. doi: 10.1186/1741-7015-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular disease, interleukin-6, and risk of mortality in older women: The Women’s Health and Aging Study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- Von Kanel R, Begre S, Abbas CC, Saner H, Gander ML, Schmid JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010;17:39–46. doi: 10.1159/000243084. [DOI] [PubMed] [Google Scholar]
- von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosomatic Medicine. 1998;60:498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstäter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA: the Journal of the American Medical Association. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor K, Bobova L, Zinbarg RE, Mineka S, Craske MG. Longitudinal investigation of the impact of anxiety and mood disorders in adolescence on subsequent substance use disorder onset and vice versa. Addictive Behaviors. 2012;37:982–985. doi: 10.1016/j.addbeh.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody EZ, Szechtman H. Adaptation to potential threat: the evolution, neurobiology, and psychopathology of the security motivation system. Neuroscience and Biobehavioral Reviews. 2011;35:1019–1033. doi: 10.1016/j.neubiorev.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Sciences. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Jr., Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. The Journal of Nervous and Mental Disease. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yin WH, Chen JW, Jen HL, Chiang MC, Huang WP, Feng AN, Young MS, Lin SJ. Independent prognostic value of elevated high-sensitivity C-reactive protein in chronic heart failure. American Heart Journal. 2004;147:931–938. doi: 10.1016/j.ahj.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]