Abstract
Background
Alterations in frontal and striatal function are hypothesized to underlie risky decision-making in drug users, but how these regions interact to affect behavior is incompletely understood. We used mediation analysis to investigate how prefrontal cortex and ventral striatum together influence risk avoidance in abstinent drug users.
Method
Thirty-seven abstinent substance-dependent individuals (SDI) and 43 controls underwent fMRI while performing a decision-making task involving risk and reward. Analyses of a priori regions-of-interest tested whether activity in dorsolateral prefrontal cortex (DLPFC) and ventral striatum (VST) explained group differences in risk avoidance. Whole-brain analysis was conducted to identify brain regions influencing the negative VST-risk avoidance relationship.
Results
Right DLPFC (RDLPFC) positively mediated the group-risk avoidance relationship (p < 0.05); RDLPFC activity was higher in SDI and predicted higher risk avoidance across groups, controlling for SDI vs. controls. Conversely, VST activity negatively influenced risk avoidance (p < 0.05); it was higher in SDI, and predicted lower risk avoidance. Whole-brain analysis revealed that, across group, RDLPFC and left temporal-parietal junction positively (p ≤ 0.001) while right thalamus and left middle frontal gyrus negatively (p < 0.005) mediated the VST activity-risk avoidance relationship.
Conclusion
RDLPFC activity mediated less risky decision-making while VST mediated more risky decision-making across drug users and controls. These results suggest a dual pathway underlying decision-making, which, if imbalanced, may adversely influence choices involving risk. Modeling contributions of multiple brain systems to behavior through mediation analysis could lead to a better understanding of mechanisms of behavior and suggest neuromodulatory treatments for addiction.
Keywords: Substance dependence, Mediation, Ventral striatum (VST), Dorsolateral prefrontal cortex (DLPFC), Decision-making, Impulsivity
1. INTRODUCTION
Risky decision-making is a hallmark of substance use disorders. Individuals who abuse drugs also display impaired risk avoidance (i.e., exhibit risk-seeking behavior) on laboratory decision-making tasks that involve reward, punishment, and uncertainty (Bechara and Damasio, 2002; Grant et al., 2000). The neural circuitry of decision-making is complex, but a large body of evidence supports the roles of prefrontal cortex, striatum, and limbic structures. The dorsolateral prefrontal cortex (DLPFC) is involved in cognitive control through choice selection, interference monitoring, and pre-potent response inhibition (Blasi et al., 2006). The right DLPFC (RDLPFC), in particular, is involved in decisions requiring response inhibition (Aron, 2011; Ernst et al., 2002; Nee et al., 2007) or when choices are ambiguous (Krain et al., 2006; Rodrigo et al., 2014). It has been suggested that RDLPFC causally inhibits risky decision-making as previous work has shown that stimulation of RDLPFC increased risk avoidance (Fecteau et al., 2007) and reduced drug cravings in addicts (Camprodon et al., 2007; Fregni et al., 2008; Mishra et al., 2010) while suppression of RDLPFC activity was associated with riskier decision-making (Knoch et al., 2006).
The striatum is also important for decision-making under conditions of uncertainty and risk (Ernst et al., 2004; Matthews et al., 2004; Tom et al., 2007) and dopamine regulation in the striatum is a critical mechanism underlying this process. Higher dopamine D1 receptor mRNA expression in the ventral striatum (VST) has been associated with greater risk-taking in rats (Simon et al., 2011). In humans, VST activity is positively associated with decisions made under uncertainty (Linnet et al., 2011; Li et al., 2010) and risk (Matthews et al., 2004) and, in particular, with loss aversion during risky decisions (Tom et al., 2007).
Numerous lines of evidence indicate that frontal and striatal function is altered in drug users which may mediate increases in risky decision-making. Decision-related activity in DLPFC is attenuated in drug users compared to healthy controls, suggesting impaired inhibitory cognitive control (Ersche et al., 2005; Paulus et al., 2002). Increased striatal activity has been found in substance-dependent individuals compared to controls during reward anticipation (Nestor et al., 2010; Yamamoto et al., 2014) or notification of reward outcome (Bjork et al., 2008; Jia et al., 2011; but see Hyatt et al., 2012) suggesting heightened striatal response during decision-making is related to increased reward sensitivity in drug users.
Apart from possible independent contributions to decision-making deficits in drug users, striatum and DLPFC interact in ways that are likely important for drug related behavior. There is a close anatomical relationship between sectors of prefrontal cortex (e.g., ventral medial, dorsolateral, and orbital frontal cortex) and striatum (Haber and Knutson, 2010) and these regions appear to influence each other functionally (Staudinger et al., 2011). Lower dopamine D2 receptor binding in the striatum has been shown to correlate with lower frontal metabolism in stimulant abusers (Volkow et al., 2001, 1993) and is associated with craving (Volkow et al., 2006). In addition, impaired reward learning in alcoholic subjects has been associated with abnormal functional connectivity between VST and RDLPFC (Park et al., 2010). These previous studies reporting correlations between fronto-striatal function and behavior suggest that striatal dysregulation influences frontal function, manifesting as pathological motivation in substance dependent individuals to procure drugs despite known risks. However, the exact nature of the interactions between striatal and frontal activity, and between fMRI activity and risky behavior in substance dependent populations, remains incompletely understood.
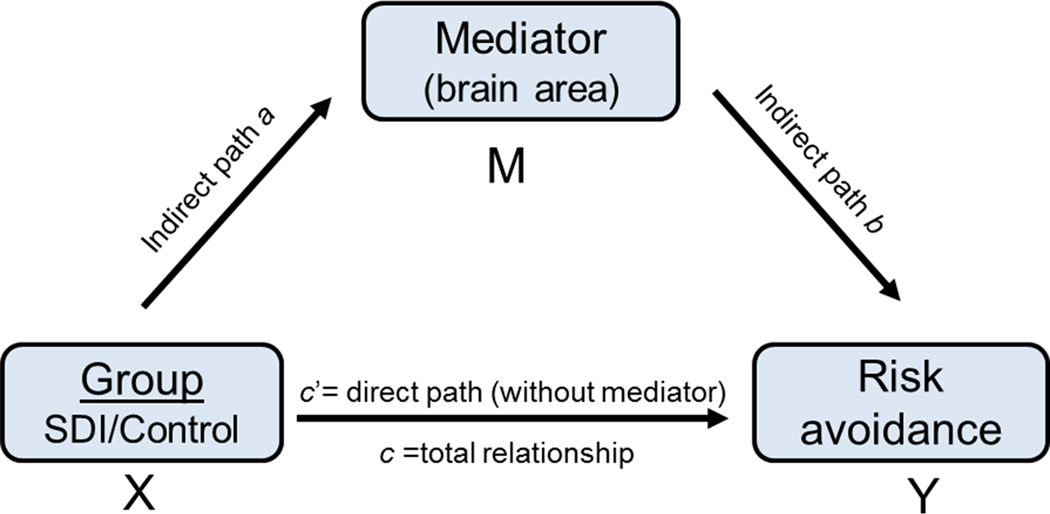
Mediation is a statistical method that can inform our understanding of how brain regions interact to result in behavior. Mediation tests whether the relationship between an independent and a dependent variable can be explained by a third variable (Figure 1) and has been used extensively in psychology research to test relational pathways among correlated variables (Baron and Kenny, 1986; MacKinnon et al., 2007). Though it has often been used to infer causality from observational data, which has been controversial (Green et al., 2010), it need not imply causal effects to provide useful models of statistical multivariate relationships. Applied to neuroimaging, studies have shown that the relationship between DLPFC activity and cognitive control of tobacco craving was mediated by decreased VST activity (Kober et al., 2010). In other words, the mediation model suggests that increases in DLPFC activity are associated with control of craving through reductions in VST activity. We use mediation analysis to investigate how DLPFC and VST activity during decision-making influence risk avoidance in long-term abstinent substance dependent individuals and controls. Because of its known contribution to addiction, impulsivity was tested as a trait mediator of risk avoidance. To our knowledge, the influence of regional and whole brain activity on risk avoidance has not been performed using these methods in drug dependence.
Figure 1. Single-level mediation model.
Path a represents the relationship of X to M. Path b represents the relationship of M to Y while controlling for X, c’ represents the relationship of X to Y controlling for M, and c represents the indirect relationship of X to Y (not adjusted for any other factors).
2. METHODS
In a prior study, we reported increased striatal activity and impaired risk avoidance in substance dependent individuals (SDI) compared to controls and a negative VST-risk avoidance relationship. The data collection has already been described and is briefly repeated here for ease of understanding. Notably, this study uses a completely different analysis technique to determine if DLPFC and VST activity have different mediation effects on increased risky behavior in long-term abstinent SDI.
2.1 Subjects
The sample population included 80 subjects: 37 SDI (18M/19F) and 43 controls (23M/20F). SDI with lifetime DSM-IV stimulant dependence were recruited from a residential treatment program at the University of Colorado Denver Addiction Research and Treatment Service (ARTS). SDI were abstinent from drugs and alcohol an average of 14 months (range=2–65, standard deviation=14.33). Most SDI were referred to ARTS from the criminal justice system where they were abstinent from drugs, alcohol, and tobacco. SDI were recruited to this study 2–4 months after admission to ARTS, where abstinence from drugs, alcohol and tobacco is monitored by direct supervision and random drug screening. These factors contributed to the long abstinence duration. Controls were recruited from the community and excluded if they met DSM-IV criteria for lifetime abuse or dependence on drugs or alcohol. Exclusions for all subjects included neurological illness, schizophrenia, bipolar disorder, major depression within the last 2 months, head trauma resulting in >15 minutes loss of consciousness, or IQ ≤ 80. All subjects provided written informed consent approved by the Colorado Multiple Institutional Review Board.
2.2 Behavioral measures
2.2.1. Screening Assessment
All subjects received structured interviews and behavioral measures administered by trained lay professionals. Drug dependence was assessed using the computerized Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM; Cottler et al., 1989). DSM-IV dependence diagnoses are listed in Table 2. The Computerized Diagnostic Interview Schedule–Version IV (C-DIS-IV) was administered to exclude schizophrenia, bipolar disorder, and current major depression (within 2 months). IQ was assessed with matrix and verbal reasoning Wechsler Abbreviated Scale of Intelligence subtests (WASI; Psychological Corporation, 1999). Impulsivity was measured using the Barratt impulsiveness scale (BIS-11), a 30-item self-report questionnaire (Patton et al., 1995).
Table 2.
Substance Dependence Diagnoses in SDI (n = 37)
| Individual Substance | Number with diagnosis |
Percent with diagnosis |
|---|---|---|
| Stimulants Total | 37 | 100 |
| Stimulants (Cocaine) | 21 | 57 |
| Stimulants (Amphetamines) | 31 | 84 |
| Alcohol | 27 | 73 |
| Tobacco | 26 | 70 |
| Cannabis | 15 | 41 |
| Opioids | 10 | 27 |
| Combination of Dependence Diagnoses | ||
| Stimulants only | 2 | 5 |
| Stimulants plus alcohol and/or tobacco | 32 | 86 |
| Stimulants plus cannabis | 15 | 41 |
| Stimulants plus opioids | 10 | 27 |
2.2.2. Decision-making test of risk avoidance
Subjects played a modified version of the computerized Iowa Gambling Task (IGT) during fMRI scanning. This decision-making task is sensitive to differences in risk avoidance (Thompson et al., 2012) and loss sensitivity (Tanabe et al., 2013) in SDI compared to healthy controls. Subjects were presented four decks of cards and instructed to earn as much pretend money as possible by choosing to either play or pass on a given deck. A “Play” response resulted in a single positive or negative monetary value, along with the running total. “Pass” response resulted in no change. To perform well, subjects had to learn to “Pass” on the two bad decks that resulted in net loss and “Play” on the two good decks that resulted in net gain over time. Risk avoidance was defined as number of passes on bad decks. For each trial the card was presented for 2 seconds, during which time the subject responded. Outcomes were shown for 4 seconds. There were 50 trials of each deck, 200 trials total, plus 50 fixation crosses presented in pseudorandom order. Total task scan time was 26 minutes (2 13 minute runs).
2.3. MRI acquisition, pre-processing, and fMRI data analysis
Functional MR images were acquired on a 3T scanner with an 8-channel head coil using GRE-EPI sequence (TR 2s, TE 30 ms, matrix 64 × 64, FOV 220 mm2, 3.4 × 3.4 mm2, slice thickness 3 mm, gap 1 mm). Data were analyzed with Statistical Parametric Mapping (SPM8). Pre-processing included motion correction, normalization to MNI space, and spatial smoothing with 6 mm FWHM Gaussian kernel. Motion exceeding 1-voxel was excluded from further analysis. First level analysis consisted of filtering low frequency noise, correcting for temporal autocorrelation, and convolving the stimulus function with a canonical hemodynamic response function. Nine conditions were modeled: decision and outcome for each of the four decks plus fixation. The contrast of interest was decision>fixation.
2.4. Region-of-interest (ROI) definition
VST was manually traced in MNI standard space using the anatomical landmarks from Mawlawi et al. (2001). The DLPFC ROI was based upon coordinates obtained from the metaanalysis framework Neurosynth (http://neurosynth.org; Yarkoni et al., 2011), which identifies neuroimaging studies reporting significant activity associated with a given feature, in this case, “decision-making”. Coordinates are given a z-score based on representation over multiple studies. Among 66 studies showing significant activity associated with “decision-making” (downloaded on 07-22-2014), the coordinate with the highest z-score (5.47) localized to the RDLPFC (MNI: 34, 32, 38). This coordinate was used to construct a 6 mm diameter sphere representing a decision-making node. Although evidence points preferentially to the RDLPFC as important for cognitive control of impulses in risky decision-making, the LDLPFC was also examined. LDLPFC was constructed to mirror the RDLPFC ROI (MNI: −34, 32, 38). Two ROIs were used as controls: primary sensory cortex (PSC; Brodmann area 1) because PSC is not known to be involved in risk avoidance; and dorsal striatum (DST) to assure that mediation results were not simply due to the group differences in VST and DST activity seen in our prior paper. DST was manually traced in MNI standard space according to anatomical landmarks from Mawlawi et al. (2001). For each ROI, fMRI signal during decision-making was extracted using the Marsbar toolbox.
2.5. Statistical analyses
2.5.1. Single level mediation
To test whether RDLPFC and VST activity mediated group differences in risk avoidance, analysis was performed using a mediation toolbox (http://wagerlab.colorado.edu/tools; Wager et al., 2008). A standard mediation model was used, with a bootstrap test (10,000 iterations) for statistical significance of the mediators (Efron and Tibshirani, 1994; Shrout and Bolger, 2002). Mediation quantifies the degree in which a relationship between two variables, X and Y, can be explained by another variable, M (Figure 1). We defined X as group (SDI or control), Y as risk avoidance and M as RDLPFC or VST activity during decision-making. When testing for two simultaneous mediators, the significance of each one is assessed while controlling for effects of the other. For example, the significance of paths a, b, and a*b are assessed controlling for VST activity, and the significance of paths a2, b2, and a2*b2 are assessed controlling for RDLPFC activity (Figure 2). Paths a and a2 measure the association between group (SDI vs. control) and the mediator (RDLPFC or VST activity). Paths b and b2 measure the association between mediator and risk avoidance while controlling for group. Controlling for group in paths b and b2 tests whether RDLPFC or VST activity predict variations in risk avoidance conditionally independent of group. Path c measures the total relationship between group and risk avoidance including direct and indirect effects. Path c’ measures the direct effect of relationship between group and risk avoidance, controlling for RDLPFC and VST activity. Finally, products a*b and a2*b2 separately test the significance of the mediators (Wager et al., 2009, 2008).
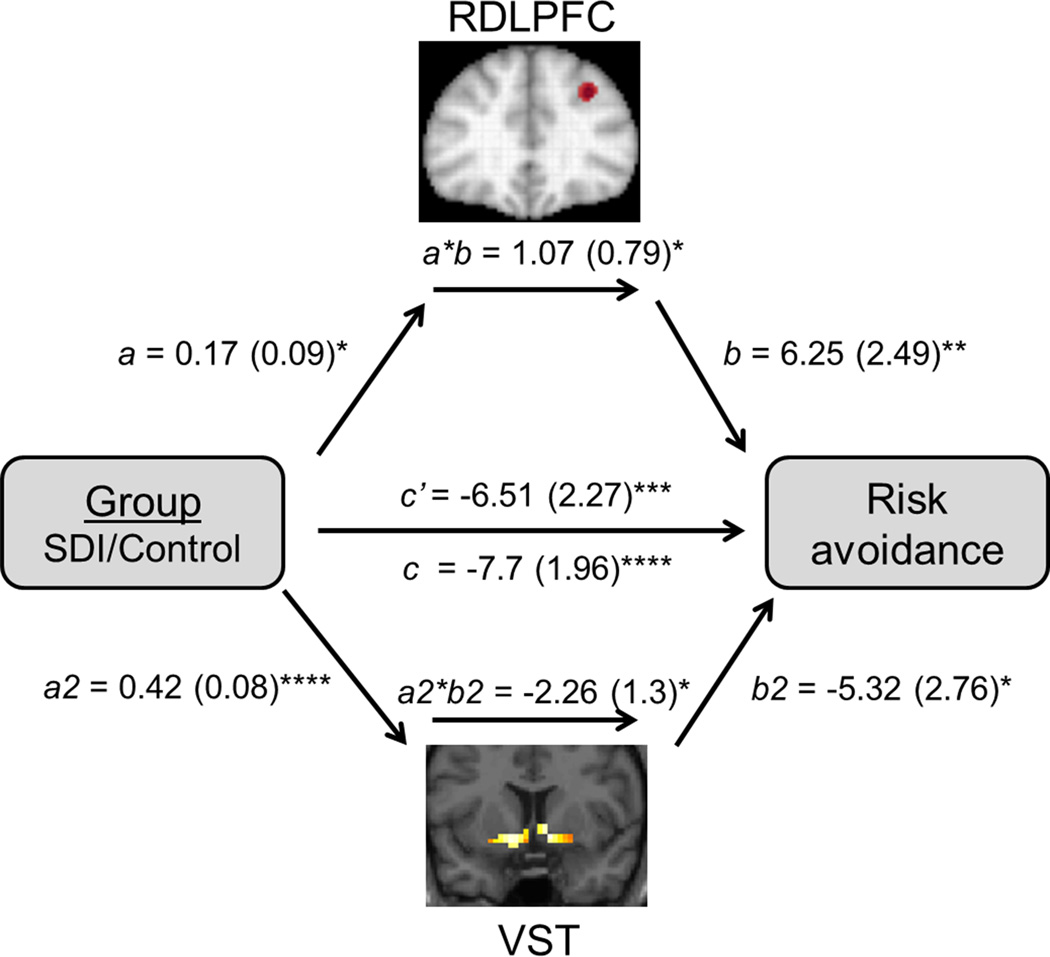
Figure 2. Single-level mediation analysis.
RDLPFC and VST oppositely mediate group differences in risk avoidance. Path coefficients are shown next to arrows with standard errors in parentheses. Path a is from the group (X) to the RDLPFC (M1). Path b is from RDLPFC (M1) to risk avoidance (Y). Path a2 is from the group (X) to the VST (M2). Path b2 is from VST (M2) to risk avoidance (Y). Paths b and b2 are calculated controlling for group (X). Paths a, b, and a*b control for VST (M2), and Paths a2, b2, and a2*b2 control for RDLPFC (M1). The direct path c’ is calculated controlling for both mediators. ****p<0.001, ***p<0.005, **p<0.01, *p<0.05, one-tailed; right dorsolateral prefrontal cortex (RDLPFC), ventral striatum (VST), mediator (M1, M2)
2.5.2. Whole-brain mediation analyses
Mediation Effect Parametric Mapping (MEPM; Wager et al., 2008) is a form of structural equation modeling that makes it possible to map multiple brain mediators of group differences in behavior or of a brain/behavior relationship. MEPM was used to explore brain regions not hypothesized to be mediators a priori. Here, group was variable X, risk avoidance was variable Y, and voxels across the brain were serially tested as mediation variable M in order to form a brain map of mediation effects. To investigate mediators of the relationship between VST activity and risk avoidance across group, another MEPM test was conducted, in which VST was variable X, risk avoidance was variable Y, and voxels across the brain were tested as candidate mediators (M). Bootstrap tests (1,000 iterations) for statistical significance were performed for each voxel. For the exploratory whole-brain analyses, voxels were considered to be significant mediators if statistical significance reached p<0.005, two-tailed, uncorrected, and at least five contiguous voxels in paths a, b, and a*b.
2.5.3. Impulsivity and risk avoidance
Impulsivity has been strongly correlated with addiction and poor decision-making, thus, impulsivity measured by BIS-11 was compared between SDI and controls using analysis of covariance (ANCOVA) controlling for education. Impulsivity was correlated with risk avoidance using Pearson’s R in SPSS. To test if impulsivity mediated group difference in risk avoidance, single level mediation was performed: group was variable X, risk avoidance was variable Y, and impulsivity was variable M.
2.5.4. Drug symptom count and brain activity
SDI were recruited for stimulant-dependence, however most exhibited comorbid dependence with other drugs (Table 2). Drug use severity was measured by DSM-IV symptom counts (11 for each drug). Total symptoms were calculated for stimulants alone and all drugs combined, then correlated with activity within each ROI.
3. RESULTS
3.1. Demographics
SDI and controls were similar in age (34.4±8.4 vs. 31.6±9.3 years, p=0.17) and gender (18M/19F vs. 23M/20F, chi-squared=0.19, p=0.67). SDI had fewer years of education than controls (12.8±1.4 vs. 14.7±1.5, p<0.001).
3.2. Drug characteristics
Please see Table 2.
3.3. Single-level mediation
When RDLPFC and VST were entered simultaneously as candidate mediators, there were opposing, significant influences on the relationship between group (SDI vs. control) and risk avoidance (Figure 2). The total relationship between group and risk avoidance was highly significant, (path c, coefficient=-7.7, z=-3.79, p<0.001), indicating reduced risk avoidance in SDI. SDI showed greater RDLPFC activity (path a, coefficient=0.17, z=1.66, p<0.05). Greater RDLPFC activity predicted increased risk avoidance controlling for group and VST activity (path b, coefficient=6.25, z=2.5, p<0.01). The mediation effect was significant (a*b, coefficient=1.07, z=1.77, p<0.05). VST activity, by contrast, was associated with decreased risk avoidance. VST activity was higher in SDI (path a2, coefficient=0.42, z=3.81, p<0.001), predicted reduced risk avoidance (path b2, coefficient=-5.32, z=-2.1, p<0.05) and was a significant mediator (a2*b2, coefficient=-2.26, z=-2.09, p<0.05). Thus, both RDLPFC and VST activity were higher in SDI, but whereas VST reduced risk-avoidant behavior, RDLPFC acted as a suppressor variable (MacKinnon et al., 2000) predicting enhanced risk-avoidance. There was no mediating effect of LDLPFC on group differences in risk avoidance. The control regions, PSC and DST, did not demonstrate any mediating effects separately or combined.
3.4. Whole brain mediation effect parametric mapping (MEPM)
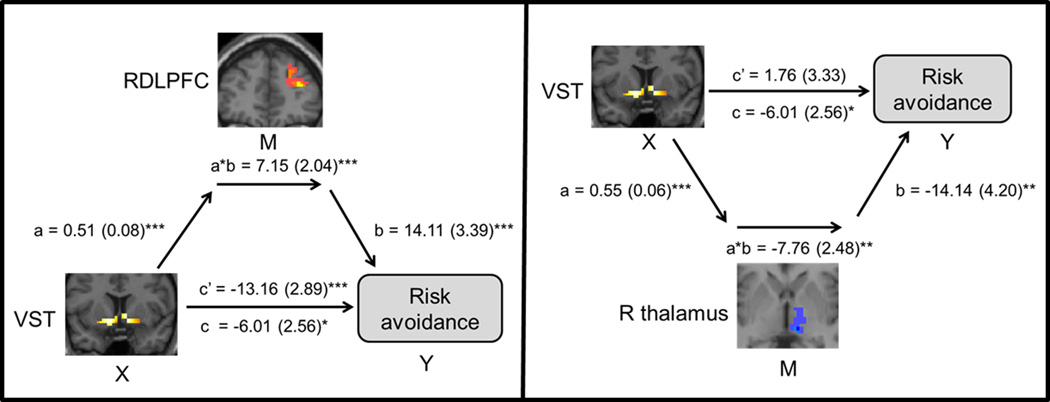
Whole brain MEPM found no regions mediating between-group differences in risk avoidance. Across group, MEPM revealed positive mediation in RDLPFC and left temporoparietal junction and negative mediation in the right thalamus and left middle frontal gyrus (Figure 3, table 1) of the VST-risk avoidance relationship.
Figure 3. Mediation Effect Parametric Mapping (MEPM).
Across group, RDLPFC positively mediated and the right thalamus negatively mediated the VST-risk avoidance relationship. The a path is from the VST (X) to each mediating region. The b path is from the mediating region (M) to risk avoidance (Y). The b path is calculated controlling for VST (X) and for other mediators. Path coefficients are shown next to arrows with standard errors in parentheses. The direct path c’ is calculated controlling for the mediator. Coefficients and p values were calculated using maxstat. ***p<0.001, **p<0.005, two-tailed. Ventral striatum (VST), right dorsolateral prefrontal cortex (RDLPFC), right thalamus (R thalamus)
Table 1.
Mediators of the VST relationship with risk avoidance
| Coordinates | a Path | b Path | ab Path | Conjunction (voxels) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mediators | Name | x | y | z | Z | p | Z | p | Z | p | p<0.005 | p<0.01 | p<0.05 |
| Positive | RDLPFC | 30 | 38 | 34 | 5.81 | <0.001 | 3.94 | <0.001 | 3.37 | <0.001 | 5 | 18 | 185 |
| LTPJ | −42 | −22 | 37 | 5.69 | <0.001 | 3.71 | <0.001 | 3.20 | 0.001 | 11 | 24 | 288 | |
| Negative | Rthal | 9 | −16 | −2 | 7.12 | <0.001 | −3.24 | 0.001 | −3.03 | 0.003 | 6 | 12 | 108 |
| LMFG | −27 | 2 | 55 | 5.70 | <0.001 | −3.10 | 0.002 | −2.79 | 0.005 | 14 | 22 | 48 | |
LTPJ, left temporoparietal junction; RDLPFC, right dorsolateral prefrontal cortex; Rthal, right thalamus; LMFG, left middle frontal gyrus; p values calculated using maxstat.
3.5. Impulsivity and risk avoidance: Group effects and mediation
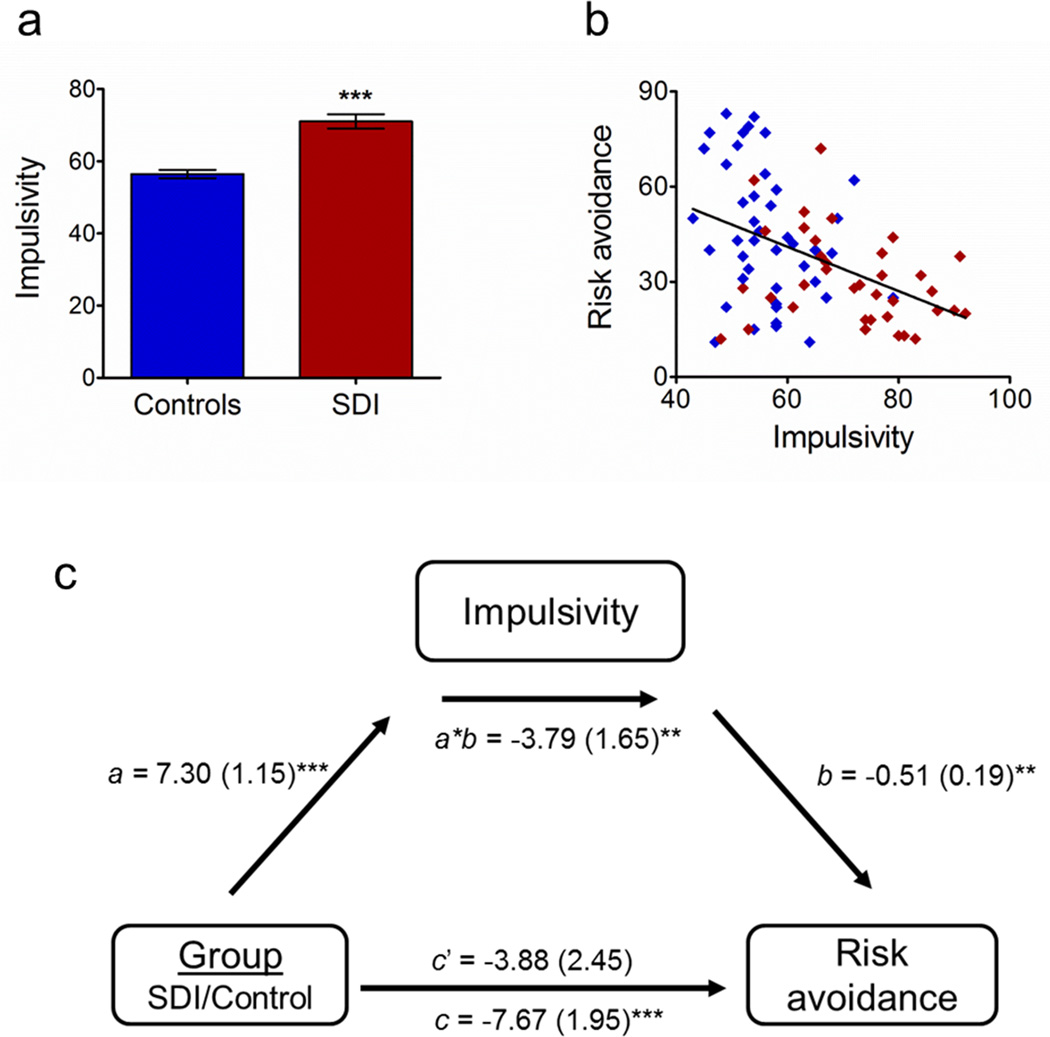
Impulsivity was greater in SDI than controls (71.05±12.0 vs. 56.49±7.58; F[1,78]=18.92, p<0.001; figure 4a). Across group, impulsivity negatively correlated with risk avoidance (r=-0.435, p<0.001; figure 4b). Impulsivity mediated group differences in risk avoidance (figure 4c). The total relationship between group and risk avoidance was highly significant (path c, coefficient=-7.67, z=-3.75, p<0.001), indicating SDI displayed reduced risk avoidance. Impulsivity was greater in SDI (path a, coefficient=7.30, z=3.61, p<0.001), predicted reduced risk avoidance (path b, coefficient=-0.51, z=-3.19, p<0.005) and was a significant mediator (a*b, coefficient=-3.79, z=-3.21, p<0.005). Impulsivity was a full mediator, meaning that group differences in risk avoidance were no longer significant after controlling for impulsivity (path c’, coefficient=-3.88, z=-1.42, p=0.15).
Figure 4. Impulsivity affects risk avoidance.
a) Controls (blue) were less impulsive than SDI (red; p<0.001). b) Risk avoidance and impulsivity were negatively correlated across groups (r=-0.435, p<0.001). c) Mediation analysis results depicting the mediating effect of impulsivity on group differences in risk avoidance. After controlling for the mediating effect of impulsivity, the relationship between group and risk avoidance was no longer significant. ***p<0.001, **p<0.005, two-tailed
3.6 Drug symptom count and brain activity
Neither stimulant symptom count nor total symptom count correlated with regional brain activity.
4. DISCUSSION
The current study sought to determine how frontal and striatal brain activity influences risk avoidance in long-term abstinent substance dependent individuals (SDI). Mediation analysis revealed opposing influences of right dorsolateral prefrontal cortex (RDLPFC) and ventral striatal (VST) activity on group differences in decision-making. RDLPFC acted as a positive mediator associated with improved risk avoidance while VST was a negative mediator associated with decreased risk avoidance. The findings are consistent with the proposed role of DLPFC in response inhibition (Blasi et al., 2006). A recent study on self-control showed that DLPFC was activated during successful response inhibition that involved foregoing an immediate smaller reward in favor of a delayed larger reward (Crockett et al., 2013). DLPFC is also involved in reward valuation and goal-directed decision-making, processes that play a role in mediating group differences in risk avoidance (Fecteau et al., 2007; Hare et al., 2009; Mohr et al., 2010). While RDLPFC appears to be involved in impulsive choice inhibition during decision-making (Ersche et al., 2005; Schonberg et al., 2012) the left DLPFC has been implicated in deliberative and inter-temporal cognitive processing (Hayashi et al., 2013; Pripfl et al., 2013). Our results are consistent with hemispheric specialization in that the decision-making task requires successful inhibition of a pre-potent response to ‘Play’ and win hypothetical money. Because the response must be made in less than 2 seconds, there is no time for deliberation. Our findings suggest that greater RDLPFC activity is associated with more successful inhibition of ‘Play’ responses on the risky decks.
Mediation analysis provides stronger tests than the component parts, as a*b is not just the conjunction of path a and path b. It differs from simple correlation in that it brings in a third variable to explain the correlation (Baron and Kenny, 1986; MacKinnon et al., 2007; Preacher and Hayes, 2004). Mediation differs from another technique, psychophysiological interaction (PPI). PPI assesses the significance of differential connectivity between brain regions depending on task state (O’Reilly et al., 2012), but does not attempt to determine whether a connection is direct or mediated by another factor. Rather, PPI analysis is a special form of moderation, a test of whether connectivity differs based on the level of a 3rd variable (Friston et al., 1997). It is therefore suited for identifying conditional functional connectivity between two regions, but not functional pathways that involve more than two regions in ‘series.’ PPI analyses also do not allow multiple brain regions to simultaneously predict or explain a behavioral effect. Our goal was thus to use mediation to study how brain activity can explain the relationship between group and risk avoidance. Mediation can be used to build models in which multiple brain systems contribute to behavior (Lim et al., 2009; Wager et al., 2009, 2008). Those systems may have multiple separable effects, as seen here; the effects of RDLPFC and VST are opposing and separable.
The suppressive effect of RDLPFC activity on risky choices has potential implications for treatment. For example, using transcranial direct current stimulation, investigators demonstrated that increasing RDLPFC activity during decision-making decreased risky choices and increased error awareness (Fecteau et al., 2007; Harty et al., 2014) while disruption of RDLPFC by low-frequency repetitive transcranial magnetic stimulation (TMS) resulted in controls making riskier choices and showing worse performance (Knoch et al., 2006). Incorporating these neuromodulatory techniques in future work could bolster causality claims, either by inducing a functional lesion with low frequency TMS or inducing functional augmentation with high frequency TMS.
VST activity was associated with decreased risk avoidance. Impulsive decisions have been associated with VST activity in healthy subjects (McClure et al., 2004; Plichta and Scheres, 2014). Thus, greater VST activity in abstinent SDI compared to controls may be associated with impulsive decision-making that is exacerbated if unopposed or weakly opposed by insufficient RDLPFC activity. Our results are consistent with reports of reductions in alcohol craving and improved abstinence in three patients treated with deep brain stimulation of the nucleus accumbens (Heinze et al., 2009; Müller et al., 2009) since DBS is thought to suppress neural activity. These promising studies underscore the need to understand more precisely the role of striatum in the neural circuitry of drug related behavior.
Few studies have examined brain activity during decision-making in long-term abstinent SDI (Ersche et al., 2005; Patel et al., 2013). Differences in brain activity between SDI and controls after prolonged abstinence suggests that the pattern we observed may be an endophenotype that predisposes one to drug use; or, alternatively, that neurocircuitry changes resulting from drug use are long-lasting. Longitudinal studies are needed to separate these possibilities. Regardless of the causal relationships, our findings suggest that brain activity differences appear to persist even with sustained, full remission.
Whole brain analysis found no mediation of group differences in risk avoidance. Perhaps with greater power (larger sample size) an effect would survive multiple comparisons. However, across-group analysis revealed that RDLPFC and an area of the left temporoparietal junction (TPJ) positively mediated the VST activity-risk avoidance relationship. The exact role of TPJ activity in the current study is unknown. There is evidence of a role for TPJ in social cognition, memory, and attention (Carter and Huettel, 2013) suggesting that TPJ may support cognitive processing that positively influences risk avoidance. Conversely, negative mediation of the relationship between VST activity and risk avoidance was found in the medial right thalamus. Thalamus and striatum influence each other through reciprocal circuitry (Haber and Knutson, 2010). Our findings are consistent with a prior study showing striatal-thalamic activity correlated with impulsivity during decision-making in cocaine users and healthy controls (Leland et al., 2006).
The fact that MEPM revealed RDLPFC as a positive mediator of VST activity and risk avoidance across group but not at the group level is consistent with classifying mental disorders based on dimensions of “observable behavior and neurobiological measures” (National Institute of Mental Health Research Domain Criteria (RDoC; http://www.nimh.nih.gov/research-priorities/rdoc). Addiction lies on one end of the behavioral spectrum of impulsivity, compulsivity, and sensation-seeking found in non-addicted individuals (Jentsch et al., 2014; Koob and Le Moal, 2008). Familial studies have shown that non-drug using family members of SDI exhibit behaviors intermediate between SDI and unrelated non-drug using healthy controls (Ersche et al., 2012) consistent with contributions from both genes and environment and supporting a role for analysis at the individual, in addition to group, level.
Impulsivity is a dimensional trait strongly associated with addiction and poor decision-making (Dolan et al., 2008; Hariri et al., 2006; Plichta and Scheres, 2014) and is both a risk factor for and a consequence of drug addiction (Feil et al., 2010; Jentsch and Taylor, 1999). VST reward sensitivity correlates strongly with impulsivity in healthy controls (Forbes et al., 2009) and striatal activity has been associated with impulsivity in alcoholics and cocaine users (Beck et al., 2009; Leland et al., 2006). The present study extends those results, demonstrating that impulsivity fully mediates group differences in risk avoidance: when impulsivity is removed as a factor, group differences in risk avoidance are no longer significant.
To our knowledge, this is the first study to investigate the influence of neuroanatomical regions on decision making in substance dependence using mediation analysis. It should be noted that this study was not designed to test causality, thus we can only state that the mediating regions influence the group-performance relationship. Neuromodulation with DBS or TMS is one way to directly manipulate striatum, DLPFC, or other regions to test for causal relationships for the mediators. The current task could be modified in future studies to address causality. Another limitation is that it is impossible to know if alterations in brain activity in SDI preceded or were a result of drug use.
In summary, we report two separate, opposing pathways influencing group differences in risk avoidance during decision-making: a positive pathway through the RDLPFC that increased risk avoidance, and a negative pathway through the VST that decreased risk avoidance. Furthermore, impulsivity may play a role in the circuit-behavior relationships across individuals. Future studies aimed at confirming an imbalance in frontal-striatal influence on risk avoidance could lead to novel treatments in addiction.
Supplementary Material
Highlights.
Drug users display impaired risk avoidance compared to controls.
We use formal mediation analysis to investigate neural correlates of risk avoidance.
Prefrontal cortex positively and striatum negatively mediated risk avoidance.
Imbalanced fronto-striatal activity may predict risky decision-making in addiction.
Acknowledgements
The authors thank Dr. Robert Perry for helpful comments on this manuscript.
Role of funding source
Funding for this study was provided by the National Institute of Drug Abuse (NIDA) grants DA024104 (JT) and DA027748 (JT); NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
Yamamoto and Tanabe contributed to design, analysis, data interpretation and manuscript preparation. Woo and Wager contributed to analysis, data interpretation and manuscript preparation. Regner contributed to data interpretation and manuscript preparation. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors report no potential conflicts of interest.
REFERENCES
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. NeuroImage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, Bertolino A, Callicott JH, Weinberger DR, Mattay VS. Brain regions underlying response inhibition and interference monitoring and suppression. Eur. J. Neurosci. 2006;23:1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Bolla K, Eldreth D, London E, Kiehl K, Mouratidis M, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprodon JA, Martínez-Raga J, Alonso-Alonso M, Shih M-C, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Carter RM, Huettel SA. A nexus model of the temporal-parietal junction. Trends Cogn. Sci. 2013;17:328–336. doi: 10.1016/j.tics.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br. J. Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Braams BR, Clark L, Tobler PN, Robbins TW, Kalenscher T. Restricting temptations: neural mechanisms of precommitment. Neuron. 2013;79:391–401. doi: 10.1016/j.neuron.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Bechara A, Nathan PE. Executive dysfunction as a risk marker for substance abuse: the role of impulsive personality traits. Behav. Sci. Law. 2008;26:799–822. doi: 10.1002/bsl.845. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton: CRC Press; 1994. [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet J-L, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJG, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl.) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am. J. Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J. Neurosci. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yücel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci. Biobehav. Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J. Clin. Psychiatry. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Green DP, Ha SE, Bullock JG. enough already about “black box” experiments: studying mediation is more difficult than most scholars suppose. Ann. Am. Acad. Pol. Soc. Sci. 2010;628:200–208. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmpfc valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, Wit H, de, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J. Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty S, Robertson IH, Miniussi C, Sheehy OC, Devine CA, McCreery S, O’Connell RG. Transcranial direct current stimulation over right dorsolateral prefrontal cortex enhances error awareness in older age. J. Neurosci. 2014;34:3646–3652. doi: 10.1523/JNEUROSCI.5308-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ko JH, Strafella AP, Dagher A. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc. Natl. Acad. Sci. 2013;110:4422–4427. doi: 10.1073/pnas.1212185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze H-J, Heldmann M, Voges J, Hinrichs H, Marco-Pallares J, Hopf J-M, Müller UJ, Galazky I, Sturm V, Bogerts B, Münte TF. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front. Hum. Neurosci. 2009;3:22. doi: 10.3389/neuro.09.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt CJ, Assaf M, Muska CE, Rosen RI, Thomas AD, Johnson MR, Hylton JL, Andrews MM, Reynolds BA, Krystal JH, Potenza MN, Pearlson GD. Reward-related dorsal striatal activity differences between former and current cocaine dependent individuals during an interactive competitive game. PLoS ONE. 2012;7:e34917. doi: 10.1371/journal.pone.0034917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann. N. Y. Acad. Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl.) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol. Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Gianotti LRR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J. Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc. Natl. Acad. Sci. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. NeuroImage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Leland DS, Arce E, Feinstein JS, Paulus MP. Young adult stimulant users’ increased striatal activation during uncertainty is related to impulsivity. NeuroImage. 2006;33:725–731. doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-L, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet J, Møller A, Peterson E, Gjedde A, Doudet D. Inverse association between dopaminergic neurotransmission and Iowa Gambling Task performance in pathological gamblers and healthy controls. Scand. J. Psychol. 2011;52:28–34. doi: 10.1111/j.1467-9450.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Z-L, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Hum. Brain Mapp. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu. Rev. Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the Mediation, Confounding and Suppression Effect. Prev. Sci. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, 1 2, Simmons AN, 1, Lane S, Paulus MP., 1 2 Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport. 2004;15:2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D-R, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: i. accuracy and precision of d2 receptor parameter measurements in ventral striatum. J. Cereb. Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR. Neural processing of risk. J. Neurosci. 2010;30:6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller UJ, Sturm V, Voges J, Heinze H-J, Galazky I, Heldmann M, Scheich H, Bogerts B. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry. 2009;42:288–291. doi: 10.1055/s-0029-1233489. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn. Affect. Behav. Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc. Cogn. Affect. Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J. Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN, Pearlson GD. Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biol. Psychiatry. 2013;74:529–537. doi: 10.1016/j.biopsych.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Neumann R, Köhler U, Lamm C. Effects of transcranial direct current stimulation on risky decision making are mediated by “hot” and “cold” decisions, personality, and hemisphere. Eur. J. Neurosci. 2013;38:3778–3785. doi: 10.1111/ejn.12375. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Padrón I, De Vega M, Ferstl EC. Adolescents’ risky decision-making activates neural networks related to social cognition and cognitive control processes. Front. Hum. Neurosci. 2014;8:60. doi: 10.3389/fnhum.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an FMRI investigation of the balloon analog risk task. Front. Neurosci. 2012;6:80. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol. Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Bañuelos C, Vokes CM, Taylor AB, Haberman RP, Bizon JL, Setlow B. Dopaminergic modulation of risky decision-making. J. Neurosci. 2011;31:17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb. Cortex. 2011;21:2578–2588. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Reynolds J, Krmpotich T, Claus E, Thompson LL, Du YP, Banich MT. Reduced neural tracking of prediction error in substance-dependent individuals. Am. J. Psychiatry. 2013;170:1356–1363. doi: 10.1176/appi.ajp.2013.12091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LL, Claus ED, Mikulich-Gilbertson SK, Banich MT, Crowley T, Krmpotich T, Miller D, Tanabe J. Negative reinforcement learning is affected in substance dependence. Drug Alcohol Depend. 2012;123:84–90. doi: 10.1016/j.drugalcdep.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat. Part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Reynolds J, Krmpotich T, Banich MT, Thompson L, Tanabe J. Temporal profile of fronto-striatal-limbic activity during implicit decisions in drug dependence. Drug Alcohol Depend. 2014;136:108–114. doi: 10.1016/j.drugalcdep.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.