Abstract
BACKGROUND
The axillary pathologic complete response rate (pCR) and the effect of axillary pCR on disease-free survival (DFS) was determined in patients with HER2-positive breast cancer and biopsy-proven axillary lymph node metastases who were receiving concurrent trastuzumab and neoadjuvant chemotherapy. The use of neoadjuvant chemotherapy is reported to result in pCR in the breast and axilla in up to 25% of patients. Patients achieving a pCR have improved DFS and overall survival. To the authors’ knowledge, the rate of eradication of biopsy-proven axillary lymph node metastases with trastuzumab-containing neoadjuvant chemotherapy regimens has not been previously reported.
METHODS
Records were reviewed of 109 consecutive patients with HER2-positive breast cancer and axillary metastases confirmed by ultrasound-guided fine-needle aspiration biopsy who received trastuzumab-containing neoadjuvant chemotherapy followed by breast surgery with complete axillary lymph node dissection. Survival was evaluated by the Kaplan-Meier method. Clinicopathologic factors and DFS were compared between patients with and without axillary pCR.
RESULTS
Eighty-one patients (74%) achieved a pCR in the axilla. Axillary pCR was not associated with age, estrogen receptor status, grade, tumor size, initial N classification, or median number of lymph nodes removed. More patients with an axillary pCR also achieved a pCR in the breast (78% vs 25%; P < .001). At a median follow-up of 29.1 months, DFS was significantly greater in the axillary pCR group (P = .02).
CONCLUSIONS
Trastuzumab-containing neoadjuvant chemotherapy appears to be effective in eradicating axillary lymph node metastases in the majority of patients treated. Patients who achieve an axillary pCR are reported to have improved DFS. The success of pCR with concurrent trastuzumab and chemotherapy in eradicating lymph node metastases has impli cations for surgical management of the axilla in these patients.
Keywords: breast neoplasms, neoadjuvant therapy, monoclonal antibodies, lymphatic metastasis, disease-free survival
Neoadjuvant (preoperative) chemotherapy is considered standard therapy for patients with locally advanced breast cancer and patients with initially large tumors who desire breast-conserving therapy. Although neoadjuvant chemotherapy has not been shown to improve survival compared with conventional postoperative chemotherapy for patients with operable breast cancer,1 neoadjuvant chemotherapy does allow for in vivo evaluation of the response of the primary tumor and metastatic lymph nodes to chemotherapy, and this response carries prognostic significance. Neoadjuvant chemotherapy can produce a pathologic complete response (pCR) in both the breast and axilla, and such a response has been shown to correlate with improved disease-free and overall survival.1-3 In addition, as our group first reported,4 in patients with cytologically confirmed axillary metastases at diagnosis, pCR in the axilla alone after neoadjuvant chemotherapy, regardless of the primary tumor response, also correlates with improved disease-free and overall survival. In that study using the best available systemic breast cancer agents, we reported that neoadjuvant chemotherapy resulted in conversion to pathologically negative axillary lymph node status in 23% of patients.4
Systemic agents for breast cancer are now targeted toward specific subtypes and patients whose tumors over-express human epidermal growth factor receptor 2 (HER2) now routinely receive neoadjuvant chemotherapy regimens containing trastuzumab. Studies have shown that patients with pCR in both the breast and the axilla after treatment with trastuzumab-containing neoadjuvant chemotherapy have improved disease-free survival.5-7 However, to the best of our knowledge, the incidence and the prognostic significance of eradication of cytologically confirmed axillary lymph node metastases by trastuzumab-containing neoadjuvant chemotherapy have not been reported to date. The evaluation of the regional lymph node basins with ultrasonography and fine-needle aspiration biopsy of suspicious-appearing lymph nodes provides improved axillary staging over clinical examination alone. With this approach, nonpalpable axillary lymph nodes can be more appropriately characterized before the initiation of chemotherapy.8 Pathologic evaluation of the lymph nodes after chemotherapy can provide an even better assessment of the effectiveness of trastuzumab-containing neoadjuvant chemotherapy regimens when the pretreatment lymph node status has been clearly defined. Ultimately, knowledge of the expected pCR rates in the regional lymph nodes can help to guide the extent and need for surgical management of the axillary lymph nodes.
In this study, we examined the incidence and prognostic significance of the complete eradication of axillary lymph node metastases and the clinicopathologic factors associated with this finding in 109 consecutive patients. Each patient had HER2-positive breast cancer and axillary lymph node metastases confirmed by ultrasound-guided fine-needle aspiration biopsy and received trastuzumab-containing neoadjuvant chemotherapy at The University of Texas M. D. Anderson Cancer Center.
MATERIALS AND METHODS
This study was reviewed and approved by The University of Texas M. D. Anderson Cancer Center Institutional Review Board and include patients treated between 2002 and 2008. Clinical and pathologic data from 109 consecutive patients identified from the prospectively entered Breast Cancer Management System Database with HER2-positive breast cancer and axillary metastases confirmed by fine-needle aspiration biopsy were analyzed. Each patient was required to have undergone trastuzumab-containing neoadjuvant chemotherapy followed by breast surgery with complete axillary lymph node dissection at our institution. Overexpression of HER2/neu was established using immunohistochemistry and confirmed using fluorescence in situ hybridization.
Patients were evaluated before the initiation of preoperative chemotherapy by a multidisciplinary team to assess the clinical stage of disease at presentation and after neoadjuvant chemotherapy to assess response. The staging workup included a complete history and physical examination, complete blood cell count, blood chemistry analysis, chest radiography, abdominal computed tomography or abdominal ultrasonography, bone scan, bilateral mammography, and bilateral ultrasonography of the breast and lymph node basins. Mammography and ultrasonography were repeated in the middle of the course of neoadjuvant chemotherapy and again after completion of neoadjuvant chemotherapy.
Patients received neoadjuvant therapy comprised of concurrent trastuzumab in combination with other agents (anthracycline-based [n = 68] or non-anthracycline-based [taxane-based, n = 41]). After completion of neoadjuvant chemotherapy, patients underwent surgery for management of their breast cancer. The type of surgery performed was at the discretion of the patient and surgeon. Twenty-eight patients underwent segmental mastectomy with axillary lymph node dissections, whereas 81 underwent modified radical mastectomy.
The histologic response to neoadjuvant chemotherapy was characterized as a pCR in the breast if there was no evidence of residual invasive tumor in the breast and as a pCR in the axilla if there was no evidence of residual invasive tumor in the resected axillary lymph nodes. Axillary lymph node status was determined on the basis of examination of a single hematoxylin and eosin-stained section from each block of serially sectioned lymph nodes removed during surgery. Immunohistochemistry for cytokeratin was performed when suspicious cells were identified.
All 28 patients who underwent breast-conserving therapy received postoperative radiotherapy, as did 67 of the 81 patients who underwent modified radical mastectomy. Patients with hormone receptor-positive tumors received tamoxifen or aromatase inhibitor therapy after completion of radiotherapy. In the later part of the study, 61 patients also received adjuvant trastuzumab therapy for an additional 6 months, which had become the national practice guideline.
For analysis, patients were divided into 2 groups: those with and those without an axillary pCR. Data were analyzed using SPLUS 8.0 and SAS statistical software packages (version 9.1). Comparisons of the clinicopatho-logic characteristics of the groups were assessed by chi-square analysis. Overall survival was calculated from the date of diagnosis, and disease-free survival was calculated from the date of surgery using the method of Kaplan and Meier. The statistical significance level (P) was taken as a measure of the strength of evidence against the null hypothesis, and P ≤ .05 was considered statistically significant.
RESULTS
Of the 109 patients in the study, overall 81 (74%) achieved an axillary pCR after neoadjuvant chemotherapy, whereas 28 (26%) did not. There was no significant difference in the rate of axillary conversion noted in patients who received trastuzumab concurrently with anthracycline chemotherapy versus those who received trastuzumab without concurrent anthracycline (taxane-based) chemotherapy (76.8% and 70%; P = 0.43; respectively). Of the 28 patients with residual axillary disease, 14 (50%) had residual disease in 1 lymph node only, 4 (14%) had residual disease in 2 lymph nodes, 5 (18%) had residual disease in 3 lymph nodes, and 5 (18%) had residual disease in ≥ 4 lymph nodes.
Patient and tumor characteristics by axillary lymph node response to chemotherapy are summarized in Table 1. There were no differences between patients with and without an axillary pCR with regard to age, estrogen receptor status, nuclear grade, T classification, initial N classification, or median number of lymph nodes removed. However, patients with an axillary pCR were more likely to have a pCR in the breast (78% vs 25%; P < .001). In keeping with including all patients who had documented positive axillary lymph node cytology and who received trastuzumab-containing preoperative chemotherapy during the study period, there were 3 patients with inflammatory carcinoma included within the cohort. None of these patents had a pCR in the breast and/or axillary lymph nodes.
Table 1.
Patient and Tumor Characteristics by Response of Axillary Lymph Node Metastases to Trastuzumab-Containing Neoadjuvant Chemotherapy
| Characteristic | Axillary Pathologic Complete Response No. (%) | Residual Axillary Disease No. (%) | Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| No. of patients | 81 (74) | 28 (26) | ||
| Median age, y | 49 | 53 | .36 | |
| Age, y | ||||
| ≤50 | 46 (57) | 13 (46) | ||
| >50 | 35 (43) | 15 (54) | .34 | |
| ER status at diagnosis | ||||
| Positive | 29 (36) | 14 (50) | 0 56 (0 23-1 33) | .19 |
| Negative | 52 (64) | 14 (50) | ||
| Modified Black nuclear grade at diagnosis | ||||
| 1 or 2 | 16 (20) | 8 (29) | ||
| 3 (most anaplastic) | 63 (78) | 20 (71) | 1.2 (0.49-2.83) | .61 |
| Not recorded | 2 (2) | 0 | ||
| T classification at diagnosis | ||||
| T0 | 2 (2) | 0 | ||
| T1 | 14(17) | 4 (14) | ||
| T2 | 29 (36) | 10 (36) | ||
| T3 | 16 (20) | 4 (14) | ||
| T4 | 20 (25) | 10 (36) | 0.82 (0.55-1.21) | .81 |
| Initial axillary lymph node status | ||||
| N1 | 58 (72) | 21 (75) | ||
| N2 | 2 (2) | 0 | ||
| N3 | 21 (26) | 7 (25) | 1.06 (0.64-1.74) | 1.0 |
| Median no. of lymph nodes removed | 19 | 18 5 | .43 | |
| Residual tumor in the breast | ||||
| No | 63 (78) | 7 (25) | ||
| Yes | 18 (22) | 21 (75) | 10.5 (3.85-28.63) | <.001 |
95% CI indicates 95% confidence interval; ER, estrogen receptor
The median follow-up for all patients in the study was 29.1 months. In the axillary pCR group, there were no locoregional recurrences; however, distant metastases developed in 4 patients: 1 had lung metastases, 1 had both liver and brain metastases, and 2 had brain metastases only. In the group with residual axillary lymph node disease, 2 patients had brain metastases, 1 patient had bone and liver metastases, 1 patient had brain metastases and a chest wall recurrence, and 1 patient had an axillary lymph node recurrence and lung metastases.
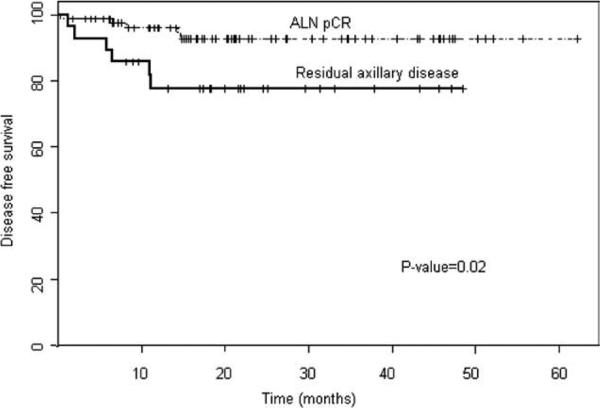
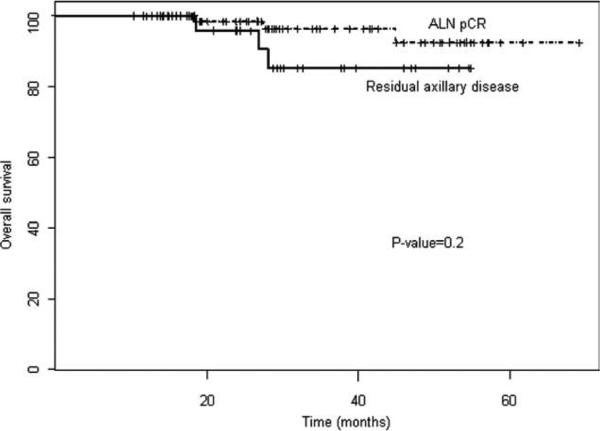
At the median follow-up, the disease-free survival rate was significantly greater in the axillary pCR group (93%; 95% confidence interval [95% CI], 86-99%) than in the group with residual axillary lymph node disease (76%; 95% CI, 61-95%) (P = .02) (Fig. 1). There was no difference in overall survival noted between the groups (P = .2), although there was a trend toward improved overall survival in the axillary pCR group (Fig. 2). No significant differences in overall and disease-free survival were noted with respect to regimen received (trastuzumab concurrently with anthracyclines vs trastuzumab without concurrent anthracycline or duration of adjuvant trastuzumab received; P = .96 and P = .81; respectively).
Figure 1.
Disease-free survival is shown by response of axillary lymph node (ALN) metastases to trastuzumab-containing neoadjuvant chemotherapy. pCR indicates pathologic complete response.
Figure 2.
Overall survival is shown by response of axillary lymph node (ALN) metastases to trastuzumab-containing neoadjuvant chemotherapy. pCR indicates pathologic complete response.
Among patients with an axillary pCR, there was no difference in disease-free survival between patients who had residual disease in the breast and those who did not (P = .33), but again, there was a trend toward improved overall survival in patients who had a pCR in both the breast and the axilla (P = .06).
DISCUSSION
The findings of the current study indicate that trastuzumab-containing neoadjuvant chemotherapy eradicated axillary lymph node metastases as assessed by standard histologic examination in 74% of patients with HER2-positive tumors with cytologically confirmed axillary disease. There has been remarkable progress since our previously published study (conducted before targeted therapies were in use), which revealed a 23% axillary clearance rate with neoadjuvant chemotherapy in patients with locally advanced breast cancer and cytologically confirmed axillary disease.4 Our findings suggest that targeted therapies not only lead to complete and partial responses in the primary tumor but are also highly effective in eradicating metastatic disease in the regional lymph nodes.
Our findings also confirm the previously reported finding that the response of the axillary lymph nodes in patients with breast cancer treated with neoadjuvant chemotherapy correlates directly with improvement in the disease-free survival rates.9-12 In the current study, with a median follow-up of only 29.1 months, patients with negative axillary lymph nodes had a projected 4-year disease-free survival rate of 93% (95% CI, 86-99%), whereas patients with residual lymph node disease had a projected 4-year disease-free survival rate of 76% (95% CI, 61-95%) (P = .02). A trend toward improved overall survival for patients with axillary pCR was also noted, although this was not yet statistically significant. Axillary pCR was associated with an excellent prognosis, despite the aggressive features of HER2-positive tumors.
The current study findings also suggest that the status of the axillary lymph nodes after neoadjuvant chemo-therapy is even more important than the amount of residual disease in the breast. When we evaluated the outcome of patients with complete eradication of axillary lymph node disease and stratified them based on pCR in the breast or residual disease in the breast, there was no difference noted with regard to disease-free survival (P = .33). However, longer follow-up is needed. Some authors have suggested that residual tumor cells within the breast may not have metastatic capability after chemo-therapy treatment and that a pCR in the primary tumor may carry prognostic significance because most of these patients also achieve an axillary pCR.13
Our finding that 74% of patients had eradication of axillary lymph node metastases after neoadjuvant chemo-therapy suggests that it may be appropriate to omit axillary dissection in some patients with HER2-positive breast cancers who receive a trastuzumab-based regimen. Our current tools for identifying which patients have residual axillary disease after neoadjuvant chemotherapy are not optimal. Axillary ultrasonography is superior to physical examination for detecting metastases, but small meta-static lymph nodes may be missed, and assessment can be difficult after a patient has received neoadjuvant chemo-therapy.8,14 An alternative to axillary lymph node dissection may be to have patients with clinically negative axillae after neoadjuvant chemotherapy who are candidates for breast conservation be treated with an additional radiation field to control subclinical axillary disease.15 In the current study, 50% of the patients with residual axillary lymph node disease after trastuzumab-containing neoadjuvant chemotherapy had only 1 positive lymph node. In addition, no patient with an axillary pCR developed a locoregional recurrence during the follow-up period. Patients who have a clinically negative axilla after neoadjuvant chemotherapy may be candidates for nonsurgical management of the axilla. This, however, would need to be examined with a prospective randomized trial.
Another alternative to axillary lymph node dissection is intraoperative lymphatic mapping and sentinel lymph node biopsy. The findings presented in the current study suggest that it may be feasible to perform intraoperative lymphatic mapping and sentinel lymph node biopsy in patients with clinical complete response to trastuzumab-containing neoadjuvant chemotherapy. Patients treated with trastuzumab-containing neoadjuvant chemo-therapy have a 74% chance of achieving an axillary pCR, and therefore complete axillary lymph node dissection represents overtreatment for many of these patients. Lymphatic mapping and sentinel lymph node biopsy is increasingly being performed by surgeons in patients who receive neoadjuvant chemotherapy and present with an initially clinically negative axilla to guide further therapy of the axilla.16 However, there have been conflicting single-institution reports regarding the feasibility and accuracy of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients who present with initial biopsy-proven axillary metastases.17-19 To address this issue, the American College of Surgeons Oncology Group has just opened a clinical trial (ACOSOG Z1071) to investigate the accuracy of lymphatic mapping and sentinel lymph node biopsy in patients who present with biopsy-proven axillary lymph node metastasis and undergo neoadjuvant chemotherapy. As better systemic agents are introduced into the breast cancer armamentarium, it will be critically important to develop minimally invasive and less morbid surgical approaches to the axilla in patients treated with neoadjuvant chemotherapy.
Acknowledgments
We thank Wei Qiao, MS, for her assistance with statistical analysis. We also thank Lajos Pusztai, MD, PhD; Savitri Krishnamurthy, MD; Isabelle Bedrosian, MD; and Vicente Valero, MD, for their assistance with accruing patients, designing the study, and analyzing the data.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
This specific study did not receive commercial sponsorship; however, some patients in this study received drugs that were paid by grants from the following companies: Genentech, Pfizer, and Bristol Myers Squibb.
REFERENCES
- 1.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 2.Mamounas EP. Overview of National Surgical Adjuvant Breast Project neoadjuvant chemotherapy studies. Semin Oncol. 1998;25:31–35. [PubMed] [Google Scholar]
- 3.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 4.Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230:72–78. doi: 10.1097/00000658-199907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Coudert BP, Largillier R, Arnould L, et al. Multicenter phase II trial of neoadjuvant therapy with trastuzumab, docetaxel, and carboplatin for human epidermal growth factor receptor-2-overexpressing stage II or III breast cancer: results of the GETN(A)-1 trial. J Clin Oncol. 2007;25:2678–2684. doi: 10.1200/JCO.2006.09.9994. [DOI] [PubMed] [Google Scholar]
- 7.Peintinger F, Buzdar AU, Kuerer HM, et al. Hormone receptor status and pathologic response of HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab. Ann Oncol. 2008;19:2020–2025. doi: 10.1093/annonc/mdn427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnamurthy S, Sneige N, Bedi DG, et al. Role of ultra-sound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 9.McCready DR, Hortobagyi GN, Kau SW, et al. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg. 1989;124:21–25. doi: 10.1001/archsurg.1989.01410010027005. [DOI] [PubMed] [Google Scholar]
- 10.Botti C, Vici P, Lopez M, et al. Prognostic value of lymph node metastases after neoadjuvant chemotherapy for large-sized operable carcinoma of the breast. J Am Coll Surg. 1995;181:202–208. [PubMed] [Google Scholar]
- 11.Kuerer HM, Newman LA, Buzdar AU, et al. Residual meta-static axillary lymph nodes following neoadjuvant chemotherapy predicts disease-free survival in locally advanced breast cancer patients. Am J Surg. 1998;176:502–509. doi: 10.1016/s0002-9610(98)00253-0. [DOI] [PubMed] [Google Scholar]
- 12.Kilbride KE, Lee MC, Nees AV, et al. Axillary staging prior to neoadjuvant chemotherapy for breast cancer: predictors of recurrence. Ann Surg Oncol. 2008;15:3252–3258. doi: 10.1245/s10434-008-0136-3. [DOI] [PubMed] [Google Scholar]
- 13.Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- 14.Vlastos G, Fornage BD, Mirza NQ, et al. The correlation of axillary ultrasonography with histologic breast cancer downstaging after induction chemotherapy. Am J Surg. 2000;179:446–452. doi: 10.1016/s0002-9610(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 15.Kuerer HM, Newman LA, Buzdar AU, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts ALN status. Cancer J Sci Am. 1998;4:230–236. [PubMed] [Google Scholar]
- 16.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–2702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Gilcrease MZ, Babiera GV, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007;109:1255–1263. doi: 10.1002/cncr.22540. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Kim EY, Kang SH, et al. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients. Breast Cancer Res Treat. 2007;102:283–288. doi: 10.1007/s10549-006-9330-9. [DOI] [PubMed] [Google Scholar]
- 19.Newman EA, Sabel MS, Nees AV, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007;14:2946–52. doi: 10.1245/s10434-007-9403-y. [DOI] [PubMed] [Google Scholar]