Abstract
Aims
In the ovary, endothelins regulate a variety of ovarian functions that include but not limited to folliculogenesis, steroidogenesis, oocyte maturation, ovulation and corpus luteum (CL) function. Two cognate receptors, EDNRA and EDNRB are constitutively expressed in the ovary, and mediate the regulatory endothelin actions. However, the physiological significance of the presence of the two receptors that often elicit opposite responses upon activation by an endothelin is yet to be determined. This study was proposed to test the hypothesis that both receptors are present in the ovary to lend an endothelin a finite regulation of ovulation.
Main methods
A rescued EDNRB knockout (rEDNRB-KO) mouse that is deficient of EDNRB expression in all cells but adrenergic cell lineage was used to test the impact of the loss of function of EDNRB on ovulation. The EDNRB gene deletion and its confirmation at mRNA level were assessed by molecular biology techniques, and the number and size of corpus lutea was determined by ovary histology.
Key findings
Female rEDNRB-KO mice had larger litter sizes (numbers of pups per birth) and their ovaries contained more corpus lutea than wild type littermates.
Significance
This result shows that without EDNRB excessive ovulation occurs, suggesting a role of EDNRB in having the extent of ovulation confined.
Keywords: endothelin, EDNRA, EDNRB, ovary, ovulation
Introduction
Endothelin (EDN) actions are mediated by G protein-coupled receptors, termed endothelin receptor type A (EDNRA) and type B (EDNRB) that have different molecular and pharmacological characteristics: EDNRA has a higher affinity to EDN1 and EDN2 than to END3, and induces prolonged vasoconstriction when activated (Arai et al., 1990; Flores et al., 1995; Gentili et al., 2001; Kedzierski and Yanagisawa, 2001; Mamluk et al., 1999b; Mancina et al., 1997; Meidan and Levy, 2002). EDNRB, on the other hand, binds to all three endothelins with equipotent affinity and induces vasodilation upon activation (Mamluk et al., 1999a). Upon agonist binding, both receptors are rapidly internalized via a common pathway from early endosomes to the lysosomes (Mamluk et al., 1998b). However, they are subsequently targeted to different intracellular localizations (Mamluk et al., 1998b): whereas the EDNRA follows the recycling pathway, reappearing at the plasma membrane, EDNRB is directed to lysosomes for degradation (Mamluk et al., 1998a). The rapid recycling of the EDNRA elicits sustained signaling response (Mamluk et al., 1998a). Conversely, lysosomal targeting of the EDNRB is consistent with its role in the clearance of endothelin from the circulation (Diamantis et al., 1998). In the ovary, both receptors are expressed and are presumed to mediate endothelin actions in regulating a variety of ovarian functions that include folliculogenesis, steroidogenesis, oocyte maturation, ovulation and corpus luteum (CL) function (Gentili et al., 2001; Meidan and Levy, 2002; Schneider et al., 2007). However, the physiological significance of having the two receptors co-expressed in the ovary is yet to be determined (Bridges et al., 2010a; Girsh et al., 1996b; Hinckley and Milvae, 2001; Levy et al., 2001; Meidan et al., 1999; Ohtani et al., 1998; Palanisamy et al., 2006). As EDNRA and EDNRB elicit mostly opposite physiological responses in an affected tissue, we hypothesized that activation of both receptors is required for a finely tuned regulation of endothelin action in regulating ovarian function. This hypothesis implicates that when the proposed ‘fine tuning’ of endothelin action is lost, an extreme physiological outcome may ensure, leading to hyper-stimulation or hypo-stimulation of a physiological event. For example, ovulation may continue when this event needs to be terminated or CL persists when this tissue needs to be demised. This study was designed to test the specific hypothesis that both receptors are present in the ovary to lend an endothelin a finite regulation of ovulation.
Genetically modified animal models such as knockout mice would provide an ideal experimental condition for testing the hypothesis. However, both EDNRA and EDNRB knockout (KO) mice do not survive to the sexual maturation due to defects in neural crest development and aganglionic megacolon, respectively (Clouthier et al., 1998; Hosoda et al., 1994), making them unavailable for determining functional roles of the receptors in regulating ovulation. As an alternative, we used a rescued EDNRB KO (rEDNRB-KO) mouse line that is deficient of EDNRB expression in all cells but adrenergic cell lineage in this study to test the hypothesis. The rEDNRB-KO mouse has a null mutation in the EDNRB gene, but survives to adulthood due to a transgenic expression of EDNRB driven by human dopamine-β-hydroxylase (DβH) promoter (Hosoda et al., 1994; Murakoshi et al., 2002; Quaschning et al., 2005). Here, we show that rEDNRB-KO mice displayed higher fecundity and their ovaries contained excessively larger numbers of CL.
Materials and Methods
Animals
The rEDNRB-KO mice were produced by successively breeding heterozygous EDNRB knockout mice (EDNRB+/−) with human DβH gene promoter-regulated EDNRB receptor transgenic mice (DβH-EDNRB) as previously described (Murakoshi et al., 2002; Quaschning et al., 2005). Three resulting genotypes that included WT mice (EDNRB+/+), heterozygous EDNRB-KO (EDNRB+/−) and rescued EDNRB-KO mice (EDNRB−/−·DβH-EDNRB) were used in this study. Fertility was determined by counting the number of pups per litter of 2–6 month old WT, EDNRB+/− and rEDNRB-KO female mice. All animal experiment procedures were reviewed and approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center.
Genomic PCR and RT-PCR of EDNRB
To verify the deletion of EDNRB gene in the ovaries of rEDNRB-KO mice, genomic DNA isolated from the ovaries of rEDNRB-KO and WT mice were analyzed by polymerase chain reaction (PCR) (Murakoshi et al., 2002). Presence of the endogenous EDNRB gene in the WT mice and the transgenic EDNRB sequence in the rEDNRB-KO mice were confirmed using primer sets that bind to exon 2 (P3; 5′-TTG CTC GCA GAG GAC TGG CCA 3′) and exon 3 (P4; 5′-AAG CAT GCA GAC CCT TAG GGG 3′) to differentiate the WT and rEDNRB-KO mice (Fig. 1A). In rEDNRB-KO mice, the introduced cDNA fragment detected by these primers does not include introns (298 bp) and therefore can be distinguished from the WT fragment which has a larger size (420 bp) due to the introns. In addition, the deletion of the EDNRB gene in the ovary of rEDNRB-KO mice was confirmed by PCR using primer sets that bind to the neomycin-resistant gene (P1; 5′-GGA TGC GGT GGG CTC TAT GGC TTC TGA - 3′) and exon 4 (P2; 5′-ATC TGC ATA CCG CTC TTC TTC CTG AGC ATT TC - 3′). The presence of a PCR product with the P1+P2 primer set was interpreted as the evidence of the deletion of exon 3 of the EDNRB gene while a 420 bp PCR product with the P3 + P4 primer set was considered proof of the presence of the wild type EDNRB gene.
Fig. 1. rEDNRB-KO mouse ovary is deficient of ENDRB gene and mRNA expression.
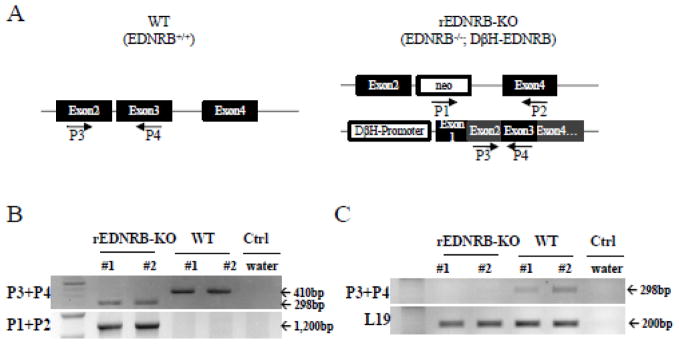
A, Schematic diagram of EDNRB genomic structure of the WT and rEDNRB-KO mice. In the rEDNRB-KO mouse, exon 3 is replaced with Neo cassette and the genome has a transgenic insertion of DbH-promoter-EDNRB cDNA expression construct. B, Confirmation of absence of exon 3 in the rEDNRB-KO ovarian genomic DNA. Genomic DNA isolated from rEDNRB-KO and WT mice were used for genotyping by PCR. PCR with P3+P4 primer combination yields 298bp product from rEDNRB-KO transgenic construct, whereas 410bp product from WT genome. P1+P2 primer combination would give rise to 1,200 bp PCR product from rEDNRB-KO genome, but no product from WT genome. C, Detection of EDNRB mRNA. Total RNA was extracted and used for the mRNA detection by RT-PCR. EDNRB bands are shown in WT but not rEDNRB-KO ovaries. Two mice for each genotype were used. L19, and ribosomal RNA was used as internal control.
For confirmation of ENDRB deletion in the rEDNRB-KO mice at mRNA levels, total RNA was also isolated from WT and rEDNRB-KO mice ovaries using Trizol (Invitrogen) and RNeasy kit (QIAGEN Inc., Valencia, CA). A DNase1 reaction buffer (Invitrogen), DNase1 (Invitrogen) and 25 mM EDTA (Invitrogen) were added to total RNA (1 μg) in 15.5 μl before heating at 65 C for 15 min, then heated at 72 C for 10 min after mixing the 12.5 μl of total RNA mixture and 1 μl oligo dT (Invitrogen). Then a cocktail of 5 × 1st strand buffer, 10 mM dNTP, 40 IU RNase out, and 200 IU Moloney leukemia virus reverse transcriptase were added to a total volume of 20 μl. The reaction mixture was incubated at 42 C for 60 min then heated at 94 C for 5 min to inactivate RNase H. And then 100 μl of DEPC H2O was added to the mixture. One microliter cDNA was added to the10 μl PCR mixture containing 1 × PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 0.2 μM primer sets that bind to exon 2 (P3) and exon 3 (P4), and Taq DNA polymerase (0.5 IU) and amplified with 34 cycles. The RT-PCR products were separated on a 1.2% agarose gel, stained with ethidium bromide, and scanned on a phosphor imager (FujiFilm FLA-5000).
Ovarian histology
Ovaries of rEDNRB-KO (n=5) and WT (n=4) mice at the ages of 6 weeks were collected and fixed in 4% paraformaldehyde. Tissues were then embedded in paraffin blocks, serially sectioned at 7 μm, and every 10th of serial sections were stained with hematoxylin and eosin (H&E) following a routine staining procedure (Al-Alem et al., 2007, Lee et al., 2009). The number of corpora lutea (CL) were counted microscopically using a BX51 microscope (Olympus, Tokyo, Japan) equipped with a digital camera (DP 70; Olympus, Tokyo, Japan). The sizes of CL were calculated by measuring the longest diameter of each CL under the assumption that a CL would have a spherical structure. The following equation was used to estimate the volume of the CL: volume (nl) = 4/3 × r (radius, um)3 × π.
Statistical Analysis
Statistical significances were assessed using Mann-Whitney rank sum test for no. of CL and numbers of pups per litter. For all statistical analysis, P < 0.05 was considered significant.
Results
rEDNRB-KO ovary is deficient of EDNRB mRNA expression
A rEDNRB-KO mouse has two independent genetic modifications. First, this mouse is deficient of EDNRB expression due to the replacement of the exon 3 of the EDNRB gene with a Neo cassette (Murakoshi et al., 2002). Second, it has a transgenic insertion of a human DβH promoter-EDNRB cDNA that expresses functional EDNRB in the adrenergic lineage cells (Murakoshi et al., 2002). Absence of exon 3 but the presence of exogenous EDNRB cDNA sequence was verified by PCR with genomic DNA extracted from rEDNRB-KO mouse ovaries (Fig 1A–B). Deficiency of EDNRB mRNA expression in the rEDNRB-KO mouse ovary was confirmed by performing RT-PCR, which produced expected cDNA bands in the WT ovaries, but not in the rEDNRB-KO ovary (Fig. 1C).
rEDNRB-KO female mice are hyper-fecund
As a way to assess the overall significance of EDNRB expression in the ovary, fecundity of the rEDNRB-KO mice was first compared with that of the rEDNRB+/− (globally heterozygous for EDNRB gene) and rEDNRB+/+ (wild type) littermates by retrospectively counting the numbers of litters and the litter sizes per mouse. Surprisingly, rather than showing a fertility defect, rEDNRB-KO mice displayed a higher fecundity over control mice as rEDNRB litter sizes (7.9 ± 0.73 pups/litter) were significantly larger than EDNRB+/− (6.19 ± 0.24 pups/litter) and WT (5.47 ± 0.11 pups/litter) (Fig. 2). No difference was however seen in the number of litters per mouse in any of the 3 genotypes compared (data not shown).
Fig. 2. rEDNRB-KO mice are hyper-fecund.
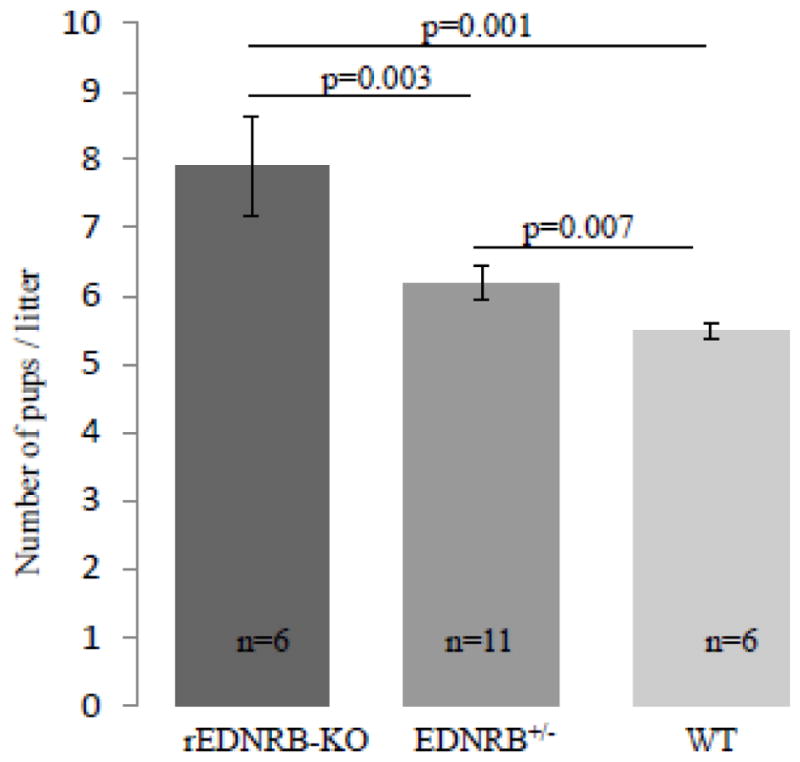
Female mice from the 3 different genotypes at the ages of 3–6 months were individually housed with proven males for the period of 2–5 months. During the breeding periods, the numbers of litters and pups were counted. Note that rEDNRB-KO mice gave births to largest numbers of pups per litter. The p values and number (n) of animals used are shown.
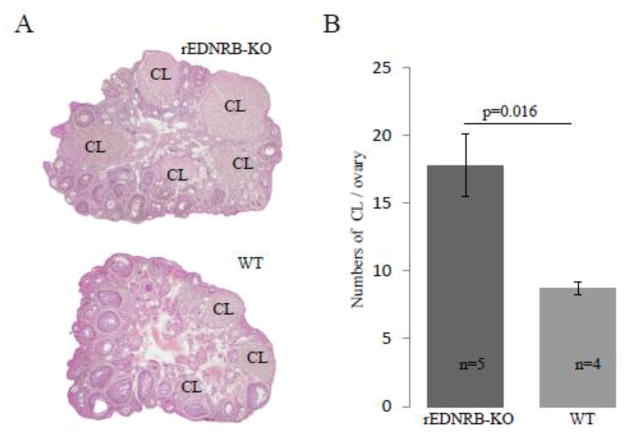
Ovaries of rEDNRB-KO mice have bigger and more corpus lutea than wild type mice
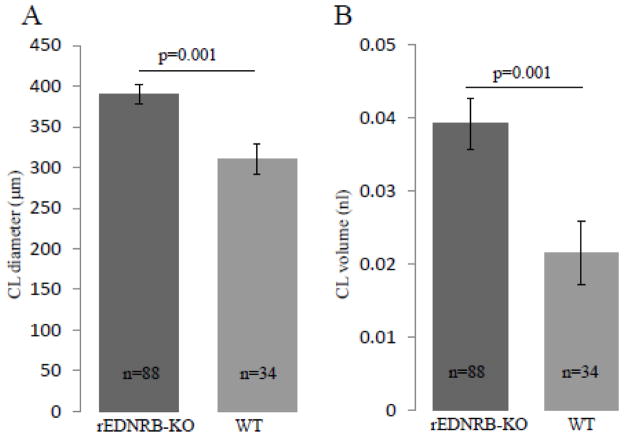
To determine whether the unexpectedly higher fecundity of rEDNRB-KO mice was an outcome of increased ovulation due to the loss of EDNRB, the numbers of the CLs of these mice were counted and compared to those of WT mice. CLs were used as markers of ovulation as a CL will be formed from a successfully ovulated follicle. For this purpose, ovaries of 6-week old rEDNRB-KO and WT mice were serially sectioned and the total numbers of CLs were counted. The data showed that the ovaries of rEDNRB-KO contained significantly larger numbers of CLs (17.8 ± 2.2/ovary) than those of WT mice (8.75 ± 0.48/ovary) (Fig. 3.). Interestingly, the mean diameter of rEDNRB-KO CL was wider (389.52 ± 12.22 μm) than that of WT (310.14 ± 18.18 μm) (Fig. 4A). This much of a difference in diameter would be equal to a 1.82 fold difference in CL volume (39 vs. 21 pico litter) (Fig. 4B.).
Fig. 3. rEDNRB-KO mouse ovary had more numbers of corpus lutea.
Ovaries were collected at the ages of 10–11 weeks, subjected to histological analysis and the numbers of CL were counted micrscopically. A, Representative sectional image of WT and rEDNRB-KO ovaries. B, Numbers of CL per ovary. Note the more and larger number of CL in the rEDNRB-KO than WT ovaries. The p values and number (n) of animals used are shown.
Fig. 4. rEDNRB-KO mouse ovary has larger corpus lutea.
Ovaries were collected at the ages of 10–11 weeks, subjected to histological analysis and the diameter of corpora lutea were measured microscopically in the ovaries of 5 rEDNRB-KO (n=5) and 4 WT mice (n=4). A, Diameter of corpus lutea of the rEDNRB-KO and WT ovaries. B, Diameter of corpus lutea of the rEDNRB-KO and WT ovaries. Note that while rEDNRB-KO ovaries are 1.26 times larger in diameter, they are 1.82 times bigger in their volume compared to WT ovaries. The p values and number (n) of CL examined are shown.
Discussion
This study was an attempt to gain insight into the significance of expressing both receptors in the ovary in regulating each of the ovarian functions. In the ovary, most of physiological events occur sequentially and cyclically: follicles grow and rupture, hormone levels rise and fall, oocyte maturation begins and stops and CL are formed and regressed. This phenomena implicate that there must be drivers that either initiate or accelerate the processes and stoppers that may attenuate and terminate the biological events. The most extensively studied role of endothelin system as contraction regulator in vasculature fits well with this logic as EDN1 triggers constriction via EDNRA (Kedzierski and Yanagisawa, 2001; Luscher and Barton, 2000), whereas EDNRB facilitates the clearance of endothelin (Diamantis et al., 1998). Therefore, it was hypothesized that having both receptors present in the ovary may lend an endothelin a finite regulation of the ovarian events, including ovulation, EDNRA as trigger of ovulation and EDNRB as a terminator of ovulation. This hypothesis presumes that loss of EDNRB would results in faulty termination of the endothelin-regulated biological events. In this study, ovulation was chosen as a model subject for testing the hypothesis because we have expertise in studying ovulation.
The proven fertility of the rEDNRB-KO mice clearly demonstrates that EDNRB is not required for ovulation to occur, but the increased fecundity and larger CL numbers in the rEDNRB-KO mice (Figs. 2–4) show that EDNRB is necessary for limiting ovulation. While being speculative, the more CLs and higher fecundity (larger litter size) seen in the rEDNRB-KO mice indicate that EDNRB may play an anti-ovulatory role and therefore ovulation rate may increase when the antagonism is compromised. Presently, the nature of antagonism is not known. However, one can speculate that EDNRB may ‘actively’ antagonize the EDNRA-driven ovulatory events (Bridges et al., 2010b; Ko et al., 2006) as a scavenger of endothelins as is in circulation system (Diamantis et al., 1998). Without EDNRB, the EDNRA-driven ovulatory events could be stronger and may last longer, resulting in ovulating follicles that would not otherwise be.
Meanwhile, EDNRB is not required for ovulation in rats as rescued EDNRB-KO rats are fertile (Gariepy et al., 1998). Successful ovulation of the rEDNRB-KO mice (this study) and rats (Gariepy et al., 1998) leaves EDNRA as the endothelin receptor that drives ovulation. This may be true as we recently showed that EDNRA is responsible for EDN2-induced ovarian constriction (Bridges et al., 2010a) that is shown to play critical role in ovulation (Bridges et al., 2010b; Ko et al., 2006).
The antagonism may continue during CL formation as CLs are larger in size and in numbers in the rEDNRB-KO than wild type ovary (Fig. 4). Or, the larger CL size may have nothing to do with CL formation process, but pertain to CL regression that this luteolytic process may be delayed when EDNRB is absent. It was shown that PGF2α, a trigger of luteolysis, initiate the luteolytic pathway by inducing EDN1 synthesis in old CL (Boiti et al., 2007; Girsh and Dekel, 2002; Girsh et al., 1996a; Girsh et al., 1996b). While the endothelin receptor type that is responsible for this CL regression pathway is not known, it would be worth to see whether EDNRB is the EDN1 receptor for an initiating the luteolytic pathway; loss of EDNRB may deter the luteolytic pathway, leaving the CL live longer and keep it larger. Interestingly, it was shown that luteolysis was inhibited by EDNRA antagonism but not by EDNRB antagonist (Doerr et al., 2008; Keator et al., 2008; Watanabe et al., 2006), indicating EDNRA plays as an initiator of luteolysis. Therefore, further study is warranted to determine specific endothelin-driven physiological events that are mediated by EDNRA and EDNRB in regulating the luteolytic pathway.
Conclusion
Female rEDNRB-KO mice had larger litter sizes (numbers of pups per birth) and their ovaries contained more corpus lutea than wild type littermates. Therefore, EDNRB expression is not required for ovulation, but may be necessary for terminating ovulation.
Acknowledgments
This work was supported by the NIH (R01HD052694 to C. Ko) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0013971 to J. Cho)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C. Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J Endocrinol. 2007;193:383–91. doi: 10.1677/JOE-07-0089. [DOI] [PubMed] [Google Scholar]
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–2. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Boiti C, Maranesi M, Dall’aglio C, Pascucci L, Brecchia G, Gobbetti A, et al. Vasoactive peptides in the luteolytic process activated by PGF2alpha in pseudopregnant rabbits at different luteal stages. Biol Reprod. 2007;77:156–64. doi: 10.1095/biolreprod.106.055889. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Jo M, Al-Alem L, Na G, Su W, Gong MC, et al. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction. Reprod Fertil Dev. 2010a;22:8. doi: 10.1071/RD09194. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Jo M, Al Alem L, Na G, Su W, Gong MC, et al. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction. Reprod Fertil Dev. 2010b;22:780–7. doi: 10.1071/RD09194. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–24. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Diamantis I, Van de Casteele M, Hurni B, Reichen J. Detection of endothelin-1 and its receptors in rat liver endothelial cells by in situ reverse transcriptase-polymerase chain reaction. J Hepatol. 1998;28:157–60. doi: 10.1016/s0168-8278(98)80215-8. [DOI] [PubMed] [Google Scholar]
- Doerr MD, Goravanahally MP, Rhinehart JD, Inskeep EK, Flores JA. Effects of endothelin receptor type-A and type-B antagonists on prostaglandin F2alpha-induced luteolysis of the sheep corpus luteum. Biol Reprod. 2008;78:688–96. doi: 10.1095/biolreprod.107.064105. [DOI] [PubMed] [Google Scholar]
- Flores JA, Winters TA, Knight JW, Veldhuis JD. Nature of endothelin binding in the porcine ovary. Endocrinology. 1995;136:5014–9. doi: 10.1210/endo.136.11.7588236. [DOI] [PubMed] [Google Scholar]
- Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest. 1998;102:1092–101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili M, Obermuller N, Schleich HG, Melchert F, Weigel M. Distinct expression of endothelin receptor subtypes A and B in luteinized human granulosa cells. Horm Metab Res. 2001;33:573–6. doi: 10.1055/s-2001-17902. [DOI] [PubMed] [Google Scholar]
- Girsh E, Dekel N. Involvement of endothelin-1 and its receptors in PGF2alpha-induced luteolysis in the rat. Mol Reprod Dev. 2002;63:71–8. doi: 10.1002/mrd.10159. [DOI] [PubMed] [Google Scholar]
- Girsh E, Milvae RA, Wang W, Meidan R. Effect of endothelin-1 on bovine luteal cell function: role in prostaglandin F2alpha-induced antisteroidogenic action. Endocrinology. 1996a;137:1306–12. doi: 10.1210/endo.137.4.8625904. [DOI] [PubMed] [Google Scholar]
- Girsh E, Wang W, Mamluk R, Arditi F, Friedman A, Milvae RA, et al. Regulation of endothelin-1 expression in the bovine corpus luteum: elevation by prostaglandin F 2 alpha. Endocrinology. 1996b;137:5191–6. doi: 10.1210/endo.137.12.8940334. [DOI] [PubMed] [Google Scholar]
- Hinckley ST, Milvae RA. Endothelin-1 mediates prostaglandin F(2alpha)-induced luteal regression in the ewe. Biol Reprod. 2001;64:1619–23. doi: 10.1095/biolreprod64.6.1619. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–76. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Keator CS, Schreiber DT, Hoagland TA, McCracken JA, Milvae RA. Intrauterine infusion of BQ-610, an endothelin type A receptor antagonist, delays luteolysis in dairy heifers. Domest Anim Endocrinol. 2008;34:411–8. doi: 10.1016/j.domaniend.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–76. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, et al. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–9. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, et al. Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology. 2009;150:3855–62. doi: 10.1210/en.2008-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N, Gordin M, Mamluk R, Yanagisawa M, Smith MF, Hampton JH, et al. Distinct cellular localization and regulation of endothelin-1 and endothelin-converting enzyme-1 expression in the bovine corpus luteum: implications for luteolysis. Endocrinology. 2001;142:5254–60. doi: 10.1210/endo.142.12.8550. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–40. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- Mamluk R, Chen D, Greber Y, Davis JS, Meidan R. Characterization of messenger ribonucleic acid expression for prostaglandin F2 alpha and luteinizing hormone receptors in various bovine luteal cell types. Biol Reprod. 1998a;58:849–56. doi: 10.1095/biolreprod58.3.849. [DOI] [PubMed] [Google Scholar]
- Mamluk R, Greber Y, Meidan R. Hormonal regulation of messenger ribonucleic acid expression for steroidogenic factor-1, steroidogenic acute regulatory protein, and cytochrome P450 side-chain cleavage in bovine luteal cells. Biol Reprod. 1999a;60:628–34. doi: 10.1095/biolreprod60.3.628. [DOI] [PubMed] [Google Scholar]
- Mamluk R, Levy N, Rueda B, Davis JS, Meidan R. Characterization and regulation of type A endothelin receptor gene expression in bovine luteal cell types. Endocrinology. 1999b;140:2110–6. doi: 10.1210/endo.140.5.6690. [DOI] [PubMed] [Google Scholar]
- Mamluk R, Wolfenson D, Meidan R. LH receptor mRNA and cytochrome P450 side-chain cleavage expression in bovine theca and granulosa cells luteinized by LH or forskolin. Domest Anim Endocrinol. 1998b;15:103–14. doi: 10.1016/s0739-7240(97)00085-4. [DOI] [PubMed] [Google Scholar]
- Mancina R, Barni T, Calogero AE, Filippi S, Amerini S, Peri A, et al. Identification, characterization, and biological activity of endothelin receptors in human ovary. J Clin Endocrinol Metab. 1997;82:4122–9. doi: 10.1210/jcem.82.12.4447. [DOI] [PubMed] [Google Scholar]
- Meidan R, Levy N. Endothelin-1 receptors and biosynthesis in the corpus luteum: molecular and physiological implications. Domest Anim Endocrinol. 2002;23:287–98. doi: 10.1016/s0739-7240(02)00164-9. [DOI] [PubMed] [Google Scholar]
- Meidan R, Milvae RA, Weiss S, Levy N, Friedman A. Intraovarian regulation of luteolysis. J Reprod Fertil Suppl. 1999;54:217–28. [PubMed] [Google Scholar]
- Murakoshi N, Miyauchi T, Kakinuma Y, Ohuchi T, Goto K, Yanagisawa M, et al. Vascular endothelin-B receptor system in vivo plays a favorable inhibitory role in vascular remodeling after injury revealed by endothelin-B receptor-knockout mice. Circulation. 2002;106:1991–8. doi: 10.1161/01.cir.0000032004.56585.2a. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Kobayashi S, Miyamoto A, Hayashi K, Fukui Y. Real-time relationships between intraluteal and plasma concentrations of endothelin, oxytocin, and progesterone during prostaglandin F2alpha-induced luteolysis in the cow. Biol Reprod. 1998;58:103–8. doi: 10.1095/biolreprod58.1.103. [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, et al. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20:2784–95. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Quaschning T, Rebhan B, Wunderlich C, Wanner C, Richter CM, Pfab T, et al. Endothelin B receptor-deficient mice develop endothelial dysfunction independently of salt loading. J Hypertens. 2005;23:979–85. doi: 10.1097/01.hjh.0000166838.55688.7e. [DOI] [PubMed] [Google Scholar]
- Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol. 2007;47:731–59. doi: 10.1146/annurev.pharmtox.47.120505.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Shirasuna K, Matsui M, Yamamoto D, Berisha B, Schams D, et al. Effect of intraluteal injection of endothelin type A receptor antagonist on PGF2alpha-induced luteolysis in the cow. J Reprod Dev. 2006;52:551–9. doi: 10.1262/jrd.18018. [DOI] [PubMed] [Google Scholar]