Abstract
Background
To identify risk factors for lower quality of life (QOL) among low-income women with breast cancer (BC), with an emphasis on the impact of patient-physician communication. In addition, we examined ethnic/racial group differences in QOL change over time.
Methods
A longitudinal study was conducted among 921 low-income women with BC. Patients were interviewed at 6-, 18-, 36- and 60- months after BC diagnosis. Mixed-effect regression models were performed to investigate predictors for and time effects on QOL. The main outcomes included the Medical Outcomes Study Health Survey Short Form 36 Mental Component Summary score (SF-36 MCS), SF-36 Physical Component Summary score (SF-36 PCS) and the Ladder of Life scale. Chief independent variables included physician information-giving and patient self-efficacy in interacting with physicians.
Results
There were no significant changes over time in QOL except for physical functioning, with survivors reporting a significant decrease over time (P<0.0001). Mean SF-36 MCS and PCS scores were lower than national general population norms at all time points. Both patient self-efficacy in interacting with physicians and physician information-giving were positively associated with SF-36 MCS (P=0.03, P=0.02, respectively) and Ladder of Life (P=0.01, P=0.03, respectively). Less acculturated Latinas reported higher SF-36 MCS and PCS scores (P<0.0001, P=0.01, respectively) and better global QOL (P<0.0001) than whites.
Conclusion
Low-income women with BC experienced poor physical and mental health. The results suggest that QOL among low-income women with BC would be enhanced by interventions aimed at empowering patients in communicating with physicians and increasing physician information giving.
Keywords: breast cancer, quality of life, minority health, low-income population, patient-physician communication
Introduction
Women treated for breast cancer (BC) represent the largest female cancer survivor group in the U.S.1 It is reported that there were 2.9 million breast cancer survivors in the U.S. as of January 1, 2012.2 Early detection and improved treatment have dramatically increased the life-expectancy of women with breast cancer,3,4,5 leading to an overall 5-year survival rate of 98% for local-stage disease and 81% for regional-stage disease.6 With these advances in breast cancer care, the goal of therapy has changed from simply survival to enhancing patients’ quality of life.
Quality of life (QOL) is an important outcome of disease and treatment frequently used to assess the impact of a health condition on patients' lives. Although there is no universal conceptual definition of QOL, some scholars have defined it as the difference between a person’s hope and expectations and his/her present life experiences.7 In other words, QOL is a subjective outcome from the patient’s perspective encompassing one’s objective state – people may perceive their QOL differently even though they have the same objective state of health.8 Ferrell et al suggested four primary domains of well-being (physical, psychological, spiritual and social) as measures of QOL among breast cancer survivors.9
In research among general BC patient populations, patient characteristics, such as lower income, lower education, being unpartnered, and comorbidity have been documented to be associated with reporting of lower QOL.10,11,12 Findings on the impact of age on QOL are mixed. Some reported that younger age had been shown to predict poorer QOL outcomes,13,14,15,16 while others found opposite results.17
Findings about the impact of type of breast cancer treatment received on QOL have also been inconsistent. A number of studies have shown that there were no major QOL differences in terms of treatment received among BC patients.18,19,20 Ganz et al., for example, reported longitudinal SF-36 scores did not differ by chemotherapy treatment exposure.21 However, some studies reported that systemic adjuvant treatment was associated with poorer outcomes in several QOL domains.22,23 In contrast, others have documented that completed chemotherapy and radiation therapy were associated with improved major dimensions of QOL.15
Social support has been found to be a positive predictor of better QOL, especially better emotional well-being.24,25,26 This support may come from family, friends, as well as physicians. It has been shown that healthcare providers are main sources of informational support and decision-making support for patients.27 Poor communication with physicians has been shown to be a barrier to achieving better QOL,28 and it might be overcome by enhancing patient self-efficacy in interacting with physicians to increase physician responsiveness specific to patients’ individual needs.
Patient self-efficacy in interacting with physicians is defined as the patient’s ability in obtaining needed medical information and attention regarding chief medical concerns from his/her physicians.29 Better self-efficacy in patient-physician communication has been shown to have a positive effect on BC patients’ decision-making process, treatment choices, BC-related symptom resolution, adherence to ongoing BC hormone therapy and satisfaction with care.30,31,32,33,34 Yet only one study of which we are aware has examined its impact on QOL among BC patients, specifically in a general population of older women showing a positive association.35
Physician information-giving is also a key component in effective patient-physician communication. Patients who had more discussion with their physicians demonstrated greater knowledge about their cancer in previous studies.31, 36 Research has shown that BC patients with greater knowledge of treatment options were more actively involved in the treatment decision-making process, resulting in better physical functioning and emotional well-being.37,38 Other studies also support the positive impact of information giving on QOL in BC patients.39,40, 41,42
The unequal distribution of the breast cancer burden across socioeconomic groups has been well-documented,43,44,45 and low income, less educated women may be at particular risk for poorer QOL after BC diagnosis due to decreased effectiveness in communicating with physicians.46 A better understanding of the role of patient-physician communication in QOL among low-income women may assist in identifying potential interventions to improve QOL among vulnerable BC patient populations.
The objective of this study was to identify potential risk factors for lower QOL among low-income, medically underserved women with BC. We were particularly interested in the impact of patient-physician communication, as a modifiable factor, on QOL in this vulnerable population. In addition, we were interested in examining if there were ethnic/racial differences in QOL change over time. Studies have examined ethnic/racial differences in QOL, and their findings are inconsistent.10,18,35,47,48,49,50,51,52,53,54 Some researchers have reported that ethnicity/race was not a predictor of QOL for breast cancer survivors47,52 while others found that ethnic minority women were more likely to report poorer QOL.10,18,35,48,49,50,51,54 However, these studies have limitations that include restrictions to certain racial/ethnic47,48,51,52,53 and age35, 53 groups, early stage,18,54 and small sample size.52 Importantly, most of these studies were cross-sectional studies and only two examined ethnic/racial differences in QOL prospectively beyond 18 months after BC diagnosis,53,54 albeit with large loss of follow up,54 or restriction to age less than 65 years old.53
Methods
Study sample
We conducted a longitudinal study to assess QOL among a low-income population of women with BC. The initial study recruited women who were aged 18 years and older, newly diagnosed with BC and continuously enrolled in the California Breast and Cervical Cancer Treatment Program (BCCTP) between February 2003, and September 2005. The BCCTP is funded in part by Medicaid and by the state of California to provide treatment for breast and cervical cancer for un- and under-insured, low-income women (≤ 200% Federal Poverty Level). The study was approved by the UCLA Human Subjects Protection Committee.
Potential participants were contacted and recruited as described in a previous paper.55 Women who did not speak English or Spanish, had a previous history of BC, or were receiving treatment for another cancer, were excluded from the study. Eligible women were interviewed by telephone at 6-, 18-, 36- and 60-months after their diagnosis of BC. Data collected included detailed information on patient level characteristics, clinical characteristics, psychosocial factors, patient-physician communication, and measures of quality of life. The baseline interview, 6-months after BC diagnosis, specifically targeted measures of sociodemographic characteristics and patient-physician communication, while the 18-, 36- and 60-month interviews specifically aimed to comprehensively measure QOL.
We also obtained and abstracted detailed clinical information from patients’ medical records at 18 months after BC diagnosis including clinical information about tumor characteristics, staging, and details of treatment. We had two abstractors abstract the medical records. The inter-rater reliability between abstractors for data on BC characteristics and treatment data ranged from 0.68 to 1.00, indicating good to excellent agreement.33
Measures
The chief outcome variables were QOL measures collected at 18-, 36- and 60- months after BC diagnosis. These included the Medical Outcomes Study Health Survey short form 36 (MOS SF-36) Mental Component Summary scale (MCS) and Physical Component Summary scale (PCS), and the Ladder of Life
The MOS SF-36 Health Survey56 includes 36 items that measure general areas of health-related QOL. The Mental Component Summary scale (MCS) is used to measure mental well-being and is based on fifteen questions from the SF-36 survey. The Physical Component Summary scale (PCS) is a measurement of physical well-being and is based on twenty-one questions from the SF-36 survey. Both summary scales are presented as T-scores, comparing to the general U.S. population normalized score with a mean of 50 points and a standard deviation of 10 points.57
The Ladder of Life has been demonstrated to be highly related to physical and psychosocial dimensions of QOL, and is widely used in epidemiologic studies to measure participants’ global QOL.58,59 This 10-point scale presents participants’ subjective rating of their own QOL at the present time, with 1 indicating “the worst possible life” and 10 indicating “the best possible life.”
The principal independent variables were measures of patient-physician communication: 1) interactive information-giving by physicians, and 2) patient-perceived self-efficacy in patient-physician communication. Information-giving was measured by a previously published index,60 which asked patients how many of 15 BC-related topics that any of their physicians had discussed with them. Self-efficacy was measured using the validated 5 item Perceived Efficacy in Patient-Physician Interactions (PEPPI) questionnaire.61 Cronbach’s alpha for this 5 item scale in this sample was 0.92. PEPPI measures patients’ perceived ability to obtain needed medical information and attention to their chief medial concerns from physicians. The PEPPI sum scale has a range from 0 to 50, with higher scores indicating greater self-efficacy.
Social support was measured at baseline by a sum scale from three questions asking if patient had received support from relatives and other social network (neighbors, church member, etc.) in medical appointment, daily tasks and emotional needs after BC diagnosis. This scale was validated by a previous study62 and the Cronbach’s alpha for it was 0.70 in this study sample. A higher score indicates more support. Other psychosocial factors measured included physician emotional support by asking three questions (Cronbach’s alpha was 0.91) such as “How often did your doctors show extreme compassion and caring “ (with a higher score indicating more emotional support from the physician) and to what degree physicians asked for patients’ input in decision-making by asking “How much did your breast cancer doctors ask you for your input or opinion about which treatment you preferred” (with higher score indicating more patient input).
Other potentially confounding variables included patient characteristics (from self-report), receipt of BC treatment (from self-report) including surgery, chemotherapy, radiotherapy and hormone therapy, and American Joint Commission on Cancer anatomic BC stage (from medical records).63 Patient characteristics included age, race/ethnicity, education and employment status. Major comorbidity was measured using the Katz et al. adaptation of the Charlson Comorbidity Index for patient self-report,64 and was dichotomized into any comorbidity versus none.
Language and acculturation can serve as a significant barrier to optimal communication, therefore among Latinas, language-based acculturation was determined by the five-item Short Acculturation Scale for Hispanics (SASH).65 The internal consistency reliability was 0.99 for this scale in the studied sample. “More acculturated” Latina was defined as being equally or more comfortable or conversant with English than Spanish; “less acculturated” was defined as being less comfortable or conversant with English than Spanish.
Data Analysis
Summary statistics, including means and percentages, were calculated to describe participants’ demographic and clinical characteristics and scores on QOL outcomes. Correlations between principle independent variables and QOL outcomes were also conducted.
Mixed-effects regression (Proc Mixed) models with participant-level random effects were used to assess the time effects on the QOL outcome measures and the potential predictors for QOL measures. Particularly, we were interested in ethnic differences in change of QOL over time. For each QOL measure, we first examined the time trends by constructing a base model (unadjusted model) with the following effects: time (6-, 18-, 36- and 60-months after diagnosis of BC), ethnicity (white, African-American, less- and more-acculturated Latina, and Asian/Pacific Islanders) and two-way interaction between these two factors. The model also included participant-level random effects to account for repeated observations per participant. Significant interaction was retained in the model. Next, we examined the full model (adjusted model) with three groups of characteristics that were selected a priori. These included sociodemographic factors (age, education, and employment), clinical characteristics (comorbidity, tumor stage, and breast cancer treatments) and psychosocial factors (social support, physician emotional support, PEPPI, information giving and patients’ input in decision-making). All statistical analyses were conducted using SAS, version 9.1; two-sided alpha levels with p values less than 0.05 were considered statistically significant.
Results
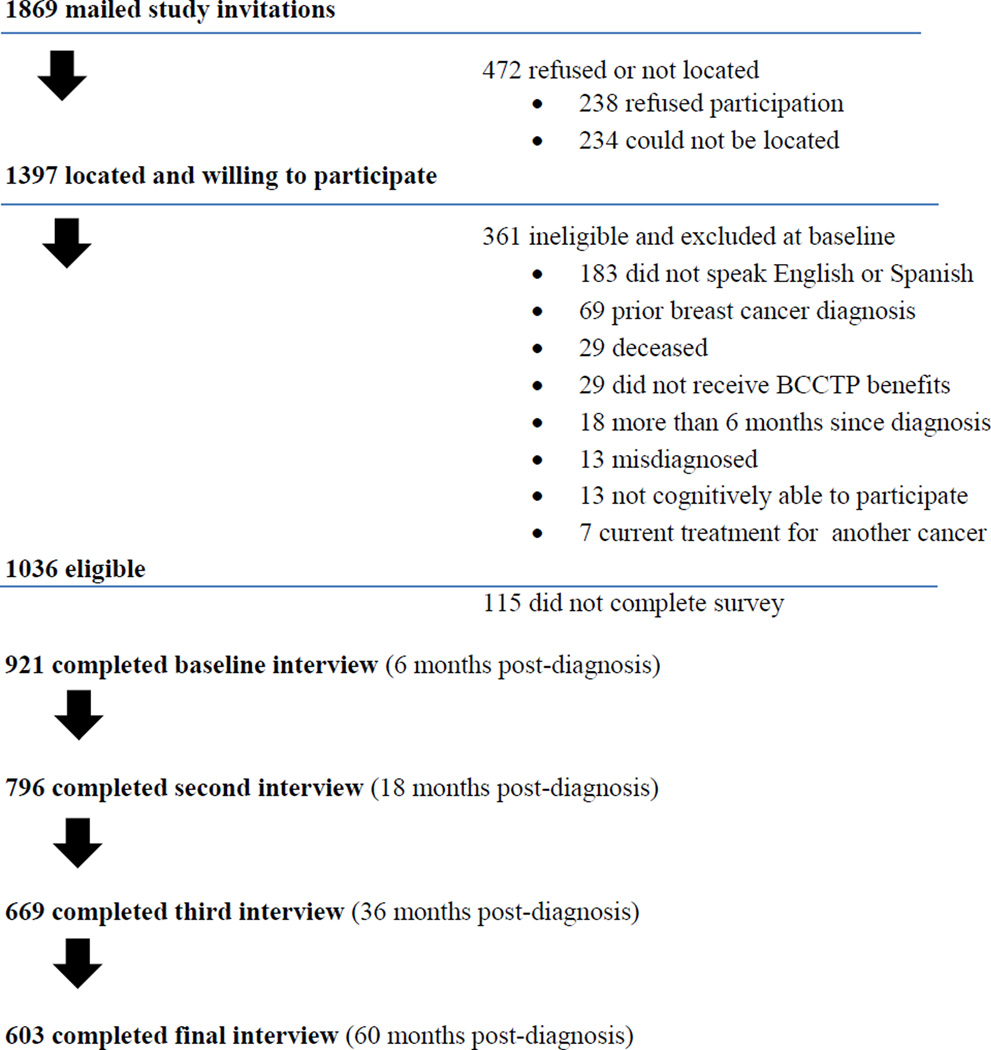
A total of 921 women of 1509 eligible women who agreed to participate completed the baseline interview at 6 months, yielding a final response rate of 61.1% (see Figure 1). Compared with survey responders, non-responders were older (52 vs. 50 years, p<0.0001), more likely to be Asian/Pacific Islanders, and less likely to be Latina and white (11.6%, 37.6%, 26.5% vs 7.4%, 53.4%, 31.7%, respectively, p<0.05). A total of 921 women were included in the initial sample of the study. Eight hundred (87%) patients consented to the medical record review and we were able to successfully retrieve and abstract medical records for 97% (n=776) of this group. Of the 921 women who participated in the first interview, response rates at 18 months, 36 months and 60 months were 86% (N=796), 73% (N=669) and 65% (N=603), respectively. A total of 569 women participated in all four interviews.
Figure 1.
Study recruitment flow
Table 1 shows the descriptive statistics among the 921 women in the study sample. Their average age was about 51 years, and they were primarily Latina (53.5%) and white (31.7%). The majority of Latinas and Asian/Pacific Islanders were born outside of the U.S. (98.6%, 95.6%, respectively). Slightly more than half graduated from high school (59.2%) and were married/partnered (51.8%). Only about 2 out of 10 women were employed at 6 months after BC diagnosis. Approximately 30% reported having at least one comorbidity. The majority of women were diagnosed either with a stage II tumor (36.6%) or a stage I (21.3%) tumor. In the case of treatment received for BC, most patients underwent breast conserving surgery (61.1%) and had receipt of chemotherapy (68.1%), radiation therapy (64.3%) and hormone therapy (64.9%). The average time to to the end of chemotherapy was 224 days after BC diagnosis and 258 days after BC diagnosis for radiation therapy. The majority of women had finished their chemotherapy (86.2%) and radiation therapy (96.4%) by 18 months after BC diagnosis. Our data indicated (Table 2) that mean SF-36 MCS and SF-36 PCS scores for the study sample were lower than national general population norms at all the time points (range 46.4–47.5, 41.8–43.4, respectively).
Table 1.
Descriptive Statistics of the Study Sample (N=921)
| Value | |
|---|---|
| Age (years) | |
| Mean(SD), [Range] | 50.8(9.5)[25.0 −85.0] |
| Ethnicity, N (%) | |
| White | 292(31.7) |
| Less-acculturated Latina | 439(47.7) |
| More-acculturated Latina | 53(5.8) |
| African-American | 54(5.9) |
| Asian/Pacific Islander | 68(7.4) |
| Other | 15(1.6) |
| Education, N (%) | |
| Less than high school | 375(40.8) |
| High school or greater | 544(59.2) |
| Married/Partnered, N (%) | |
| Yes | 444(48.2) |
| Employed, N(%) | |
| Yes | 164(17.8) |
| Comorbidity, N (%) | |
| Any | 275(29.9) |
| Stage, N (%) | |
| 0 | 81(8.8) |
| I | 196(21.3) |
| II | 337(36.6) |
| III | 154(16.7) |
| IV | 29(3.2) |
| Missing | 124(13.5) |
| Surgery, N (%)† | |
| Lumpectomy | 563(61.1) |
| Mastectomy | 358(38.9) |
| Chemotherapy, N (%) | |
| Yes | 627(68.1) |
| Radiation Therapy, N (%) | |
| Yes | 592(64.3) |
| Hormone Therapy, (%) | |
| Yes | 512(64.9) |
| Outcome Measures, Mean (SD) | |
| SF-36 MCS (at 18 months)* | 46.4(12.6) |
| SF-36 PCS (at 18 months)§ | 43.4(10.7) |
| Ladder of Life (at18 months)§§ | 6.6(2.3) |
American Joint Commission on Cancer anatomic stage.
SF-36 Mental Component Summary scale: a summary scale for mental health from the SF-36. The general U.S. population norms for SF-36MCS is a mean of 50 points with a standard deviation of 10 points
SF-36 Physical Component Summary scale: a summary scale for physical health from the SF-36. The general U.S. population norms for SF-36PCS is a mean of 50 points with a standard deviation of 10 points.
Range (1–10): 10=best possible life.
Table 2.
Mean Scores of Domains of Quality of Life
| SF-36 MCS* (Range:0–100) |
SF-36 PCS§ (Range:0–100) |
Ladder of Life Scale (Range:0–10) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| 18 months after BC diagnosis | 46.43 | 12.56 | 43.43 | 10.73 | 6.62 | 2.26 |
| 36 months after BC diagnosis | 47.47 | 11.94 | 42.31 | 11.01 | 6.60 | 2.33 |
| 60 months after BC diagnosis | 47.52 | 12.74 | 41.82 | 11.85 | 6.55 | 2.32 |
SF-36 Mental Component Summary scale: a summary scale for mental health from the SF-36. The general U.S. population norms for SF-36MCS is a mean of 50 points with a standard deviation of 10 points
SF-36 Physical Component Summary scale: a summary scale for physical l health from the SF-36. The general U.S. population norms for SF-36PCS is a mean of 50 points with a standard deviation of 10 points
Table 3 presents the results for the adjusted mixed regression models for other QOL measures. All these measures were first collected at 18- months after BC diagnosis. None of the two-way interaction terms for these QOL measures were found to be significant, meaning there was no evidence that the changes in these measures differ across ethnic groups. Overall, these women reported a significant decline in physical well-being from 18 to 36-months after their BC diagnosis (P<0.0001); even at 60 months, their physical well-being was still significantly declining compared to 18 months after diagnosis. However, there were no significant changes in mental well-being and global quality of life.
Table 3.
Mixed-effects Regression Models of Domains of Quality of Life
| Predictor | SF36 MCS§ | SF36 PCS§§ | Ladder of Life | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | S.E. | P | Estimate | S.E. | P | Estimate | S.E. | P | |
| Time Effect* (change from 18 months) | |||||||||
| At 36 months | 0.772 | 0.526 | 0.14 | −2.449 | 0.407 | <.0001 | −0.132 | 0.099 | 0.18 |
| At 60 months | 0.687 | 0.506 | 0.17 | −1.650 | 0.391 | <.0001 | −0.099 | 0.095 | 0.30 |
| Sociodemographic Factors | |||||||||
| Age | 0.483 | 0.211 | 0.02 | −0.764 | 0.193 | <.0001 | 0.009 | 0.038 | 0.82 |
| Ethnicity (ref =white) | |||||||||
| Asian/Pacific Islanders | 4.252 | 1.534 | 0.01 | 2.413 | 1.403 | 0.09 | 0.566 | 0.279 | 0.04 |
| African Americans | 1.919 | 1.763 | 0.28 | 0.647 | 1.603 | 0.69 | −0.139 | 0.321 | 0.66 |
| Less-acculturated Latinas | 4.203 | 1.025 | <.0001 | 2.359 | 0.939 | 0.01 | 0.982 | 0.186 | <.0001 |
| More-acculturated Latinas | 1.555 | 1.718 | 0.37 | 1.334 | 1.580 | 0.40 | 0.483 | 0.312 | 0.12 |
| Other | 2.427 | 2.838 | 0.39 | 0.399 | 2.610 | 0.88 | 0.992 | 0.515 | 0.05 |
| High School Graduates (Yes) | 1.591 | 0.894 | 0.08 | 0.198 | 0.819 | 0.81 | 0.149 | 0.162 | 0.36 |
| Employment (Yes) | −0.356 | 0.423 | 0.40 | −1.406 | 0.389 | 0.0003 | −0.153 | 0.077 | 0.05 |
| Clinical Characteristics | |||||||||
| Comorbidity (Yes) | −2.958 | 0.815 | 0.0003 | −4.554 | 0.746 | <.0001 | −0.500 | 0.148 | 0.0008 |
| Stage (ref=0–II) III, IV | 0.195 | 0.978 | 0.84 | −2.122 | 0.890 | 0.02 | 0.307 | 0.178 | 0.08 |
| Mastectomy (Yes) | −0.673 | 0.800 | 0.40 | 0.620 | 0.732 | 0.40 | 0.102 | 0.145 | 0.48 |
| Chemotherapy (Yes) | −1.433 | 0.902 | 0.11 | −1.625 | 0.827 | 0.05 | −0.317 | 0.164 | 0.05 |
| Radiotherapy (Yes) | 0.842 | 0.933 | 0.37 | 0.971 | 0.855 | 0.26 | 0.246 | 0.169 | 0.15 |
| Hormone Therapy (Yes) | 0.360 | 0.775 | 0.64 | 0.695 | 0.710 | 0.33 | 0.005 | 0.141 | 0.97 |
| Psychosocial Factors | |||||||||
| Social Support | 0.321 | 0.167 | 0.05 | −0.100 | 0.153 | 0.51 | 0.070 | 0.030 | 0.02 |
| Physician Emotional Support | 0.177 | 0.169 | 0.29 | 0.340 | 0.155 | 0.03 | 0.017 | 0.031 | 0.59 |
| PEPPI** | 0.079 | 0.037 | 0.03 | −0.003 | 0.033 | 0.94 | 0.016 | 0.007 | 0.01 |
| Physician Information Giving | 0.362 | 0.153 | 0.02 | 0.007 | 0.140 | 0.96 | 0.060 | 0.028 | 0.03 |
| Input in Decision-making | −0.041 | 0.382 | 0.91 | 0.610 | 0.350 | 0.08 | 0.087 | 0.069 | 0.21 |
Positive score indicates improvement
Perceived efficacy in physician-patient interactions
SF-36 Mental Component Summary scale: a summary scale for mental health from the SF-36. The general U.S. population norms for SF-36 MCS is a mean of 50 points with a standard deviation of 10 point.
SF-36 Physical Component Summary scale: a summary scale for physical health from the SF-36. The general U.S. population norms for SF-36 PCS is a mean of 50 points with a standard deviation of 10 points
Greater patient-perceived self-efficacy in patient-physician interactions (PEPPI) was significantly associated with better mental well-being (P=0.03) and better global quality of life (P=0.01). Similarly, women who received more physician information-giving reported better mental well-being (P=0.02) and better global QOL (P=0.03). Women who received more physician emotional support also reported better physical well-being (P=0.03); while women who received more social support reported better global QOL (P=0.02). Not surprising, comorbidity was inversely associated with all these QOL measures (all P<0.001), as was higher stage with SF-36 PCS (P=0.02). Older women reported better mental well-being (P=0.02) but worse physical well-being (P<0.0001). Being employed and diagnosed at a later stage (III/IV) were negatively associated with physical well-being (P=0.0003, P=0.02, respectively). Overall, ethnicity was associated with all the QOL outcomes in Table 3. Interestingly, less-acculturated Latinas reported better mental and physical well-being (P<0.0001, P=0.01, respectively), and better global QOL (P<0.0001) compared to whites. Similar findings were shown in Asian/Pacific Islanders except for SF-36 PCS.
Discussion
This study followed 921 low-income breast cancer patients from the time of 6 months after BC diagnosis to 5 years after BC diagnosis. It is one of the first studies focusing on risk factors for poor QOL in a low-income population of breast cancer patients, including mutable risk factors such as patient-physician communication. In addition, our study fills the gap in the current literature on ethnic/racial differences in QOL change over time by using longitudinal data. This study is also particularly notable for its large Latina representation, allowing a close examination of QOL in the largest ethnic/racial minority group in the U.S.,66 about which little is known.
Findings from this study overall suggest that there were no significant changes over time in QOL except for physical functioning, with survivors reporting a significant decline over 5 years. Findings on physical functioning from previous studies are mixed. Some investigators have reported restriction in physical functioning among women aged 65 years or older.67 In contrast, others have reported no changes or improvement in physical functioning over time.12,68,69 Women in our study sample experienced poor mental health and physical health compared to the general health population. The scores on both the SF-36 MCS and PCS at all the time points were all lower than population norms for the general health population.57 These are not surprising findings for a low income population; previous findings have shown that lower socioeconomic status is associated with worse health-related QOL among breast cancer patients.11,14
The negative association between employment status and SF-36 PCS in our study might also relate to the financial hardship that these women experienced. Many studies have found that being employed is positively associated with better QOL among breast cancer patients.70,71,72 This is not surprising as usually only those patients who have good enough QOL would be able to go to work. However, in our study population, women who were employed reported significantly worse SF-36 PCS. It is possible that these low income women, even though with worse reported QOL, had to go to work due to financial hardship, which has been particularly reported among Latinas,73 which form the majority of our sample.
Surprisingly, we found that less-acculturated Latinas reported both better mental and physical well-being, as well as better global quality of life status compared to whites. These findings are in contradistinction to research that has shown that Latina BC patients tend to have poorer QOL than whites.10,35,49,53,74,75,76,77 However, our study is also echoed by several nationwide studies in general populations showing that Mexican Americans, who comprise the largest proportion of Latinos in California,66 were significantly less likely to have psychiatric/anxiety disorders than whites.78, 79, 80 Also, lesser acculturation may buffer against the stress of low socioeconomic status.81
Similar to less-acculturated Latinas, Asians/Pacific Islanders also enjoyed better mental health and global quality of life than whites in our study. These findings are supported by similar findings from other studies in populations other than BC patients showing that Asian Americans report better quality of life than whites.82,83,84, 85,86 In addition, another study indicated that Asian Americans were likely to perceive a higher health-related QOL score than whites although their objective health and disease conditions were equal, perhaps indicating cultural differences in interpreting QOL and adverse events.87
Given the fact that over 95% of these two ethnic groups in our study was born outside of the U.S, the "healthy immigrant effect, "88, 89 in which healthy individuals with good health status are more likely to immigrate to US, might yet be another explanation for better QOL reported among Latinas and Asians/Pacific Islanders in our sample. In addition, the traditional cultural retention of family networks among Latinos and Asians may also play a role in their better assessed QOL.88,90
Findings about QOL among African American women with breast cancer have been mixed, with some studies showing no difference compared to whites,10,49,50,91 and others showing worse QOL for African American women compared to whites.53,92 Our study showed no difference on QOL among all measured domains between low-income African American women with breast cancer compared to white women with breast cancer.
Consistent with previous studies showing that self-efficacy in interacting with physicians is a positive predictor for many BC care outcomes in vulnerable populations,32,31,33 we found that low-income women with greater self-efficacy in interacting with physicians at the time of initial diagnosis and treatment reported better scores on global QOL and mental health than those with less self-efficacy, controlling for the potential confounders of education and language barriers. This finding was concordant with Kwan et al’s study showing that poorer patient-physician interaction was associated with worse QOL.93 Women with greater self-efficacy may be able to solicit and incorporate information in their decision-making process and adjustment to the BC diagnosis and treatment process that is personalized to their particular informational and psychosocial needs. Healthcare practitioners should be aware of the vital role of patient’s self-efficacy in interacting with physicians plays in this disempowered population.
On the other side of the patient-physician communication dyad, physician information-giving about the diagnosis and treatment of breast cancer was found to predict mental well-being and global quality of life, controlling for a wide range of confounders, including education and language barriers. This is one of the few studies to show this direct link. This population may have been particularly enabled and their sense of control increased during a stressful time by such communication, due to low levels of a priori breast cancer knowledge secondary to issues of literacy, levels of education, and language barriers, as well as concomitant life burdens associated with lower SES.
Social support has been well demonstrated to be a predictor of many QOL domains such as physical, mental well-being as well as overall QOL among BC patients.14,24 However, more social support only positively affected global QOL in our study, but not other QOL outcomes. The controlling for psychosocial factors other than social support might partially explain these diminished effects of social support in our study. The significant impacts of self-efficacy in interacting with physicians and physician information giving suggest that support from healthcare professionals might be as or a more important coping resource than support from family members or friends for patients in adjustment to breast cancer.
Age was associated with both mental and physical functioning, yet its impact on these two outcomes was opposite. Older women reported worse physical health compared to younger women, which is in accordance with prior results.94 This physical functioning decrease for older women might partly reflect the normal aging process. However, some investigators have suggested that older people tended to adjust their perceptions about their health while aging, whereas younger people may hold higher expectations concerning their sense of well-being or have increased emotional disruption due to factors such as experiencing a menopause transition as part of therapy or feeling more vulnerable after cancer.95,96 Our finding of better mental health status in older women compared with that in younger women supports this observation.
Consistent with previous studies,10,11 cormorbidity had significant negative associations with all QOL outcomes. Later stage was only negatively associated with physical functioning, but not other QOL outcomes. However, specific types of BC treatment were not associated with differences in QOL outcomes when controlling for other factors, including race/ethnicity. This suggests it was not treatment or tumor characteristic per se, but rather cormorbidity that can make an impact on QOL for long term low-income breast cancer survivors. Healthcare providers thus should focus more on primary care of chronic conditions during the long term follow-up period to help improve survivors’ QOL.
The strengths of this study include that this is the largest study of low-income women with BC, along with the largest representation of Latinas, of which we are aware. In addition, the longitudinal data allowed us to investigate ethnic/racial differences in QOL over time, with the majority of the sample completing the study, which is almost absent in the current literature. However, several limitations to this study should be noted. Because the sample was comprised of low-income, medically underserved women in a specific Medicaid BC treatment program in California, generalizability of the findings to other low-income populations may be limited. Also, generalizability to other Latina populations other than Mexican Americans may be limited due to their predominance in the California Latino population.65 Second, although we achieved a 61% response rate, the differences between responders and non-responders in terms of age and race/ethnicity might potentially have biased the observed results. Third, an additional limitation of the study is that we did not have pretreatment assessments of all the QOL measures, which were not possible due to limitations in access to patients being recruited into the study. Finally, the quality of our data depends on the accuracy of patient self-report on physician communication, thus recall bias may be an issue in our study. However, it has been noted that people who have undergone a sudden and life-threatening health crisis manifest very clear recall of the details surrounding the event.97 BC patients, example, can recall the precise time when they first noticed their symptoms.98 Moreover, a recent paper reported that self-reporting of key treatment and prognostic information is relatively accurate among these low-income women with BC.99
In summary, findings from this study suggest that the low income women in our study sample experienced poor mental health and physical health compared to the general health population. However, less acculturated Latinas reported better mental health and physical well- and Asians/Pacific Islanders reported better mental health and global quality of life than whites, which for the former group is in contradistinction to previous research. Our results further suggest that quality of life among low-income, medically underserved women with breast cancer could be enhanced by targeted interventions aimed at increasing patients' empowerment in patient-physician interactions and information giving, encouraging social ties, and by attention to comorbid medical conditions.
Acknowledgments
Research Support:
This study was funded by the American Cancer Society (# TURSG-02-081), the California Breast Cancer Research Program (# 7PB-0070), and the National Cancer Institute (# 1R01CA119197-01A1). Dr. Maly was further supported by Grant No. 1R01 CA140481-01 A1 from the National Cancer Institute.
Footnotes
Financial Disclosures: None.
References
- 1.NCI Office of Cancer Survivorship. Available from: http://cancercontrol.cancer.gov/ocs/prevalence/chart2.html.
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012 Jul-Aug;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Soerjomataram I, Louwman MWJ, Bibot JG, Roukema JA, Coebergh JWW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor R, Davis P, Boyages J. Long-term survival of women with breast cancer in New South Wales. Eur J Cancer. 2003;39:215–222. doi: 10.1016/s0959-8049(02)00486-0. [DOI] [PubMed] [Google Scholar]
- 5.Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 6.Ries LAG, Eisner MP, Kosary CL, et al., editors. SEER cancer statistics review, 1975–2002, national cancer institute. Bethesda, MD: 2005. http://seer.cancer.org/csr/1975_2002/, base on November 2004 SEER data submission, pasted to the SEER web site 2005. [Google Scholar]
- 7.Calman K. Definition and dimensions of quality of life. In: Aaronson NK, Beckman J, editors. Quality of Life of Cancer Patients. New York: Raven Press; 1987. pp. 1–10. [Google Scholar]
- 8.Bloom JR, Stewart SL, Napoles AM, et al. Quality of life of Latina and Euro-American women with ductal carcinoma in situ. Psychooncology. 2012 Jun 7; doi: 10.1002/pon.3098. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Ferrell BR, Grant M, Funk B. Quality of life in breast cancer. Cancer Pract. 1996;4:331–340. [PubMed] [Google Scholar]
- 10.Ashing-Giwa KT, Tejero JS, Kim J, et al. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Quality of life Research. 2007;16:413–428. doi: 10.1007/s11136-006-9138-4. [DOI] [PubMed] [Google Scholar]
- 11.Ashing-Giwa KT, Lim JW. Examining the impact of socioeconomic status and socioecologic stress on physical and mental health quality of life among breast cancer survivors. Oncol Nurs Forum. 2009;36(1):79–88. doi: 10.1188/09.ONF.79-88. [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. JNCI. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 13.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. JNCI. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 14.Kwan ML, Ergas IJ, Somkin CP, et al. Quality of life among women recently diagnosed with invasive breast cancer: the Pathways Study. Breast Cancer Res Treat. 2010 Sep;123(2):507–524. doi: 10.1007/s10549-010-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W, Cui Y, Chen X, et al. Changes in quality of life among breast cancer patients three years post-diagnosis. Breast Cancer Res Treat. 2008;114(2):357–369. doi: 10.1007/s10549-008-0008-3. [DOI] [PubMed] [Google Scholar]
- 16.Champion VL, Wagner LI, Monahan PO, et al. Comparison of younger and older breast cancer surviors and age-matched controls on specific and overall quality of life domains. Cancer. 2014 doi: 10.1002/cncr.28737. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park BW, Lee S, Lee AR, Lee KH, Hwang SY. Quality of life differences between younger and older breast cancer patients. J Breast Cancer. 2011 Jun;14(2):112–118. doi: 10.4048/jbc.2011.14.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janz NK, Mujahid M, Lantz PM, et al. Population-based study of the relationship of treatment and sociodemographics on quality of life for early stage breast cancer. Qual Life Res. 2005;14:1467–1479. doi: 10.1007/s11136-005-0288-6. [DOI] [PubMed] [Google Scholar]
- 19.Janni W, Rjosk D, Dimpfl TH, et al. Quality of life influenced by primary surgical treatment for stage I–III breast cancer-long-term follow-up of a matched-pair analysis. Ann Surg Oncol. 2001;8:542–548. doi: 10.1007/s10434-001-0542-2. [DOI] [PubMed] [Google Scholar]
- 20.Grimison PS, Stockler M. Quality of life and adjuvant systemic therapy for breast cancer. Expert Rev Anticancer Ther. 2007;7:1123–1134. doi: 10.1586/14737140.7.8.1123. [DOI] [PubMed] [Google Scholar]
- 21.Ganz PA, Kwan L, Stanton AL, et al. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29:1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 23.Broeckel JA, Jacobsen PB, Balducci L, et al. Quality of life after adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2000;62:141–150. doi: 10.1023/a:1006401914682. [DOI] [PubMed] [Google Scholar]
- 24.Neuling SJ, Winefield HR. Social support and recovery after surgery for breast cancer: Frequency and correlates of supportive behaviors by family, friends and surgeon. Soc Sci Med. 1988;27:385–392. doi: 10.1016/0277-9536(88)90273-0. [DOI] [PubMed] [Google Scholar]
- 25.Salonen P, Tarkka MT, Kellokumpu-Lehtinen PL, et al. Effect of social support on changes in quality of life in early breast cancer patients: a longitudinal study. Scand J Caring Sci. 2013;27(2):396–405. doi: 10.1111/j.1471-6712.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 26.Ebright PR, Lyon B. Understanding hope and factors that enhance hope in women with breast cancer. Oncol Nurs Forum. 2002;29:561–568. doi: 10.1188/02.ONF.561-568. [DOI] [PubMed] [Google Scholar]
- 27.Arora NK, Rutten LJF, Gustafson DH, Moser R, Hawwkins RP. Perceived helpfulness and impact of social support provided by family, friends, and health care providers to women newly diagnosed with breast cancer. Psychoooncology. 2007;16:474–486. doi: 10.1002/pon.1084. [DOI] [PubMed] [Google Scholar]
- 28.Silliman RA, Dukes KA, Sullivan LM, et al. Breast cancer care in older women: Sources of information, social support, and emotional health outcomes. Cancer. 1998;83:706–711. [PubMed] [Google Scholar]
- 29.Maly RC, Umezawa Y, Leake B, et al. Determinants of participation in treatment decision-making by older breast cancer patients. Breast Cancer Res Treat. 2004;85:201–209. doi: 10.1023/B:BREA.0000025408.46234.66. [DOI] [PubMed] [Google Scholar]
- 30.Maly R, Liu Y, Kwong E, et al. Breast Reconstructive surgery in medically underserved women with breast cancer: the role of patient-physician communication. Cancer. 2009;115(20):4819–4827. doi: 10.1002/cncr.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maly RC, Liu Y, Leake B, et al. Treatment-related symptoms among underserved women with breast cancer: the impact of physician-patient communication. Breast Cancer Res Treat. 2010;119(3):707–716. doi: 10.1007/s10549-009-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maly RC, Leake B, Silliman RA. Breast cancer treatment in older women: impact of the patient-physician interaction. J Am Geriatr Soc. 2004;52(7):1138–1145. doi: 10.1111/j.1532-5415.2004.52312.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Malin JL, Diamant AL, Thind A, Maly RC. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat. 2013 Feb;137(3):829–836. doi: 10.1007/s10549-012-2387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thind A, Diamant A, Liu Y, Maly R. Factors that determines satisfaction with surgical treatment of low income women with breast cancer. Archives of Surgery. 2009;144(11):1068–1073. doi: 10.1001/archsurg.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maly RC, Stein JA, Umezawa Y, et al. Racial/ethnic differences in breast cancer outcomes among older patients: effects of physician communication and patient empowerment. Health Psychol. 2008;27(6):728–736. doi: 10.1037/0278-6133.27.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siminoff LA, Ravdin P, Colabianchi N, Sturm CM. Doctor-patient communication patterns in breast cancer adjuvant therapy discussions. Health Expect. 2000;3(1):26–36. doi: 10.1046/j.1369-6513.2000.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moyer A, Salovey P. Patient participation in treatment decision making and the psychological consequences of breast cancer surgery. Womens Health. 1998;4(2):103–116. [PubMed] [Google Scholar]
- 38.Street RL, Jr, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Med Decis Making. 1997;17(3):298–306. doi: 10.1177/0272989X9701700306. [DOI] [PubMed] [Google Scholar]
- 39.Davies NI, Kinman G, Thomas RJ, Bailey T. Information satisfaction in breast and prostate cancer patients: Implications for quality of life. Psycho-Oncology. 2008;17(10):1048–1052. doi: 10.1002/pon.1305. [DOI] [PubMed] [Google Scholar]
- 40.Engel J, Kerr J, Schlesinger-Rabb A, et al. Predictors of quality of life of breast cancer patients. Acta Oncologica. 2003;42(7):710–718. doi: 10.1080/02841860310017658. [DOI] [PubMed] [Google Scholar]
- 41.Vogel BA, Leonhart R, Helmes AW. Communication matters: The impact of communication and participation in decision making on breast cancer patients’ depression and quality of life. Patient Educ and Counseling. 2009;77(3):391–397. doi: 10.1016/j.pec.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Kerr J, Engel J, Schlesinger-Raab A, et al. Communication, quality of life and age: Results of a 5-year prospective study in breast cancer patients. Annals of Oncology. 2003;14(3):421–427. doi: 10.1093/annonc/mdg098. [DOI] [PubMed] [Google Scholar]
- 43.Smedley BD, Smith AY, Nelson AR, editors. IOM of the National Academies. 2003. Unequal treatment: Confronting Racial and Ethnic Disparities in Healthcare. [PubMed] [Google Scholar]
- 44.Newman LA. Disparities in breast cancer. Curr Problems in Cancer. 2007;31(3):134–156. doi: 10.1016/j.currproblcancer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Breen N, Kessler LG, Brown ML. Breast cancer control among the underserved-an overview. Breast Cancer Res Treat. 1996;40:105–15. doi: 10.1007/BF01806006. [DOI] [PubMed] [Google Scholar]
- 46.Siminoff LA, Graham GC, Gordon NH. Cancer communication patterns and the influence of patient characteristics: disparities in information-giving and affective behaviors. Patient Educ Couns. 2006 Sep;62(3):355–360. doi: 10.1016/j.pec.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Ashing-Giwa KT, Ganz PA, Petersen L. Quality of life of African-Americans and white long term breast carcinoma survivors. Cancer. 1999;85:418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Christie KM, Meyerowitz B, Maly RC. Depression and sexual adjustment following breast cancer in low-income Hispanic and non-Hispanic White women. Psychooncology. 2010 Oct;19(10):1069–1077. doi: 10.1002/pon.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janz NK, Mujahid MS, Hawley ST, et al. Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv. 2009;3(4):212–222. doi: 10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallicchio L, Calhoun C, Helzlsouer KJ. Association between race and physical functioning limitations among breast cancer survivors. Support Care Cancer. 2014 Apr;22(4):1081–1088. doi: 10.1007/s00520-013-2066-2. [DOI] [PubMed] [Google Scholar]
- 51.Yanez B, Stanon AL, Maly RC. Breast cancer treatment decision making among Latinas and non-Latina whites: A communication model predicting decisional outcomes and quality of life. Health Psychol. 2012 Sep;31(5):552–561. doi: 10.1037/a0028629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedman LC, Kalidas M, Elledge R, et al. Optimism, social support and psychosocial functioning among women with breast cancer. Psychooncology. 2006;15:595–603. doi: 10.1002/pon.992. [DOI] [PubMed] [Google Scholar]
- 53.Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007 Nov;106(1):85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carver CS, Smith RG, Antoni MH, Petronis VM, Weiss S, Derhagopian RP. Optimistic personality and psychosocial well-being during treatment predict psychosocial well-being among long-term survivors of breast cancer. Health Psychol. 2005 Sep;24(5):508–516. doi: 10.1037/0278-6133.24.5.508. [DOI] [PubMed] [Google Scholar]
- 55.Chen JY, Diamant AL, Thind A, Maly RC. Determinants of breast cancer knowledge among newly diagnosed, low-income, medically underserved women with breast cancer. Cancer. 2008;112(5):1153–1161. doi: 10.1002/cncr.23262. [DOI] [PubMed] [Google Scholar]
- 56.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 57.Ware JE, Jr, Kosinski M, Keller SD. SF-36 physical and mental health summary scales: a user’s manual. Boston (MA): The Health Institute; 1994. [Google Scholar]
- 58.Cantril H. The pattern of human concerns. New Brunswick (NJ): Rutgers University Press; 1965. [Google Scholar]
- 59.Schag CA, Heinrich RL, Aadland RL, Ganz PA. Accessing problems of cancer patients: psychometric properties of the cancer inventory of problem situations. Health Psychol. 1990;9:83–102. doi: 10.1037//0278-6133.9.1.83. [DOI] [PubMed] [Google Scholar]
- 60.Maly RC, Leake B, Silliman R. Health care disparities in older patients with breast carcinoma. Cancer. 2003;97:1517–1527. doi: 10.1002/cncr.11211. [DOI] [PubMed] [Google Scholar]
- 61.Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc. 1998;46:889–894. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 62.Seeman TE, Berkman LF. Structural characteristics of social networks and their relationship with social support in the elderly: Who provides support? Social Science and Medicine. 1988;26(7):737–749. doi: 10.1016/0277-9536(88)90065-2. [DOI] [PubMed] [Google Scholar]
- 63.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: pringer; 2010. pp. 347–376. [Google Scholar]
- 64.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Marin G, Sabogal F, Marin B, et al. Development of a short acculturation scale for Hispanics. Hsp J Behav Sci. 1987;9:183–205. [Google Scholar]
- 66.Humes KR, Jones NA, Ramirez RR. Overview of Race and Hispanic Origin: 2010 Census Briefs. [acceessd July 24, 2014]; Available from URL: http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf.
- 67.Sehl M, Lu X, Silliman R, Ganz PA. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv. 2013 Mar;7(1):20–31. doi: 10.1007/s11764-012-0239-5. Epub 2012 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sagen A, Karesen R, Sandvik L, Risberg MA. Changes in arm morbidities and health-related quality of life after breast cancer surgery – a five-year follow-up study. Acta Oncol. 2009;48(8):1111–1118. doi: 10.3109/02841860903061691. [DOI] [PubMed] [Google Scholar]
- 69.Hsu T, Ennis M, Hood N, Graham M, Goodwin PJ. Quality of life in long-term breast cancer survivors. J Clin Oncol. 2013 Oct 1;31(28):3540–3348. doi: 10.1200/JCO.2012.48.1903. [DOI] [PubMed] [Google Scholar]
- 70.Mahar KK, BrintzenhofeSzoc K, Shields JJ. The impact of changes in employment status on psychosocial well-being: a study of breast cancer survivors. J Psychosoc Oncol. 2008;26(3):1–17. doi: 10.1080/07347330802115400. [DOI] [PubMed] [Google Scholar]
- 71.Timperi AW, Ergas IJ, Rehkopf DH, et al. Employment status and quality of life in recently diagnosed breast cancer survivors. Psychooncology. 2013 Jun;22(6):1411–1420. doi: 10.1002/pon.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundh MH, Lampic C, Nordin K, et al. Changes in health-related quality of life by occupational status among women diagnosed with breast cancer-a population-based cohort study. Psychooncology. 2013 Apr 14; doi: 10.1002/pon.3285. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 73.Ashing-Giwa KT, Padilla GV, Bohórquez DE, Tejero JS, Garcia M. Understanding the breast cancer experience of Latina women. J Psychosoc Oncol. 2006;24(3):19–52. doi: 10.1300/J077v24n03_02. [DOI] [PubMed] [Google Scholar]
- 74.Sammarco A, Konecny LM. Quality of life, social support, and uncertainty among Latina and Caucasian breast cancer survivors: a comparative study. Oncol Nurs Forum. 2010 Jan;37(1):93–99. doi: 10.1188/10.ONF.93-99. [DOI] [PubMed] [Google Scholar]
- 75.Graves KD, Jensen RE, Canar J, et al. Through the lens of culture: quality of life among Latina breast cancer survivors. Brest Cancer Res Treat. 2012;136:603–613. doi: 10.1007/s10549-012-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carver CS, Smith RG, Petronis VM, Antoni MH. Quality of life among long-term survivors of breast cancer: Different types of antecedents predict different classes of outcomes. Psycho-Oncology. 2006;15:749–758. doi: 10.1002/pon.1006. [DOI] [PubMed] [Google Scholar]
- 77.Ashing-Giwa KT, Rosales M, Lai L, Weitzel J. Depressive symptomatology among Latina breast cancer survivors. Psycho-Oncology. 2013;22:845–853. doi: 10.1002/pon.3084. [DOI] [PubMed] [Google Scholar]
- 78.Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, Anderson K. Immigration and lifetime prevalence of DSM-IV psychiatric disorders among Mexican Americans and Non-Hispanic Whites in the United States. Arch Gen Psychiatry. 2004;61:1226–1233. doi: 10.1001/archpsyc.61.12.1226. [DOI] [PubMed] [Google Scholar]
- 79.Robin LN, Regier DA, editors. Psychiatric disorders in America: the Epidemiologic Catchment Area Study. New York, NY: Free Press; 1991. [Google Scholar]
- 80.Kessler RC, Walters EE. The National Comorbidity survey. In: Tsaung MT, Tohen M, editors. Textbook in psychiatric epidemiology. 2nd ed. New York, NY: John Wiley & Sons; 2002. pp. 343–362. [Google Scholar]
- 81.Gallo LC, de Los Monteros KE, Allison M, et al. Do socioeconomic gradients in subclinical atherosclerosis vary according to acculturation level? Analyses of Mexican-Americans in the multi-ethnic study of atherosclerosis. Psychosom Med. 2009 Sep;71(7):756–766. doi: 10.1097/PSY.0b013e3181b0d2b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Avis NE, Ory M, Matthews KA, et al. Health-related quality of life in a multiethnic sample of middle-aged women: study of Women’s Health across the Nation (SWAN) Med Care. 2003;41:1262–1276. doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 83.Lopes AA, Bragg-Gresham JL, Satayathum S, et al. Health related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2003;41:605–615. doi: 10.1053/ajkd.2003.50122. [DOI] [PubMed] [Google Scholar]
- 84.Chowdhury PP, Balluz L, Strine TW. Health-related quality of life among minority populations in the United States, BRFSS 2001–2002. Ethnicity & Disease. 2008;18:483–487. [PubMed] [Google Scholar]
- 85.WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- 86.Takeuchi DT, Zane N, Hong S, et al. Immigration-related factors and mental disorders among Asian Americans. American J of Public Health. 2007;97(1):84–90. doi: 10.2105/AJPH.2006.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu AZ, Kattan MW. Racial and ethnic differences in preference-based health status measure. Curr Med Res Opin. 2006;22(12):2439–2449. doi: 10.1185/030079906X148391. [DOI] [PubMed] [Google Scholar]
- 88.Burnam MA, Hough RL, Karno M, Escobar JI, Telles CA. Acculturation and life-time prevalence of psychiatric disorders among Mexican Americans in Los Angeles. J Health Soc Behav. 1987;28:89–102. [PubMed] [Google Scholar]
- 89.Landale NS, Oropesa RS, Bradatan C. Hispanics and the Future of America. In: Tienda M, Mitchell F, editors. National Research Council (US) Panel on Hispanics in the United States. Washington (DC): National Academies Press (US); 2006. [PubMed] [Google Scholar]
- 90.Juang LP, Cookston JT. Acculturation, discrimination, and depressive symptoms among Chinese American adolescents: a longitudinal study. J Primary Prevent. 2009;30:475–496. doi: 10.1007/s10935-009-0177-9. [DOI] [PubMed] [Google Scholar]
- 91.Giedzinska AS, Meyerowitz BE, Ganz PA, Rowland JH. Health-related quality of life in a multiethnic sample of breast cancer survivors. Ann Behav Med. 2004 Aug;28(1):39–51. doi: 10.1207/s15324796abm2801_6. [DOI] [PubMed] [Google Scholar]
- 92.Paskett ED, Alfano CM, Davidson MA, et al. Breast cancer survivors’ health-related quality of life : racial differences and comparisons with noncancer controls. Cancer. 2008 Dec 1;113(11):3222–3230. doi: 10.1002/cncr.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwan ML, Tam EK, Ergas IJ, et al. Patient-physician interaction and quality of life in recently diagnosed breast cancer patients. Breast Cancer Res Treat. 2013;139:581–595. doi: 10.1007/s10549-013-2569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ganz PA, Day R, Ware JE, Jr, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995 Sep 20;87(18):1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 95.Bloom JR, Kang SH, Petersen DM, Stewart SL. Quality of life in long-term cancer survivors. In: Feuerstein M, editor. Handbook of Cancer Survivorship. Bethesda, MD: Springer; 2007. pp. 43–66. [Google Scholar]
- 96.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003 Nov 15;21(22):4184–4493. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 97.Brown R, Kulik J. Flashbulb memories. In: Neisser U, editor. Memory Observed. San Francisco, CA: Freeman and Co.; 1992. pp. 23–40. [Google Scholar]
- 98.Burgess C, Ramirez A, Richards M, et al. Who and what influences delayed presentation in breast cancer? Br J Cancer. 1998;77:1343–1348. doi: 10.1038/bjc.1998.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y, Diamant A, Thind A, Maly R. Validity of self-reported of breast cancer treatment in low-income, medically underserved women with breast cancer. Breast Cancer Res Treat. 2010 Feb;119(3):745–751. doi: 10.1007/s10549-009-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]