Abstract
Background
Litter decomposition greatly influences soil structure, nutrient content and carbon sequestration, but how litter decomposition is affected by climate change is still not well understood.
Methodology/Principal Findings
A field experiment with increased temperature and nitrogen (N) addition was established in April 2007 to examine the effects of experimental warming, N addition and their interaction on litter decomposition in a temperate meadow steppe in northeastern China. Warming, N addition and warming plus N addition reduced the residual mass of L. chinensis litter by 3.78%, 7.51% and 4.53%, respectively, in 2008 and 2009, and by 4.73%, 24.08% and 16.1%, respectively, in 2010. Warming, N addition and warming plus N addition had no effect on the decomposition of P. communis litter in 2008 or 2009, but reduced the residual litter mass by 5.58%, 15.53% and 5.17%, respectively, in 2010. Warming and N addition reduced the cellulose percentage of L. chinensis and P. communis, specifically in 2010. The lignin percentage of L. chinensis and P. communis was reduced by warming but increased by N addition. The C, N and P contents of L. chinensis and P. communis litter increased with time. Warming and N addition reduced the C content and C:N ratios of L. chinensisand P. communis litter, but increased the N and P contents. Significant interactive effects of warming and N addition on litter decomposition were observed (P<0.01).
Conclusion/Significance
The litter decomposition rate was highly correlated with soil temperature, soil water content and litter quality. Warming and N addition significantly impacted the litter decomposition rate in the Songnen meadow ecosystem, and the effects of warming and N addition on litter decomposition were also influenced by the quality of litter. These results highlight how climate change could alter grassland ecosystem carbon, nitrogen and phosphorus contents in soil by influencing litter decomposition.
Introduction
Litter provides important energy and nutrient sources for microbial metabolism [1] and stores most of the belowground carbon in an ecosystem [2]. The factors associated with litter decomposition are primarily driven by micro- and macro-organismic activities (i.e., microbes, arthropods), litter quality (i.e., litter carbon (C), nitrogen (N), phosphorus (P), C:N ratios and lignin), climate (i.e., temperature and moisture), and the abundance of decomposers [3,4]. High quality litter (i.e., with low lignin content, high N and P contents and narrow C:N ratios) exhibits relatively fast decomposition rates [5,6,7]. Thus, litter decomposition rates are positively correlated with litter N and P contents and cellulose [8,9,10]. Therefore, any changes in litter quality and climate will affect litter decomposition. In addition, potential changes in decomposition rates and associated C loss and N and P release and/or immobilization from litter under global climate change will influence plant productivity and global C, N and P cycling [3,11].
It is predicted that global mean surface temperatures will rise by 1.1–6.4°C [12]. As a consequence, climate warming may directly alter soil temperature and moisture and the activity of soil organisms in ecosystems [3]. Nadelhoffer et al. reported that increased temperature enhances the rate of litter decomposition by stimulating microbial activity [13]. However, other studies revealed the opposite result, where the increase in temperature hinders the rate of decomposition because of moisture decline [14], as well as decreasing plant litter quality [15] and altering plant species composition of different litter qualities [14]. These changes in litter quality and species composition under warming have an indirect impact on litter decomposition [11]. Though the influence of climate warming on litter production, decomposition, and quality has been examined [16–18], the effects of warming on litter decomposition to C, N and P dynamics in litter pools at the ecosystem scale remain unclear.
Nitrogen deposition caused by anthropogenic activities is currently 30% greater than deposition from natural terrestrial inputs and is substantially greater than it was a hundred years ago [19]. This increase in N inputs is expected to affect ecosystem processes such as plant growth, plant species diversity, biogeochemical cycles, and net ecosystem C-accumulation [20], specifically in temperate terrestrial ecosystems where N deposition is limited. Though the effects of N added to soil through litter decomposition are inconsistent (i.e., positive [21–24], negative [9,25], and neutral effects [5,6] have been reported), N deposition still plays a key role in litter decomposition dynamics. Further, N addition can increase inorganic N availability and decrease litter C:N ratios [17,25] due to more rapid litter decomposition [21]. Currently, the effects of N deposition on litter decomposition remain controversial.
Numerous studies have focused on the individual effects of N addition and warming on litter quality; however, little attention has been paid to the interactive effects of warming and N addition on litter decomposition. Concurrent changes in global temperature and N addition may have potentially interactive effects on litter decomposition. Songnen Grassland lies in the eastern edge of the Eurasian grassland biome, which is the most typical and largest meadow steppe in China. The average temperature of the Songnen meadow steppe has risen 2°C in the last two decades [26], and average atmospheric N deposition is approximately 10.5 g m-2 a-1 [27]. To examine the influence of experimental warming and N addition on litter decomposition, we conducted a 3-year artificial warming and N addition experiment in the Songnen meadow steppe in northeast China. In this study, we addressed these questions: (1) to what extent do warming and N addition affect litter decomposition? and (2) are there interactive effects between warming and N addition on litter decomposition?
Materials and Methods
Study Site
The study was conducted in the Songnen Grassland at the Grassland Ecosystem Field Station of the Northeast Normal University in northeast China (123°44′ E and 144°40′ N). The long-term mean annual temperature is 6.4°C with a frost-free period of 141 days. The mean annual rainfall is 470 mm, which occurs between June and August. The annual potential evapotranspiration is 2–3 times higher than the annual rainfall [28]. The growing season is limited from late April to early October. The vegetation is dominated by the perennial grass Leymus chinensis (Trin.) Tzvel. and Phragmites communis, accompanying vegetation are Kalimeris integrifolia Turcz. Ex DC., Carex duriuscula C. A. Mey. and Rhizoma phragmitis. The height of the plant communities is 60 cm, and total community cover exceeds 80%. The primary soil type is Chernozem. The total soil N, organic C and pH, measured at a depth of 0 to 25 cm, are 19.6 ± 1.32 g kg-1, 29.39 ± 2.96 g kg-1, and 8.14 ± 0.2, respectively. The soluble salt content of the soil is high; the main cation is Na+, and the main anion is HCO3 -[28].
Experimental Design
The experiment was designed as a randomized complete block with warming and N addition as fixed factors. Each factor had two levels. There were four treatments: control (C), warming (W), N addition (N), and warming plus N addition (WN), with six replicates of each treatment. The size of each plot was 2 × 3 m. The warming plots were heated continuously using infrared radiators (Kalglo Electronics Inc. Bethlehem, PA, MSR-2420, USA) suspended at a height of 2.25 m over the center of each plot. In each control and N addition plot, a ‘dummy’ heater with the same shape and size was installed to simulate the shading effects of the infrared radiator. The heaters were set at a radiation output of approximately 1700 W.
He et al. estimated that airborne N up to 80–90 g m-2 yr-1 and higher N deposition would occur in the future owing to land-use change and anthropogenic activities [29]. In addition, Bai et al. estimated that the community saturation rate of N deposition was approximately 10.5 g m-2 yr-1 for a temperate grassland ecosystem [27]. Thus, in the N addition treatment plots in the current study, a pulse of aqueous ammonium nitrate (10 g m-2 yr-1) was added on the first day of May each year. The same amount of water (equivalent to ~ 2 mm of rainfall) was applied to N addition and ambient N plots (i.e., without N addition).
Soil Temperature and Water Content Measurements
Soil temperature and water content were measured using an ECH2O Dielectric Aquameter (EM50/R Decagon Ltd, Pullman, WA, USA). In each subplot, soil temperature and water content (0–15 cm) were measured daily between 08:00 and 09:00 A.M. in late May, mid-June, mid-July, early August, mid-September and mid-October in 2008, 2009 and 2010.
Litter Decomposition
In September 2007, we collected Leymus chinensis and Phragmites communis litter from areas outside the experimental blocks. The litter had recently been dropped on the ground surface and was dead but still connected to living plants. The litter samples were stored under ventilated conditions in the laboratory prior to decomposition experiments. In April 2008, the samples were air-dried in a ventilation oven to constant weight and cut into 10 cm long segments. The litter was placed in 25 × 15 cm bags constructed using 1 mm mesh nylon wire, and each litterbag contained 10 g of litter per species. The litterbags were fixed on the ground surface of the appropriate plots using metal pins to prevent movement from wind. The initial chemical composition of L. chinensis and P. communis differed (Table 1). L. chinensis had 55.4% higher N content, 10.3% higher P content, 18.5% lower C content, 47.5% lower C:N ratio, 11.3% lower cellulose and 24.2% lower lignin content compared with P. communis.
Table 1. Initial litter chemistry component of L. chinensis and P. communis .
| Species | C (mg/g) | N (mg/g) | P (mg/g) | C:N | Cellulose (%) | Lignin (%) |
|---|---|---|---|---|---|---|
| L. chinensis | 321.4±6.0b | 6.272±0.3a | 0.86±0.02a | 51.24±3b | 36.58±2b | 6.22±0.2b |
| P. communis | 394.12±7.2a | 4.037±0.2b | 0.78±0.01b | 97.63±7a | 41.24±5a | 8.21±0.3a |
C = total C; N = total N; P = total P. Values presented are the means±S.E. (n = 6). Statistically significant differences (P < 0.05) between treatments are indicated by different lowercase letters.
The litterbags were retrieved in October of 2008, 2009 and 2010 and transported to the laboratory for further analyses. Living plants and plant tissues were removed, and soil particles were carefully wiped off. Samples were dried for 48 hours at 65°C and then weighed.
Chemical Analysis
The dried samples were ground and passed through a 1 mm mesh. Total C and N contents of the samples were determined using the dichromate oxidation [30] and Kjeldahl methods [31]. Total P was first digested in sulfuric acid and subsequently quantified using an ICP Elemental Analyzer (Bruker Analysis Instrument Ltd., Karlsruhe, BW Germany). Specifically, 10 ml of sulfuric acid and 5 ml of perchloric acid were added to 0.7 g of soil and boiled for 1 hour at 420°C. Then, the solution was filtered, made up to a constant volume, and quantified using an ICP Elemental Analyzer. The cellulose and lignin contents were analyzed using the acid-detergent fiber method [32]. In addition, six litter samples from each plant species were analyzed for total C, N, P, cellulose and lignin to determine the initial litter chemistry.
Statistical Analysis
The dry residual mass of the litter (R) during decomposition was calculated as follows:
where M0 is the initial dry mass of the litter before decomposition, Mt is the dry residual mass of litter in the litterbag after a specific time period (t) of decomposition.
A one-way ANOVA and repeated measures ANOVAs were used to assess the temporal (inter- or intra-annual) variation and effects of warming and N addition on soil temperature, soil water content, litter C, N, P, cellulose and lignin contents. Warming, N addition, and their interaction were treated as between-subject factors. Linear regression analyses were used to determine the relationships between litter decomposition and soil temperature, soil water content, litter C, N, P, cellulose and lignin contents. Post-hoc Tukey’s tests were performed to compare treatment differences. Statistical analyses were conducted using SPSS 16.0 software (SPSS Institute Inc., Chicago, IL, USA). Data are reported as the mean±SE.
Results
Soil Temperature and Water Content
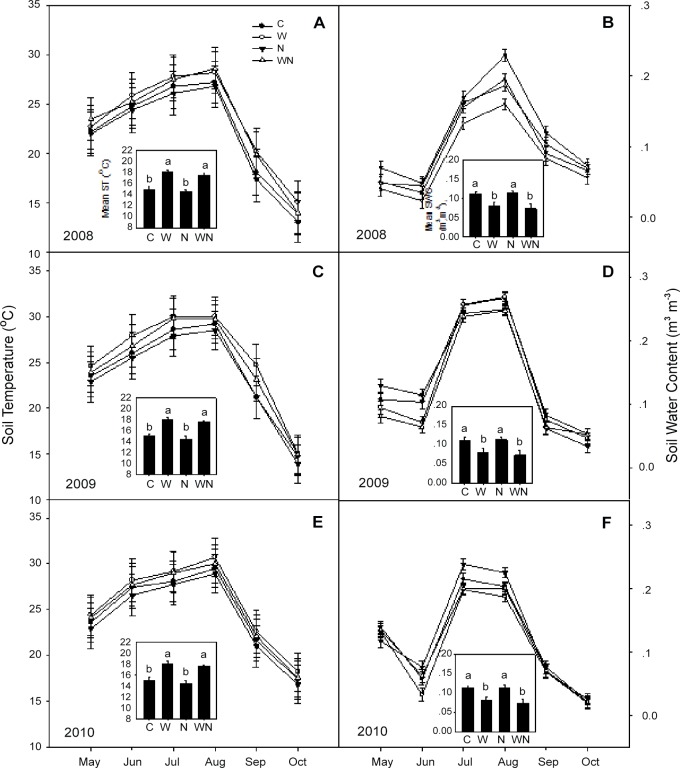
Soil temperature displayed a seasonal response to treatments over the three growing seasons. Within a single growing season, the soil temperature (measured at a depth of 0–15 cm) exhibited a unimodal peak in August (Fig. 1A, C, E). In general, warming increased the soil temperature (P<0.05) by an average of 1.1°C, but N addition did not affect soil temperature. Warming in combination with N addition significantly increased soil temperature (P<0.05), but there was no difference between this combined treatment and warming alone.
Fig 1. Effects of experimental warming and N addition on soil temperature and soil volumetric water content at a depth of 15 cm over the period 2008 to 2010.
C: Control; W: Warming; N: N addition; WN: Warming plus N addition. Vertical bars indicate the standard error of the mean (n = 6). Different lowercase letters indicate significant differences (P < 0.05) between treatments.
Similar to the soil temperature response, the soil water content displayed a seasonal trend, with peak values in July and August (Fig. 1B, D, F). Warming significantly reduced the soil water content (P<0.05), whereas the addition of N alone had no effect compared with the control. The combination of warming and N addition reduced the soil water content (P<0.05); however, the difference between this combined treatment and warming alone was not significant.
Litter Decomposition
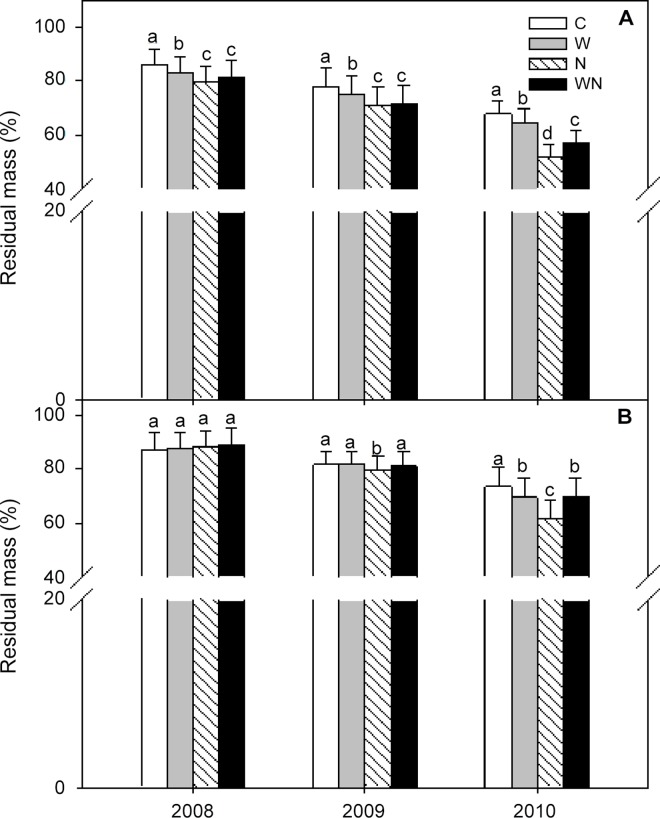
L. chinensis litter decomposed faster than P. communis litter (Fig. 2). After 3 years, litter loss was 21.8% higher (P<0.05) in L. chinensis compared with P. communis in the control plots.
Fig 2. Effects of warming and N addition on the litter decomposition of L. chinensis (A) and P. communis (B) from 2008 to 2010.
C: Control; W: Warming; N: N addition; WN: Warming plus N addition. Vertical bars represent the standard error of the mean (n = 6). Different lowercase letters indicate significant differences (P < 0.05) between treatments.
Warming, N addition and warming plus N addition reduced the residual mass of L. chinensis litter by 3.78%, 7.51% and 4.53% (all P < 0.05), respectively, in 2008 and 2009, and by 4.73%, 24.08% and 16.1% (all P < 0.05), respectively, in 2010 (Fig. 2A). Warming, N addition and warming plus N addition had no effect on the decomposition of P. communis litter in 2008 or 2009, but reduced the residual litter mass by 5.58% (P < 0.05), 15.53% (P< 0.01) and 5.17% (P < 0.05), respectively, in 2010 (Fig. 2B). Therefore, the effect of N addition on litter decomposition was greater than the effect of warming, and warming, N addition and warming plus N addition had different effects on L. chinensis and P. communis litter. The residual mass of L. chinensis and P. communis litter was significantly (P < 0.001) affected by warming, N addition and the interaction between warming and N addition (Table 2).
Table 2. Results (F-value) of two-way factorial ANOVA on the effects of specie (S), warming (W), nitrogen addition (N) and their interaction on P-values from mixed statistics on the residual mass of litter, litter total carbon (C), total N (N), total P (P), cellulose and lignin.
| Residual mass | C | N | P | C:N | Cellulose | Lignin | |
|---|---|---|---|---|---|---|---|
| S | 117.312*** | 147.449*** | 27.618*** | 14.14* | 18.361** | 90.156*** | 12.145** |
| W | 97.491*** | 100.216*** | 12.138* | 13.701* | 39.259** | 111.874*** | 21.718** |
| N | 268.84*** | 114.404*** | 9.047* | 32.996** | 31.649** | 160.537*** | 74.849*** |
| W×N | 64.83*** | 223.229*** | 12.256* | 0.875 | 68.969*** | 61.458*** | 13.651* |
| S×W | 7.81* | 20.346** | 5.507 | 3.715 | 16.215** | 4.433 | 15.889** |
| S×N | 6.231 | 25.913** | 16.024** | 9.83* | 7.301* | 15.447** | 47.898*** |
| S×W×N | 4.908 | 63.328*** | 7.215* | 2.337 | 10.014* | 3.728 | 10.356* |
* denotes significant difference at P < 0.05;
** denotes significant difference at P < 0.01;
*** denotes significant difference at P < 0.001.
Litter quality
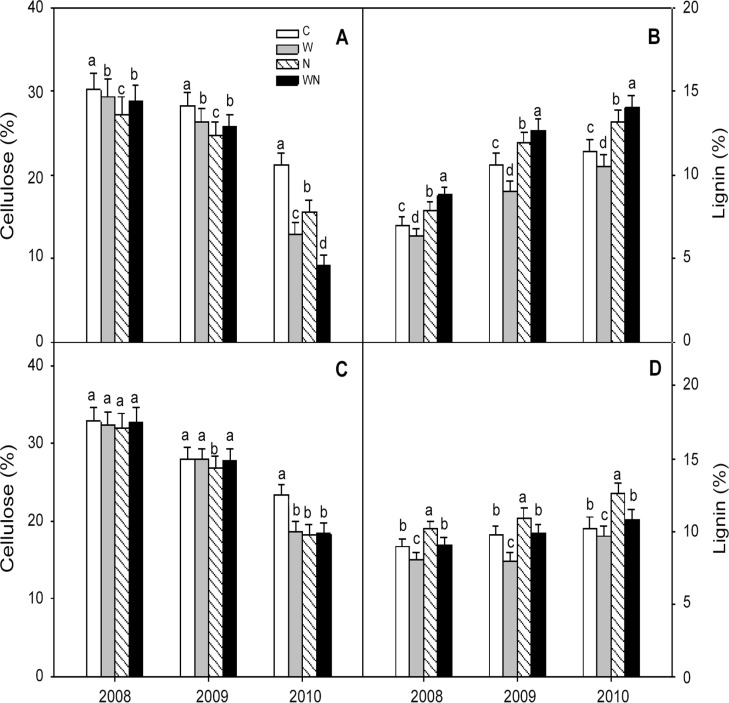
After 3 years, the cellulose contents of L. chinensis and P. communis in the control plots decreased by 29.51% and 28.48% (all P < 0.05), respectively, and the lignin contents increased by 60.03% and 14.38% (all P < 0.05), respectively (Fig. 3).
Fig 3. Effects of warming and N addition on cellulose and lignin contents of L. chinensis (A and B) and P. communis (C and D) from 2008 to 2010.
C: Control; W: Warming; N: N addition; WN: Warming plus N addition. Vertical bars represent the standard error of the mean (n = 6). Different lowercase letters indicate significant differences (P < 0.05) between treatments.
Warming, N addition and warming plus N addition reduced the cellulose content of L. chinensis litter by 2.54%, 9.61% and 4.66% (all P < 0.05), respectively, in 2008 and 2009, and by 39.28%, 26.57% and 56.93% (all P < 0.05), respectively, in 2010 (Fig. 3A). Warming, N addition and warming plus N addition had no effect on the cellulose content of P. communis litter in 2008 and 2009 but reduced the cellulose content by 20.31% (P < 0.05), 22.16% (P< 0.01) and 21.56% (P < 0.05), respectively, in 2010 (Fig. 3C). Therefore, N addition had a greater effect on the cellulose decomposition of L. chinensis litter compared with warming in 2008 and 2009, but the effect of warming was greater than that of N addition in 2010.
During the 3 years of decomposition, warming reduced the lignin content of L. chinensis and P. communis litter by 9.88% and 9.41% (all P < 0.05), respectively (Fig. 3B, D), N addition increased the lignin contents by 12.52% and 14.15% (all P < 0.05), respectively (Fig. 3B, D), and warming plus N addition increased the lignin content of L. chinensis litter by 25.63% (P < 0.05), but had no effect on the lignin content of P. communis litter.
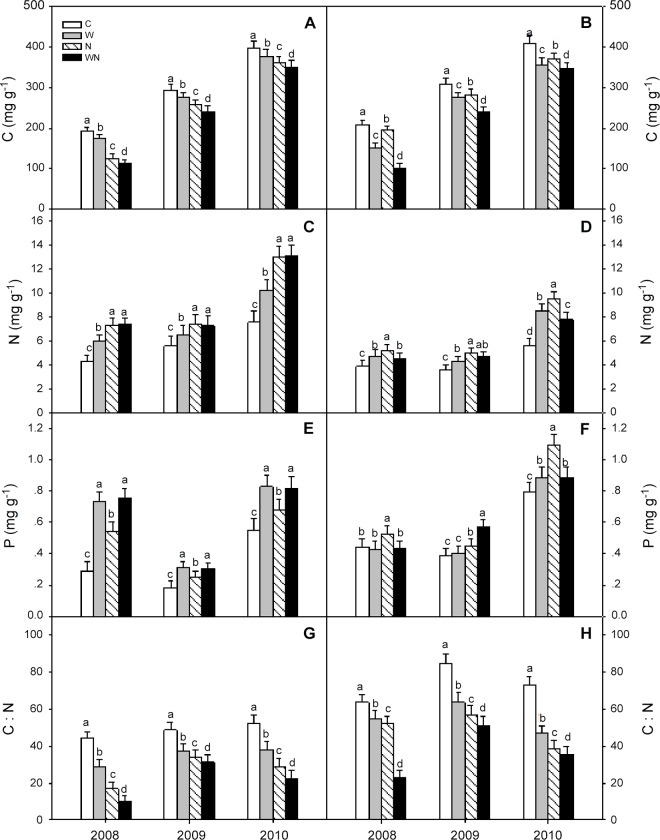
The C and N contents of L. chinensis and P. communis litter increased over time in the control plots (Fig. 4). During the 3 years of decomposition, warming, N addition and warming plus N addition reduced the C contents and C:N ratios of L. chinensis and P. communis litter (P < 0.001) (Fig. 4 A, B, G, H), but increased the N and P contents of L. chinensis (Fig. 4C, E) and N content of P. communis litter (Fig. 4D) (P < 0.05). Warming increased the P content of P. communis litter in 2010 (P < 0.05), but had no effect in 2008 or 2009, and N addition increased the P content of P. communis litter (P < 0.05) (Fig. 4F).
Fig 4. Effects of warming and N addition on litter quality of L. chinensis (A, C, E and G) and P. communis (B, D, F and H) from 2008 to 2010.
C: Control; W: Warming; N: N addition; WN: Warming plus N addition. Vertical bars represent the standard error of the mean (n = 6). Different lowercase letters indicate significant differences (P < 0.05) between treatments.
The C, N and P content, C:N ratio, and the cellulose and lignin contents of the L. chinensis and P. communis litter were significantly (P < 0.001) affected by warming, N addition and the interaction between warming and N addition (Table 2).
Correlation of litter decomposition with soil microclimate and litter quality
Correlation analysis showed that the residual mass of L. chinensis and P. communis litter was positively correlated with soil temperature, water content, cellulose and N, P content (P < 0.05), but negatively correlated with the C:N ratio (P < 0.01) and lignin content (P < 0.05). The correlation coefficients ranged from 0.286 to 0.984 (Table 3).
Table 3. Correlations between the residual mass of litter and litter quality, soil temperature, and soil water content.
| Residual mass | ST | SWC | C | N | P | C:N | Cellulose | Lignin |
|---|---|---|---|---|---|---|---|---|
| L. chinensis | 0.823** | 0.920** | 0.929* | 0.944** | 0.382* | -0.936** | 0.984* | -0.884*** |
| P. communis | 0.642* | 0.565* | 0.286* | 0.676** | 0.983*** | -0.550** | 0.960* | -0.722* |
C = total C; N = total N; P = total P; ST = soil temperature, SWC = soil water content.
*denotes significant difference at P < 0.05;
** denotes significant difference at P < 0.01;
*** denotes significant difference at P < 0.001.
Discussion
Effects of Warming on Litter Decomposition
A stimulatory effect of warming on decomposition rates has been found in other studies [10,14,33,34,35]. In our experiment, warming increased litter decomposition rates although the effect was weak. An increase in temperature can increase litter decomposition rates directly by affecting the soil microclimate and stimulating microbial activity [13], or indirectly by improving litter quality [11]. After 3 years of warming, most of the initial litter chemistry parameters had changed considerably, e.g., warming increased N and P contents, and reduced C contents, C:N ratios, cellulose and lignin contents. These results indicate that warming improved litter quality, resulting in increased litter decomposition rates. And warming increased litter decomposition rates by changing grassland community structure and plant tissue quality have also been reported [36,37]. These studies found that warming increased the annual aboveground biomass, increased the output of litter, and warming increased the N, P contents of plant tissue, improved the quality of plant tissue, which benefited decomposition of litter. Warming had a greater effect on the decomposition of L. chinensis litter compared with P. communis litter. The decomposition rate and temperature sensitivity of high quality litter quality is higher than low litter quality [11,34,35]. In the current study, the litter quality of L. chinensis was higher than P. communis.
Nitrogen is frequently immobilized during litter decomposition [38], and in the current study, warming enhanced this phenomenon and consequently increased N contents. An increase in C leaching and a reduction in the C:N ratio has been observed in response to warming [39]. Warming stimulates the litter decomposition rate, which reduces cellulose and lignin contents, and subsequently decreases C storage [14,17].
The negative correlations observed between the litter decomposition rates and C:N ratios and lignin agree with the results of previous studies [14,15,40]. L. chinensis litter had higher N and P contents, but lower C contents, C:N ratios, cellulose and lignin contents than P. communis. Therefore, L. chinensis litter decomposed faster than P. communis. Though elevated soil temperatures under climate warming may accelerate litter decomposition, warming also decreases soil moisture, which suppresses soil microbial activity [14,41]. It is possible that the more rapid decomposition associated with improved litter quality in the warming plots may have counteracted slow decomposition rates caused by decreased soil moisture and soil microbial activity.
Warming had little effect on P. communis litter decomposition in 2008 and 2009, possibly because losses in the early phase of decomposition consist of easily degraded soluble compounds and celluloses [42], after which P. communis likely had higher lignin and C contents.
Effects of N Addition on Litter Decomposition
The mass loss of litter was accelerated by N addition, indicating that an increase in N availability accelerated litter decomposition, which is consistent with previous results [21,22,43]. However, other studies found that N addition had no effect [6], or even depressive effects [8,25,44] on litter decomposition. These findings can be attributed to differences in the amount of N added, fertilizer types and species [6,45].
After 3 years of N addition, most of the initial litter chemistry parameters had changed considerably. N addition increased N, P and lignin contents, but decreased C, cellulose contents and C:N ratios. These results indicate that N addition improved litter quality, resulting in increased litter decomposition rates. Similar results have been observed in other studies [17,46]. Nitrogen addition had a greater effect on the decomposition of L. chinensis litter compared with P. communis litter. Specifically, N addition reduced the residual mass of L. chinensis in all 3 years, whereas this treatment did not affect the decomposition of P. communis litter in 2008 and 2009, but reduced the residual mass of P. communis litter in 2010. L. chinensis litter with a higher N content and lower C content and C:N ratio decomposed faster than low-quality P. communis litter [47]. In addition, P. communis litter had a high lignin content, and cellulose and compounds that are easily degraded are decomposed during the early phase of decomposition [42]. Further, N addition may indirectly stimulate litter decomposition rates by increasing the N content of the litter. The balance between N and P contents is important because several nutrient contents in litter are frequently correlated, and nutrient balance is necessary for microbial growth [7,40]. N addition can inhibit the decomposition of lignin by suppressing the synthesis of ligninolytic enzymes or promoting the formation of additional recalcitrant compounds through interactions between N and lignin breakdown products. In addition, added N can promote the decomposition of cellulose by increasing the activity of cellulose-degrading enzymes because shifts in enzyme activity can influence litter decomposition [5,7,8,25]. It is generally believed that N addition accelerates the decomposition of rapidly decomposing substrates (i.e., cellulose) but slows the breakdown of slowly decomposing materials (i.e., lignin) [45,48]. Dias et al. accounted for the integrated effects of N enrichment on litter decomposability taking into consideration the N-driven changes in the whole plant community (changes in plant species composition and litter quality), perhaps by altering the competitive interactions between species [49]. Maybe N additions reduced the abundance of P. communis and benefited L. chinensis, which resulted in greater biomass stimulation of L. chinensis, greater plant tissue quality and greater quality of litter inputs [49,50]. Therefore, N addition had a greater effect on L. chinensis litter decomposition compared with P. communis.
Interactive Effects between Warming and N Addition on Litter Decomposition
Litter decomposition rates were stimulated by the interactive effects of warming and N addition. Warming and N addition may increase litter decomposition rates directly by stimulating microbial activity (data not shown) or indirectly by improving litter quality. The interactive effect between warming and N addition reduced C contents and C:N ratios, but increased N and P contents and therefore improved litter quality. High-quality litter exhibited relatively faster decomposition, which is consistent with earlier studies [7]. There was a greater interactive effect between warming and N addition on L. chinensis litter decomposition compared with P. communis. This result is because the interactive effect resulted in higher N and P and lower cellulose contents in L. chinensis litter compared with P. communis, resulting in litter that was more easily decomposed.
Conclusions
Changes in abiotic (soil temperature and moisture) environments and litter quality play a vital role in litter decomposition in temperate meadow grasslands. As rates of litter decomposition increase with warming and N addition in temperate meadow grasslands, the turnover times of C, N and P from the litter to soil will decrease. This has the potential to increase the storage of C, N and P in the soil, and might alter the timing and availability of these nutrients for plant growth.
Our findings suggest that plant species with different litter qualities should be considered when modeling C and N cycles and nutrient dynamics in grassland ecosystems. In addition, L. chinensis is expected to contribute more than P. communis to C and N cycling and nutrient dynamics in the semi-arid Songnen grasslands. Moreover, our results further the current understanding of litter decomposition in response to multiple global change drivers in temperate grassland ecosystems.
Acknowledgments
We thank two anonymous reviewers for their constructive comments, which helped in improving the manuscript; Dr. Ling Wang and Baotian Zhang for supporting the laboratory work and field work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the National Natural Science Foundation of China (No. 31170303, 31300097 and 31100332), the Fundamental Research Funds for the Central Universities (No. 12QNJJ017), and State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, ChineseAcademy of Sciences.
References
- 1. Magill AH, Aber JD (2000) Dissolved organic carbon and nitrogen relationships in forest litter as affected by nitrogen deposition. Soil Biology & Biochemistry 32: 603–613. [Google Scholar]
- 2. Milchunas DG, Lauenroth WK (2001) Belowground primary production by carbon isotope decay and longterm root biomass dynamics. Ecosystems 4: 139–150. [Google Scholar]
- 3. Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. Journal of Ecology 94: 713–724. [Google Scholar]
- 4. Li LJ, Zeng DH, Yu ZY, Fan ZP, Yang D, et al. (2011) Impact of litter quality and soil nutrient availability on leaf decomposition rate in a semi-arid grassland of Northeast China. Journal of Arid Environments 75(9): 787–792. [Google Scholar]
- 5. Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic-matter. Biological Reviews 63: 433–462. [Google Scholar]
- 6. Zhang CH, Li SG, Zhang LM, Xin XP, Liu XR (2013) Effects of species and low dose nitrogen addition on litter decomposition of three dominant grasses in Hulun Buir Meadow Steppe. Journal of Resources and Ecology 4(1): 20–26. [Google Scholar]
- 7. Berg B, Matzner E (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environmental Reviews 5: 1–25. [Google Scholar]
- 8. Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of Long-Term Nitrogen Addition on Microbial Enzyme Activity in Eight Forested and Grassland Sites: Implications for Litter and Soil Organic Matter Decomposition. Ecosystems 12: 1–15. [Google Scholar]
- 9. Hobbie SE (2005) Contrasting effects of substrate and fertilizer nitrogen on the early stages of decomposition. Ecosystems 8: 644–656. [Google Scholar]
- 10. Salah YMS, Scholes MC (2011) Effect of temperature and litter quality on decomposition rate of Pinus patula needle litter. Procedia Environmental Sciences 6: 180–193. [Google Scholar]
- 11. Cornelissen JHC, van Bodegom PM, Aerts R, Callaghan TV, van Logtestijn RSP, et al. (2007) Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecology Letters 10: 619–627. [DOI] [PubMed] [Google Scholar]
- 12. IPCC (Intergovernmental Panel on Climate Change) (2007) Climate Change 2007 The Physical Science Basis. Contribution of the Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Camebridge, UK. [Google Scholar]
- 13. Nadelhoffer KJ, Giblin AE, Shaver GR, Linkins AE (1992) Microbial processes and plant nutrient availability in arctic soils In: Chapin F.S., Jeffries R.L. III, Reynolds J.F., Shaver G.R., Svoboda J. (Eds.), Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective. Academic Press, San Diego, California, USA: pp. 281–300. [Google Scholar]
- 14. Shaw MR, Harte J (2001) Control of litter decomposition in a subalpine meadow sagebrush species ecotone under climate change. Ecology Applied 11: 1206–1223. [Google Scholar]
- 15. Bontti E, Decant JP, Munson SM, Gathany MA, Przeszlowska A, et al. (2009) Litter decomposition in grasslands of Central North America (US Great Plains). Global Change Biology 15: 1356–1363. [Google Scholar]
- 16. Butenschoen O, Stefan S, Eisenhauer N (2011) Interactive effects of warming, soil humidity and plant diversity on litter decomposition and microbial activity. Soil Biology & Biochemistry 43: 1902–1907. [Google Scholar]
- 17. Henry HAL, Cleland EE, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on plant litter quality in a California annual grassland. Oecologia 142: 456–473. [DOI] [PubMed] [Google Scholar]
- 18. Saura-Mas S, Estiarte M, Penuelas J, Lloret F (2012) Effects of climate change on leaf litter decomposition across post-fire plant regenerative groups. Environmental and Experimental Botany 77: 274–282. [Google Scholar]
- 19. Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, et al. (2004) Nitrogen cycles: Past, present, and future. Biogeochemistry 70(2): 153–226. [Google Scholar]
- 20. Johansson O, Palmqvist K, Olofsson J (2012) Nitrogen deposition drives lichen community changes through differential species responses. Global Change Biology 18(8): 2626–2635. [Google Scholar]
- 21. Liu P, Huang JH, Han XG, Sun OJ, Zhou ZY (2006) Differential responses of litter decomposition to increased soil nutrients and water between two contrasting grassland plant species of Inner Mongolia, China. Applied Soil Ecology 34(2–3): 266–275. [Google Scholar]
- 22. Deng XW, Liu Y, Han SJ (2009) Carbon and nitrogen dynamics in early stages of forest litter decomposition as affected by nitrogen addition. Journal of Forestry Research 20(2): 111–116. [Google Scholar]
- 23. Song CC, Liu DY, Yang GS, Song YY, Mao R (2011) Effect of nitrogen addition on decomposition of Calamagrostis angustifolia litters from freshwater marshes of Northeast China. Ecological Engineering 37(10): 1578–1582. [Google Scholar]
- 24. Bragazza L, Buttler A, Habermacher J, Brancaleoni L, Gerdol R, et al. (2012) High nitrogen deposition alters the decomposition of bog plant litter and reduces carbon accumulation. Global Change Biology 18(3): 1163–1172. [Google Scholar]
- 25. Sjoberg G, Bergkvist B, Berggren D, Nilsson SI (2003) Long-term N addition effects on the C mineralization and DOC production in mor humus under spruce. Soil Biology & Biochemistry 35: 1305–1315. [Google Scholar]
- 26. Wang ZM, Song KS, Zhang B, Liu DW (2006) Analyses of features of agroclimatic changes in Songnen plain in the past 40 years. Chinese Agricultural Science Bulletin 22: 241–246. [Google Scholar]
- 27. Bai YF, Wu JG, Clark CM, Naeem S, Pan QM, et al. (2010) Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from Inner Mongolia grasslands. Global Change Biology 16: 358–372. [Google Scholar]
- 28. Qu G, Guo J (2003) The relationship between different plant communities and soil characteristics in Songnen grassland. Acta Prataculturae Sinica 12: 18–22. [Google Scholar]
- 29. He CE, Liu XJ, Andreas F, Zhang FS (2007) Quantifying the total airborne nitrogen input into agroecosystems in the North China Plain. Agriculture Ecosystems and Environment 121: 395–400. [Google Scholar]
- 30. Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter In: Page A.L., Miller R.H., Keeney D.R. (Eds.), Methods of soil analysis, Madison: American Society of Agronomy; pp. 539–579. [Google Scholar]
- 31. Parkinson JA, Allen SE (1975) A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Communications in Soil Science and Plant Analysis 6: 1–11. [Google Scholar]
- 32. Rowland AP, Roberts JD (1994) Lignin and cellulose fractionation in decomposition studies using acid-detergent fiber methods. Communications in Soil Sciences and Plant Analysis 25: 269–277. [Google Scholar]
- 33. Meeteren MJM, Tietema A, Loon EE, Verstraten JM (2008) Microbial dynamics and litter decomposition under a changed climate in a Dutch heathland. Applied Soil Ecology 38: 119–127. [Google Scholar]
- 34. Xu GP, Hu YG, Wang SP, Zhang ZH, Chang XF, et al. (2010) Effects of litter quality and climate change along an elevation gradient on litter mass loss in an alpine meadow ecosystem on the Tibetan plateau. Plant Ecol 209: 257–268. [Google Scholar]
- 35. Xu GP, Chao ZG, Wang SP, Hu YG, Zhang ZH, et al. (2010) Temperature sensitivity of nutrient release from dung along elevation gradient on the Qinghai-Tibetan plateau. Nutrient Cycling in Agroecosystems 87:49–57. [Google Scholar]
- 36. Cantarel AAM, Bloor JMG, Soussana J (2013) Four years of simulated climate change reduces aboveground productivity and alters functional diversity in a grassland ecosystem. Journal of Vegetation Science 24: 113–126. [Google Scholar]
- 37. Flury S, Gessner MO (2014) Effects of experimental warming and nitrogen enrichment onleaf and litter chemistry of a wetland grass, Phragmitesaustralis . Basic and Applied Ecology 15: 219–228. [Google Scholar]
- 38. Gallardo A, Merino J (1992) Nitrogen immobilization in leaf litter at two Mediterranean ecosystems of SW Spain. Biogeochemistry 15: 213–228. [Google Scholar]
- 39. Sardans J, Penuelas J, Estiarte M (2008) Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrub land. Applied Soil Ecology 39: 223–235. [Google Scholar]
- 40. Kuperman RG (1999) Litter decomposition and nutrient dynamics in oak-hickory forests along a historic gradient of nitrogen and sulfur deposition. Soil Biology & Biochemistry 31: 237–244. [Google Scholar]
- 41. Cheng XL, Luo YQ, Su B, Zhou XZ, Niu SL, et al. (2010) Experimental warming and clipping altered litter carbon and nitrogen. Agriculture, Ecosystems and Environment 138: 206–213. [Google Scholar]
- 42. Couteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends in Ecology and Evolution 10: 63–66. [DOI] [PubMed] [Google Scholar]
- 43. Sjoberg G, Nilsson SI, Persson T, Karlsson P (2004) Degradation of hemicellulose, cellulose and lignin in decomposing spruce needle litter in relation to N. Soil Biology & Biochemistry 36: 1761–1768. [Google Scholar]
- 44. Fang H, Mo JM, Peng SL, Li ZA, Wang H (2007) Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern China. Plant Soil 297: 233–242. [Google Scholar]
- 45. Knorr M, Frey SD, Curtio PS (2005) Nitrogen additions and litter decomposition: a meta analysis. Ecology 86: 3252–3253. [Google Scholar]
- 46. Pregitzer KS, Zak DR, Burton AJ, Ashby JA, MacDonald NW (2004) Chronic nitrate additions dramatically increase the export of carbon and nitrogen from northern hardwood ecosystems. Biogeochemistry 68: 179–197. [Google Scholar]
- 47. Sanchez FG (2001) Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. Forest Ecology Management 152: 85–96. [Google Scholar]
- 48. Waldrop MP, Zak DR, Singsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecological Applications 14: 1172–1177. [Google Scholar]
- 49. Dias T, Oakley S, Alarcon-Gutierrez E, Ziarelli F, Trindade H, et al. (2013) N-driven changes in a plant community affect leaf-litter traits and may delay organic matter decomposition in a Mediterranean maquis. Soil Biology and Biochemistry 58: 163–171. [Google Scholar]
- 50. Knops JMH, Naeem S, Reich PB (2007) The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Global Change Biology 13: 1960–1971. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.