Abstract
B cells play a critical role in the pathogenesis of autoimmune diabetes. To investigate the mechanisms by which B cell depletion therapy attenuates islet β cell loss and particularly to examine the effect of B cells on both diabetogenic and regulatory Ag-specific T cells, we generated a transgenic BDC2.5NOD mouse expressing human CD20 on B cells. This allowed us to deplete B cells for defined time periods and investigate the effect of B cell depletion on Ag-specific BDC2.5 T cells. We depleted B cells with anti-human CD20 Ab using a multiple injection protocol. We studied two time points, before and after B cell regeneration, to examine the effect on BDC2.5 T cell phenotype and functions that included antigenic response, cytokine profile, diabetogenicity, and suppressive function of regulatory T (Treg) cells. We found unexpectedly that B cell depletion induced transient aggressive behavior in BDC2.5 diabetogenic T cells and reduction in Treg cell number and function during the depletion period. However, after B cell reconstitution, we found that more regenerated B cells, particularly in the CD1d− fraction, expressed immune regulatory function. Our results suggest that the regenerated B cells are likely to be responsible for the therapeutic effect after B cell depletion. Our preclinical study also provides direct evidence that B cells regulate both pathogenic and Treg cell function, and this knowledge could explain the increased T cell responses to islet Ag after rituximab therapy in diabetic patients in a recent report and will be useful in design of future clinical protocols.
Type 1 diabetes (T1D) results from autoimmune destruction of insulin-producing β cells of the pancreas. The immune attack involves both CD4+ and CD8+ T cells that react to islet autoantigens. However, B cells are very important specific APC for this process, in addition to macrophages and dendritic cells. It has been known for decades that T cells, in particular CD4+ T cells, influence the function of B cells; however, less is known about how B cells affect the function of T cells, particularly in the setting of autoimmune disease. This is important, as B cell-targeted immunotherapy is in clinical practice (1, 2) and has been used in recent clinical trials of treatment of multiple sclerosis and T1D, both of which are believed to be T cell-mediated autoimmune diseases (2, 3).
Rituximab (anti-human CD20) induces rapid and specific B cell depletion. We have generated human CD20 transgenic (Tg) NOD (hCD20NOD) mice, which allowed us to use the mAb, 2H7 (mouse anti-human CD20 that recognizes the same epitope as rituximab), to deplete B cells in vivo. We demonstrated that temporary B cell depletion both delayed and prevented spontaneous diabetes when administered to prediabetic NOD mice and importantly also reversed disease in some diabetic NOD mice (4). This was mediated, at least in part, through the induction of Treg and B cells and modulation of APC function analyzed in the B cell regeneration phase (4). Other studies using different reagents targeting B cells reported similar findings (5–7). In parallel with these efforts in animal models, a phase II clinical trial using rituximab was carried out in newly diagnosed patients with T1D that showed that temporary B cell depletion delayed β cell loss, and β cell function was preserved in rituximab-treated T1D patients (3). However, it is interesting that some patients with T1D have shown stronger T cell responses in vitro to islet autoantigen(s) after rituximab treatment, even though their β cell function was preserved because of the treatment (8).
TCR Tg mice have facilitated the study of Ag-specific T cells in autoimmune diabetes, among which the BDC2.5 TCR Tg mouse has been studied extensively (9, 10). The BDC2.5 TCR recognizes a posttranslationally modified peptide of chromogranin A (11), and the BDC2.5 TCR Tg cells are also stimulated by a large number of mimotope peptides (12). The cells are highly diabetogenic when transferred to NOD.SCID or NOD.RAG−/− recipients. Thus, the BDC2.5 Tg mouse is a very useful model for the study of Ag-specific CD4 T cells in autoimmune diabetes.
The mechanisms underlying the effect of B cell depletion on diabetogenic T cells are largely unknown. Furthermore, it is puzzling why patients with T1D who responded better to rituximab treatment have shown stronger T cell responses in vitro to islet autoantigen(s) (8). To facilitate our understanding of this paradoxical phenomenon and to further investigate the effect of B cell depletion on Ag-specific diabetogenic and regulatory CD4 T cells, we generated hCD20/BDC2.5 double Tg mice. Using this double Tg NOD mouse model system, we found that B cells have a basal role in regulating diabetogenic T cells, because prolonged depletion of B cells increases pathogenicity of diabetogenic CD4 T cells. However, protective regulation is increased following B cell regeneration, which suggests that the major immune regulatory effect comes from the regeneration phase of the treatment.
Materials and Methods
Mice
To generate hCD20-BDC2.5 Tg NOD mice, we bred our hCD20NOD mice (4) with BDC2.5 NOD mice purchased from The Jackson Laboratory. Foxp3 reporter (Fir) mice were generated as described previously (13) and provided by R. Flavell (Yale University, New Haven, CT). Fir mice were backcrossed onto the NOD background for >10 generations, then bred with hCD20-BDC2.5NOD mice to obtain hCD20-BDC2.5-Fir NOD mice. NOD/Caj and NOD.SCID mice have been maintained at Yale University for >20 y. All the mice were kept in specific pathogen-free conditions in a 12-h dark/light cycle and housed in individually ventilated filter cages with autoclaved food. All studies were approved by the Institutional Animal Care and Use Committee of Yale University.
Abs and reagents
All fluorochrome-conjugated purified mAbs used in this study were purchased from eBioscience or BioLegend. The mAb hybridoma supernatants were provided by the late Charles Janeway, Jr. (Yale University). Anti-hCD20 mAb 2H7 (14), recognizing the same epitope as rituximab, was affinity purified by BioXCell from the hybridoma cell line 2H7 (kindly provided by E.A. Clark, University of Washington). Control IgG used in the in vivo studies was purchased from Rockland. Magnetic beads conjugated with goat anti-mouse IgG and IgM and goat anti-rat IgG were purchased from Qiagen. Bruff’s medium and heat-inactivated FCS were purchased from Invitrogen and Gemini, respectively. The BDC2.5 mimotope peptide (RTRPLWVRME) was synthesized at the Keck facility at Yale University.
B cell depletion protocol
HCD20-BDC2.5 Tg NOD mice were treated with four doses of anti-hCD20 (2H7) or control IgG, weekly for the first two doses and once every other week for the remaining three doses. This protocol was modified from the protocol for the rituximab clinical trial in T1D patents (four weekly doses of anti-CD20 treatment) (8).
Cell purification
Whenever possible, cell purification was performed by negative selection. B cells were purified by removing CD4+ (clone GK1.5), CD8+ T cells (clone T1B105), and macrophages (F4/80) using mAb hybridoma supernatants, followed by magnetic bead (conjugated with anti–Rat-IgG) separation. CD4 T cells were purified by removing CD8+ T cells (mAb T1B105), B cells (anti-mouse Igs), and I-Ag7 MHC class II+ cells (mAb 10.2.16) using mAb hybridoma supernatants and magnetic bead separation. The purity of the cells was routinely≥90%, analyzed by FACS. For in vivo experiments, cells were purified by flow cytometric sorting using positive selection.
Proliferation assays
For BDC2.5 T cell proliferation assays, purified CD4+ BDC2.5 T cells from 2H7- or mouse IgG (mIgG)-treated hCD20-BDC2.5NOD mice were cocultured (105/well) with irradiated APCs (5 × 104/well) from 6-wk-old NOD mice with or without BDC2.5 mimotope peptide. For tests of Treg cell function, purified CD4+CD25− BDC2.5 T effector (Teff) cells (105/well) were cocultured with irradiated Foxp3+ Treg cells (5 × 104/well) from 2H7- or mIgG-treated hCD20-BDC2.5NOD mice. For B cell suppression tests, purified CD4+ BDC2.5 T cells (105/well) were cultured with (3 × 105/well) irradiated B cells (CD19+B220+), regulatory B cell (Breg) (CD1d+CD19+B220+), or non-Breg cells (CD1d−CD19+B220+) from NOD mice. Proliferation of BDC2.5 T cells was assessed by [3H]thymidine incorporation during the last 14–18 h of the 3-d culture. Cell proliferation is presented either by cpm or as stimulation index calculated as [3H]thy-midine incorporation (cpm) with Ag/[3H]thymidine incorporation (cpm) without Ag.
CFSE-labeled proliferation assay
Purified splenic CD4+ BDC2.5 T cells were labeled with CFSE and i.v. injected (107/mouse) into hCD20NOD mice that had been treated with 2H7 or mIgG. Recipient mice were sacrificed 5 d later, and lymphocytes from spleen, mesenteric lymph nodes (MLN), and pancreatic lymph nodes (PLN) were harvested and stained with fluorochrome-conjugated mAbs against surface markers and intracellular cytokines.
Adoptive transfer experiments
Purified BDC2.5 T cells from hCD20-BDC2.5NOD mice treated with 2H7 or mIgG were i.v. injected into 6- to 8-wk-old NOD.SCID mice (106/mouse). The mice were screened for diabetes development with Diastix daily for glycosuria for the first 3 wk and then twice a week. Diabetes was confirmed by blood glucose measurement (≥250 mg/dl) with a glucose meter (One Touch; Johnson and Johnson). For cotransfer experiments, purified CD4+ BDC2.5 T cells (106/mouse) were coinjected with purified B cells (total B cells or B cell subsets) into NOD.SCID mice.
Luminex assay to determine cytokines
Plasma cytokines were detected using Luminex beads, following the manufacturer’s protocol (Bio-Rad). Plates were read in a Luminex reader (Bio-Rad 200).
Statistics
Statistical analysis was performed using GraphPad Prism software. The incidence of diabetes was compared using the log-rank test. Mean values for flow cytometric analysis were compared by unpaired Student t tests.
Results
hCD20/BDC2.5NOD mice and B cell depletion
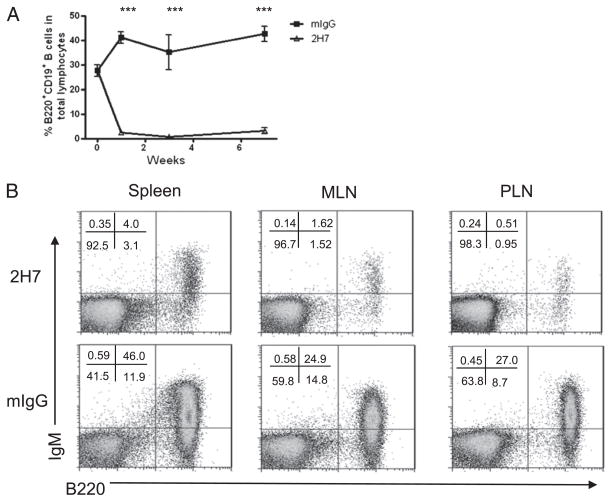
BDC2.5/NOD mice develop a very low incidence of spontaneous diabetes, although BDC2.5 cells used ex vivo are highly diabetogenic (9, 10). Introducing the hCD20 transgene did not change the phenotype, nor did it affect the function of BDC2.5 T cells (Supplemental Fig. 1). Injection of mouse IgG also did not have any noticeable effect in the hCD20-BDC2.5NOD, BDC2.5/NOD, and NOD mice used in this study (data not shown). To study the effect of B cells on diabetogenic BDC2.5 CD4+ T cells, we depleted B cells using 2H7 as described in Materials and Methods. Fig. 1 shows depletion of B cells (IgM+B220+) in peripheral blood (Fig. 1A) and in different lymphoid tissues (Fig. 1B). B cell depletion was most effective in blood (Fig. 1A) and draining PLN that had up to 40 times reduction of B cells (Fig. 1B). It was interesting that B cell depletion also reduced the number of BDC2.5 T cells (Table I). This indicates that BDC2.5 T cells did not undergo homeostatic expansion because of B cell depletion.
FIGURE 1.
B cell depletion in hCD20BDC2.5 NOD mice. (A) B cell profile in peripheral blood after depletion of B cells. After the first injection (500 μg), 250 μg was administered a week later, followed by three additional injections at 2-wk intervals. After removing erythrocytes, peripheral blood cells were costained with anti-CD19 and anti-B220 and analyzed by flow cytometry prior to the next injection. B cell (B220+CD19+) percentage in total lymphocytes is shown at different time points. Mean values ± SEM of six to eight mice are presented. (B) B cell depletion in different lymphoid organs. HCD20-BDC2.5NOD mice were treated with 2H7 (upper panel) or mIgG (lower panel). (B) shows representative depletion profiles of B cells (IgM+B220+) from spleen, MLN, and PLN when the mice shown in (A) were terminated (10 d after the last Ab injection).
Table I.
Cell numbers in lymphoid tissues following B cell depletion
| Cell Type | Tissue | 2H7 | mlgG |
|---|---|---|---|
| Total cells (× 106) | SPL | 37.88 ± 8.11 | 83.63 ± 3.68b |
| MLN | 2.78 ± 0.76 | 7.830 ± 0.40c | |
| PLN | 1.03 ± 4.24 | 4.73 ± 8.75a | |
| Vβ4+CD4+ T cells (× 106) | SPL | 17.61 ± 3.77 | 22.58 ± 0.99 |
| MLN | 2.67 ± 0.12 | 3.61 ± 0.18a | |
| PLN | 0.75 ± 0.31 | 1.84 ± 0.34 | |
| B220+IgM+ B cells (× 106) | SPL | 6.42 ± 1.38 | 49.34 ± 2.17c |
| MLN | 0.06 ± 0.003 | 1.30 ± 0.065c | |
| PLN | 0.02 ± 0.01 | 0.85 ± 0.16b |
Mice were sacrificed after B cell depletion. Values (mean ± SEM) indicate cell number (× 106) present in each tissue (n = 5/group).
p < 0.05.
p < 0.01.
p < 0.001, significant differences between 2H7-treated versus control mIgG-treated mice.
Enhanced proliferative responses of BDC2.5 diabetogenic T cells soon after B cell depletion
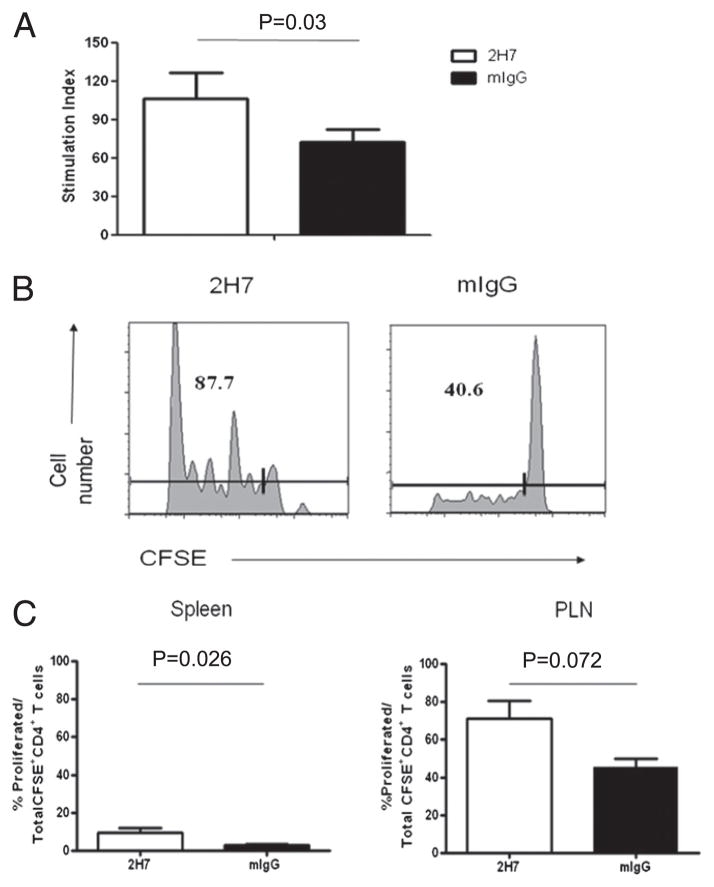
Our previous study was mostly focused on the regenerated B cells and the effect on polyclonal pathogenic and Treg cells, long after B cell depletion (4). However, it was not clear what the immediate effect of B cell depletion was on Ag-specific T cells. To investigate the function of BDC2.5 T cells following B cell depletion, but before B cell regeneration, we tested their response to the cognate Ag in vitro. As shown in Fig. 2A, purified BDC2.5 T cells from B cell-depleted hCD20-BDC2.5NOD mice responded more strongly to mimotope antigenic peptide than BDC2.5 CD4+ T cells from control IgG-treated mice (p = 0.03; Fig. 2A). This was further confirmed using CFSE-labeled purified BDC2.5 CD4+ T cells from BDC2.5NOD mice injected into hCD20NOD hosts that had been treated with anti-CD20 or control IgG (Fig. 2B, 2C). Our results suggest that BDC2.5 T cells were more responsive to their cognate Ag early after B cell depletion, before B cell repopulation.
FIGURE 2.
Enhanced proliferation of BDC2.5 diabetogenic T cells after B cell depletion. (A) BDC2.5 T cell proliferation in vitro. Purified BDC2.5 CD4+ T cells from treated hCD20-BDC2.5NOD mice (n = 5) cocultured with irradiated NOD APCs in the presence or absence of BDC2.5 mimotope. Cell proliferation was shown as stimulation index = [3H]thymidine incorporation (cpm) in the presence of mimotope/[3H]thymidine incorporation in the absence of mimotope stimulation. The average [3H]thymidine incorporation (cpm) without mimotope stimulation was 513.3 ± 72.76. Similar results were obtained from three independent experiments. (B) Proliferation of BDC2.5 T cells in vivo. hCD20NOD mice were treated with 2H7 or mIgG as described in Materials and Methods. CFSE-labeled BDC2.5 CD4+ T cells (107) were injected into treated mice. Spleen and PLN were harvested from recipient mice 5 d later, and cell proliferation was determined by flow cytometric detection of CFSE dilution. Numbers indicate relative percentage of CFSE-diluted BDC2.5 T cells in PLN. (C) Summary of proliferation of BDC2.5 T cells in vivo. Numbers indicate the percentage of proliferated cells in total CFSE-labeled BDC2.5 T cells (n = 3/group). Similar results were obtained from two independent experiments.
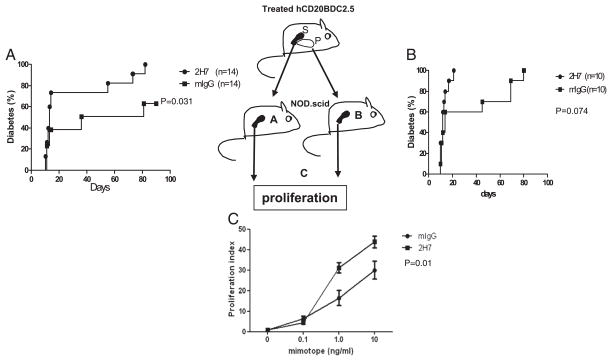
BDC2.5 T cells from newly B cell-depleted mice accelerate diabetes in adoptive transfer
To further investigate the function of BDC2.5 diabetogenic T cells related to B cell depletion, and also to confirm that the in vivo results presented in Fig. 2 were not due to homeostatic proliferation, we performed adoptive transfer experiments using purified splenic BDC2.5 CD4+ T cells from B cell-depleted or control IgG-treated hCD20-BDC2.5NOD mice. We found that BDC2.5 cells from newly B cell-depleted donors, whereas the mice remained B cell-depleted, more potently induced diabetes in NOD.SCID mice compared with cells from control IgG-treated donors (p = 0.031; Fig. 3A). To check for possible homeostatic proliferation, we harvested the BDC2.5 cells from all the NOD. SCID recipients when the mice were terminated. We did not find more BDC2.5 cells from NOD.SCID mice that were transferred with BDC2.5 cells from B cell-depleted donor mice compared with those that had been treated with control IgG. The number of BDC2.5 cells recovered from the spleen and lymph nodes was very similar in all the recipients (data not shown). In addition, we purified CD4+ T cells from the islets of B cell-depleted or control hCD20-BDC2.5 mice and transferred these cells to NOD.SCID recipients. Consistent with the earlier results, islet-infiltrating BDC2.5 T cells from B cell-depleted donor mice also induced more rapid diabetes onset (100% in <20 d) in the NOD. SCID recipients compared with the mice transferred with BDC2.5 T cells from control IgG-treated donors (100% by 80 d; Fig. 3B).
FIGURE 3.
Adoptive transfer of diabetes by BDC2.5 T cells. The cartoon indicates the scheme of transfer and analysis. (A) NOD.SCID mice were injected i.v. with 106 purified splenic BDC2.5 CD4+ T cells from 2H7- or mIgG-treated hCD20-BDC2.5NOD mice. Diabetes was significantly accelerated in the group transferred with BDC2.5 T cells from 2H7-treated mice (p = 0.031). (B) NOD.SCID mice were injected i.v. with 5 × 105 purified pancreas-infiltrating BDC2.5 CD4+T cells from 2H7- or mIgG-treated hCD20-BDC2.5NOD mice. There was acceleration of the onset of diabetes in the recipients that were transferred with cells from 2H7-treated mice, although this was not statistically significant (p = 0.074). (C) Purified BDC2.5 CD4+T cells from the spleens of recipient NOD.SCID mice were cocultured with irradiated APCs (NOD splenocytes) in the presence or absence of BDC2.5 mimotope. Cell proliferation is presented as stimulation index. The average background of [3H]thymidine incorporation cpm in the absence of mimotope was 137.53 ± 47.65. Overall, p = 0.011. P, Pancreas; S, spleen.
In line with our in vivo results, BDC2.5 T cells extracted from NOD.SCID recipients given BDC2.5 T cells from B cell-depleted hCD20-BDC2.5 donors expressed a stronger response to the antigenic peptide than cells retrieved from NOD.SCID recipients transferred with BDC2.5 T cells from control-treated hCD20BDC2.5NOD mice (Fig. 3C). Thus, our results support the notion that B cell depletion could lead to enhanced potency of proliferation and diabetes induction by diabetogenic CD4+ T cells.
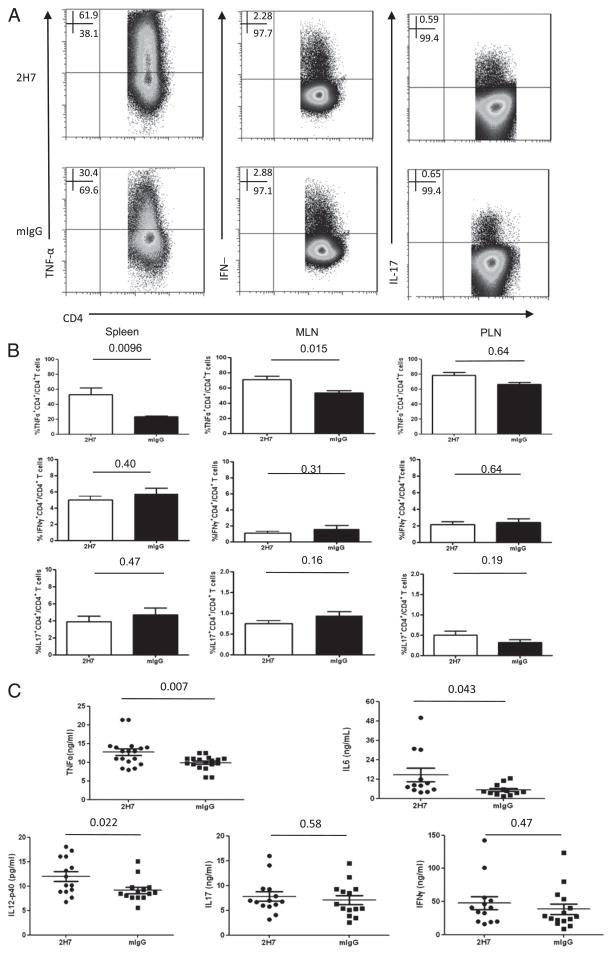
More proinflammatory cytokines are produced early after B cell depletion
To further investigate the function of BDC2.5 diabetogenic T cells after B cell depletion, we examined the inflammatory cytokine profile of BDC2.5 T cells from spleen, MLN, and PLN of 2H7 or control IgG-treated hCD20-BDC2.5 mice (Fig. 4A, spleen). B cell depletion resulted in a significant increase in TNF-α production by CD4+ BDC2.5 T cells from spleen and MLN but not from PLN (Fig. 4B). To confirm this finding, we also measured multiple circulating cytokines in the plasma samples from anti-CD20 or control IgG-treated mice. Cytokine profiles in the plasma also showed that there were more proinflammatory cytokines that included TNF-α, IL-6, and IL-12p40 in the samples taken soon after depletion from B cell-depleted mice (Fig. 4C).
FIGURE 4.
BDC2.5 T cells produce more inflammatory cytokines after B cell depletion. HCD20-BDC2.5NOD mice were sacrificed after 2H7 or mIgG treatment. Spleen, MLN, and PLN cells were stimulated with BDC2.5 mimotope (10 ng/ml) overnight, followed by intracellular staining for cytokines. (A) Representative dot plots for inflammatory cytokine production by splenic BDC2.5 T cells after 2H7 and mIgG treatment. (B) Summary of percentage of inflammatory cytokine-producing BDC2.5 T cells (TNF-α+CD4+, IFN-γ+CD4+, and IL-17+CD4+) on gated CD4+ T cells (mean ± SEM; n = 4) in B cell-depleted (□) or control IgG (■)-treated hCD20-BDC2.5NOD mice. Similar results were obtained from three independent experiments. (C) Circulating plasma cytokines including TNF-α, IL-6, IL-12p40, IL-17, and IFN-γ were measured by Luminex assay from B cell-depleted and control IgG-treated mice (n > 12/group). All the numbers above the lines in (B) and (C) are p values.
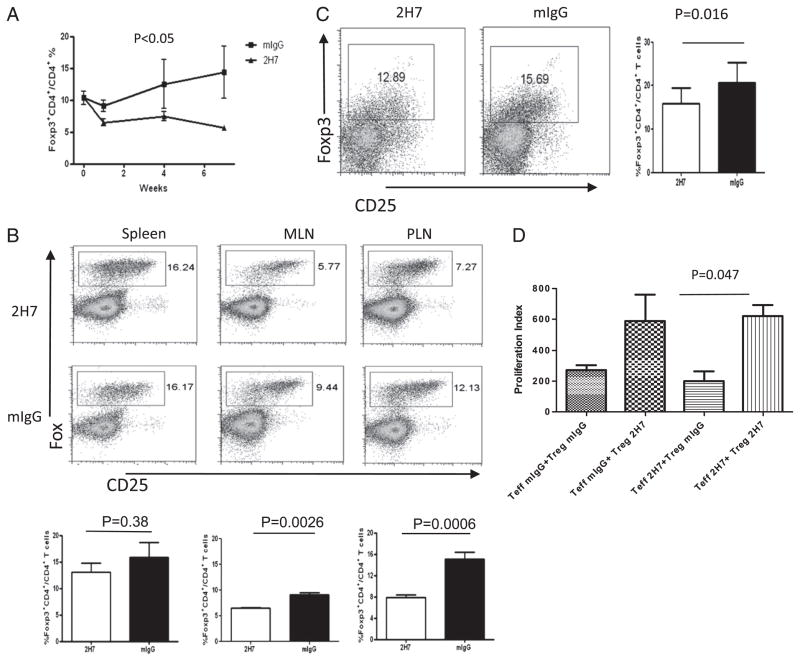
B cell depletion modifies the number and function of Treg cells
Our previous study showed that Treg cells were increased long after B cell depletion (4). However, the immediate effect of B cell depletion on Treg cells was not known. In this study, we found that B cell depletion resulted in a significant, maintained reduction of Ag-specific BDC2.5 Treg cells, during the period of B cell depletion, compared with control IgG-treated mice, in peripheral blood (p < 0.05; Fig. 5A), MLN (p = 0.0026; Fig. 5B), and PLN (p = 0.0006; Fig. 5B). To explore whether there was enhanced Ag-specific Treg cell migration to islets, contributing to the reduction of Treg cells in other lymphoid organs, we analyzed islet-infiltrating lymphocytes from 2H7- or control IgG-treated hCD20-BDC2.5NOD mice by flow cytometry. However, we found that concordant with fewer BDC2.5 Treg cells in other lymphoid tissues, there was also a decrease of BDC2.5 Treg cells in islets of 2H7-treated mice (Fig. 5C). To study whether B cell depletion affected the function of the Treg cells, we bred hCD20-BDC2.5NOD mice with Foxp3 RFP reporter (Fir) NOD mice to obtain triple Tg hCD20-BDC2.5-FirNOD mice. We treated hCD20-BDC2.5-FirNOD mice with anti-CD20 or control IgG and FACS-sorted BDC2.5 (Vβ4+) Fir+ (Foxp3+) Treg cells and Vβ4+ CD25−Fir− Teff cells. To our surprise, Treg cells from islets of B cell-depleted donor mice could not suppress the proliferation of Teff cells in response to antigenic peptide (BDC2.5 mimotope) as effectively as their counterparts from control IgG-treated mice (data not shown). To test whether this was due to resistance of Teff cells to regulation by Treg cells (15), we performed cross-culture experiments in which Teff cells were from 2H7- or IgG-treated donor mice, and Treg cells were obtained from control IgG- or 2H7-treated donor mice (Fig. 5D). Our results from these cross-culture experiments further supported the notion that the poor suppression was not due to the unresponsiveness of Teff cells but the functional impairment of Treg cells soon after B cell depletion. These results suggested that although the B cells were depleted, the suppressive function of Foxp3+ Treg cells was also reduced in this model system.
FIGURE 5.
B cell depletion reduces BDC2.5 Treg number and function. (A) Foxp3+ Treg cells from PBMC were analyzed by flow cytometry following treatment of hCD20-BDC2.5NOD mice with 2H7 or mIgG. The percentage of BDC2.5 Treg cells decreased in 2H7-treated mice (n = 6–8 mice/group; p < 0.05). (B) Percentages of Treg in BDC2.5 T cells in different lymphoid organs were variable. Representative dot plots show the percentage of BDC2.5 Treg (Foxp3+CD25high+low) cells. The bar charts show the pooled data from at least six mice per group (mean ± SEM). (C) BDC2.5 Tregs in islets from 2H7- or mIgG-treated hCD20-BDC2.5NOD mice. After islet isolation, the islet cells were dispersed, and infiltrating cells were stained with the corresponding mAbs and analyzed by flow cytometry. There were fewer infiltrating Treg cells in islets after B cell depletion. Dot plots are shown after gating on CD4+ cells. At least two mice were pooled for islet isolation. Experiments were performed three times and summarized in the bar graphs on the right. (D) Cross-suppression assay. Foxp3+ BDC2.5 Treg cells were sorted from 2H7- or mIgG-treated hCD20-BDC2.5-FirNOD mice and used as Treg (Foxp3+) and Teff cells (Fir−CD25−CD4+) were also sorted from 2H7- or mIgG-treated hCD20-BDC2.5-FirNOD mice. As indicated in (D), the cross-suppression assay was performed by coculturing Teff cells from 2H7-treated mice with Treg cells from mouse IgG-treated mice or Teff cells from IgG-treated mice with Treg cells from 2H7-treated mice. Cocultures of 2H7-treated Teff and Treg cells or IgG-treated Teff and Treg cells were used as controls. A cell ratio of 2:1 (Teff/Treg) was used in the cocultures. Proliferation is shown as stimulation index. Treg cells from 2H7-treated mice do not suppress Teff cell proliferation as effectively as Treg cells from mIgG-treated mice. The average [3H]thymidine incorporation in the absence of mimotope = 74.65 ± 13.48 cpm. Similar results were obtained from three independent experiments.
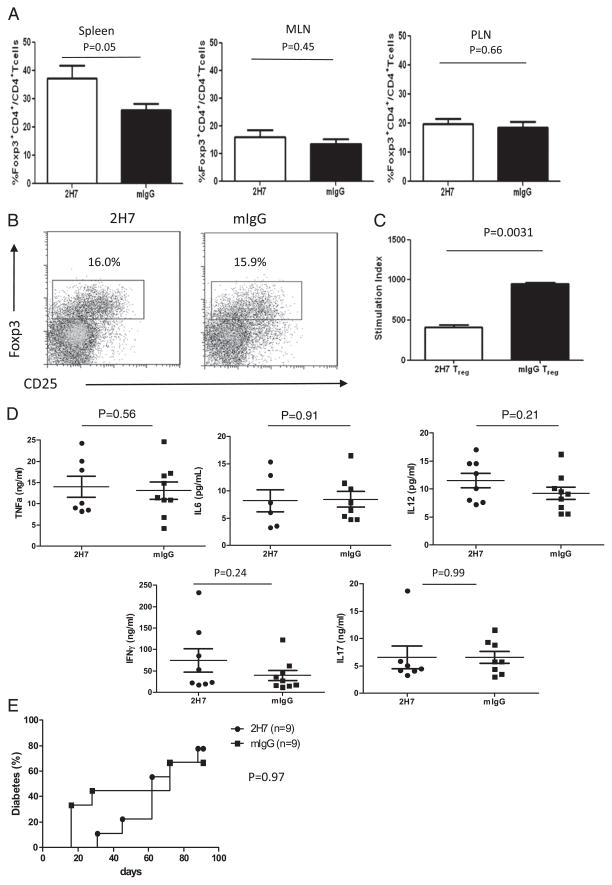
Newly generated B cells can modify the function of Teff and Treg cells
The data presented earlier showed enhanced Teff function and reduced Treg cell function soon after B cell depletion (before B cell regeneration). However, our previous study showed that both number and the function of Treg and Breg cells were increased after B cell regeneration in NOD mice (4). We next investigated whether this increase in regulatory cells after B cell regeneration was seen in cells with a defined TCR (i.e., the Ag-specific model used in this study). HCD20-BDC2.5NOD mice were sacrificed 10 wk after the last injection, when ~60–70% of B cells had regenerated, and examined regulatory (Foxp3+) BDC2.5 CD4+ T cells. In contrast to the results presented earlier (Fig. 5), where BDC2.5 T cells were evaluated before B cell repopulation, we found that after B cell repopulation, the numbers of splenic Foxp3+ Treg cells were significantly increased in anti–CD20-treated mice compared with control mice (p = 0.05; Fig. 6A), similar to our previous report (4). Foxp3+ Treg cells in MLN and PLN were comparable in both groups (Fig. 6A), which was in sharp contrast to the results prior to B cell repopulation shown in Fig. 5B. In addition, the number of infiltrating Treg cells in pancreas was also restored to the level similar to the control group (Fig. 6B). We further tested Treg function in the B cell regeneration period (10 wk after last Ab injection) using purified Teff cells (Foxp3−CD25−CD4+) and Treg cells (Foxp3+CD4+) from these mice. As shown in Fig. 6C, Treg cells from the 2H7-treated mice suppressed proliferation of Teff cells better than cells from the control group (p = 0.003), whereas Treg cells taken from the B cell-depleted mice before repopulation (2 wk after the last Ab injection when ≥90% of B cells were still absent; data not shown) did not suppress as shown earlier (Fig. 5D). Furthermore, in contrast to the results presented in Fig. 4, the percentage of TNF-α–producing BDC2.5 T cells was not elevated (data not shown), and the elevated inflammatory cytokines in plasma had returned to the levels seen in the control group (Fig. 6D).
FIGURE 6.
Regenerated B cells affect T cell phenotype and function. HCD20-BDC2.5NOD mice were sacrificed 10 wk after the last injection of 2H7 or mIgG. (A) Bar graphs indicate the percentage of Treg cells (Foxp3+CD4+) in gated CD4+ T cells in different lymphoid organs (mean ± SEM; n = 4–5). Similar results were obtained from two independent experiments. (B) Treg cells in islets after B cell regeneration. Islets were isolated from hCD20-BDC2.5NOD mice following B cell reconstitution after B cell depletion. Treg cells (Foxp3+CD25high+low) are shown as percentage of BDC2.5 T cells (CD4+ Vβ4+). Four mice were pooled for islet isolation. (C) Treg functional recovery with B cell reconstitution. HCD20-BDC2.5-Fir+NOD mice were sacrificed 2 mo after the end of the 2H7 or mIgG treatment. Fir+ T cells were sorted and used as Treg cells (Foxp3+) in coculture with Teff cells (Fir−CD25−CD4+) and irradiated splenic APC from normal NOD mice. [3H]Thymidine incorporation is shown as stimulation index. Treg cells from 2H7-treated mice after B cell reconstitution show increased suppression of Teff cells compared with the control group. Average [3H]thymidine incorporation without mimotope stimulation = 103.25 ± 14.58. Similar results were obtained from at least three independent experiments. (D) Circulating plasma cytokine profiles including TNF-α, IL-6, IFN-γ, and IL-17 were measured by Luminex from B cell reconstituted mice and control mice (n = 6–9). (E) Adoptive transfer of diabetes by BDC2.5 T cells. NOD.SCID mice were injected i.v. with 106 purified splenic BDC2.5 T cells taken after B cell reconstitution from 2H7- or mIgG-treated hCD20-BDC2.5NOD mice (10 wk after the end of treatment). Diabetes development was delayed and reduced up to the first 50 d in the recipients given BDC2.5 T cells from 2H7-treated donor mice; however, there was no statistical difference. n = 9/group; p = 0.97.
To test whether this increased regulation had effects on effector function of BDC2.5 T cells after B cell regeneration, we purified BDC2.5 T cells from hCD20-BDC2.5 mice in the B cell repopulation period (over 2 mo after anti-CD20 treatment) and adoptively transferred the T cells to NOD.SCID recipients. In contrast to the results presented in Fig. 3, diabetes following adoptive transfer of BDC2.5 CD4 T cells from the mice with newly repopulated B cells was delayed, although overall, this was not statistically significant (Fig. 6E). This suggests that BDC2.5 CD4+ T cells were less aggressively pathogenic after B cell reconstitution, and this seemed to be at the disease initiation stage as diabetes development was clearly delayed. However, the final incidence of diabetes was comparable.
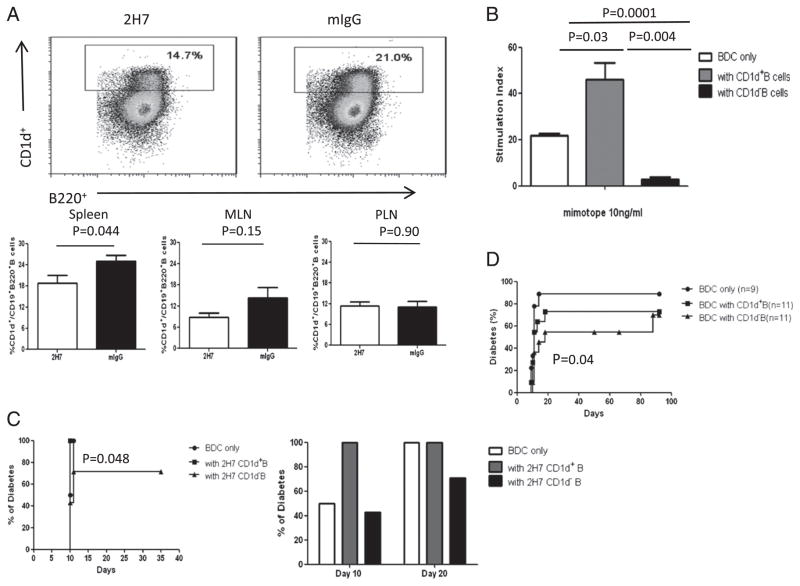
The role of CD1d B cell subpopulation in regenerated B cells
The newly regenerated B cells appeared to be regulating BDC2.5 T cells. To further investigate whether a regulatory subset could be identified, we examined CD1d+ Breg cells (16–18). It was interesting that there were proportionally fewer CD1d+ B cells in regenerated B cells (Fig. 7A). To test the function of this B cell subset, we cocultured purified BDC2.5 T cells with purified CD1d+ or CD1d− B cells (B220+CD19+CD5low/−) from either the 2H7- or mIgG-treated group together with irradiated APCs in the presence of BDC2.5 mimotope. Surprisingly, we found that CD1d− B (CD19+B220+) cells suppressed BDC2.5 T cell proliferation in vitro, whereas CD1d+ B cells enhanced the BDC2.5 T cell response (Fig. 7B). To test the function of these B cells in vivo, we transferred BDC2.5 T cells (0.5 × 106) with purified CD1d+ B or CD1d− B cells (1.5 × 106) from either 2H7- or mIgG-treated mice, after B cell repopulation, to NOD.SCID recipients. In line with the in vitro data, we found that cotransfer of BDC2.5 T cells with CD1d+ B cells from 2H7 as well as mIgG-treated mice accelerated diabetes development, whereas cotransfer with CD1d− B cells from either the mIgG- or 2H7-treated groups reduced diabetes development (Fig. 7C). All the recipients cotransferred with BDC2.5 T cells and CD1d+ B cells developed diabetes by 10 d, whereas it took 20 d for the recipients transferred with only BDC2.5 T cells to develop diabetes. Furthermore, 30% of recipients cotransferred with BDC2.5 T cells and CD1d- B cells remained diabetes free at the time the experiment was terminated (p = 0.048; Fig. 7C).
FIGURE 7.
CD1d-B cells are more protective than CD1d+ B cells. HCD20-BDC2.5NOD mice were sacrificed 2 mo after the last treatment. (A) Representative dot plots (top panels) showing regenerated B cells (gated on CD19+) from 2H7- or mIgG-treated hCD20-BDC2.5NOD mice as determined by inmmunofluorescent staining with CD1d, CD19, and B220. At least three mice per group were used for each experiment, and the experiments were performed three times as summarized in the lower panels. (B) Purified BDC2.5 T cells were cocultured with three times the number of sorted CD1d+ B cells (CD19+B220+) or CD1d− B cells (irradiated after sorting) in the presence of 10 ng/ml mimotope. Stimulation index was calculated as described earlier. Background cpm in the absence of mimotope was 80.17 ± 7.1. p = 0.0012 for three groups overall. Similar results were obtained from three independent experiments. (C) Purified BDC2.5 T cells (0.5 × 106) were cotransferred with sorted CD1d+ or CD1d− B cells (1.5 × 106) taken from 2H7-treated hCD20-BDC2.5NOD mice in the B cell regeneration phase (2 mo after the treatment). Mice were screened daily for diabetes. Mice transferred with BDC2.5 T cells only (n = 6) were compared with mice transferred with BDC2.5 T cells together with CD1d+ 2H7-treated B cells (n = 10); p = 0.08. Mice transferred with BDC2.5 T cells together with CD1d+ 2H7-treated B cells were compared with mice transferred with BDC2.5 T cells together with CD1d- 2H7 B cells (n = 10); p = 0.048. (D) Purified BDC2.5 T cells (106) with sorted CD1d+CD19+B220+ B cells or CD1d−CD19+B220+ B cells from 6-wk-old NOD mice were transferred (3 × 106) into NOD.SCID recipient mice. For comparison between BDC2.5 T cells with or without CD1d-B cells, p = 0.04; for comparison of BDC2.5 T cells with or without CD1d+B cells, p = 0.88.
As depletion of B cells led to an increase in pathogenicity of diabetogenic BDC2.5 cells when tested soon after depletion was completed, but before regeneration of B cells, this suggested that there is an endogenous subset of B cells that has a suppressive effect on diabetogenic CD4 T cells, although this is not sufficient to prevent NOD mice from diabetes in normal circumstances. To investigate whether CD1d+ and CD1d− B cells from normal NOD mice could regulate BDC2.5 T cells, we sorted these two populations from unmanipulated NOD mice. The sorted B cells (1.5 × 106) were cotransferred with purified BDC2.5 T cells (0.5 × 106) into NOD.SCID recipients. CD1d+ B cells from NOD mice did not show much effect on diabetes development (p = 0.88; Fig. 7D); however, cotransfer of CD1d− B cells from NOD mice with BDC2.5 cells led to a significant reduction in diabetes development (p = 0.04; Fig. 7D), similar to the results using regenerated CD1d− B cells from 2H7-treated mice (Fig. 7C) and the in vitro results shown in Fig. 7B.
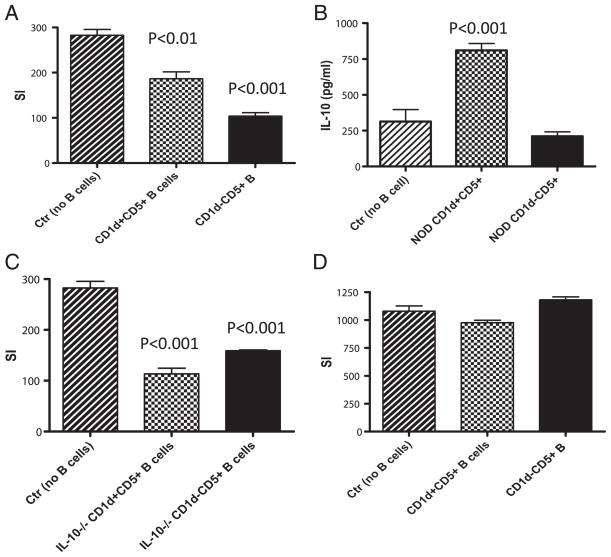
Yanaba et al. (19) reported that CD1d+CD5+ B cells were potent Breg cells. To examine whether CD5 plays a role in our findings described above, we sorted CD1d+CD5+ and CD1d−CD5+ B cells (gated on B220+CD19+) from splenocytes of normal NOD mice. In contrast to the results presented in Fig. 7B, where sorted CD1d+ CD5low/− B cells were used, CD1d+CD5+ B cells suppressed the proliferation of BDC2.5 T cells (Fig. 8A). However, in line with the results showed in Fig. 7B, CD1d− B cells, although CD5+, showed stronger suppression of the antigenic response by BDC2.5 T cells (Fig. 8A). We also examined IL-10 production by these two B cell subsets. Consistent with the report by Yanaba et al. (19), CD1d+CD5+ B cells produced a large amount of IL-10, whereas CD1d−CD5+ B cells did not (Fig. 8B). Interestingly, the suppression mediated by either CD1d+CD5+ or CD1d−CD5+ B cells was not dependent on IL-10 as IL-10–deficient CD1d+ CD5+ or CD1d−CD5+ B cells were still strongly suppressive (Fig. 8C). Next, we tested whether the suppression was cell–cell contact dependent. We repeated the above suppression assay but separated purified CD1d+CD5+ or CD1d−CD5+ B cells from BDC2.5 T cells within the culture by Transwell. As shown in Fig. 8D, the suppression was completely abolished without cell–cell contact.
FIGURE 8.
Immune regulation by CD1d− B cells is not dependent on IL-10, but on cell–cell contact. (A) Purified BDC2.5 T cells were cocultured with the same number of sorted CD1d+CD5+ or CD1d−CD5+ B cells (all gated on CD19+B220+) from NOD mice in the presence of 10 ng/ml BDC2.5 mimotope peptide and irradiated APC (NOD splenocytes). Stimulation index (SI) was calculated as described earlier. Background cpm in the absence of mimotope was 74 ± 11. The experiments were performed twice, and similar results were obtained. (B) IL-10 production from the culture supernatants of (A) was examined by IL-10 ELISA kit (R&D Systems) following the manufacturer’s instructions. (C) Purified BDC2.5 T cells were cocultured with the same number of sorted CD1d+CD5+ or CD1d−CD5+ B cells (all gated on CD19+B220+) from IL-10−/− NOD mice in the presence of 10 ng/ml BDC2.5 mimotope peptide and irradiated APC (NOD splenocytes). Stimulation index was calculated as described earlier. Background cpm in the absence of mimotope was 128 ± 26. The experiments were repeated twice, and similar results were obtained. (D) Purified BDC2.5 T cells were cocultured with the same number of sorted CD1d+CD5+ or CD1d−CD5+ B cells (all gated on CD19+B220+), in 0.4-μm Transwells (BD Biosciences), from NOD mice in the presence of 10 ng/ml mimotope and irradiated APC (NOD splenocytes). Stimulation index was calculated as described earlier. Background cpm in the absence of mimotope was 52 ± 9. The experiments were performed twice, and similar results were obtained.
Discussion
We and others showed previously that B cell depletion could prevent and reverse diabetes development in NOD mice (4–7). Importantly, results from anti-CD20 (rituximab) clinical trial revealed a similar outcome (3). However, it is interesting that patients who responded better to the therapy showed stronger T cell responses to islet Ags in vitro (8). To investigate this paradoxical phenomenon and the mechanism behind it, we generated hCD20-BDC2.5NOD mice to examine the effects of B cell depletion specifically on both Ag-specific pathogenic and regulatory CD4 T cells in T1D. The introduction of the hCD20 transgene, of itself, did not alter the phenotype or functions of BDC2.5 T cells. However, we found that during the B cell depletion period, before regeneration of B cells, islet Ag-specific BDC2.5 T cells responded to the Ag more robustly in vitro and more aggressively induced diabetes in vivo, compared with non-B cell–depleted control mice. During this time, more inflammatory cytokines were produced, including TNF-α, IL-6, and IL-12p40, and there was also a reduction in Foxp3+ Treg cell number and suppressive function in the mice, whereas B cells were depleted. It is interesting that this did not promote hCD20-BDC2.5 TCR Tg mice to develop spontaneous diabetes even with continued B cell depletion (data not shown). However, after B cell regeneration, this enhanced inflammatory phenotype was controlled. The number and function of Treg cells were restored. Our results support, at least in part, the human studies showing that some patients had increased T cell responses to islet Ags 6 mo after anti-CD20 treatment, whereas these increased T cell responses were decreased at a later time point (12 mo) after the treatment (8). Thus, B cell depletion treatment has biphasic effects on Ag-specific diabetogenic and Treg cells, increasing pathogenicity of diabetogenic T cells and reducing Treg cells, whereas B cells are depleted, an effect that is reversed after B cell repopulation. Our results support a regulatory role for B cells in influencing T cell phenotype and function, and this is enhanced once B cell populations are restored after depletion.
The clinical trial results using rituximab to treat newly diagnosed patients with T1D showed that one course of short-term B cell depletion could delay β cell loss and preserve β cell function (3), as was also shown in the preclinical models of diabetes (4–7). Results from further prolonged follow up have indicated that significant differences in various parameters compared with control groups were less sustained (20). Furthermore, it is not clear why some patients with T1D showed stronger T cell responses in vitro to islet autoantigen(s) after rituximab treatment (8). Thus, understanding how the treatment affects diabetogenic T cells may provide an insight into how B cell treatment could be optimized. In our study, the use of the hCD20-BDC2.5 double Tg mouse allowed us to investigate the mechanisms underlying the effects of B cell depletion specifically on well-characterized, highly diabetogenic CD4 T cells both during the period of B cell depletion as well as after repopulation of B cells. Our results showing that during and soon after B cell depletion, diabetogenic BDC2.5 CD4 T cells became more inflammatory and aggressive. This was manifested by a more robust response to cognate Ag stimulation and accompanied by more proinflammatory cytokine production in vitro and in vivo, and the cells also caused more rapid diabetes when adoptively transferred to immunodeficient NOD.SCID mice. This was not due to homeostatic proliferation within the hCD20-BDC2.5 double Tg mice, prior to harvesting the cells for adoptive transfer, as the number of BDC2.5 T cells was, in fact, reduced in hCD20-BDC2.5 hosts after B cell depletion. Interestingly, patients with T1D showed a transient decrease of T cells (CD4+ and CD8+) not long after anti-CD20 treatment (8). In addition, we also observed that Foxp3+ BDC2.5 Treg cells were significantly reduced in number and function soon after B cell depletion in the lymphoid tissues and pancreatic islets, before B cell regeneration. It should be noted that when we used a shorter B cell depletion protocol, similar to the protocol we used in the previous study (4), we found comparable but less pronounced effects as described above (data not shown). However, when the majority of B cells had regenerated, regardless of which depletion protocol was used, the inflammatory phenotype of BDC2.5 T cells was reduced and the number and function of BDC2.5 Treg cells recovered. This is clear evidence that B cells regulate not only the pathogenic BDC2.5 CD4+ T cell immune responses, but that this effect may be mediated in part by an effect on Ag-specific Treg cells.
Immunoregulatory properties of subsets of B cells have been known for years (21–23). Recently there has been a focus on CD1d+ B cells as having immunoregulatory function (16, 17, 19, 24). Matsushita et al. (18) also reported that these CD1dhi B cells expressed CD5+ and could suppress EAE and contact hypersensitivity (19). The regulation was mediated by IL-10 (19). In contrast to these findings, in our diabetes model, we showed that the CD1dhi B cell frequency was reduced in newly regenerated B cells and the CD1d+ B cells from the mice used in this study (BDC2.5NOD-, 2H7-, or mIgG-treated hCD20-BDC2.5NOD and normal NOD mice) did not demonstrate regulatory effects on pathogenic BDC2.5 T cells in vitro and in vivo, compared with CD1d-B cells, although CD1d+CD5+ B cells showed some suppression in vitro. In addition, the suppression was independent of IL-10 but dependent on cell–cell contact. We suggest that there may be at least two factors that account for these differences. First, there are genetic differences in the mouse strains namely C57BL/6 versus NOD mice. Compared with the C57BL/6 mice used in the previous studies, NOD mice have differences in function of APCs, NK cells, Treg cells and deficiency of complement C5 (25–27). Second, there are differences in the type of model used—immunization-induced disease versus spontaneous autoimmune disease; immunization induced EAE is a very different autoimmune disease model from the NOD mice that develop spontaneous T1D. It is clear that more studies are required to further understand the differences in Breg cells in different disease models and whether immunoregulatory cytokine(s) are involved in the function of Breg cells in our model presented in this study.
In summary, using an islet Ag-specific T cell model system, we have found that following B cell depletion, diabetogenic T cells were more aggressive; however, this aggressive phenotype was diminished after B cell repopulation. Thus, there may be a biphasic response to B cell depletion–exacerbation of diabetogenic T cell activity, followed by increased regulation, which may help to explain why more complete protection is not achieved with B cell depletion later on in the course of disease. It is important to note that our current study extends our previous findings where we investigated immune regulation long after B cell repopulation (4). In the current study, we also identified a CD1d− B cell subset that showed strong immunoregulatory function, which was independent of IL-10 but dependent on cell–cell contact. Our results suggest that anti-CD20 treatment increases B cell regulation of pathogenic Ag-specific T cells and enhances Treg cells, particularly after B cell regeneration and one of the beneficial effects of rituximab therapy likely lies in the postregeneration period. Thus, we suggest that the shortest possible B cell depletion period should be considered such that the period of increased pathogenicity of Ag-specific T cells should be minimized and reinforces the notion that the recovery phase after B cell depletion leads to increased regulation and the therapeutic benefits of this treatment. It will be necessary to take this into account in the design of future clinical protocols.
Supplementary Material
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation International (Grants 1-2007-586 and 4-2007-1059) and the Diabetes Endocrinology Research Center transgenic and animal core (National Institute of Diabetes and Digestive and Kidney Diseases). Y.X. was a recipient of a Ph.D. fellowship from the China Scholarship Council (2008637071).
We thank Xiaojun Zhang and Bin Lu for expert animal care. We thank Kevan Herold for critical reading of the manuscript.
Abbreviations used in this article
- Breg
regulatory B cell
- hCD20NOD
human CD20 transgenic NOD
- mIgG
mouse IgG
- MLN
mesenteric lymph node
- PLN
pancreatic lymph node
- T1D
type 1 diabetes
- Teff
T effector
- Treg
regulatory T
Footnotes
Y.X. designed some of the experiments, researched data, and wrote the manuscript; J.P. researched data; N.T. researched data; C.H. contributed to discussions; Z.Z. contributed to discussions; F.S.W. contributed to discussions and reviewed/edited the manuscript; and L.W. designed the experiments, wrote the manuscript, contributed to discussions, and reviewed/edited the manuscript.
This work was presented in abstract form at the 97th Annual Meeting of the American Association of Immunologists, May 7–11, 2010, Baltimore, MD, and at the American Diabetes Association, 70th Scientific Sessions, June 25–29, 2010, Orlando, FL.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 2.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 3.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, et al. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in FcγR effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 6.Mariño E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4+CD25+ T-cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58:1568–1577. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, Wu E, Tian Z, Abdi R, Guleria I, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57:3013–3024. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold KC, Pescovitz MD, McGee P, Krause-Steinrauf H, Spain LM, Bourcier K, Asare A, Liu Z, Lachin JM, Dosch HM, et al. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011;187:1998–2005. doi: 10.4049/jimmunol.1100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D. Genetic control of diabetes progression. Immunity. 1997;7:873–883. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- 11.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 13.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 15.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10–producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 17.Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, Gadola SD, Mizutani A, Kakumanu SR, et al. Immuno-regulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol. 2003;171:2142–2153. doi: 10.4049/jimmunol.171.4.2142. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Pescovitz MD. Treatment of type 1 diabetes with rituximab: 2-year follow-up. [Accessed: March 8, 2011];American Diabetes Association 70th Scientific Sessions. 2010 Jun 25–29; Available at: http://professional.diabetes.org/presentations_details.aspx?session=3463.
- 21.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 23.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, Cao X, Lu L. Novel function of B cell-activating factor in the induction of IL-10–producing regulatory B cells. J Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Faustman D. Defective function of the proteasome in autoimmunity: involvement of impaired NF-κB activation. Diabetes Technol Ther. 2000;2:415–428. doi: 10.1089/15209150050194288. [DOI] [PubMed] [Google Scholar]
- 26.Fan H, Longacre A, Meng F, Patel V, Hsiao K, Koh JS, Levine JS. Cytokine dysregulation induced by apoptotic cells is a shared characteristic of macrophages from nonobese diabetic and systemic lupus erythematosus-prone mice. J Immunol. 2004;172:4834–4843. doi: 10.4049/jimmunol.172.8.4834. [DOI] [PubMed] [Google Scholar]
- 27.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.