Abstract
Background: While there is widespread dissemination of patient navigation programs in an effort to reduce delays in cancer care, little is known about the impact of barriers to care on timely outcomes.
Methods: We conducted a secondary analysis of the Boston Patient Navigation Research Program (PNRP) to examine the effect that the presence of barriers had on time to diagnostic resolution of abnormal breast or cervical cancer screening tests. We used multivariable Cox proportional hazards regression with time to diagnostic resolution as the outcome to examine the effect of the number of barriers, controlling for demographic covariates and clustered by patients' primary navigator.
Results: There were 1481 women who received navigation; mean age was 39 years; 32% were White, 27% Black, and 31% Hispanic; 28% had private health insurance; and 38% did not speak English. Overall, half (n=745, 50%) had documentation of one or more barriers to care. Women with barriers were more likely to be older, non-White, non-English language speakers, and on public or no health insurance compared with women without barriers. In multivariable analyses, we found less timely diagnostic resolution as the number of barriers increased (one barrier, adjusted hazard ratio [aHR] 0.81 [95% CI 0.56–1.17], p=0.26; two barriers, aHR 0.55 [95% CI 0.37–0.81], p=0.0025; three or more barriers, aHR 0.31 [95% CI 0.21–0.46], p<0.0001)].
Conclusion: Within a patient navigation program proven to reduce delays in care, we found that navigated patients with documented barriers to care experience less timely resolution of abnormal cancer screening tests.
Introduction
Underserved populations bear a disproportionate burden of poor cancer-related outcomes, in part due to well-documented delays in the delivery of evidence-based cancer care.1–3 Patient-level barriers, or obstacles to care delivery, have been well described in the literature.4 Psychosocial barriers include cancer fatalism, fear or anxiety about procedures, embarrassment about testing, or mistrust of the medical system.5–7 Logistic barriers include lack of transportation and competing employment or childcare demands.8–10 Commonly reported health care system barriers include lack of health insurance, inconvenient appointment times or locations, and inadequate patient–provider communication.10–12 These barriers may operate in concert to impede access to appropriate cancer care.13,14
Patient navigation is a form of care coordination designed to reduce cancer health disparities by addressing such barriers to obtaining timely, quality care.15 The central premise of patient navigation is that the identification and elimination of patient-level barriers to care can help to close the delivery gap.4,16 Indeed, mounting evidence documents the benefits of navigation on the delivery of timely, quality cancer care.17–21 Despite increasing adoption of navigation practices, there is limited understanding of the impact of barriers to care on clinical outcomes within patient navigation programs.
In order to understand the effect of barriers on cancer care delivery in the presence of patient navigation, we conducted a secondary analysis of the Boston Patient Navigation Research Program (PNRP), a multicenter clinical trial sponsored by the National Cancer Institute's Center to Reduce Cancer Health Disparities and the American Cancer Society that was designed to investigate the effectiveness of patient navigation. We examine the relationship between the number of barriers identified among women navigated for abnormal breast or cervical cancer screening tests and the timeliness of their follow-up care. We hypothesize that the more barriers identified by navigators, the longer the time interval until follow-up diagnostic care.
Materials and Methods
The Boston Patient Navigation Research Program
The Boston PNRP conducted a quasi-experimental patient navigation intervention program across six federally qualified urban community health centers (CHCs) targeting women with breast and cervical cancer screening abnormalities from January 2007 to December 2010. Details of the study design and results for the primary outcomes have been previously published.18 We conducted a secondary analysis of the intervention subjects to examine the association between barriers and timeliness of care. The Boston University Institutional Review Board approved the study.
Study site and subjects
Boston Medical Center is the largest safety-net medical center in New England, and the hospital and its CHCs serve a predominantly low-income, racially diverse patient population, many of whom have limited material and social resources. To be eligible for the PNRP study, female patients at the selected CHCs had an abnormal breast or cervical cancer screening test documented in the electronic health record (EHR). Abnormal breast cancer screening included abnormal clinical breast exams suspicious for malignancy or imaging such as mammogram or ultrasound with breast imaging-reporting and data system (BI-RADS) scores of 0, 3, 4, or 5 according to the Breast Imaging Reporting and Data System.22 Abnormal cervical cancer screening included Papanicolaou test results of low grade squamous intraepithelial lesion or high grade squamous intraepithelial lesion, atypical squamous cells of undetermined significance that also tested positive for high-risk human papillomavirus, or atypical glandular cells of undetermined significance.
The navigation intervention
Patient navigators were women from the community with some health care experience and at least a high school level education. All spoke English and some spoke additional languages, and all navigators had access to robust interpretation services. To ensure uniform navigation practices, navigators participated in annual national training on patient navigation, received bimonthly local training by the research team supplemented by periodic national webinars, and underwent semiannual on-site competency evaluations.23
After physicians had notified subjects of their abnormal cancer screening test results, navigators contacted subjects and used the care management model to determine what impediments, if any, there were to obtaining the necessary diagnostic follow-up (i.e., any barriers to care).24 For each subject, navigators (1) identified what barriers existed to accessing care, (2) developed a care plan to address these barriers, and (3) tracked progress toward recommended care over time. Navigators were trained to ask open-ended questions to elicit barriers to care, and to record these barriers in a template with predefined categories of 20 common barriers that had been established by the PNRP investigators based on literature review and expert consensus. A category was also available for barriers that the navigator did not feel fit within one of the existing categories. With each separate navigation contact, barriers were documented in this structured template in the EHR; if subjects had more than one barrier, then each unique barrier type was noted. Subjects could have a single encounter or multiple encounters with navigators during the course of the study, including in-person visits and telephone calls. In order to reduce potential confounding by indication, unique barriers were counted only once, even if noted on multiple visits.
Data sources
All data were captured from the EHR, including demographic information from registration and billing data. As described above, the navigator template for documenting both the encounter with subjects and the identified barriers was also embedded in the EHR and was designed to allow for electronic abstraction from the EHR. Manual abstraction collected clinical care delivery information on type and dates of tests ordered, tests completed, and test results.18
Study measures
For our independent variable, we categorized subjects dichotomously as those with navigator-identified barriers to care and those without barriers to care. We also classified subjects according to the number of unique barriers (zero, one, two, or three or more barriers) identified by navigators in each subject's case.
The outcome of interest was timeliness of care, defined in two ways. First, time to diagnostic resolution of screening abnormality was defined as a continuous variable as the number of days from the index screening abnormality to the date of the definitive diagnostic test or evaluation. In the case of breast cancer screening abnormalities, diagnostic resolution could be immediate follow-up imaging (for BI-RADS 0), short-interval serial imaging (for BI-RADS 3), or biopsy (for BI-RADS 4 or 5). For cervical cancer screening abnormalities, the definitive diagnostic test was usually colposcopy. Second, timeliness of care was defined as a dichotomous variable of achievement of diagnostic resolution by 365 days after the date of the index screening abnormality. The time point of 365 days was chosen because the national PNRP analysis demonstrated a benefit of navigation during the entire follow-up period of up to one year.21
Analysis
Among subjects with barriers, we calculated the frequency of specific barriers from among the 20 predefined barrier types. Once classified into groups according to the presence of barriers or their number of barriers, subjects were compared on descriptive statistics and time to diagnostic resolution using analysis of variance for means of measurement variables, the Kruskal-Wallis test for median time to resolution, and chi-squared for categorical variables. If a subject did not achieve diagnostic resolution by 365 days, she was considered unresolved at 365 days. Unadjusted Kaplan-Meier cumulative incidence curves were calculated to examine the effect of the number of barriers to care on time to diagnostic resolution. Time to resolution was compared through log-rank tests.
Cox proportional hazards regression models compared time to diagnostic resolution across the four groups (none, one, two, or three or more barriers to care) controlling for continuous age, race/ethnicity, language, and insurance type. These covariates were chosen based on prior studies examining sociodemographic factors associated with completion of diagnostic testing.8,25,26 The proportional hazards assumption was tested for possible violation; where it was violated (for age only), a log(time) interaction term was added. To account for individual navigator effects, subjects were clustered according to the subject's navigator. Where more than one navigator was involved in care, we clustered by the navigator who had the greatest number of encounters with the subject. Because navigators were assigned to particular sites, this clustering also accounted for the effects of the subject's CHC. Because age and cancer type are collinear in our sample (women are screened for cervical cancer at a younger age than breast cancer), we controlled for age but not cancer type in our primary analysis. A secondary analysis was also performed controlling for both age and cancer type. Two-tailed p<0.05 was used for statistical significance, and all analyses were conducted in SAS 9.3 (SAS Institute, Cary, North Carolina).
Results
The Boston PNRP recruited a diverse, disadvantaged population of women for navigation of screening abnormalities (Table 1). There were 1481 subjects navigated during the study period, and the mean age was 39 years. Consistent with the patient population served across the CHCs, 32% of women were White, 27% were Black, 31% were Hispanic, and 10% were from other racial/ethnic groups. Only 62% of women spoke English as their primary language, while 21% spoke Spanish and 16% spoke another language. The majority of women had publically funded insurance (44%), while the remainder had private insurance (28%) or were uninsured (28%).
Table 1.
Demographic Characteristics of Navigated Subjects in the Boston Patient Navigation Research Program, 2007–2010, by Presence of Barriers to Care
| Total n (%) | Barrier(s) present n (%) | No barriers n (%) | p | |
|---|---|---|---|---|
| Total (N) | 1481 | 745 (50) | 736 (50) | |
| Age (years) | 0.0005 | |||
| 18–29 | 486 (33) | 224 (30) | 262 (36) | |
| 30–39 | 245 (17) | 100 (13) | 145 (20) | |
| 40–49 | 396 (27) | 219 (29) | 177 (24) | |
| 50–59 | 213 (14) | 123 (17) | 90 (12) | |
| 60–69 | 87 (6) | 51 (7) | 36 (5) | |
| 70+ | 54 (4) | 28 (4) | 26 (4) | |
| Race/ethnicity | <0.0001 | |||
| White | 468 (32) | 172 (23) | 296 (40) | |
| African American | 405 (27) | 203 (27) | 202 (27) | |
| Hispanic | 460 (31) | 263 (35) | 197 (27) | |
| Other | 148 (10) | 107 (14) | 41 (6) | |
| Language | <0.0001 | |||
| English | 925 (62) | 405 (54) | 520 (71) | |
| Spanish | 317 (21) | 191 (26) | 126 (17) | |
| Other | 239 (16) | 149 (20) | 90 (12) | |
| Insurance | <0.0001 | |||
| Private | 410 (28) | 156 (21) | 254 (35) | |
| Public | 657 (44) | 370 (50) | 287 (39) | |
| No insurance | 414 (28) | 219 (29) | 195 (26) | |
| Screening abnormality | 0.0001 | |||
| Breast | 760 (51) | 419 (56) | 341 (46) | |
| Cervical | 721 (49) | 326 (44) | 395 (54) |
Values may not sum to 100% due to rounding.
Of the navigated women, 745 (50%) had one or more unique barrier to care identified by navigators. Compared to those without barriers, women with barriers were more likely to be older (p<0.0005), of minority race/ethnicity (p<0.0001), and non-English speaking (p<0.0001). They were also more likely to have public insurance than private insurance (p<0.0001) and to be enrolled for a breast cancer screening abnormality than for a cervical cancer screening abnormality (p=0.0001).
A total of 1044 barriers were identified among 745 women (Table 2). The most common barriers identified were language (25%), system problems with scheduling care (14%), and fear (12%). For nearly a quarter of the barriers identified, navigators did not classify them into one of the 20 prespecified categories. We found that 541 (37%) subjects had one barrier to care, 140 (9%) had two barriers, and 64 (4%) had three or more barriers to care (Table 3).
Table 2.
Frequency of Barriers Identified Among Navigated Subjects in the Boston Patient Navigation Research Program
| Barrier | Description | Frequency (%) |
|---|---|---|
| Language/interpreter | Health care personnel and patient do not share a common language for communication | 25.2 |
| Not otherwise specified | Barrier other than one defined in the PNRP framework | 24.5 |
| System problems with scheduling care | Care provided to patient is not convenient/efficient to patient's needs | 14.4 |
| Fear | Fear about any aspect of medical care or their health | 11.8 |
| Employment issues | Work demands make getting health care difficult | 5.0 |
| Out of town/country | Patient known to be out of area during their care | 4.3 |
| Location of health care facility | Distance from health care facility is a barrier even if patient has transportation | 2.8 |
| Insurance | Paying for all aspects of health care is a problem | 2.0 |
| Medical and mental health co-morbidities | Medical health problems or mental health problems that make getting health care difficult | 1.9 |
| Communication concerns with medical personnel | Barriers to understanding the information given to a patient by medical personnel | 1.5 |
| Transportation | Difficulty getting from home to where the patient obtains her health care | 1.3 |
| Perceptions/beliefs about tests/treatment | Personal or cultural beliefs that affect receiving health care | 1.3 |
| Attitudes toward providers | Perceptions and beliefs about the health care providers that impact receiving care | 0.9 |
| Childcare | Not having childcare when the patient needs medical care | 0.7 |
| Housing | Worrying about where the patient lives during her health care | 0.6 |
| Adult care | Difficulty finding support for other family when the patient needs medical care | 0.6 |
| Patient disability | Disability that makes getting health care difficult | 0.5 |
| Financial problems | Dealing with financial problems is interfering with receiving health care | 0.4 |
| Literacy | Difficulty understanding written communication from the health care setting | 0.3 |
| Support/practical support | Lacks a person/community to help them through the care | 0.2 |
n=1044 barriers identified in 745 subjects.
PNRP, Patient Navigation Research Program.
Table 3.
Median Time to Diagnostic Resolution and Percent Resolved by 365 Days, According to Number of Unique Barriers, for Navigated Subjects in the Boston Patient Navigation Research Program
| Number of barriers n (%) | Time to resolution, in days, median (interquartile range) | Percent resolved by 365 days (%) | |
|---|---|---|---|
| No barriers | 736 (50%) | 50 (27–92) | 94 |
| One barrier | 541 (37%) | 51 (28–113) | 90* |
| Two barriers | 140 (9%) | 63 (34–219) | 84* |
| Three or more barriers | 64 (4%) | 265 (71–365) | 69* |
N=1481 subjects.
p<0.0001 for comparison of percent resolved by 365 days to subjects with no barriers.
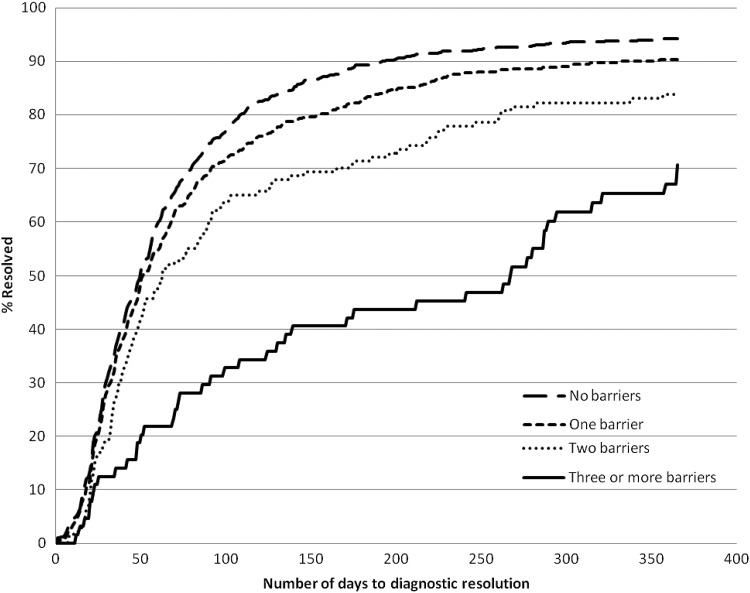
The outcome of interest and timeliness of care varied according to the number of unique barriers (Figure 1). Median time to resolution for women without barriers was 50 days, with longer median times as the number of barriers increased (51, 63, and 265 days for women with one, two, and three or more barriers to care, respectively) (Table 3). There was a negative association between the number of barriers and the percent of screening abnormalities resolved by 365 days.
FIG. 1.
Barriers were identified by patient navigators during navigation encounters. The number of days to diagnostic resolution was the time interval between the index screening abnormality (e.g., abnormal mammogram, abnormal Papanicolaou test) and the definitive diagnostic test (e.g., biopsy, colposcopy).
We looked at the bivariate association between the presence of the three most common barriers and median time to completion of diagnostic care (Table 4). The finding was not consistent by type of barrier. Subjects with system problems with scheduling care had greater delays in care, those with language barriers had fewer delays in care, and patient reports of fear were not associated with time to completion of care.
Table 4.
Median Time to Diagnostic Resolution and Percent Resolved by 365 Days, According to Type of Barrier, Among Subjects with Documented Barriers in the Boston PNRP
| Barrier | Presence of barrier n (%) | Time to resolution, in days Median (IQR) | Percent resolved by 365 days (%) | |
|---|---|---|---|---|
| Language/interpreter | Yes | 263 (35) | 50 (29–92)* | 92 |
| No | 482 (65) | 68 (32–195) | 84 | |
| System problems with scheduling care | Yes | 150 (20) | 199 (73–365)* | 71* |
| No | 595 (80) | 49 (27–102) | 91 | |
| Fear | Yes | 123 (17) | 64 (34–185) | 85 |
| No | 622 (83) | 57 (29–141) | 87 | |
N=745 subjects.
p<0.001 for comparison of median time to diagnostic resolution or percent resolved by 365 days between subjects with and without barrier of interest.
IQR, interquartile range.
Table 5 presents the adjusted hazards ratios (aHR) for reaching diagnostic resolution according to number of unique barriers, where aHRs less than 1.0 indicate less timely resolution. As the number of barriers increases, the likelihood of timely resolution decreases. Compared to women with no barriers to care, the aHR for diagnostic resolution for women with one barrier to care was 0.81 (95% confidence interval [95% CI] 0.56–1.17), with two barriers to care was 0.55 (95% CI 0.37–0.81), and with three or more barriers to care was 0.31 (95% CI 0.21–0.46).
Table 5.
Cox Proportional Adjusted Hazard Ratios for Time to Resolution, According to Number of Unique Barriers, for Navigated Subjects in the Boston Patient Navigation Research Program
| Variable | Levels | aHR (95% CI) | p |
|---|---|---|---|
| Number of barriers | None | 1.00 (reference) | |
| 1 | 0.81 (0.56, 1.17) | 0.2611 | |
| 2 | 0.55 (0.37, 0.81) | 0.0025 | |
| 3+ | 0.31 (0.21, 0.46) | <0.0001 | |
| Age | Years (continuous) | 1.07 (1.04, 1.11) | <0.0001 |
| Age x log (time to resolution) | 0.99 (0.98, 1.00) | 0.0023* | |
| Race | White | 1.00 (reference) | |
| African American | 0.76 (0.58, 0.98) | 0.0317 | |
| Hispanic | 1.15 (0.91, 1.47) | 0.2463 | |
| Other | 1.47 (1.25, 1.73) | <0.001 | |
| Language | English | 1.00 (reference) | |
| Spanish | 1.05 (0.85, 1.31) | 0.6531 | |
| Other | 0.91 (0.68, 1.20) | 0.4839 | |
| Insurance | Private | 1.00 (reference) | |
| Public | 0.93 (0.80, 1.08) | 0.3449 | |
| Uninsured | 1.01 (0.81, 1.25) | 0.9495 |
N=1481 subjects.
Adjusted hazard ratio (aHR) <1.0 indicates a lower likelihood of timely resolution.
Cox proportional analysis adjusted for age, race/ethnicity, language, and insurance, with clustering by primary patient navigator.
The time by age interaction indicates that the effect of age changes with time. Combining the slopes for the age and interaction term gives aHRs for a 10-year difference in age of 1.26, 1.09, and 1.00 at 30, 90, and 180 days, respectively.
After adjusting for the number of unique barriers, race/ethnicity was significantly associated with likelihood of achieving diagnostic resolution in the model. Compared to White subjects, African-American women were less likely to have timely resolution (aHR 0.76, 95% CI 0.58–0.98), while women of other race/ethnicity were more likely to have timely resolution (aHR 1.47, 95% CI 1.25–1.73). Hispanic ethnicity was not predictive of the outcome (aHR 1.15, 95% 0.91–1.47, p=0.25). There was violation of the proportional hazards assumption for age, where older women were initially more likely to resolve than younger women, although this advantage diminished over follow-up (Table 5 footnote). After adjusting for the number of barriers, language and insurance status were not predictors of timely resolution. A secondary analysis adding cancer type to the control variables showed very similar effects of barriers on time to resolution.
Discussion
In this analysis, we found that the greater the number of unique barriers to care identified by a patient navigator, the longer the time to diagnostic resolution for women with an abnormal breast or cervical cancer screening test. According to our adjusted model, the number of barriers exerted an apparent dose-dependent impact on the likelihood of achieving diagnostic resolution – the more barriers identified for a subject, the less likely she was to resolve her screening abnormality. These findings have face validity and confirm clinical observations that the more obstacles a patient faces, the more time she will require to obtain needed health care.
Only half of the at-risk women enrolled in the navigation program had at least one barrier to care identified. Other patient navigation interventions have noted no barriers to care in 50% or fewer of disadvantaged patients.17,27 Subjects without barriers had the shortest time to resolution, and nearly all had resolved by one year after index screening abnormality. Meanwhile, the women with barriers were among the most vulnerable members of society: racial/ethnic minorities, non-English language speakers, and those with public health insurance. This finding suggests that barrier identification could be used to identify patients at greatest risk for delays in need of these intensive care management services. If the mechanism of action of navigation is through identification and elimination of barriers, then arguably patients without barriers would derive limited benefit from such care coordination. Looking at specific types of barriers, some appear to have a greater influence on the outcomes; systems barriers with scheduling care were associated with delays, while language barriers and fear were not.
Even with navigation, our study found that women with barriers were less likely to resolve screening abnormalities compared to women without barriers. The impact of barriers was most apparent for women with multiple barriers. Women with two barriers experienced delays of approximately two weeks compared to women with no barriers; the corresponding delay was nearly seven months for women with 3 or more barriers. Although the latter group was small (n=64, 4% of the total sample), it represents the subset of patients at greatest risk of not completing care, and thus the principal target of navigation.
These persistent delays may relate to the kinds of barriers identified in this sample and how easily these barriers can be addressed. For example, many (38%) of our subjects spoke a language other than English, and language was the most frequently documented barrier in this study. Navigators could address this barrier using telephone interpreter services or personal language skills, or by scheduling interpreters to be present at future appointments. In those in whom language was identified as a barrier, median time to resolution was the same as those with no barriers (50 days), suggesting that navigators were able to overcome this barrier. For other barriers, like system problems with scheduling care, navigators could potentially reschedule appointments at a more convenient date and time, but may be limited in how much they can completely eliminate the barrier. Underlying behavioral health issues can also contribute to delays in care if not detected and addressed; navigator training could enhance skills in recognizing mental health needs and referring appropriately. Certain barriers frequently identified in this study, such as employment issues or being out of town/country, are even less amenable to modification by navigators. These findings support our previously published analyses documenting the relationship between multiple social service barriers, such as lack of adequate housing or income supports, and timely resolution.28
Half of our navigated population had documented barriers to care, similar to some navigation programs17,27 while other navigation programs have found a greater frequency of barriers29 using the same methodology in barrier identification. Such differences between sites reinforces the importance of tailoring navigation training and implementation to the local needs and barriers of the community served.
A quarter of barriers could not be categorized into the predefined barrier types by navigators. Documentation space was available for navigators to write additional details about these “Not otherwise specified” barriers, but navigators rarely did so; therefore, we do not know what obstacles these barriers may represent. It is worth noting that navigators chose to document a barrier in this “not otherwise specified” category rather than choosing “no barrier identified,” suggesting that navigators detected a barrier but could not readily classify it among the predefined categories. Navigators received rigorous training and competency evaluation on the identification and documentation of barriers.23 Barriers in the PNRP were identified by navigators during encounters with subjects; future studies could instead or additionally collect patient-reported information on barriers. This approach may reduce the number of unclassifiable barriers but is susceptible to social desirability bias and may make it more difficult to compare definitions of barriers across navigation programs.
We recognize certain limitations of this study. We do not have data on barriers in the control population to compare the impact of navigation on types or subgroups of barriers. Future research should collect information on barriers in patients not receiving navigation for comparison to navigated patients in order to understand the differential benefits of navigation according to barrier number and type.
With greater delays in care and more encounters with navigators, there is greater opportunity to identify barriers. We mitigated against this confounding by indication by only counting a unique barrier once, even when identified on subsequent visits. More than half of all barriers were identified within the first two encounters, also reducing the possibility of confounding by indication. However, this strategy does not allow us to examine persistence of barriers over time or to estimate when barriers were removed. Although we know that navigators took certain actions to address barriers, we do not know whether a barrier was eliminated or not. Furthermore, we treated all barriers as equally important in their magnitude of influence on time to resolution in the multivariate models, even though our bivariate results on the most common barriers indicated differences in association with the outcome. Other studies have shown that barriers negatively impact timeliness of care regardless of type.27 This study was conducted only among women seeking breast and cervical cancer screening at health centers in one city, and therefore results may not be generalizable to other populations and settings. Finally, as a secondary analysis, this study is particularly useful for generating hypotheses rather than causal associations.
Conclusions
Our findings provide more detailed understanding of the importance of barriers to care on the delivery of cancer-care related outcomes, and are further evidence of the impact of social determinants of health on care delivery. Patient navigation is an increasingly adopted form of care coordination to improve the delivery of health care to disadvantaged patients, eliminate barriers to care, and address persistent health care disparities. Our findings support targeting patient navigation to vulnerable patients who need it most—patients with identifiable barriers to care. The challenge for future patient navigation programs will be recognizing such at-risk patients even within an overall disadvantaged group and tailoring navigation to the particular needs and prevalent barriers of the communities they serve.
Acknowledgments
This research was funded by the National Cancer Institute Center to Reduce Cancer Health Disparities (NCI U01 CA116892), American Cancer Society Physician Training Award in Cancer Prevention (PTAPM-97-185-16), and American Cancer Society (CRP-12-219-01-CPPB).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Desantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin 2013;63:151–166 [DOI] [PubMed] [Google Scholar]
- 2.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic Status. CA Cancer J Clin 2004:54:78–93 [DOI] [PubMed] [Google Scholar]
- 3.Elmore JG, Nakano CY, Linden HM, Reisch LM, Ayanian JZ, Larson EB. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care 2005:43;141–148 [DOI] [PubMed] [Google Scholar]
- 4.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: A review of barriers to quality care. Cancer 1999;86:2378–2390 [PubMed] [Google Scholar]
- 5.Schoenberg NE, Studts CR, Hatcher-Keller J, Buelt E, Adams E. Patterns and determinants of breast and cervical cancer non-screening among Appalachian women. Women Health 2013;53:552–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sly JR, Edwards T, Shelton RC. Jandorf L. Identifying barriers to colonoscopy screening for nonadherent African American participants in a patient navigation intervention. Health Educ Behav 2013;40:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lasser KE, Ayanian JZ, Fletcher RH, Good M-J. Barriers to colorectal cancer screening in community health centers: A qualitative study. BMC Fam Pract 2008;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggleston K. S., Coker, A. L., Das, I. P., Cordray, S. T. Luchok, K. J. Understanding Barriers for Adherence to Follow-Up Care for Abnormal Pap Tests. J Womens Health 2007;16:311–330 [DOI] [PubMed] [Google Scholar]
- 9.Rojas M, Mandelblatt J, Cagney K, Kerner J, Freeman H. Barriers to follow-up of abnormal screening mammograms among low-income minority women. Cancer Control Center of Harlem Ethn Health 1996;1:221–228 [DOI] [PubMed] [Google Scholar]
- 10.Percac-Lima S, Aldrich LS, Gamba GB, Bearse AM, Atlas SJ. Barriers to follow-up of an abnormal Pap smear in Latina women referred for colposcopy. J Gen InternMed 2010;25:1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Malley AS, Beaton E, Yabroff KR, Abramson R, Mandelblatt J. Patient and provider barriers to colorectal cancer screening in the primary care safety-net. Prev Med 2004;39:56–63 [DOI] [PubMed] [Google Scholar]
- 12.Woloshin S, Schwartz LM, Katz SJ, Welch HG. Is language a barrier to the use of preventive services? J Gen Intern Med 1997;12:472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X-C, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol 2012;30:142–150 [DOI] [PubMed] [Google Scholar]
- 14.Gorey KM, Haji-Jama S, Bartfay E, Luginaah IN, Wright FC, Kanjeekal SM. Lack of access to chemotherapy for colon cancer: Multiplicative disadvantage of being extremely poor, inadequately insured and African American. BMC Health Serv Res 2014;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund KM, Battaglia TA, Calhoun E, et al. National Cancer Institute Patient Navigation Research Program. Cancer 2008;113:3391–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient navigation: State of the art or is it science? Cancer 2008;113:1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paskett ED, Katz ML, Post DM, et al. The Ohio Patient Navigation Research Program: Does the American Cancer Society Patient navigation model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiol Biomarkers Prev 2012;21:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battaglia TA, Bak SM, Heeren T, et al. Boston Patient Navigation Research Program: The impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomarkers Prev 2012;21:1645–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D. Patient navigation improves cancer diagnostic resolution: An individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev 2012;21:1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley DJ, Drake J, Quinlan J, et al. Beneficial effects of a combined navigator/promotora approach for Hispanic women diagnosed with breast abnormalities. Cancer Epidemiol Biomarkers Prev 2012;21:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freund KM, Battaglia TA, Calhoun E, et al. Impact of patient navigation on timely cancer care: The patient navigation research program. J Natl Cancer Inst 2014;2014(48):106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Radiology. Illustrated breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology, 2003 [Google Scholar]
- 23.Calhoun EA, Whitley EM, Esparza A, et al. A national patient navigator training program. Health Promot Pract 2010;11:205–215 [DOI] [PubMed] [Google Scholar]
- 24.Longest B, Young GJ. Coordination and communication. In: Shortell S, Kaluzny AD, eds. Health Care Management: Organization Design and Behavior. Clifton Park, NY: Thomson Delmar Learning, 2000:210–243 [Google Scholar]
- 25.Tabnak F, Müller H-G, Wang J-L, Zhang W, Howell LP. Timeliness and follow-up patterns of cervical cancer detection in a cohort of medically underserved California women. Cancer Causes Control 2010;21:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglia TA, Santana MC, Bak S, et al. Predictors of timely follow-up after abnormal cancer screening among women seeking care at urban community health centers. Cancer 2010;116: 913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz ML, Young GS, Reiter PL, et al. Barriers reported among patients with breast and cervical abnormalities in the patient navigation research program: Impact on timely care. Womens Health Issues 2014;24:e155–e162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Primeau SW, Freund KM, Ramachandran A, et al. Social service barriers delay care among women with abnormal cancer screening. J Gen Intern Med 2014;29:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tejeda S, Darnell JS, Cho YI, Stolley MR, Markossian TW, Calhoun EA. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health 2013;22:507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]