Abstract
Cardiopulmonary exercise testing (CPET) is a common method of evaluating patients with a Fontan circulation. Equations to calculate predicted CPET values are based on children with normal circulation. This study aims to create predictive equations for CPET variables solely based on patients with Fontan circulation. Patients who performed CPET in the multicenter Pediatric Heart Network Fontan Cross-Sectional Study were screened. Peak variable equations were calculated using patients who performed a maximal test (RER > 1.1) and anaerobic threshold (AT) variable equations on patients where AT was adequately calculated. Eighty percent of each cohort was randomly selected to derive the predictive equation and the remaining served as a validation cohort. Linear regression analysis was performed for each CPET variable within the derivation cohort. The resulting equations were applied to calculate predicted values in the validation cohort. Observed versus predicted variables were compared in the validation cohort using linear regression. 411 patients underwent CPET, 166 performed maximal exercise tests and 317 had adequately calculated AT. Predictive equations for peak CPET variables had good performance; peak VO2, R2 = 0.61; maximum work, R2 = 0.61; maximum O2 pulse, R2 = 0.59. The equations for CPET variables at AT explained less of the variability; VO2 at AT, R2 = 0.15; work at AT, R2 = 0.39; O2 pulse at AT, R2 = 0.34; VE/VCO2 at AT, R2 = 0.18; VE/VO2 at AT, R2 = 0.14. Only the models for VE/VCO2 and VE/VO2 at AT had significantly worse performance in validation cohort. Of the 8 equations for commonly measured CPET variables, six were able to be validated. The equations for peak variables were more robust in explaining variation in values than AT equations.
Keywords: Fontan, Cardiopulmonary exercise testing, Congenital heart disease
Introduction
Cardiopulmonary exercise testing (CPET) is a long established means of evaluating patients with heart disease providing invaluable diagnostic, physiological, and prognostic information [12, 18, 23]. CPET has been used in the clinical management of congenital heart disease as well as endpoint for therapeutic clinical trials, especially in patients with single ventricles who have had a Fontan surgery [13, 20, 28]. This has led to an ever growing knowledge of the factors that influence CPET variables in the Fontan population [10]. Despite the growing knowledge regarding CPET in Fontan patients, it is frequent for authors to use calculated predicted CPET variables using formulas developed for children without heart disease [6].
The aim of this study was to develop predictive equations for CPET variables specific for patients with Fontan circulation.
Methods
The study was approved by the Institutional Review Board of the Medical University of South Carolina. Data from the multi-institutional NIH/NHLBI Pediatric Heart Network (PHN) Fontan Cross-Sectional Study was downloaded from the PHN website (www.pediatricheartnetwork.org). The methods and results of PHN Fontan study have been previously reported [1]. Briefly, 7 US and Canadian centers recruited 546 Fontan survivors between ages 6 and 18.
Exercise Testing
The exercise protocols employed during the study have been previously published [26]. Patients underwent maximal exercise testing using a cycle ergometer with a ramp protocol. Peak oxygen consumption (peak VO2) was defined as the maximal oxygen consumption obtained during exercise. Anaerobic threshold (AT) was calculated by the V-slope method. Ventilatory equivalents for carbon dioxide production (VE/VCO2) and oxygen consumption (VE/VO2) were measured at the AT. O2 pulse at peak exercise and AT was calculated by dividing VO2 at each time point by heart rate. Work measured by Watts was recorded at AT and peak exercise.
Equation Development
Linear regression was performed to create predictive equations for the following CPET variables at peak exercise: peak VO2, O2 pulse, and work; and the following variables at AT: VO2, O2 pulse, work, VE/VCO2 and VE/VO2. The patient characteristics and anatomical variables used for analysis were chosen due to previously reported or likely association with CPET variables (Table 1) [9, 10, 21, 24–26]. For categorical variables, the key denotes the value for a variable given when developing the equation. Peak VO2 was similar between the left and mixed ventricular morphology patients; therefore, these two groups were combined.
Table 1. Variable evaluated in the multivariable model.
| Categorical variables |
| Gender (0 = female, 1 = male) |
| Ventricular looping (0 = d-loop, 1 = l-loop) |
| Dominant ventricle (0 = left and mixed, 1 = right) |
| Rhythm at rest (0 = non-sinus rhythm, 1 = sinus rhythm) |
| Fenestration present at study (0 = no, 1 = indeterminate, 2 = yes) |
| Continuous variables |
| Age at exercise testing |
| Age at Fontan |
| Height |
| Weight |
For linear regression analysis of peak exercise CPET variables only patients who reached peak exercise, defined as respiratory exchange ratio (RER) greater than 1.1 at peak exercise, were included. For analysis of AT variables only patients who were reported to have an identifiable AT were included.
In each analysis, a derivation and validation cohorts were created randomly. The derivation cohort consisted of 80 % of the study population; the validation cohort was the remaining 20 %. Random selection was performed by the statistical program, IBM SPSS® v. 21 (New York, USA). Covariates associated with the CPET variable in univariate analysis (p ≤ 0.1) were placed into the linear multivariable regression analysis. In order to create an efficient as well as accurate equation, covariates were removed in a stepwise fashion from the multivariable regression analysis if the partial R2 for the covariate was less than 0.01 or covariate p value was > 0.05.
Linear regression was then performed between the predicted CPET variables and observed values in validation cohort. To determine the performance of the equation in the validation cohort, two statistical tests were performed. First, the difference between R2 (R2 difference) between the both cohorts was calculated (R2 of derivation cohort— R2 of validation cohort). A priori, a difference between R2 of ≤0.05 was set as acceptable, i.e., the R2 of the validation cohort could be no more than 0.05 lower than the R2 of the derivation cohort to be acceptable, but could be higher. Secondly, to determine if there was a significant tendency in the equation to over or under estimate variables, a single sample T test was performed to see if the mean difference in the entire validation cohort between predicted and observed variables differed significantly (p < 0.05) from zero.
All statistical analysis was performed using IBM SPSS® v.21 (New York, USA).
Results
Of the 546 patients who were recruited, 411 underwent exercise testing, in which 166 (40 %) had maximal exercise tests and 317 (77 %) had adequate AT calculated. The patient characteristics of each group are listed in Table 2.
Table 2. Patient characteristics in each cohort.
| Characteristic | Total (n = 411) | Maximal exercise (n = 166) | Adequate anaerobic threshold (n = 317) |
|---|---|---|---|
| Age | |||
| At Fontan | 3.4 ± 2.0 | 3.4 ± 2.1 | 3.5 ± 2.2 |
| At exercise test | 12.4 ± 3.2 | 13.9 ± 2.9 | 12.9 ± 3.1 |
| Height (cm) | 146.9 ± 16 | 154.5 ± 14 | 150 ± 15 |
| Weight (kg) | 42.5 ± 16 | 48.5 ± 16 | 44.9 ± 16 |
| Male, n (%) | 242 (59) | 94 (57) | 190 (60) |
| Dominant ventricle, n (%) | |||
| Right | 126 (31) | 51 (30) | 99 (31) |
| Left and mixed | 211 (68) | 115 (69) | 216 (69) |
| Sinus rhythm at rest, n (%) | 283 (69) | 116 (70) | 216 (69) |
| Fenestration present at time of study, n (%) | |||
| Not present | 327 (80) | 127 (77) | 244 (78) |
| Indeterminate | 46 (11) | 21 (13) | 40 (13) |
| Present | 37 (9) | 15 (9) | 29 (9) |
| L-loop, n (%) | 79 (19) | 41 (25) | 66 (21) |
For the maximal exercise cohort, 136 (82 %) cases were randomly selected for the derivation cohort of peak exercise variables. Associations between covariate and peak variables using univariate statistics for the derivation cohort are shown in Table 3. Table 4 outlines how the final estimating equations were created. The final models yielded the equations outlined in Table 5.
Table 3. Univariate statistics for derivation cohort of peak CPET variables (n = 136).
| Covariate | Peak VO2 (L/min) | Peak work (W) | Peak 02 pulse (mL O2/beat) | |||
|---|---|---|---|---|---|---|
| Age | ||||||
| At Fontan | r = −0.07 | p = 0.41 | r = −0.08 | p = 0.35 | r = −0.33 | p = 0.70 |
| At exercise | r = 0.51 | p < 0.01 | r = 0.60 | p < 0.01 | r = 0.52 | p < 0.01 |
| Height | r = 0.72 | p < 0.01 | r = 0.73 | p < 0.01 | r = 0.72 | p < 0.01 |
| Weight | r = 0.69 | p < 0.01 | r = 0.70 | p < 0.01 | r = 0.67 | p < 0.01 |
| Gender | ||||||
| Male | 1.45 ± 0.52 | p < 0.01 | 110 ± 42 | p < 0.01 | 9.24 ± 3.36 | p < 0.01 |
| Female | 1.11 ± 0.27 | 85 ± 27 | 7.12 ± 1.79 | |||
| Rhythm at rest | ||||||
| Sinus | 1.31 ± 0.48 | p = 0.74 | 99 ± 39 | p = 0.91 | 8.20 ± 3.02 | p = 0.33 |
| Non-sinus | 1.29 ± 0.42 | 100 ± 36 | 8.74 ± 2.87 | |||
| Fenestration | ||||||
| Present | 1.01 ± 0.31 | 80 ± 30 | 6.58± 2.54 | |||
| Indeterminate | 1.23 ± 0.24 | p = 0.02 | 88 ± 22 | p = 0.07 | 7.73 ± 1.42 | p = 0.32 |
| Not present | 1.35 ± 0.48 | 103 ± 40 | 8.67 ± 3.12 | |||
| Dominant ventricle | ||||||
| Left and mixed | 1.35 ± 0.48 | p = 0.09 | 103 ± 39 | p = 0.11 | 8.71 ± 3.08 | p = 0.04 |
| Right | 1.21 ± 0.39 | 92 ± 34 | 7.60 ± 2.63 | |||
| Ventricular looping | ||||||
| d-loop | 1.29 ± 0.45 | p = 0.21 | 98 ± 37 | p = 0.34 | 8.24 ± 2.95 | p = 0.38 |
| l-loop | 1.40 ± 0.47 | 105 ± 42 | 8.78 ± 3.10 | |||
Table 4. Derivation of predictive equations.
| Peak variables | |||
|---|---|---|---|
|
|
|||
| Peak VO2 | Maximum work | Max O2 pulse | |
| Initial model | Age at exercise, height, weight, gender, fenestration, dominant ventricle | Age at exercise, height, weight, gender, fenestration, dominant ventricle | Age at exercise, weight, height, gender, dominant ventricle |
| Covariate removed | |||
| Step 1 | Dominant ventricle* | Dominant ventricle* | Age at exercise* |
| Step 2 | Age at exercise* | Fenestration* | Dominant ventricle* |
| Step 3 | Fenestration* | Age at exercise* | |
| Covariates in final model | Gender, height, weight | Gender, height, weight | Gender, height, weight |
|
| |||
| Anaerobic threshold variables | |||
|
|
|||
| VO2 at AT | Work at AT | O2 pulse at AT | |
|
| |||
| Initial model | Age at exercise, height, weight, gender | Ventricular looping, gender, weight, height, age at exercise, dominant ventricle, fenestration | Ventricular looping, gender, weight, height, age at exercise, dominant ventricle, fenestration |
| Covariate removed | |||
| Step 1 | Weight* | Dominant ventricle* | Ventricular looping* |
| Step 2 | Age at exercise* | Age at exercise* | Age at exercise* |
| Step 3 | Weight* | Fenestration* | |
| Step 4 | Ventricular looping** | Dominant ventricle* | |
| Covariates in final model | Gender, height | Height, gender, fenestration | Gender, height, weight |
|
| |||
| Ventilatory equivalents at anaerobic threshold | |||
|
|
|||
| VE/VCO2 at AT | VE/VO2 at AT | ||
|
| |||
| Initial model | Ventricular looping, weight, height, age at exercise, dominant ventricle, fenestration | Ventricular looping, weight, height, age at exercise, dominant ventricle, fenestration | |
| Covariate removed | |||
| Step 1 | Height* | Height* | |
| Step 2 | Dominant ventricle* | Age at exercise* | |
| Step 3 | Age at exercise* | Dominant ventricle* | |
| Covariates in final model | Weight, ventricular looping, fenestration | Weight, ventricular looping, fenestration | |
Removed due to partial R2 < 0.02 and p value > 0.05
Removed due to p value > 0.05
Table 5. Final predictive equations.
| CPET variable | Constant | Height (cm) | Weight (kg) | Gender | Fenestration | Ventricular looping | R2 | Standard error of estimate |
|---|---|---|---|---|---|---|---|---|
| Peak VO2 (L/min) | −1.71 | 0.016 | 0.008 | 0.243 | 0.61 | 0.29 | ||
| Maximum O2 pulse (mL O2/beat) | −11.89 | 0.111 | 0.043 | 1.51 | 0.59 | 1.92 | ||
| Maximum work (W) | −153.47 | 1.35 | 0.66 | 17.72 | 0.61 | 24.2 | ||
| VO2 at AT (L/min) | −0.933 | 0.011 | 0.218 | 0.15 | 0.46 | |||
| Work at AT (W) | −3.97 | 0.926 | 9.976 | −4.762 | 0.39 | 18.9 | ||
| O2 pulse at AT (mL O2/beat) | −9.581 | 0.088 | 0.044 | 1.271 | 0.34 | 2.8 | ||
| VE/VCO2 at AT | 50.91 | −0.169 | 1.851 | −4.784 | 0.18 | 8.21 | ||
| VE/VO2 at AT | 48.04 | −0.134 | 2.086 | −5.164 | 0.18 | 9.01 |
Final equations are: CPET variable = constant + [Coefficient1*Covariate1] + [Coefficient2*Covariate2]… Example: For a 170 cm and 70 kg male, fenestration present and l-looped ventricle: predicted peak VO2 (L/min) ± one SD = −1.71 + [0.016*170 cm] + [0.008*70 kg] + [0.243*1] = 1.813 L/min ± 0.29. Predicted VE/VCO2 at AT ± one SD = 50.91 + [−0.169*70 kg] + [1.851*2] + [−4.784*1) = 38 ± 9. For categorical variables: gender (0 = male, 1 = male); fenestration (0 = no, 1 = indeterminate, 2 = yes); Ventricular looping (0 = d-loop, 1 = l-loop)
All constant and coefficients of covariates were significant (p < 0.05), blank fields represent covariates not in final equation CPET cardiopulmonary exercise testing, AT anaerobic threshold
Comparisons between the validation and derivation (n = 30) cohort are shown in Table 6. The cohorts were similar in possible covariates as well as peak CPET variables except that the validation cohort was younger at time of Fontan (2.7 ± 1 0.2 vs. 3.7 ± 2.3, p = 0.04). For all three peak variable equations the R2 difference was <0.05 and showed no bias toward over or underestimated peak variable. Predicted peak VO2 correlated well with observed peak VO2 in the derivation cohort, R2 = 0.67, SEM = 0.26, p < 0.01, with a R2 difference of −0.06, and the mean difference between predicted and observed did not differ from zero (p = 0.59), 0.02 L/min ± 0.25. Predicted maximal work showed good correlation with observed maximum work, R2 = 0.61, SEM = 21.2, p < 0.01 when comparing the predictive equation to observed work. The mean difference between observed and predicted peak work was 4.3 ± 21.1 W and did not differ from zero (p = 0.27) and R2 difference was 0. There was significant correlation between predicted peak O2 pulse and observed O2 pulse in the derivation cohort (R2 = 0.79, p < 0.01), observed 02 pulse did not differ from zero (−0.07 ± 1.62, p = 0.81), and the R2 difference was −0.2. Therefore, all three equations for maximal CPET variables were validated (Fig. 1).
Table 6. Comparisons between derivation and validation cohorts.
| Peak CPET | Anaerobic threshold | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | Derivation cohort (n = 136) | Validation cohort (n = 30) | p value | Derivation cohort (n = 246) | Validation cohort (n = 69) | p value |
| Patient characteristics | ||||||
| Height (cm) | 155.3 ± 13.1 | 151.1 ± 15.5 | 0.13 | 149.7 ± 14.5 | 151.3 ± 15.7 | 0.64 |
| Weight (kg) | 49.3 ± 15.9 | 44.2 ± 13.9 | 0.11 | 45.0 ± 15.8 | 44.6 ± 16.6 | 0.6 |
| Age at exercise (years) | 13.9 ± 2.9 | 13.9 ± 2.9 | 0.92 | 12.9 ± 3.0 | 13.1 ± 3.3 | 0.24 |
| Age at Fontan (years) | 3.7 ± 2.3 | 2.7 ± 1.2 | 0.04 | 3.5 ± 2.2 | 3.3 ± 2.0 | 0.44 |
| Male, n (%) | 78 (57) | 16 (53) | 0.69 | 141 (57) | 49 (71) | 0.04 |
| d-looped ventricles, n (%) | 104 (77) | 20 (67) | 0.23 | 190 (77) | 59 (86) | 0.14 |
| Right dominant ventricle, n (%) | 44 (32) | 8 (17) | 0.09 | 81 (33) | 18 (26) | 0.28 |
| Sinus rhythm at rest, n (%) | 98 (72) | 18 (60) | 0.19 | 164 (67) | 52 (75) | 0.17 |
| Fenestration present, n (%) | 14 (10) | 1 (3) | 0.22 | 22 (9) | 7 (10) | 0.78 |
| CPET variables | ||||||
| Peak VO2 (L/min) | 1.31 ± 0.46 | 1.17 ± 0.44 | 0.13 | |||
| Maximum work (W) | 99.6 ± 38 | 93.5 ± 34 | 0.42 | |||
| Maximum O2 pulse (mL 02/min) | 8.35 ± 2.98 | 7.52 ± 2.63 | 0.16 | |||
| VO2 at AT (L/min) | 0.82 ± 0.50 | 0.83 ± 0.34 | 0.83 | |||
| Work at AT | 48.9 ± 24.0 | 55.0 ± 26.7 | 0.64 | |||
| 02 pulse at AT | 6.23 ± 3.4 | 6.56 ± 3.7 | 0.63 | |||
| VE/VCO2 at AT | 42.8 ± 3.9 | 44.5 ± 12.3 | 0.03 | |||
| VE/VO2 at AT | 41.5 ± 9.6 | 43.0 ± 11.3 | 0.19 | |||
Fig 1.
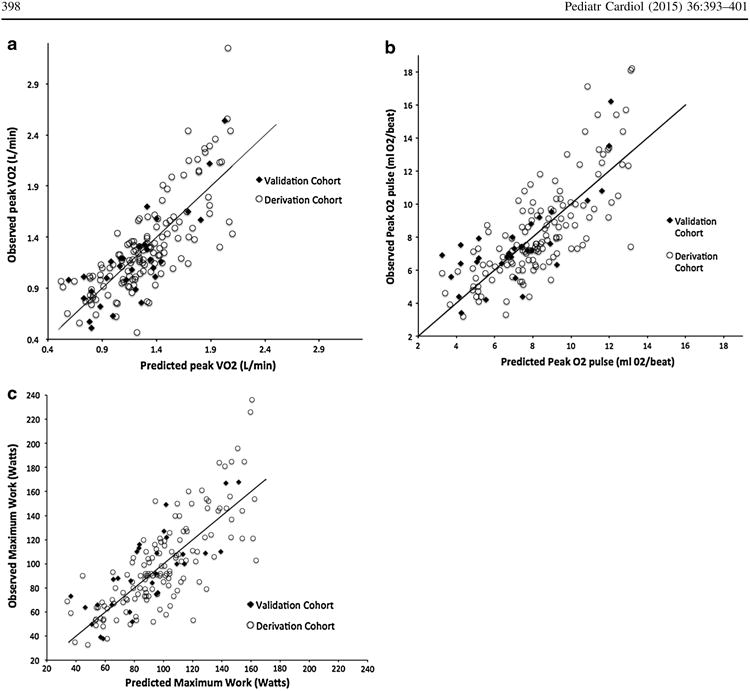
Graphs depicting correlation between predicted CPET variables and observed values of both cohorts. The validation cohort is noted by closed diamonds, the derivation cohort by open circles. Reference line shows 1:1 correlation
For the group with adequately calculate AT, 246 (78 %) were randomly selected for the derivation cohort. Univariate statistics between possible covariates and AT CPET variables are shown in Table 7. The initial models and steps to reach final models are outlined in Table 4. Of note, model explanation of variation of AT variables was lower than peak CPET variables, with R2 ranging from 0.13 to 0.39. The final equations are shown in Table 5.
Table 7. Univariate statistics for derivation cohort of AT CPET variables (n = 246).
| Covariate | AT VO2 (L/min) | AT work (W) | AT 02 pulse (mL O2/beat) | AT VE/VCO2 | AT VE/VO2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||
| At Fontan | r = 0.04 | p = 0.58 | r = 0.32 | p = 0.61 | r = 0.06 | p = 0.35 | r = -0.02 | p = 0.74 | r = -0.06 | p = 0.36 |
| At exercise | r = 0.17 | p < 0.01 | r = 0.46 | p < 0.01 | r = 0.41 | p < 0.01 | r = -0.27 | p < 0.01 | r = -0.21 | p < 0.01 |
| Height | r = 0.32 | p < 0.01 | r = 0.57 | p < 0.01 | r = 0.55 | p < 0.01 | r = -0.32 | p < 0.01 | r = -0.23 | p < 0.01 |
| Weight | r = 0.31 | p < 0.01 | r = 0.52 | p < 0.01 | r = 0.52 | p < 0.01 | r = -0.33 | p < 0.01 | r = -0.25 | p < 0.01 |
| Gender | ||||||||||
| Male | 0.92 ± 0.61 | p < 0.01 | 53.4 ± 25 | p < 0.01 | 6.76 ± 3.8 | p < 0.01 | 42.8 ± 10 | p = 0.98 | 41.6 ± 10 | p = 0.94 |
| Female | 0.70 ± 0.23 | 42.9 ± 21 | 5.51 ± 2.7 | 42.8 ± 9 | 41.5 ± 8 | |||||
| Rhythm at rest | ||||||||||
| Sinus | 0.85 ± 0.57 | p = 0.33 | 49.3 ± 24 | p = 0.73 | 6.24 ± 3.0 | p = 0.95 | 42.8 ± 9 | p = 0.92 | 41.8 ± 10 | p 0.54 |
| Non-sinus | 0.78 ± 0.32 | 48.2 ± 24 | 6.20 ± 4.1 | 42.8 ± 9 | 41.0 ± 8.9 | |||||
| Fenestration | ||||||||||
| Present | 0.91 ± 1.3 | 36.6 ± 22 | 5.13 ± 1.7 | 47.3 ± 10 | 46.1 ± 11 | |||||
| Indeterminate | 0.73 ± 0.2 | p = 0.38 | 43.1 ± 21 | p = 0.08 | 5.21 ± 4.0 | p = 0.04 | 43.8 ± 7 | p = 0.03 | 42.9 ± 10 | p = 0.03 |
| Not present | 0.83 ± 0.34 | 51.3 ± 24 | 6.53 ± 3.4 | 42.1 ± 9 | 40.7 ± 9 | |||||
| Dominant ventricle | ||||||||||
| Left and mixed | 0.80 ± 0.32 | p = 0.35 | 50.3 ± 23 | p = 0.18 | 6.47 ± 3.3 | p = 0.11 | 42.2 ± 9 | p = 0.13 | 40.6 ± 9 | p = 0.04 |
| Right | 0.87 ± 0.74 | 46.0 ± 26 | 5.73 ± 3.7 | 44.1 ± 10 | 43.3 ± 11 | |||||
| Ventricular looping | ||||||||||
| d-loop | 0.81 ± 0.53 | p = 0.61 | 46.7 ± 22 | p = 0.02 | 6.01 ± 3.5 | p = 0.07 | 44.0 ± 9 | p < 0.01 | 42.8 ± 10 | p < 0.01 |
| l-loop | 0.85 ± 0.37 | 55.7 ± 28 | 6.97 ± 3.1 | 38.7 ± 6 | 37.2 ± 7 | |||||
The validation and derivation cohort were similar in patient characteristics except that the derivation cohort were more likely to be male (71 vs. 54 %, p = 0.04) and had slightly higher VE/VCO2 at AT (44.5 vs. 42.8, p = 0.03). Linear regression comparing calculated VO2 at AT versus observed values showed similar model performance as the derivation cohort, R2 = 0.18, SEM = 0.43, p < 0.01. The mean difference between observed and peak values did not differ from zero (−0.23 ± 0.43, p = 0.35). R2 difference was −0.02. Similarly, calculated predicted work and 02 pulse at AT correlated with observed variables in similar fashion in the validation cohort as the derivation cohort, and the mean difference between calculated and observed values did not differ from zero; (Watts; R2 = 0.42, SEM = 18.6, p < 0.01, mean difference 0.9 ± 18.8, p = 0.40, R2 difference = −0.03) (02 pulse, R2 = 0.38, SEM = 2.7, p < 0.01, mean difference −0.04 ± 2.6, p = 0.90, R2 difference = −0.04). However, the correlation between predicted VE/VCO2 and VE/VO2 at AT and observed values was lower in the validation cohort; VE/VCO2, R2 = 0.10, p = 0.01, R2 difference = 0.08; and VE/VO2, R2 = 0.04, p = 0.09, R2 difference = 0.09. Therefore, the equations for VO2 at AT, Work at AT and O2 pulse at AT were validated; however, VE/VCO2 and VE/VO2 at AT were not validated.
Discussion
To the authors' knowledge, this study represents the first development and validation of predictive equations for CPET variables specific for patients with Fontan physiology. The data used to derive the equations are from a multicenter database with a heterogeneous group of Fontan patients. Therefore, the equations that showed good performance in the validation cohort are applicable to routine clinical practice. These equations will help the congenital cardiologist interpret the results of CPET testing in Fontan patients by benchmarking the CPET results to other Fontan patients while taking into account relevant patient characteristics, such as height, weight, and gender. The equations can be easily added to existing CPET software, and therefore, the clinician can quickly compare a Fontan patient's performance to normal children (using previous published equations) as well as other Fontan patients.
The equations can be used in clinical practice to help identify patients who may benefit from therapeutic interventions. Low skeletal mass has been associated with poorer worse exercise performance in Fontan patients and exercise training programs have been associated with improved exercise capacity [2, 7, 8]. However, referring all Fontan patients for exercise training programs is unfeasible in clinical practice. Using the Fontan CPET equations, pediatric cardiologists could easily identify those Fontan patients who have significantly reduced exercise capacity, and make selective referral for exercise training. Secondly, phosphodiesterase 5 inhibitors have been associated with improved exercise capacity in Fontan patients with the best benefit seen in patients with the lowest exercise capacity [11, 15]. Therefore, by comparing CPET results to other patients with similar circulation and adjusting for height, weight, and gender, clinicians can use the equations to easily identify patients who would theoretically most likely benefit from phosphodiesterase 5 inhibitors.
The predictive equations predict lower VO2 at peak and AT, as well as work and 02 pulse when compared to standard pediatric formulas derived from normal children [6]. This is consistent with previous reports, and not surprising given that the Fontan patient have decreased skeletal muscle, lower lean body mass, and no subpulmonary ventricle to help augment systemic ventricular stroke volume during exercise [3, 8, 14, 19]. The equations developed incorporate both height and weight in predicting peak VO2, while other commonly used pediatric equations only use height or weight [5, 6, 17]. In the equations to predict peak VO2, O2 pulse, and Watts, height is given more influence on predicted peak VO2 than body weight. Two commonly used pediatric predictive equations rely solely on height to calculate predicted peak VO2, supporting the correlation between height and peak CPET variables [5, 17]. However, our equation does incorporate weight as well. Since weight does influence lean body mass, and lean body mass is associated with maximal exercise capacity, the incorporation of body mass is physiologically appropriate [4, 16].
Of the eight equations, only VE/VCO2 and VE/VO2 at AT did not show similar performance in validation cohort as the derivation cohort and, therefore, may not be applicable. However, in both VE/VCO2 and VE/VO2 at AT equations, fenestration was a significant covariate. Persistent fenestration was not associated with peak exercise variables. This is consistent with previous reports that showed a decrease in VE/VCO2 slope after fenestration closure and, therefore, removal of a significant right to left shunt, but no change in peak exercise variables [25]. Patients with elevated VE/VCO2 at AT may have a residual right to left shunt. Therefore, the equations give a benchmark which the clinician can help determine when VE/ VCO2 at AT is elevated in the Fontan population.
The peak exercise predictive equations had a higher R2 than the equations for AT variables. This is likely from multiple etiologies. First, the peak variable cohort was smaller and only consisted of patients who participated and reached maximal exercise (RER > 1.1 at maximal exercise). Fontan patients who were able to perform maximal exercise testing are more likely to be healthier and, therefore, may have less variability than patients who only reach AT. However, when we performed linear regression on just participants who performed maximal exercise tests, resulting models showed similar R2 values. Given that it was a multicenter database, there is possible practice variation in determining AT that would lead to variability that could not be accounted for in the multivariable model.
The equations that showed similar performance in the validation cohort, consistently only required the following covariates: gender, height, weight, and fenestration. While this makes the equations more practical to implement, it does leave out covariates that have been previously associated with CPET variables. Specifically, age and age at Fontan completion were not used in the final equations despite being associated with peak VO2 in previous reports [10, 22]. Unlike Giardini et al. and Fernandes et al., who reported an association between age and decreasing peak VO2, the current database was from a cross-sectional study, while the previous studies were longitudinal studies. Secondly, Giardini's study included Fontan patients into their third decade of life, where as the database only included patients into their second decade. Therefore, it is possible that equations derived from a longitudinal database that included patients into their third decade of life would include the covariate of age. A previous study by Madan et al. [22] showed that age at time of Fontan is independently associated with CPET results. However, that study utilized percent of predicted VO2 as the primary outcome, while our study investigated absolute peak VO2. The difference in primary outcome between studies is the likely etiology for the differing results in regard to age at Fontan surgery.
Limitations
The dataset only included Fontan patients into their second decade of life, therefore, the developed equations may not be applicable to older patients. The equations developed for VE/VCO2 and VE/VO2 at AT did not show similar R2 in the validation cohort and, therefore, may not be applicable to wide population. There is significant variation not explained in the AT equations. Lastly, due to a lack of collected information or in order to derive equations that are clinically relevant, patient specific factors that have been associated with exercise performance were not included [27]. The analysis of peak variables is based upon the 166 patients who were able to perform maximal exercise tests. Therefore, more than half of the patients enrolled in the study were not included in this analysis. The equations are derived from patients with ages that ranged from 7 to 18 years, weight range of 23–97 kg and height range of 126–183 cm. Therefore, the equations may not be applicable to Fontan patients who fall within these ranges.
Conclusion
Using multivariable analysis, equations to predict CPET variables specific for Fontan population were derived. Six of the eight equations showed similar performance in a validation cohort. Only, VE/VCO2, and VE/VO2 equations may not be applicable. These equations can assist the pediatric cardiologist in interpreting CPET results for patients with Fontan circulation. These equations should further be refined as this cohort of patients continues to age through the current PHN Fontan 3 longitudinal study.
Footnotes
Disclosures The NIH/NHLBI Pediatric Heart Network Fontan Cross-Sectional Study dataset was used in preparation of this work. Accessed from https://www.pediatricheartnetwork.org. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Contributor Information
Ryan J. Butts, Email: butts@musc.edu, Division of Cardiology, Department of Pediatrics, Medical University of South Carolina, 165 Ashley Avenue, MSC 915, Charleston, SC 29425, USA.
Carolyn T. Spencer, Division of Cardiology, Department of Pediatrics, Medical University of South Carolina, 165 Ashley Avenue, MSC 915, Charleston, SC 29425, USA
Lanier Jackson, Division of Cardiology, Department of Pediatrics, Medical University of South Carolina, 165 Ashley Avenue, MSC 915, Charleston, SC 29425, USA.
Martha E. Heal, Division of Cardiology, Department of Pediatrics, Medical University of South Carolina, 165 Ashley Avenue, MSC 915, Charleston, SC 29425, USA
Geoffrey Forbus, Division of Cardiology, Department of Pediatrics, Medical University of South Carolina, 165 Ashley Avenue, MSC 915, Charleston, SC 29425, USA.
Thomas C. Hulsey, Division of Epidemiology, Department of Pediatrics, Medical University of South Carolina, 165 Ashley Avenue, Charleston, SC 29485, USA
Andrew M. Atz, Division of Cardiology, Department of Pediatrics, Medical University of South Carolina, 165 Ashley Avenue, MSC 915, Charleston, SC 29425, USA
References
- 1.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ., 3rd Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avitabile CM, Leonard MB, Zemel BS, Brodsky JL, Lee D, Dodds K, Hayden-Rush C, Whitehead KK, Goldmuntz E, Paridon SM, Rychik J, Goldberg DJ. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart. 2014 doi: 10.1136/heartjnl-2014-305723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal M, Fiutem JJ, Hill JA, O'Riordan MA, Zahka KG. Oxygen pulse kinetics in Fontan patients during treadmill ramp protocol cardiopulmonary exercise testing. Pediatr Cardiol. 2012;33:1301–1306. doi: 10.1007/s00246-012-0308-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen JK, Chen TW, Chen CH, Huang MH. Oxygen uptake for cycling in relation to body composition: a pilot study. The Kaohsiung J Med Sci. 2009;25:544–551. doi: 10.1016/S1607-551X(09)70547-9. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–S48. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Growth-related changes in oxygen uptake and heart rate during progressive exercise in children. Pediatr Res. 1984;18:845–851. doi: 10.1203/00006450-198409000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Cordina RL, O'Meagher S, Karmali A, Rae CL, Liess C, Kemp GJ, Puranik R, Singh N, Celermajer DS. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Cordina R, O'Meagher S, Gould H, Rae C, Kemp G, Pasco JA, Celermajer DS, Singh N. Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Heart. 2013;99:1530–1534. doi: 10.1136/heartjnl-2013-304249. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes SM, McElhinney DB, Khairy P, Graham DA, Landzberg MJ, Rhodes J. Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatr Cardiol. 2010;31:175–180. doi: 10.1007/s00246-009-9580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giardini A, Hager A, Pace Napoleone C, Picchio FM. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg. 2008;85:818–821. doi: 10.1016/j.athoracsur.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–1687. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 12.Giardini A, Fenton M, Derrick G, Burch M. Impairment of heart rate recovery after peak exercise predicts poor outcome after pediatric heart transplantation. Circulation. 2013;128:S199–S204. doi: 10.1161/CIRCULATIONAHA.112.000369. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, Wernovsky G, Paridon SM, Rychik J. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123:1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg DJ, Avitabile CM, McBride MG, Paridon SM. Exercise capacity in the Fontan circulation. Cardiol Young. 2013;23:823–829. doi: 10.1017/S1047951113001649. [DOI] [PubMed] [Google Scholar]
- 15.Hager A, Weber R, Muller J, Hess J. Predictors of sildenafil effects on exercise capacity in adolescents and adults with Fontan circulation. Clin Res Cardiol. 2014;103:641–646. doi: 10.1007/s00392-014-0694-2. [DOI] [PubMed] [Google Scholar]
- 16.Hume R. Prediction of lean body mass from height and weight. J Clin Pathol. 1966;19:389–391. doi: 10.1136/jcp.19.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James FW, Kaplan S, Glueck CJ, Tsay JY, Knight MJ, Sarwar CJ. Responses of normal children and young adults to controlled bicycle exercise. Circulation. 1980;61(5):902–912. doi: 10.1161/01.cir.61.5.902. [DOI] [PubMed] [Google Scholar]
- 18.Kato TS, Collado E, Khawaja T, Kawano Y, Kim M, Farr M, Mancini DM, Schulze PC. Value of peak exercise oxygen consumption combined with B-type natriuretic peptide levels for optimal timing of cardiac transplantation. Circ Heart Fail. 2013;6:6–14. doi: 10.1161/CIRCHEARTFAILURE.112.968123. [DOI] [PubMed] [Google Scholar]
- 19.Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, Diller GP. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life-single centre experience and review of published data. Eur Heart J. 2012;33:1386–1396. doi: 10.1093/eurheartj/ehr461. [DOI] [PubMed] [Google Scholar]
- 20.Kouatli AA, Garcia JA, Zellers TM, Weinstein EM, Mahony L. Enalapril does not enhance exercise capacity in patients after Fontan procedure. Circulation. 1997;96:1507–1512. doi: 10.1161/01.cir.96.5.1507. [DOI] [PubMed] [Google Scholar]
- 21.Loomba RS, Danduran ME, Dixon JE, Rao RP. Effect of Fontan fenestration on regional venous oxygen saturation during exercise: further insights into Fontan fenestration closure. Pediatr Cardiol. 2014;35:514–520. doi: 10.1007/s00246-013-0817-y. [DOI] [PubMed] [Google Scholar]
- 22.Madan P, Stout KK, Fitzpatrick AL. Age at Fontan procedure impacts exercise performance in adolescents: results from the Pediatric Heart Network Multicenter study. Am Heart J. 2013;166(365–372):e361. doi: 10.1016/j.ahj.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 24.Mays WA, Border WL, Knecht SK, Gerdes YM, Pfriem H, Claytor RP, Knilans TK, Hirsch R, Mone SM, Beekman RH., 3rd Exercise capacity improves after transcatheter closure of the Fontan fenestration in children. Congenit Heart Dis. 2008;3:254–261. doi: 10.1111/j.1747-0803.2008.00199.x. [DOI] [PubMed] [Google Scholar]
- 25.Meadows J, Lang P, Marx G, Rhodes J. Fontan fenestration closure has no acute effect on exercise capacity but improves ventilatory response to exercise. J Am Coll Cardiol. 2008;52:108–113. doi: 10.1016/j.jacc.2007.12.063. [DOI] [PubMed] [Google Scholar]
- 26.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 27.Prakash A, Travison TG, Fogel MA, Hurwitz LM, Powell AJ, Printz BF, Puchalski MD, Shirali GS, Yoo SJ, Geva T Pediatric Heart Network I. Relation of size of secondary ventricles to exercise performance in children after Fontan operation. Am J Cardiol. 2010;106:1652–1656. doi: 10.1016/j.amjcard.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes J, Ubeda-Tikkanen A, Clair M, Fernandes SM, Graham DA, Milliren CE, Daly KP, Mullen MP, Landzberg MJ. Effect of inhaled iloprost on the exercise function of Fontan patients: a demonstration of concept. Int J Cardiol. 2013;168:2435–2440. doi: 10.1016/j.ijcard.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


