Abstract
Lynch syndrome (LS) is an autosomal dominant inherited disorder caused by germline mutations in DNA mismatch repair (MMR) genes. Mutation carriers are at substantially increased risk of developing cancers of the colorectum and endometrium, among others. Given recent recommendations for universal, cost-effective screening of all patients with newly diagnosed colorectal cancer using MMR protein immunohistochemistry, we evaluated MMR protein expression in a series of endometrial cancers in the general population. A total of 605 consecutive cases of primary endometrial cancer at a single institution (1997 to 2013) were evaluated regardless of age, family history, or histologic features. Evaluation methods consisted of immunohistochemistry for theMMR proteins MLH1,MSH2, MSH6, and PMS2, followed by DNA methylation analysis for cases with MLH1/PMS2 deficiency. Germline mutation testing was performed on a subset of cases. Forty MMR-deficient, nonmethylated endometrial cancers were identified: 3 MLH1/PMS2 and 37 MSH6/MSH2 protein deficiencies. Only 25% occurred in women below 50 years of age (range, 39 to 88 y), 1 of which was in a risk-reducing hysterectomy specimen. Only 15% of patients had a prior history of carcinoma, including only 2 patients with prior colorectal carcinoma. Most (80%) of the endometrial cancers were purely endometrioid; there were 2 mixed endometrioid/mucinous, 1 mucinous, 1 serous, 2 clear cell, and 2 carcinosarcoma cases. When grading was applicable, 40% of the endometrial malignancies were FIGO grade 1, 34% grade 2, and 26% grade 3. Thirteen percent arose in the lower uterine segment, and 23% had tumor-infiltrating lymphocytes. Of the tumors with known germline testing, 41% with a LS-associated germline mutation were not associated with any of the traditional indicators that have been recommended for LS screening (ie, age 50 y or younger, personal/family cancer pedigree that meets Bethesda guideline criteria, presence of MMR-associated tumor morphology, or location in the lower uterine segment). These data suggest that a significant number of LS-associated endometrial carcinomas are missed using clinical, histologic, and locational screening parameters and provide support for universal screening of all newly diagnosed endometrial cancers.
Keywords: Lynch syndrome, mismatch-repair deficiency, endometrial adenocarcinoma, microsatellite instability
Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer syndrome, is caused by germline defects in DNA mismatch repair (MMR) genes and is characterized by a predilection for oncogenesis at a variety of sites including the colon, rectum, small bowel, endometrium, ovary, stomach, pancreas, renal pelvis and ureter, and brain.1-3 Although first identified in patients with familial lower gastrointestinal cancers, the incidence of gynecologic cancers equals or exceeds that of colorectal cancers in LS patients. Endometrial cancer is particularly common in these patients and represents the harbinger or index malignancy in over half of patients.4 Germline defects in DNA MMR have been reported in 1.8% to 2.1% of unselected endometrial carcinoma patients5,6 and in up to 9% of endometrial carcinoma patients under age 50.7,8
The strong association between endometrial cancer and LS has led to increased screening of endometrial carcinoma specimens for abnormalities in the DNA MMR system.7,9-12 LS screening can use a variety of techniques to directly and indirectly identify mutations in MMR genes. The most conclusive method is germline mutation analysis, although germline testing misses a subset of LS. Germline testing is focused on the 4 primary MMR genes implicated in LS: MSH2, MSH6, MLH1, and PMS2. In a small subset of patients, mutations in the gene EPCAM can also lead to an LS phenotype by causing hypermethylation and inactivation of the MSH2 promoter.13,14
Immunohistochemical (IHC) staining to identify the loss of MSH2, MSH6, MLH1, and PMS2 protein expression serves as a more cost-effective screening approach and has been shown to be sensitive and, in the absence of sporadic MLH1 promoter methylation, specific for underlying germline defects.15-19 Microsatellite instability testing also serves as a surrogate for abnormalities in the MMR system, but this test has been shown to be less sensitive than IHC, in large part due to failure to detect many MSH6 germline mutation carriers. 19 In addition, microsatellite instability fails to identify the putative causative gene/protein deficiency. Importantly, loss of MLH1/PMS2 expression and high microsatellite instability are not necessarily due to germline mutations, as they can occur in tumors with sporadic methylation of the MLH1 promoter. Approximately 10% to 20% of endometrial carcinomas show loss of MLH1/PMS2 expression, but this loss is attributable to germline mutations in only a small subset.20,21 IHC, microsatellite instability, and germline mutation analysis can be used in varying combinations to screen for and solidify a diagnosis of LS.
Regardless of the testing approach utilized, it is incumbent upon gynecologic oncologists and pathologists to appropriately screen endometrial cancer patients for defects in the DNA MMR system. At present, screening is predominantly based on patient age (below 50 y), family history, and/or patient history of prior or concurrent malignancies22-24; however, it is clear that clinical screening criteria have imperfect efficacy in identifying MMR-deficient cases.25 Family history–based screening is inadequate in part because there are inconsistencies in patient and clinician reporting of family cancer history. 26,27 Some screening recommendations give importance to histologic features such as tumor-infiltrating lymphocytes and high-grade histology28,29 or anatomic location,30 although these have not been standardized. A similar approach was initially used for colon cancer patients; however, subsequent work has shown that limiting screening on the basis of these criteria misses a substantial number of LS patients.16,31 For instance, restricting MMR testing to colorectal carcinoma patients under age 50 fails to identify 56% of LS patients.31 On the basis of these findings, many institutions, including our own, now routinely test all colorectal carcinomas for loss of MMR protein expression on IHC irrespective of patient age, patient history, and tumor histology. It has been well established that endometrial cancer often precedes colorectal and other LS-associated malignancies in women, and it follows that age-based screening is also likely to be inappropriate in this patient population. MSH6 mutations, in particular, have been implicated in endometrial cancers arising in LS patients above 50 years of age21,32 and are known to be missed using current clinical screening criteria.33 Buchanan et al34 recently demonstrated that restricting MMR testing to women under 50 years of age misses a significant proportion of MMR mutation–positive cases and proposed expanding testing recommendations to endometrial cancers arising in women below 60 years of age. Here, we evaluate a large number of unselected endometrial carcinomas for loss of DNA MMR protein expression and, on the basis of the results herein, propose universal screening of endometrial carcinomas for LS.
MATERIALS AND METHODS
The internal files of the Stanford University Department of Pathology were searched for endometrial cancers diagnosed between 1997 and 2013. A total of 605 cases of primary endometrial cancer were identified and were evaluated for LS regardless of age, family history, or histologic features (IRB-11663). All cases were assessed using IHC for the MMR proteins MLH1, MSH2, MSH6, and PMS2. Primary monoclonal antibodies against MLH1 (clone G168-728; BD PharMingen, San Diego, CA; 1:200), MSH2 (clone FE11; Oncogene Research Products, Cambridge, MA; 1:100), MSH6 (clone 44; BD Transduction, San Jose, CA; 1:200), and PMS2 (clone MRQ-28; Cell Marque, Rocklin, CA; 1:10) were applied to 4-mm-thick formalin-fixed, paraffin-embedded sections. Antigen retrieval was performed using Leica’s proprietary antigen retrieval solution at pH 6.0 (MLH1, PMS2, and MSH2) or pH 9.0 (MSH6). Normal expression was defined as nuclear staining within tumor cells, using nuclei of infiltrating lymphocytes, and/or normal stromal cells as positive internal control. Negative protein expression was defined as complete absence of nuclear staining within tumor cells with concurrent positive labeling of the internal control.
Mutational analysis for BRAF V600E was performed on an early, initial subset of 30 cases but discontinued due to absence of detectable BRAF mutation in the tumors tested. For these cases, DNA was extracted from paraffinembedded tissue at the Stanford Molecular Laboratory. In brief, sections cut from paraffin blocks underwent paraffin removal using Histoclear (National Diagnostics, Atlanta, GA). This was followed by ethanol washing, complete drying, and submersion in Protein K buffer solution. The tissue specimens were incubated overnight, and digestion was then stopped by immersion in boiling water. Agarose gel electrophoresis was performed to assess the DNA quality and concentration. Real-time polymerase chain reaction (PCR) (Methylight) for DNA methylation was performed on all cases with MLH1/PMS2 deficiency in the retrospective cohort. The prospective cohort was assessed for promoter hypermethylation by Mayo Medical Laboratories (Rochester, MN) and (for a subset of cases) GoPath Laboratories (Chicago, IL).
Cases with loss of MMR protein expression by IHC and no evidence of methylation by PCR were reviewed for histologic features including histologic type, grade, and the presence of tumor-infiltrating lymphocytes. Tumor-infiltrating lymphocytes were considered increased when they equaled or exceeded 40/10 high-powered fields. Tumor location was assigned on the basis of the gross description recorded in the pathology report as well as histologic assessment for lower uterine segment. Germline data and history of prior or subsequent carcinoma were obtained, when available. Cases with MLH1 promoter methylation were reviewed as part of a separate study.
Microsatellite instability testing, when performed, was conducted by Stanford Clinical Laboratories using a commercially available primer set (Promega MSI Analysis System; Promega BioSciences, San Luis Obispo, CA) that includes 5 mononucleotide (BAT25, BAT26, NR21, NR24, and MONO27) and 2 pentanucleotide (Penta C and Penta D) repeats on both tumor and normal tissue for each patient. A semiquantitative expression plot for tumor tissue was obtained and compared with that for normal tissue for each patient in accordance with the NCI guidelines.35
RESULTS
Forty MMR-deficient, nonmethylated endometrial cancers were identified in women with an age range of 39 to 88 years. The vast majority (37/40) of the cases showed loss of MSH6 and/or MSH2 (Fig. 1). Isolated loss of MSH6 was seen in 12 (Fig. 2). Two of the remaining cases had dual loss of MLH1/PMS2 without evidence of DNA methylation on real-time PCR analysis, whereas 1 showed isolated loss of PMS2. In 2 cases, MSH6 expression was equivocal. Loss of MLH1/PMS2 was initially identified in 97 cases, which showed methylation of the MLH1 gene promoter; although some of the patients with these tumors may have LS not detected by the current protocol, these cancers were considered to be sporadic for the purposes of this study and were excluded from further analysis. Only 25% (10/40) of MMR protein–deficient endometrial cancers occurred in women under 50 years of age; one of these cases was identified in a risk-reducing hysterectomy specimen from a woman with a prior diagnosis of LS. A history of prior malignancy was identified in a minority of patients (15%, 6/40): 2 patients had a history of colorectal carcinoma, 1 had a history of transitional cell carcinoma of the renal pelvis/ureters, 2 had a history of ductal carcinoma of the breast (1: in situ, 1: invasive), and 1 had a history of cervical squamous cell carcinoma. One patient had a simultaneous ovarian carcinoma. One patient developed lobular carcinoma of the breast within 1 month of diagnosis, whereas another developed sebaceous skin carcinoma within 2 months of diagnosis. Colorectal adenomas, including 1 with intramucosal carcinoma, were identified in 1 patient who was enrolled in a cancer surveillance program following diagnosis of LS-associated endometrial cancer (Fig. 3). Lower uterine segment tumors accounted for only 5 cases (13%). High-risk histologies comprised a small proportion of tumors, with only 1 serous carcinoma, 2 clear cell carcinomas, and 2 carcinosarcomas identified. The majority (80%, 32/40) of tumors showed pure conventional endometrioid histology. Mucinous histology was seen in 3 cases; 2 mucinous tumors had admixed endometrioid components, whereas 1 showed pure mucinous morphology. Histologic grading of endometrioid and mucinous tumors showed a similar distribution to MMR-intact carcinomas and consisted of 40% (14/35) FIGO grade 1, 34% (12/35) grade 2, and 26% (9/35) grade 3 cases. Tumor-infiltrating lymphocytes were appreciated in 23% (9/40) of cases. In the tumors studied for microsatellite instability, 85% were microsatellite instability high, whereas 15% were stable.
FIGURE 1.
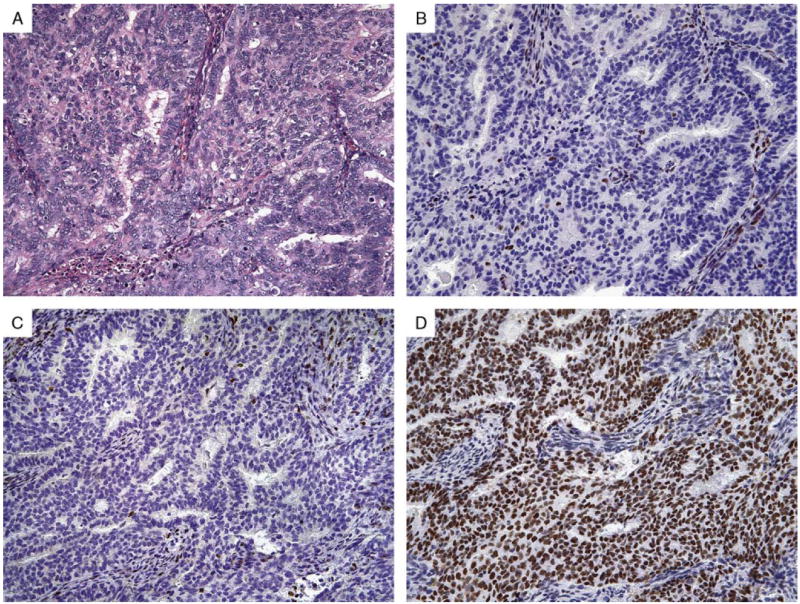
Conventional endometrioid adenocarcinoma (A) in a 61-year-old woman with dual loss of MSH2 (B) and MSH6 (C) but intact MLH1 (not shown) and PMS2 (D). Subsequent genetic testing confirmed a germline mutation in MSH2.
FIGURE 2.
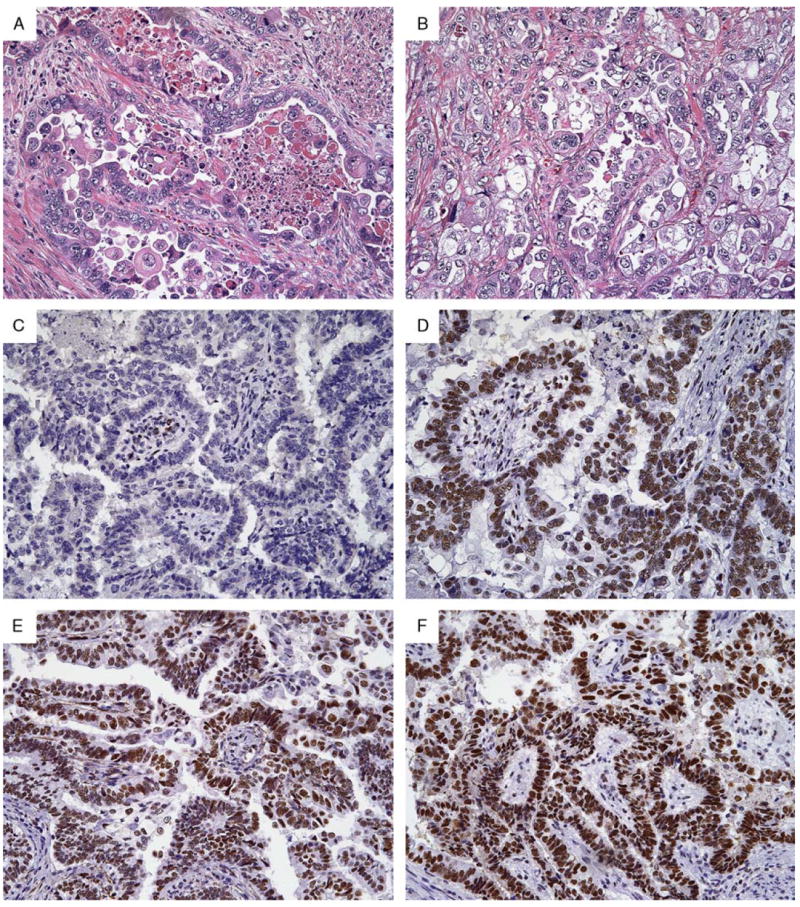
High-grade endometrial adenocarcinoma (A and B) in a 77-year-old woman showing isolated loss of MSH6 (C) but intact MSH2 (D), MLH1 (E), and PMS2 (F). This tumor was associated with a germline mutation in MSH6.
FIGURE 3.
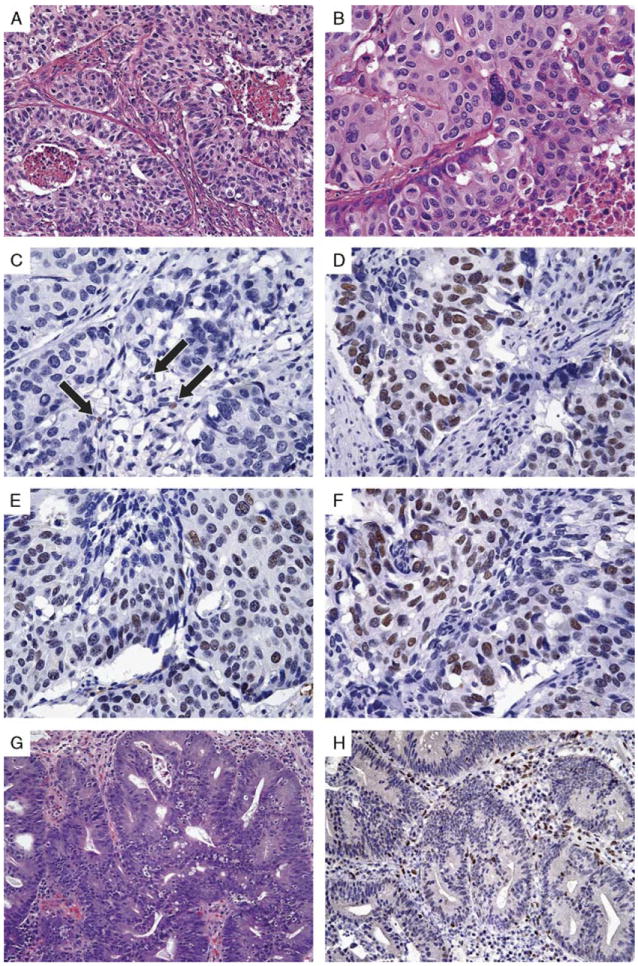
Endometrial adenocarcinoma (A and B) in a 58-year-old woman with isolated loss of MSH6 (C) but intact MSH2 (D), MLH1 (E), and PMS2 (F). Subsequent genetic testing confirmed a germline mutation in MSH6. Intramucosal carcinoma in a subsequent surveillance polypectomy specimen (G) also demonstrated loss of MSH6 protein (H). Note intact MSH6 expression in admixed lymphocytes (highlighted by arrows in C).
Germline testing confirmed LS in 17/21 patients. Most had germline mutations in MSH2 (n = 12), followed by MSH6 (n = 3) and MLH1 (n = 2). One tumor with loss of MLH1/PMS2 was associated with an MLH1 germline mutation variant of uncertain significance, whereas another tumor with loss of MSH6 was associated with an MSH6 germline mutation variant of uncertain significance. No germline mutation was identified in association with 2 tumors with loss of MSH2/MSH6 expression and 1 tumor with loss of PMS2. One of the tumors with equivocal MSH6 protein expression was not associated with a known germline mutation in MSH6. Of the 17 tumors with known germline mutations, 7 (41%) were not associated with any of the traditional screening parameters that have been recommended for LS (ie, age 50 y or younger, personal/family cancer pedigree that meets Bethesda guideline criteria, presence of MMR-associated tumor morphology, or location in the lower uterine segment).
DISCUSSION
Our findings demonstrate that a directed screening approach informed by Bethesda guidelines and recently suggested histopathologic parameters misses a substantial proportion of patients with MMR protein–deficient endometrial cancer and many potential LS patients. Although clinical confirmation and/or germline testing was not performed on all patients in this study, prior work has shown that loss of MMR protein expression is a reasonably reliable proxy for identifying potential LS patients. In addition (and based predominantly on colorectal cancer studies), although MLH1 promoter hypermethylation may rarely occur in LS, the presence of MLH1 hypermethylation in tumors with loss of MLH1 and PMS2 expression can be used as a surrogate marker for sporadic carcinoma.36
A proposal to extend LS testing to all patients has obvious monetary implications. One approach is to initially screen using microsatellite instability testing. However, using microsatellite instability as the first line of screening has several disadvantages. First, it cannot identify which genes are likely to be mutated, therefore it must either be followed by IHC for the protein products of MLH1, PMS2, MSH2, and MSH6 to direct gene sequencing, or by direct germline sequencing of all 4 commonly implicated genes, a costly option. Alternatively, methylation testing may be performed as a follow-up to microsatellite instability; however, if methylation is negative it still remains unclear which gene is involved, and IHC and/or germline sequencing remains necessary. Microsatellite instability testing is also poor at identifying possible MSH6 germline mutation carriers, which account for a higher proportion of LS endometrial cancer patients when compared with colorectal cancer patients.21
A more effective approach that ameliorates cost and streamlines the screening approach is to begin with IHC staining for MMR proteins as the initial screen, followed by directed germline mutation testing in cases with MMR protein loss or strong clinical suspicion despite apparently intact protein expression (Fig. 4). It has been demonstrated that IHC is a highly sensitive technique to identify mutations in MMR genes in colorectal cancer, therefore one would predict that an IHC-based screening approach would not miss significant numbers of LS patients.37 Cost can be further reduced by limiting initial screening to MSH6 and PMS2 proteins.38,39 Because MSH6 and PMS2 are obligate binding partners of MSH2 and MLH1, respectively, they are not expressed at the protein level in the absence of their partner proteins. Thus, loss of MSH6 expression can detect both defects in MSH6 and MSH2, whereas PMS2 loss is a stand-in for both PMS2 and MLH1 defects. Loss of both MSH2 and MSH6 protein expression with preservation of both these genes on germline testing may indicate an underlying EPCAM deletion, therefore IHC for EPCAM and/or germline testing of EPCAM should be considered in this setting.
FIGURE 4.
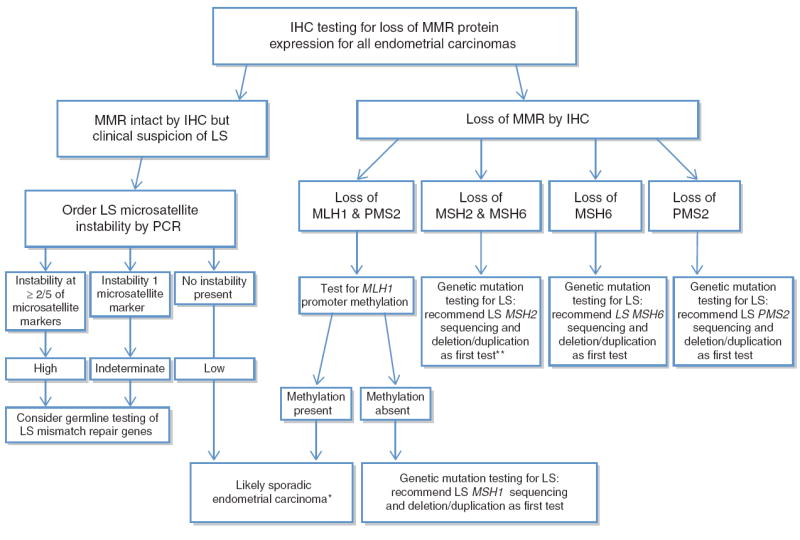
Algorithm for testing of endometrial adenocarcinomas for MMR deficiency. A 2-antibody approach utilizing MSH6 and PMS2 as preliminary screening with subsequent MSH2 or MLH1 IHC for cases that show loss of MSH6 or PMS2 can also be performed.
*If strong clinical suspicion for LS, consider MLH1 promoter methylation analysis of non-neoplastic tissue/peripheral blood to evaluate for germline epigenetic MLH1 promoter methylation.
**If MSH2 and MSH6 unmutated, consider LS EPCAM, sequencing and deletion/duplication.
As loss of MLH1 and PMS2 protein expression can also be seen with sporadic methylation of the MLH1 gene, PCR for MLH1 hypermethylation must be performed on all cases with apparent MLH1 defects. Unlike colorectal cancer, BRAF mutations do not generally occur in association with sporadic methylation of MLH1 in endometrial cancer.40 In evaluating MLH1 hypermethylation, it must be borne in mind that rare cases of LS are attributed to germline mutations in promoter methylation. If MLH1 promoter methylation is identified, but the patient’s family or personal history is strongly compelling for a heritable process, both tumor and normal tissue should be tested to exclude germline defects in promoter methylation. Germline testing can also be considered in cases for which there is strong clinical suspicion of LS despite preserved expression for all 4 proteins on IHC.
Although broader LS screening may increase the immediate financial burden on the health care system, in the long term the prevention and early detection of cancers in these patients should decrease net health care costs and transition health care delivery to a more optimal approach of screening and prevention. The fiscal arguments against universal screening become even less tenable in the era of personalized medicine, when individualized testing is increasingly requested and recognized as the standard of care by many patients and treating physicians. LS is the most common heritable cancer syndrome in the United States and Europe, and increased screening will likely disclose a higher prevalence than has been suspected. The emergence of affordable whole-genome sequencing may further impact the approach to hereditable disorders.
Identification of LS patients is critical for a variety of reasons. First, they are at substantial increased risk for synchronous and metachronous malignancies when compared with the general population. Intensive cancer surveillance should therefore be used for these patients, and risk-reducing surgeries (eg, hysterectomy, colectomy and oophorectomy) should be considered.41,42 If established at the time of initial biopsy, a diagnosis of LS can also influence the extent of resection; for instance, removal of a larger portion of bowel could be considered in colon cancer patients. Recent work has shown that IHC for MMR proteins is a reliable LS screen in small colorectal biopsy specimens, although staining heterogeneity can rarely lead to false-negative cases.43 Similar studies have not been performed in endometrial biopsies but it has been our experience that some cases of low-grade endometrial carcinoma may be difficult to interpret in small biopsy specimens because of the presence of admixed atypical hyperplasia. Although testing for MMR defects has been suggested in these precursor lesions, the vast majority of precursor lesions in the endometrium and in the colon (with the exception of polyps that harbor a focus of high-grade dysplasia) may retain MMR protein expression even in the presence of a germline mutation.
In addition to influencing health care decisions for individual cancer patients, a diagnosis of LS affects screening strategies for related family members. LS demonstrates autosomal dominant inheritance; therefore the identification of one patient can precipitate the unveiling of many affected relatives.5 Involvement of genetic counselors is critical for advising both individual cancer patients and family members about the implications of testing.
A secondary function of MMR testing in colorectal cancers is the identification of sporadic carcinomas with MLH1 promoter methylation. Although not associated with familial cancer syndromes, this defect nonetheless leads to microsatellite instability indistinguishable from what is seen with germline MMR gene mutations. In the colon, microsatellite instability is associated with improved overall prognosis but decreased response to fluorouracil. 44 The prognostic implications of sporadic MLH1 methylation have not been well characterized, but as we learn more about the behavior of sporadic endometrial cancer with MLH1 methylation, it is possible that this information may have independent value for guiding clinical treatment.
In summary, at least 41% with LS-associated germline mutations are not associated with any of the traditional indicators that have been recommended for LS screening in women with endometrial adenocarcinoma (ie, age 50 y or younger, personal/family cancer pedigree that meets Bethesda guideline criteria, presence of MMRassociated tumor morphology, or location in the lower uterine segment). These data suggest that a significant number of LS-associated endometrial carcinomas are missed using these clinical, histologic, and locational screening parameters and provide support for universal screening of all newly diagnosed endometrial cancers. The clinical utility of LS testing depends on the MMR gene mutated. Although it has been suggested that the benefit is lower for those diagnosed at older ages and with lesspenetrant MSH6 mutations, data are limited.45 In our opinion, a large, prospective study on unselected endometrial cancer patients that includes a rigorous healtheconomic analysis is needed before premature adoption of more limited screening recommendations.46
Footnotes
Preliminary results from this work were presented at the 100th Annual Meeting of the United States & Canadian Academy of Pathology, San Antonio, TX 2011.
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 2.Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 3.Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu KH, Broaddus RR. Gynecologic cancers in Lynch syndrome/HNPCC. Fam Cancer. 2005;4:249–254. doi: 10.1007/s10689-005-1838-3. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 6.Ollikainen M, Abdel-Rahman WM, Moisio AL, et al. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J Clin Oncol. 2005;23:4609–4616. doi: 10.1200/JCO.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 7.Berends MJ, Wu Y, Sijmons RH, et al. Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol. 2003;21:4364–4370. doi: 10.1200/JCO.2003.04.094. [DOI] [PubMed] [Google Scholar]
- 8.Lu KH, Schorge JO, Rodabaugh KJ, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 9.Clarke BA, Cooper K. Identifying Lynch syndrome in patients with endometrial carcinoma: shortcomings of morphologic and clinical schemas. Adv Anat Pathol. 2012;19:231–238. doi: 10.1097/PAP.0b013e31825c6b76. [DOI] [PubMed] [Google Scholar]
- 10.Resnick K, Straughn JM, Jr, Backes F, et al. Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstet Gynecol. 2009;114:530–536. doi: 10.1097/AOG.0b013e3181b11ecc. [DOI] [PubMed] [Google Scholar]
- 11.Moline J, Mahdi H, Yang B, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecol Oncol. 2013;130:121–126. doi: 10.1016/j.ygyno.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Heald B, Plesec T, Liu X, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J Clin Oncol. 2013;31:1336–1340. doi: 10.1200/JCO.2012.45.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempers MJ, Kuiper RP, Ockeloen CW, et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol. 2011;12:49–55. doi: 10.1016/S1470-2045(10)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligtenberg MJ, Kuiper RP, Geurts van Kessel A, et al. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam Cancer. 2013;12:169–174. doi: 10.1007/s10689-012-9591-x. [DOI] [PubMed] [Google Scholar]
- 15.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampel H. Point: justification for Lynch syndrome screening among all patients with newly diagnosed colorectal cancer. J Natl Compr Canc Netw. 2010;8:597–601. doi: 10.6004/jnccn.2010.0044. [DOI] [PubMed] [Google Scholar]
- 17.Kalloger SE, Allo G, Mulligan AM, et al. Use of mismatch repair immunohistochemistry and microsatellite instability testing: exploring Canadian practices. Am J Surg Pathol. 2012;36:560–569. doi: 10.1097/PAS.0b013e31823f3b28. [DOI] [PubMed] [Google Scholar]
- 18.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backes FJ, Leon ME, Ivanov I, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009;114:486–490. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 22.Lancaster JM, Powell CB, Kauff ND, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007;107:159–162. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh CS, Blum A, Walts A, et al. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol Oncol. 2010;116:516–521. doi: 10.1016/j.ygyno.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan P, Mulligan AM, Aronson M, et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer. 2012;118:681–688. doi: 10.1002/cncr.26323. [DOI] [PubMed] [Google Scholar]
- 26.Lanceley A, Eagle Z, Ogden G, et al. Family history and women with ovarian cancer: is it asked and does it matter?: an observational study. Int J Gynecol Cancer. 2012;22:254–259. doi: 10.1097/IGC.0b013e3182392714. [DOI] [PubMed] [Google Scholar]
- 27.Tan YY, McGaughran J, Ferguson K, et al. Improving identification of lynch syndrome patients: a comparison of research data with clinical records. Int J Cancer. 2013;132:2876–2883. doi: 10.1002/ijc.27978. [DOI] [PubMed] [Google Scholar]
- 28.Carcangiu ML, Radice P, Casalini P, et al. Lynch syndrome—related endometrial carcinomas show a high frequency of non-endometrioid types and of high FIGO grade endometrioid types. Int J Surg Pathol. 2010;18:21–26. doi: 10.1177/1066896909332117. [DOI] [PubMed] [Google Scholar]
- 29.Garg K, Leitao MM, Jr, Kauff ND, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol. 2009;33:925–933. doi: 10.1097/PAS.0b013e318197a046. [DOI] [PubMed] [Google Scholar]
- 30.Westin SN, Lacour RA, Urbauer DL, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol. 2008;26:5965–5971. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de la Chapelle A, Palomaki G, Hampel H. Identifying Lynch syndrome. Int J Cancer. 2009;125:1492–1493. doi: 10.1002/ijc.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner A, Hendriks Y, Meijers-Heijboer EJ, et al. Atypical HNPCC owing to MSH6 germline mutations: analysis of a large Dutch pedigree. J Med Genet. 2001;38:318–322. doi: 10.1136/jmg.38.5.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjursen W, Haukanes BI, Grindedal EM, et al. Current clinical criteria for Lynch syndrome are not sensitive enough to identify MSH6 mutation carriers. J Med Genet. 2010;47:579–585. doi: 10.1136/jmg.2010.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchanan DD, Tan YY, Walsh MD, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014;32:90–100. doi: 10.1200/JCO.2013.51.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 36.Rahner N, Friedrichs N, Steinke V, et al. Coexisting somatic promoter hypermethylation and pathogenic MLH1 germline mutation in Lynch syndrome. J Pathol. 2008;214:10–16. doi: 10.1002/path.2263. [DOI] [PubMed] [Google Scholar]
- 37.Musulen E, Sanz C, Munoz-Marmol AM, et al. Mismatch repair protein immunohistochemistry: a useful population screening strategy for Lynch syndrome. Hum Pathol. 2014;45:1388–1396. doi: 10.1016/j.humpath.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Mojtahed A, Schrijver I, Ford JM, et al. A two-antibody mismatch repair protein immunohistochemistry screening approach for colorectal carcinomas, skin sebaceous tumors, and gynecologic tract carcinomas. Mod Pathol. 2011;24:1004–1014. doi: 10.1038/modpathol.2011.55. [DOI] [PubMed] [Google Scholar]
- 39.Shia J, Tang LH, Vakiani E, et al. Immunohistochemistry as firstline screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol. 2009;33:1639–1645. doi: 10.1097/PAS.0b013e3181b15aa2. [DOI] [PubMed] [Google Scholar]
- 40.Metcalf AM, Spurdle AB. Endometrial tumour BRAF mutations and MLH1 promoter methylation as predictors of germline mismatch repair gene mutation status: a literature review. Fam Cancer. 2014;13:1–12. doi: 10.1007/s10689-013-9671-6. [DOI] [PubMed] [Google Scholar]
- 41.Kwon JS, Sun CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer. 2008;113:326–335. doi: 10.1002/cncr.23554. [DOI] [PubMed] [Google Scholar]
- 42.Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 43.Shia J, Stadler Z, Weiser MR, et al. Immunohistochemical staining for DNA mismatch repair proteins in intestinal tract carcinoma: how reliable are biopsy samples? Am J Surg Pathol. 2011;35:447–454. doi: 10.1097/PAS.0b013e31820a091d. [DOI] [PubMed] [Google Scholar]
- 44.Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res. 2012;18:1506–1512. doi: 10.1158/1078-0432.CCR-11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart A. Genetic testing strategies in newly diagnosed endometrial cancer patients aimed at reducing morbidity or mortality from lynch syndrome in the index case or her relatives. PLoS Curr. 2013 doi: 10.1371/currents.eogt.b59a6e84f27c536e50db4e46aa26309c. Online article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabban JT, Calkins SM, Karnezis AN, et al. Association of tumor morphology with mismatch-repair protein status in older endometrial cancer patients: implications for universal versus selective screening strategies for Lynch syndrome. Am J Surg Pathol. 2014;38:793–800. doi: 10.1097/PAS.0000000000000177. [DOI] [PubMed] [Google Scholar]


