Conspectus
Many non-protein coding RNAs fold into intricate three-dimensional shapes in order to act in protein synthesis, splicing, and many other facets of gene regulation and expression. Hydroxyl radical footprinting probes the solvent accessibility of the RNA backbone at each residue in as little as 10 ms, providing detailed views of RNA folding pathways in real time. Time-resolved footprinting of ribozymes showed, in conjunction with other methods such as solution scattering and single-molecule FRET, that stable domains of RNA tertiary structure fold in less than 1 s. However, the free energy landscapes for RNA folding are rugged, and individual molecules kinetically partition into folding pathways that lead through metastable intermediates, stalling the folding or assembly process.
Time-resolved footprinting was used to follow the formation of tertiary structure and protein interactions in the 16S rRNA during the assembly of 30S ribosomes. As previously observed in much simpler ribozymes, assembly occurs in stages, with individual molecules taking different routes to the final complex. Interactions occur concurrently in all domains of the 16S rRNA, and multi-stage protection of binding sites of individual proteins suggests initial encounter complexes between the rRNA and ribosomal proteins are remodeled during assembly.
Equilibrium footprinting experiments showed that one primary binding protein was sufficient to stabilize the tertiary structure of the entire 16S 5′ domain. The rich detail available from the footprinting data showed that the secondary assembly protein S16 suppresses non-native structures in the 16S 5′ domain. In doing so, S16 enables a conformational switch distant from its own binding site, that may play a role in establishing interactions with other domains of the 30S subunit. Together, the footprinting results show how protein-induced changes in RNA structure are communicated over long distances, ensuring cooperative assembly of even very large RNA-protein complexes such as the ribosome.
Non-coding RNAs play many roles in gene expression and genome maintenance. Not surprisingly, the folded structures of many non-protein coding transcripts are vitally important for their biological function, often more so than their primary sequence. Long non-coding RNAs fold into diverse and complex tertiary structures that allow them to act as enzymes or metabolic sensors, often in complex with one or more proteins. 1,2,3–5 A confluence of biophysical and biochemical methods are now revealing the details of how ribosomal subunits and other ribonucleoprotein complexes (RNP) assemble in real time from their molecular components. 6,7
In this review, I discuss how we have used hydroxyl radical footprinting and other experimental methods to study pathways of RNA folding and 30S ribosome assembly. The detailed information on RNA folding intermediates provided by footprinting experiments is providing evidence for induced-fit in RNA-protein recognition, and revealing how RNA-binding proteins can communicate RNA structural changes over long distances. In the future, a combination of in vivo footprinting, isotopic labeling, and genetic approaches should reveal how assembly is coordinated with RNA processing, intracellular trafficking, and quality control pathways.
Visualizing RNA folding pathways with hydroxyl radical footprinting
Over the past two decades, many laboratories have studied the folding pathways and dynamics of RNA, using a wide range of experimental methods including NMR, footprinting, fluorescence and absorption spectroscopy, single molecule techniques, small angle X-ray scattering (SAXS) and molecular simulations.8–10 Double helices are known to form rapidly, allowing stable elements of the RNA secondary structure to become established early during the folding process.11 How these double helices pack together to form the final tertiary structure, however, is a more difficult problem, as the tertiary interactions are distributed through the RNA sequence, and intermediates can have diverse structures.
Hydroxyl radical is a particularly useful reagent for monitoring helix packing and protein binding in RNA, because cleavage of the RNA backbone in the presence of hydroxyl radical is proportional to the solvent accessibility of the C4′ and C5′ positions in the ribose (Figure 1).12 Moreover, the strand scission chemistry is relatively insensitive to the base sequence, and can be determined for every residue. Consequently, protection from hydroxyl radical cleavage can be used to map the inside of a folded RNA with single nucleotide precision, and to quantify the extent of protein binding.13,14
Figure 1. Hydroxyl radical footprinting of RNA and protein interactions.
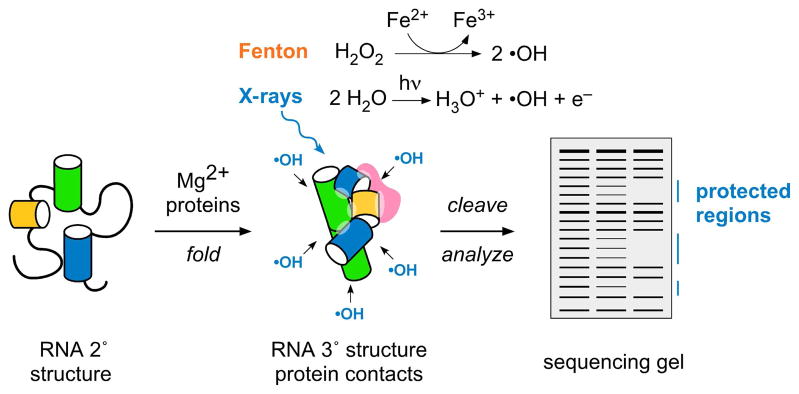
Ribose sugars buried by RNA tertiary interactions or protein contacts are protected from attack by hydroxyl radical, producing a gap or “footprint” in the distribution of cleaved products.12 Radicals are produced by the Fenton reaction or X-ray photolysis of water; strand scission products are analyzed and quantified by primer extension with reverse transcriptase. Base oxidation products are not readily detected by this method.
To follow RNA folding in real time, it is necessary to carry out the hydroxyl radical cleavage reaction in a short time, to obtain “snap shots” of the RNA tertiary interactions as they form. Mark Chance and Michael Brenowitz’s labs developed a synchrotron X-ray beamline for footprinting that in collaboration we used to follow the folding pathway of the Tetrahymena ribozyme.15 In “X-ray footprinting”, hydroxyl radical is generated by irradiating aqueous samples for 5–10 ms, which permits the dynamics of the structures to be examined.16,17 Brenowitz and co-workers subsequently developed a time-resolved footprinting protocol that uses the Fenton reaction (Fe(II)-EDTA) to produce hydroxyl radical, and a rapid quench instrument to mix reagents within a few milliseconds.18 Both methods produce comparable RNA cleavage patterns, although the X-ray beam can also be used to footprint RNA molecules in vivo.19,20
Since footprinting methods probe the average conformation of the molecules in the solution at a given time, a clear footprint is only visible when >10% of molecules form exactly the same molecular contacts. Consequently, this method does not detect compact intermediates that are dynamic in structure, or regions of the structure that are heterogeneously folded within the population. As discussed below, such compact intermediates can be detected by SAXS or by FRET, providing a more complete picture of the folding process.
Folding pathway of the Tetrahymena ribozyme
Hydroxyl radical footprinting of the Tetrahymena group I ribozyme after different folding times showed that tertiary interactions in its stable P4–P6 domain formed at a rate of 1 s−1, while tertiary interactions around the periphery of the RNA structure formed at an intermediate rate, and interactions in the P3–P9 domain, which form the catalytic center of the ribozyme, appeared most slowly, at ~1 min−1 (Figure 2).15 These results agreed with the folding pathway revealed by equilibrium footprinting21 and antisense oligonucleotide probes, 22,23 and showed that large domains of RNA tertiary structure can form in a short time.
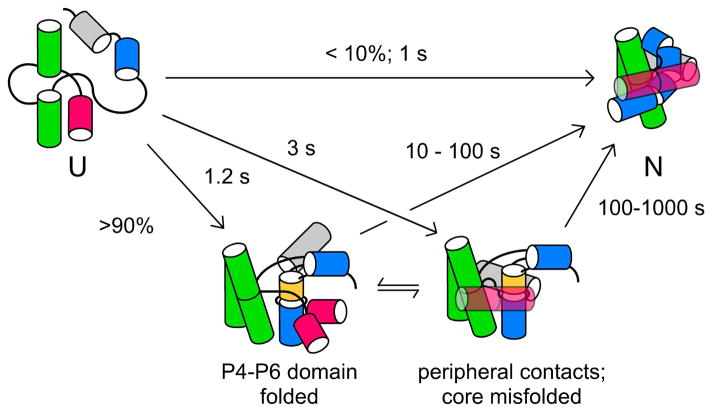
Figure 2. Folding pathway of the Tetrahymena ribozyme.
Time-resolved footprinting30,31 showed that tertiary interactions in the P4–P6 domain (green) form in ~1 s, more rapidly than contacts in peripheral helices (pink and grey) and in the P3–P9 domain (blue), due to mispairing of the P3 helix (gold).24 The ensemble of unfolded structures (U) partitions among parallel pathways, with 5–10% of molecules folding directly to the native state (N).25
As the domains of the Tetrahymena ribozyme become protected in stages, it is tempting to think of the folding process as a linear path towards the native state. However, other experiments showed that delayed protection of the P3–P9 domain is due to mispairing of the P3/P7 pseudoknot,24 trapping the RNA in misfolded structures in which the P3–P9 domain does not pack correctly against its neighbors.25 Folding times from misfolded structures are long because incorrect RNA structures must unfold before they can refold correctly.
Importantly, while ≥ 90% of ribozyme molecules become trapped in misfolded intermediates in vitro, a few molecules avoid this fate and fold correctly with a rate of ~1 s−1 at 37 °C.25–27 Thus, the RNA population partitions among parallel folding pathways, some leading directly to the native structure while others detour through metastable intermediates.28,29 The footprinting results reflect the superposition of these folding pathways, or the probability that a particular region of RNA becomes ordered within a given time window (Figure 2). If the pathways are not too varied, the dominant folding intermediates can be inferred by deconvoluting the changes in backbone accessibility over time.30
The “outside-in” folding pattern of the Tetrahymena ribozyme revealed by the time-resolved footprinting studies31 suggested that the late folding intermediates are compact, as later shown by SAXS experiments.32 The initial collapse transition to compact structures begins within 15 ms after Mg2+ is added to the RNA.33 However, in the Tetrahymena ribozyme34 and nearly all other ribozymes studied so far, the initial collapse transition, which is barely visible by footprinting, is followed by further conformational adjustments that allow the double helices to pack tightly and specifically with one another.10 This collapse transition is driven by electrostatic interactions with cations,10 and sequence-specific tertiary interactions that bring the helical domains together.34–37
Free energy landscapes in RNA folding
In general, the overall folding time correlates directly with the fidelity of helix assembly and the heights of the energy barriers that separate the intermediates from the native state.28,29 For example, the Azoarcus group I ribozyme, which has the same core architecture as the Tetrahymena ribozyme, largely avoids becoming trapped in non-native intermediates above 40 °C and folds in 5–30 ms.38 The heterogeneity of the Azoarcus ribozyme collapse kinetics depends on the concentration of Mg2+ and interactions in the unfolded ensemble.39
The stretched folding kinetics and heterogeneous folding intermediates in RNA can be described by free energy landscape models, in which the folding process is represented as a multi-dimensional search for low free energy structures.29,40,41 Non-native metastable structures in RNA are represented as local free energy minima in free energy landscapes that trap individual RNA molecules as they traverse the free energy surface. Individual molecules in a population can follow different routes across the folding landscape, and the kinetic partitioning of the population among different, parallel folding pathways has been visualized for several different RNAs using single molecule FRET and single molecule force denaturation.26,42,43 The same perspective is useful for understanding the assembly of RNPs, in which protein binding simply becomes an additional variable that alters the energy landscape of the system.7
Multistage assembly of 30S ribosome by time-resolved hydroxyl radical footprinting
As the rRNA structure defines the overall architecture of the ribosome, and its actives sites are comprised almost entirely of RNA,44 it seemed reasonable to expect that rRNA folding is a critical force in assembly of the ribosomal subunits. Few proteins contact each other directly in the mature bacteria 30S subunit.45 Instead, the hierarchical addition of proteins to ribosomal subunits described by the Nomura assembly map 46 arises from structural changes in the rRNA due to protein binding.47 Although each protein stabilizes the folded structure of its immediate binding site, it also stimulates structural changes in adjacent rRNA residues that help recruit other proteins to the complex.
The basis for cooperative assembly was illustrated by biophysical studies showing that protein S15 captures and stabilizes the junction between helices 20, 21 and 22 in the central domain, holding them in the correct orientation.48,49 S15 binding also stabilizes interactions with helix 23 that form the binding site of the S6–S18 dimer,50 which in turn helps organize the binding site for S11. The thermodynamic cooperativity for binding of central domain proteins is associated with a reduction in the unfavorable entropy change associated with protein binding, consistent with increased pre-organization of the rRNA.51 As we shall see below, ribosomal proteins can smooth the folding and assembly landscape by preferentially stabilizing productive folding intermediates.52 Moreover, ribosomal proteins are positively charged and contain intrinsically disordered regions, features associated with RNA chaperones.53,54
Although ribosomal proteins stabilize the folded structure of the rRNA, how do the RNA and protein interactions evolve over time as the RNP complexes come together? We used time-resolved hydroxyl radical footprinting to visualize the folding of the rRNA during 30S assembly in real time.55 E. coli 16S rRNA was first refolded in 20 mM MgCl2, then 30S subunit proteins were added using a rapid quench instrument. Complexes were exposed at various intervals to a synchrotron X-ray beam for 10 ms (Figure 3). This approach captured “snapshots” of the emerging RNA tertiary interactions and RNA-protein interactions from 20 ms to 180 s of assembly.
Figure 3. X-ray footprinting of 30S ribosome assembly.
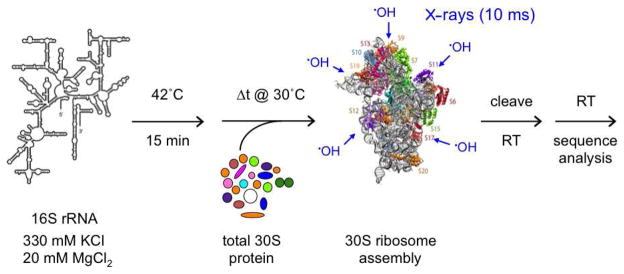
The evolution of RNA and RNA-protein interactions was probed from 20 ms to 120 s, using a 10 ms X-ray pulse.55 Native E. coli 16S rRNA was pre-folded in reconstitution buffer before addition of native E. coli 30S proteins (TP30). Quantitation of primer extension products revealed multi-stage folding of individual residues throughout the 16S rRNA.
As in the Tetrahymena ribozyme, some 16S residues became more than 65% protected within the first 50 ms of the assembly reaction, while other residues required several minutes to fold.55 Not only were residues in different domains of the rRNA protected at different times, but individual residues were protected with more than one rate constant. For many residues, a burst of protection in the first 50 ms was followed by much slower saturation of the backbone interactions. These results suggested not only that assembly involves different intermediate complexes, but also that different rRNA molecules assemble along different paths, as inferred earlier from the binding kinetics of 30S proteins.52
Residues in every domain of the 16S rRNA were protected rapidly, showing that the domains of the 16S rRNA assembly concurrently in vitro.55 Some of the fast folding regions corresponded to helix junctions that are partly stable in the absence of proteins, while others mapped to protein binding sites, including primary assembly proteins S4, S8, S15, and S7. Regions of the 16S rRNA that were protected most slowly included irregularly folded regions of the rRNA, residues that form long-range interactions between structural domains, and residues that form the central pseudoknot and the mRNA decoding site in the mature 30S subunit. These last results agreed with reconstitution experiments showing that the pseudoknot forms in the late stages of 30S assembly.56
Direction of assembly during transcription
In nature, ribosomal subunits are assembled during transcription, and assembly is intimately coupled with processing and maturation of the pre-rRNA.57 At 30 °C, the gradient of assembly flows in a 5′ to 3′ direction, presumably reflecting sequential assembly of the pre-16S rRNA during transcription.52,58,59 Our footprinting experiments suggested that the 5′ to 3′ direction of assembly correlates with the propensity of each 16S domain to misfold. While the 5′ domain of the E. coli 16S rRNA can fold independently of proteins in a few seconds,60 the central and 3′ domains do not fold properly without their protein ligands. The folding time of the 3′ domain became 100 times longer when the 16S rRNA was renatured at 30 °C instead of 42 °C, and was more sensitive to base modification,61 suggesting that assembly can proceed more rapidly if metastable conformations in the rRNA are overcome. The propensity of the 16S 3′ domain to misfold may explain why many of the 3′ domain proteins do not productively join the complex until after a heat activation step 46, and why several 30S assembly factors act on the 3′ domain.
Induced fit in RNA-protein recognition
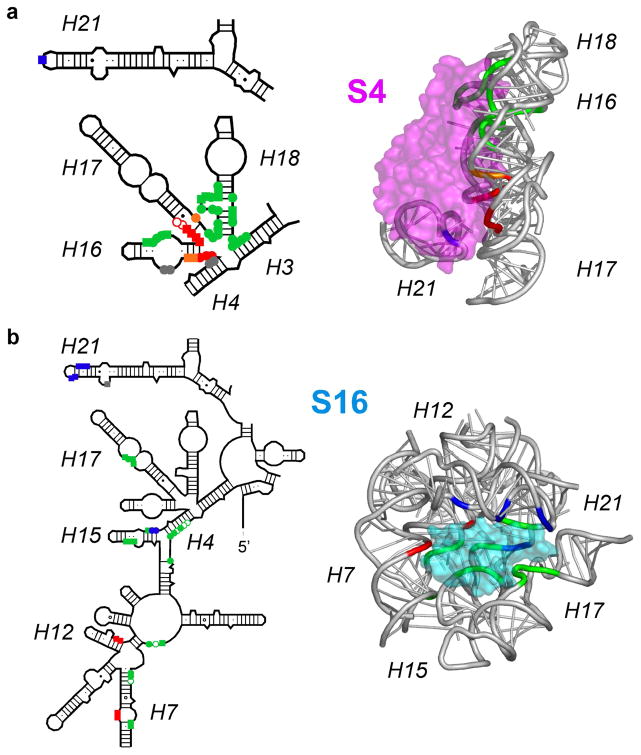
One of the most striking observations from time-resolved footprinting of 30S ribosome assembly was that the interfaces between the rRNA and individual ribosomal proteins form at different rates. For example, residues in helices 16 and 17 that contact the C-terminal domain of protein S4 were protected in 0.1 to 1 s, while residues in helices 16 and 18 that interact with the N-terminal region of S4 were protected more slowly (Figure 4).55 This suggested that initial encounter complexes between the ribosomal proteins and the RNA contain only a subset of the native contacts and must undergo further structural changes. In general, the slowest rates of protection from hydroxyl radical cleavage were most similar to protein binding rates measured by pulse chase mass spectrometry,52 suggesting that it is these slower steps that commit proteins to further steps of 30S assembly.
Figure 4. Induced fit in rRNA-protein interactions.
Different residues in a single 30S protein binding site are protected with different rate constants, when probed by X-ray footprinting. (a) Residues contacted by protein S4 in mature 30S subunits colored according to the rate of protection: red, ≥ 20s−1; orange, 2–20 s−1; green, 0.2 –2 s−1; blue, 0.01 – 0.2 s−1. (b) Residues contacted by protein S16, colored as in (a). Reproduced from Ref. 55 with permission.
Interestingly, the N-terminal region of S4 is disordered in the free protein,62 raising the possibility that changes in protein structure occur after the initial binding event. Although RNA binding proteins may promote folding simply by selecting conformations already present in the ensemble of unliganded structures, non-specific protein binding may induce new conformations or increase the dynamics of the RNA, lowering the energy barriers to refolding.63–65 Most RNA binding proteins contain large numbers of basic amino acid residues, and electrostatic attraction to the RNA may drive the initial formation of non-specific and dynamic encounter complexes, as suggested for the RNA recognition motif (RRM) protein U1A.66,67
Global stabilization of rRNA structure by ribosomal proteins
To understand in detail how proteins stabilize the tertiary structure of the rRNA, we footprinted the structure of the 16S 5′ domain RNA in the presence of one or more proteins. The 16S 5′ domain forms the body of the 30S subunit, and stably binds three primary assembly proteins (S4, S17 and S20), as well as one secondary assembly protein, S16.68–70 S4, S17 and S20 each organize a different helix junction in the 5′ domain (Figure 5), while S16 binds at the interface between the “upper” and “lower” halves of the 5′ domain. Protein S12 also forms part of 30S body, but binds 16S 5′ domain fragments weakly.
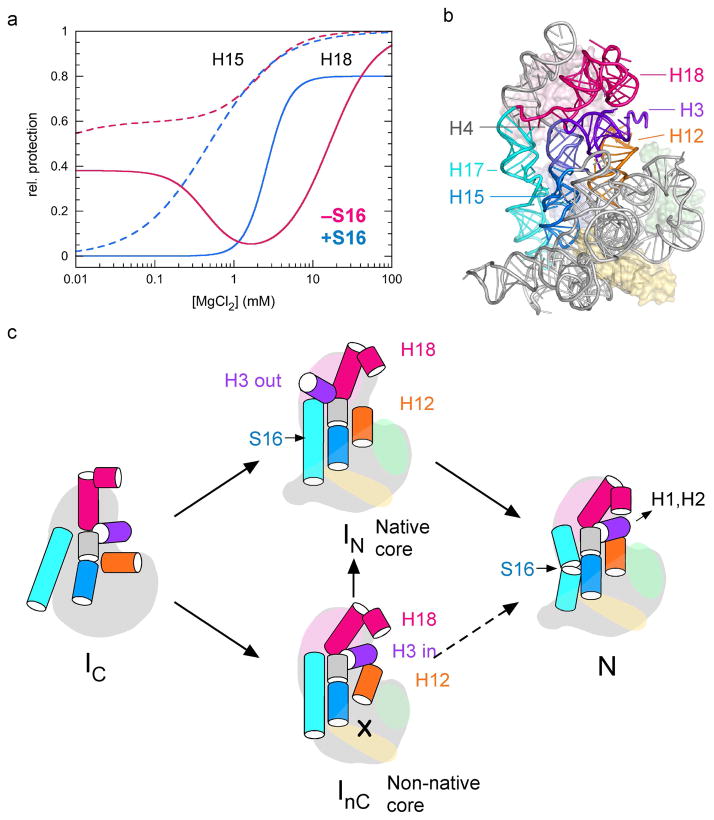
Figure 5. S16-dependent conformational switch.
(a) Proteins that bind the 16S 5′ domain (body) stabilize RNA tertiary interactions, lowering the Mg2+ concentration required for protection from hydroxyl radical cleavage.71,72 Fitted curves for protection of residues in helix 15 (nt 378; dashed lines) and helix 18 (nt 501–502; solid lines) in the presence of S4, S17 and S20, without S16 (pink) or with S16 (blue). (b) Exposure of helix 18 can be explained by movement of helix 3 (purple) during assembly. Ribbon (pdb 2avy)81 shows 16S nt 24–562 with proteins as pastel surfaces; S4 (pink), S17 (green), S20 (yellow), S16 (blue). (c) Minimal model for 5′ domain assembly, involving intermediate RNPs with native (IN) or non-native (InC) configuration in the lower half of the domain near helices 6, 6a, 10, 15. Helix 3 is displaced in IN, then moves back into position in the native complex (N). S16 smooths the path of assembly by favoring IN over InC, and by stabilizing N. Reproduced from Ref. 71 with permission.
The Mg2+ concentration required to refold RNA generally correlates with the stability of its tertiary structure. Therefore, by reconstituting 5′ domain RNP complexes in a range of Mg2+ concentration, we could determine how much the proteins stabilize specific RNA tertiary contacts.71 Although the 16S 5′ domain RNA can fold in the absence of proteins in high MgCl2,60 the ribosomal proteins stabilized the RNA structure as expected, allowing tertiary interactions in the 5′ domain RNA to form in 5 mM MgCl2.71
Surprisingly, S4 or S17 alone stabilized tertiary interactions throughout the 5′ domain, allowing many of the expected RNA tertiary contacts to form in low Mg2+.72 Indirect stabilization of an entire RNA tertiary domain has been observed for other non-coding RNAs, such as group I introns and RNase P, that form a strong network of interactions able to propagate the effects of protein binding to distant regions of the complex.73–75 In these examples, the RNA can usually form compact folding intermediates in the absence of protein. By contrast, RNAs with weaker tertiary interactions, such as signal recognition particle (SRP) 76,77 or telomerase,78 may recruit their protein ligands in a stricter hierarchy, because one protein is needed induce compact structures capable of being recognized by the other proteins.
A conformational switch in the 16S 5′ domain
The detail in the footprinting data revealed how the ribosomal proteins change the structure of the rRNA, altering the free energy landscape for assembly. Although the primary assembly proteins S4, S17 and S20 each stabilized the 16S 5′ domain globally, they selected a different subset of RNA conformations that were revealed as different footprints in the intermediate Mg2+ concentrations.72 When all three proteins were added to the reaction, the RNA became more ordered, even in very low (0.01–0.1 mM) Mg2+ concentrations. However, in ~1 mM MgCl2, some residues were only partially protected, while residues in helix 18 and at the tip of helix 12, which both contact either side of helix 3 in the upper half of the 5′ domain, were transiently exposed in moderate MgCl2 concentrations.71 These results suggested first that assembly intermediates with different structures accumulate simultaneously, and second, that interactions surrounding helix 3 are remodeled during the assembly process.71
Nucleotides with similar folding behavior were clustered to yield a minimal equilibrium model for 5′ domain assembly (Figure 5).71 When S4, S17 and S20 are present, at least two assembly intermediates have similar free energies: InC in which helix 3 interacts with helices 18 and 12 but the lower half of the 5′ domain is in a non-native conformation, and IN in which the lower half of the 5′ domain is folded correctly but helix 3 is displaced from helices 18 and 12. The secondary assembly protein S16 depopulates InC, presumably by preferentially stabilizing the native configuration of the lower domain in IN. As the Mg2+ concentration is raised further, the native RNP forms, containing the pseudoknot in helix 18, and normal tertiary interactions between helix 3 and helices 12 and 18. Thus, protein S16 smoothes the 5′ domain assembly pathway, suppressing the InC complex and stabilizing the final, native RNP.
Remarkably, the S16 binding site straddles the interface between helices 15 and 17, ~30 Å away from helix 3. S16 binding may influence the conformational switch at helix 3 by correctly orienting helix 12 and helix 5, and by stabilizing a tertiary interaction with a C-loop motif in helix 15. The ability of S16 to influence the conformation of helices 3, 12 and 18 over this distance helps explain its important role in 30S assembly, and demonstrates how the architecture of the rRNA transmits ligand interactions over long distances, even in immature complexes.79
Although our footprinting experiments were done on a fragment of the 16S rRNA containing only the 5′ domain, helix 3 is likely to influence interdomain interactions late in 30S assembly. In the mature 30S subunit, helix 3 stacks co-axially with helix 1,45 which in turn participates in the central pseudoknot that is critical for 30S function. The central pseudoknot and residues around the tip of helix 12 form the binding site for protein S12, and become protected from modification late in assembly, after the heat-dependent RI to RI* step of 30S reconstitution.56 As helix 1 can exchange with an alternative helix in the pre-16S rRNA,80 these folding steps may be linked to processing of the 5′ end of the pre-16S rRNA.
Conclusions
Experimental methods and theoretical models for studying RNA folding pathways are being adapted to reveal how large cellular complexes are assembled from their molecular components. Given the many non-coding RNAs that function as cellular enzymes or that participate in regulatory pathways, methods for interrogating RNA conformation will remain in demand. Hydroxyl radical footprinting probes the solvent accessibility of individual residues in RNA in a few milliseconds, allowing RNP assembly reactions to be followed in real time. The detailed information from footprinting studies complements results from fluorescence spectroscopy, microscopy, solution scattering and mass spectrometry.
Consistent with studies on smaller ribozymes, time resolved footprinting of the 16S rRNA showed that 30S subunit assembly is multiphase, suggesting that individual complexes take different routes to the final structure. Each domain of the 16S rRNA assembles concurrently, with many stable RNA and RNA-protein interactions forming within the first 50–100 ms. Individual proteins protect their binding sites with more than one rate constant, suggesting that induced fit of RNA-protein contacts contributes to the hierarchy of 30S assembly. Finally, the connectivity in the rRNA structure allows bound proteins to influence the structure of the rRNA over long distances, driving specific conformational changes that help create the mRNA decoding site of the 30S subunit. In the future, in vivo footprinting methods promise further insight into how the folding and assembly of RNA-protein complexes coordinate with each phase of RNP biogenesis.
Acknowledgments
The authors thanks the many people in her laboratory who contributed to this work over the years, and collaborations past and present with M. Brenowitz, R. Briber, M. Chance, and D. Thirumalai, without whom this work would not have been possible. This work was funded by the NIGMS.
References
- 1.Serganov A. Determination of riboswitch structures: light at the end of the tunnel? RNA Biol. 2010;7:98–103. doi: 10.4161/rna.7.1.10756. [DOI] [PubMed] [Google Scholar]
- 2.Pyle AM. The tertiary structure of group II introns: implications for biological function and evolution. Crit Rev Biochem Mol Biol. 2010;45:215–32. doi: 10.3109/10409231003796523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan V. Ribosome structure and the mechanism of translation. Cell. 2002;108:557–72. doi: 10.1016/s0092-8674(02)00619-0. [DOI] [PubMed] [Google Scholar]
- 4.Fedor MJ. Comparative enzymology and structural biology of RNA self-cleavage. Annu Rev Biophys. 2009;38:271–99. doi: 10.1146/annurev.biophys.050708.133710. [DOI] [PubMed] [Google Scholar]
- 5.Newman AJ, Nagai K. Structural studies of the spliceosome: blind men and an elephant. Curr Opin Struct Biol. 2010;20:82–9. doi: 10.1016/j.sbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Woodson SA. RNA folding and ribosome assembly. Curr Opin Chem Biol. 2008;12:667–73. doi: 10.1016/j.cbpa.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson JR. Biophysical studies of bacterial ribosome assembly. Curr Opin Struct Biol. 2008;18:299–304. doi: 10.1016/j.sbi.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokinsky G, Zhuang X. Single-molecule RNA folding. Acc Chem Res. 2005;38:566–73. doi: 10.1021/ar040142o. [DOI] [PubMed] [Google Scholar]
- 9.Sosnick TR. Kinetic barriers and the role of topology in protein and RNA folding. Protein Sci. 2008;17:1308–18. doi: 10.1110/ps.036319.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodson SA. Compact intermediates in RNA folding. Annu Rev Biophys. 2010;39:61–77. doi: 10.1146/annurev.biophys.093008.131334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crothers DM. RNA conformational dynamics. In: Söll D, Nishimura S, Moore P, editors. RNA. Elsevier; Oxford, UK: 2001. pp. 61–70. [Google Scholar]
- 12.Tullius TD, Greenbaum JA. Mapping nucleic acid structure by hydroxyl radical cleavage. Curr Opin Chem Biol. 2005;9:127–34. doi: 10.1016/j.cbpa.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Latham JA, Cech TR. Defining the inside and outside of a catalytic RNA molecule. Science. 1989;245:276–282. doi: 10.1126/science.2501870. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh M, Brenowitz M. Quantitative kinetics footprinting of protein-DNA association reactions. Methods Enzymol. 1996;274:478–92. doi: 10.1016/s0076-6879(96)74038-7. [DOI] [PubMed] [Google Scholar]
- 15.Sclavi B, Sullivan M, Chance MR, Brenowitz M, Woodson SA. RNA folding at millisecond intervals by synchrotron hydroxyl radical footprinting. Science. 1998;279:1940–1943. doi: 10.1126/science.279.5358.1940. [DOI] [PubMed] [Google Scholar]
- 16.Brenowitz M, Chance MR, Dhavan G, Takamoto K. Probing the structural dynamics of nucleic acids by quantitative time-resolved and equilibrium hydroxyl radical “footprinting”. Curr Opin Struct Biol. 2002;12:648–53. doi: 10.1016/s0959-440x(02)00366-4. [DOI] [PubMed] [Google Scholar]
- 17.Maleknia SD, Ralston CY, Brenowitz MD, Downard KM, Chance MR. Determination of macromolecular folding and structure by synchrotron x-ray radiolysis techniques. Anal Biochem. 2001;289:103–15. doi: 10.1006/abio.2000.4910. [DOI] [PubMed] [Google Scholar]
- 18.Shcherbakova I, Mitra S, Beer RH, Brenowitz M. Following molecular transitions with single residue spatial and millisecond time resolution. Methods Cell Biol. 2008;84:589–615. doi: 10.1016/S0091-679X(07)84019-2. [DOI] [PubMed] [Google Scholar]
- 19.Adilakshmi T, Soper SF, Woodson SA. Structural analysis of RNA in living cells by in vivo synchrotron X-ray footprinting. Methods Enzymol. 2009;468:239–58. doi: 10.1016/S0076-6879(09)68012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes JJ, Kam L, Tullius TD. Footprinting protein-DNA complexes with gamma-rays. Methods Enzymol. 1990;186:545–9. doi: 10.1016/0076-6879(90)86148-o. [DOI] [PubMed] [Google Scholar]
- 21.Celander DW, Cech TR. Visualizing the higher order folding of a catalytic RNA molecule. Science. 1991;251:401–407. doi: 10.1126/science.1989074. [DOI] [PubMed] [Google Scholar]
- 22.Zarrinkar PP, Williamson JR. Kinetic intermediates in RNA folding. Science. 1994;265:918–924. doi: 10.1126/science.8052848. [DOI] [PubMed] [Google Scholar]
- 23.Zarrinkar PP, Williamson JR. The kinetic folding pathway of the Tetrahymena ribozyme reveals possible similarities between RNA and protein folding. Nat Struct Biol. 1996;3:432–438. doi: 10.1038/nsb0596-432. [DOI] [PubMed] [Google Scholar]
- 24.Pan J, Woodson SA. Folding intermediates of a self-splicing RNA: mispairing of the catalytic core. J Mol Biol. 1998;280:597–609. doi: 10.1006/jmbi.1998.1901. [DOI] [PubMed] [Google Scholar]
- 25.Pan J, Thirumalai D, Woodson SA. Folding of RNA involves parallel pathways. J Mol Biol. 1997;273:7–13. doi: 10.1006/jmbi.1997.1311. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang X, Bartley LE, Babcock HP, Russell R, Ha T, Herschlag D, Chu S. A single-molecule study of RNA catalysis and folding. Science. 2000;288:2048–51. doi: 10.1126/science.288.5473.2048. [DOI] [PubMed] [Google Scholar]
- 27.Pan J, Deras ML, Woodson SA. Fast folding of a ribozyme by stabilizing core interactions: evidence for multiple folding pathways in RNA. J Mol Biol. 2000;296:133–144. doi: 10.1006/jmbi.1999.3439. [DOI] [PubMed] [Google Scholar]
- 28.Thirumalai D, Lee N, Woodson SA, Klimov D. Early events in RNA folding. Annu Rev Phys Chem. 2001;52:751–62. doi: 10.1146/annurev.physchem.52.1.751. [DOI] [PubMed] [Google Scholar]
- 29.Thirumalai D, Woodson SA. Kinetics of folding of protein and RNA. Acc Chem Res. 1996;29:433–439. [Google Scholar]
- 30.Laederach A, Shcherbakova I, Liang MP, Brenowitz M, Altman RB. Local kinetic measures of macromolecular structure reveal partitioning among multiple parallel pathways from the earliest steps in the folding of a large RNA molecule. J Mol Biol. 2006;358:1179–90. doi: 10.1016/j.jmb.2006.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sclavi B, Sullivan M, Chance MR, Brenowitz M, Woodson SA. RNA folding at millisecond intervals by synchrotron hydroxyl radical footprinting. Science. 1998;279:1940–3. doi: 10.1126/science.279.5358.1940. [DOI] [PubMed] [Google Scholar]
- 32.Russell R, Millett IS, Doniach S, Herschlag D. Small angle X-ray scattering reveals a compact intermediate in RNA folding. Nat Struct Biol. 2000;7:367–370. doi: 10.1038/75132. [DOI] [PubMed] [Google Scholar]
- 33.Russell R, Millett IS, Tate MW, Kwok LW, Nakatani B, Gruner SM, Mochrie SG, Pande V, Doniach S, Herschlag D, Pollack L. Rapid compaction during RNA folding. Proc Natl Acad Sci US A. 2002;99:4266–71. doi: 10.1073/pnas.072589599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das R, Kwok LW, Millett IS, Bai Y, Mills TT, Jacob J, Maskel GS, Seifert S, Mochrie SG, Thiyagarajan P, Doniach S, Pollack L, Herschlag D. The fastest global events in RNA folding: electrostatic relaxation and tertiary collapse of the Tetrahymena ribozyme. J Mol Biol. 2003;332:311–9. doi: 10.1016/s0022-2836(03)00854-4. [DOI] [PubMed] [Google Scholar]
- 35.Buchmueller KL, Weeks KM. Near native structure in an RNA collapsed state. Biochemistry. 2003;42:13869–78. doi: 10.1021/bi035476k. [DOI] [PubMed] [Google Scholar]
- 36.Chauhan S, Caliskan G, Briber RM, Perez-Salas U, Rangan P, Thirumalai D, Woodson SA. RNA tertiary interactions mediate native collapse of a bacterial group I ribozyme. J Mol Biol. 2005;353:1199–209. doi: 10.1016/j.jmb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Kwok LW, Shcherbakova I, Lamb JS, Park HY, Andresen K, Smith H, Brenowitz M, Pollack L. Concordant Exploration of the Kinetics of RNA Folding from Global and Local Perspectives. J Mol Biol. 2006;355:282–293. doi: 10.1016/j.jmb.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 38.Rangan P, Woodson SA. Structural requirement for Mg2+ binding in the group I intron core. J Mol Biol. 2003;329:229–38. doi: 10.1016/s0022-2836(03)00430-3. [DOI] [PubMed] [Google Scholar]
- 39.Roh JH, Guo L, Kilburn JD, Briber RM, Irving T, Woodson SA. Multistage collapse of a bacterial ribozyme observed by time-resolved small-angle X-ray scattering. J Am Chem Soc. 2010;132:10148–54. doi: 10.1021/ja103867p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onuchic JN, Luthey-Schulten Z, Wolynes PG. Theory of protein folding: the energy landscape perspective. Annu Rev Phys Chem. 1997;48:545–600. doi: 10.1146/annurev.physchem.48.1.545. [DOI] [PubMed] [Google Scholar]
- 41.Chen SJ, Dill KA. RNA folding energy landscapes. Proc Nat Acad Sci US A. 2000;97:646–651. doi: 10.1073/pnas.97.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang X, Kim H, Pereira MJ, Babcock HP, Walter NG, Chu S. Correlating structural dynamics and function in single ribozyme molecules. Science. 2002;296:1473–6. doi: 10.1126/science.1069013. [DOI] [PubMed] [Google Scholar]
- 43.Li PT, Bustamante C, Tinoco I., Jr Real-time control of the energy landscape by force directs the folding of RNA molecules. Proc Natl Acad Sci USA. 2007;104:7039–44. doi: 10.1073/pnas.0702137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noller HF. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- 45.Wimberly BT, Brodersen DE, Clemons WM, Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 46.Nomura M, Held WA. Reconstitution of ribosomes: Studies of ribosome structure, function, and assembly. In: Nomura M, Tissieres A, Lengyel P, editors. Ribosomes. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1974. pp. 193–223. [Google Scholar]
- 47.Stern S, Powers T, Changchien LM, Noller HF. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989;244:783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- 48.Batey RT, Williamson JR. Interaction of the Bacillus stearothermophilus ribosomal protein S15 with 16 S rRNA: I. Defining the minimal RNA site. J Mol Biol. 1996;261:536–49. doi: 10.1006/jmbi.1996.0481. [DOI] [PubMed] [Google Scholar]
- 49.Ha T, Zhuang X, Kim HD, Orr JW, Williamson JR, Chu S. Ligand-induced conformational changes observed in single RNA molecules. Proc Natl Acad Sci USA. 1999;96:9077–82. doi: 10.1073/pnas.96.16.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agalarov SC, Sridhar Prasad G, Funke PM, Stout CD, Williamson JR. Structure of the S15,S6,S18-rRNA complex: assembly of the 30S ribosome central domain. Science. 2000;288:107–13. doi: 10.1126/science.288.5463.107. [DOI] [PubMed] [Google Scholar]
- 51.Recht MI, Williamson JR. RNA tertiary structure and cooperative assembly of a large ribonucleoprotein complex. J Mol Biol. 2004;344:395–407. doi: 10.1016/j.jmb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Talkington MW, Siuzdak G, Williamson JR. An assembly landscape for the 30S ribosomal subunit. Nature. 2005;438:628–32. doi: 10.1038/nature04261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coetzee T, Herschlag D, Belfort M. Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev. 1994;8:1575–88. doi: 10.1101/gad.8.13.1575. [DOI] [PubMed] [Google Scholar]
- 54.Rajkowitsch L, Schroeder R. Dissecting RNA chaperone activity. RNA. 2007;13:2053–60. doi: 10.1261/rna.671807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adilakshmi T, Bellur DL, Woodson SA. Concurrent nucleation of 16S folding and induced fit in 30S ribosome assembly. Nature. 2008;455:1268–1272. doi: 10.1038/nature07298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holmes KL, Culver GM. Mapping structural differences between 30S ribosomal subunit assembly intermediates. Nat Struct Mol Biol. 2004;11:179–86. doi: 10.1038/nsmb719. [DOI] [PubMed] [Google Scholar]
- 57.Kaczanowska M, Ryden-Aulin M. Ribosome biogenesis and the translation process in Escherichia coli. Microbiol Mol Biol Rev. 2007;71:477–94. doi: 10.1128/MMBR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powers T, Daubresse G, Noller HF. Dynamics of in vitro assembly of 16 S rRNA into 30 S ribosomal subunits. J Mol Biol. 1993;232:362–374. doi: 10.1006/jmbi.1993.1396. [DOI] [PubMed] [Google Scholar]
- 59.Bunner AE, Beck AH, Williamson JR. Kinetic cooperativity in Escherichia coli 30S ribosomal subunit reconstitution reveals additional complexity in the assembly landscape. Proc Natl Acad Sci USA. 2010;107:5417–22. doi: 10.1073/pnas.0912007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adilakshmi T, Ramaswamy P, Woodson SA. Protein-independent folding pathway of the 16S rRNA 5′ domain. J Mol Biol. 2005;351:508–19. doi: 10.1016/j.jmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Xu Z, Culver GM. Differential assembly of 16S rRNA domains during 30S subunit formation. RNA. 2010;16:1990–2001. doi: 10.1261/rna.2246710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sayers EW, Gerstner RB, Draper DE, Torchia DA. Structural preordering in the N-terminal region of ribosomal protein S4 revealed by heteronuclear NMR spectroscopy. Biochemistry. 2000;39:13602–13. doi: 10.1021/bi0013391. [DOI] [PubMed] [Google Scholar]
- 63.Webb AE, Rose MA, Westhof E, Weeks KM. Protein-dependent transition states for ribonucleoprotein assembly. J Mol Biol. 2001;309:1087–100. doi: 10.1006/jmbi.2001.4714. [DOI] [PubMed] [Google Scholar]
- 64.Bokinsky G, Nivon LG, Liu S, Chai G, Hong M, Weeks KM, Zhuang X. Two distinct binding modes of a protein cofactor with its target RNA. J Mol Biol. 2006;361:771–84. doi: 10.1016/j.jmb.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewin AS, Thomas J, Jr, Tirupati HK. Cotranscriptional splicing of a group I intron is facilitated by the Cbp2 protein. Mol Cell Biol. 1995;15:6971–8. doi: 10.1128/mcb.15.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin F, Chen Y, Wu M, Li Y, Zhang J, Chen HF. Induced fit or conformational selection for RNA/U1A folding. RNA. 2010;16:1053–61. doi: 10.1261/rna.2008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katsamba PS, Myszka DG, Laird-Offringa IA. Two functionally distinct steps mediate high affinity binding of U1A protein to U1 hairpin II RNA. J Biol Chem. 2001;276:21476–81. doi: 10.1074/jbc.M101624200. [DOI] [PubMed] [Google Scholar]
- 68.Stern S, Changchien LM, Craven GR, Noller HF. Interaction of proteins S16, S17 and S20 with 16 S ribosomal RNA. J Mol Biol. 1988;200:291–9. doi: 10.1016/0022-2836(88)90241-0. [DOI] [PubMed] [Google Scholar]
- 69.Weitzmann CJ, Cunningham PR, Nurse K, Ofengand J. Chemical evidence for domain assembly of the Escherichia coli 30S ribosome. FASEB J. 1993;7:177–80. doi: 10.1096/fasebj.7.1.7916699. [DOI] [PubMed] [Google Scholar]
- 70.Brodersen DE, Clemons WM, Jr, Carter AP, Wimberly BT, Ramakrishnan V. Crystal structure of the 30 S ribosomal subunit from Thermus thermophilus: structure of the proteins and their interactions with 16 S RNA. J Mol Biol. 2002;316:725–68. doi: 10.1006/jmbi.2001.5359. [DOI] [PubMed] [Google Scholar]
- 71.Ramaswamy P, Woodson SA. S16 throws a conformational switch during assembly of 30S 5′ domain. Nat Struct Mol Biol. 2009;16:438–45. doi: 10.1038/nsmb.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramaswamy P, Woodson SA. Global Stabilization of rRNA Structure by Ribosomal Proteins S4, S17, and S20. J Mol Biol. 2009:666–677. doi: 10.1016/j.jmb.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caprara MG, Mohr G, Lambowitz AM. A tyrosyl-tRNA synthetase protein induces tertiary folding of the group I intron catalytic core. J Mol Biol. 1996;257:512–531. doi: 10.1006/jmbi.1996.0182. [DOI] [PubMed] [Google Scholar]
- 74.Weeks KM, Cech TR. Protein facilitation of group I intron splicing by assembly of the catalytic core and the 5′ splice site domain. Cell. 1995;82:221–30. doi: 10.1016/0092-8674(95)90309-7. [DOI] [PubMed] [Google Scholar]
- 75.Biswas R, Ledman DW, Fox RO, Altman S, Gopalan V. Mapping RNA-protein interactions in ribonuclease P from Escherichia coli using disulfide-linked EDTA-Fe. J Mol Biol. 2000;296:19–31. doi: 10.1006/jmbi.1999.3443. [DOI] [PubMed] [Google Scholar]
- 76.Doudna JA, Batey RT. Structural insights into the signal recognition particle. Annu Rev Biochem. 2004;73:539–57. doi: 10.1146/annurev.biochem.73.011303.074048. [DOI] [PubMed] [Google Scholar]
- 77.Menichelli E, Isel C, Oubridge C, Nagai K. Protein-induced conformational changes of RNA during the assembly of human signal recognition particle. J Mol Biol. 2007;367:187–203. doi: 10.1016/j.jmb.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 78.Stone MD, Mihalusova M, O’Connor CM, Prathapam R, Collins K, Zhuang X. Stepwise protein-mediated RNA folding directs assembly of telomerase ribonucleoprotein. Nature. 2007;446:458–61. doi: 10.1038/nature05600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Held WA, Nomura M. Escherichia coli 30 S ribosomal proteins uniquely required for assembly. J Biol Chem. 1975;250:3179–84. [PubMed] [Google Scholar]
- 80.Dammel CS, Noller HF. A cold-sensitive mutation in 16S rRNA provides evidence for helical switching in ribosome assembly. Genes Dev. 1993;7:660–70. doi: 10.1101/gad.7.4.660. [DOI] [PubMed] [Google Scholar]
- 81.Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–34. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]