Abstract
Cancer stem cells (CSCs) are a small subset of cells that may be responsible for initiation, progression and recurrence of tumors. Recent studies have demonstrated that CSCs are highly tumorigenic and resistant to conventional chemotherapies, making them a promising target for the development of preventive/therapeutic agents. A single or combination of various markers, such as CD44, EpCAM, CD49f, CD133, CXCR4, ALDH-1 and CD24, were utilized to isolate CSCs fromvarious types of human cancers. Notch, Hedgehog, Wnt, and TGF-β signalingregulate self-renewal and differentiation of normal stem cells andare aberrantly activated in CSCs. In addition, many studies have demonstrated that these stem cell-associated signaling pathways are required for the maintenance of CSCs in differentmalignancies, including breast, colorectal, prostate and pancreatic cancers. Accumulating evidence hasshowninhibitory effects of vitamin D and its analogs on the cancer stem cell signaling pathways, suggesting vitamin D as a potential preventive/therapeutic agent against CSCs.In this review, we summarize recent findings about the roles of Notch, Hedgehog, Wnt, and TGF-β signaling in CSCs as well as the effects of vitamin D on these stem cell signaling pathways.
Keywords: Vitamin D, Cancer stem cell, Hedgehog, Notch, TGF-β, Wnt
1 Introduction
The observation of intratumoral heterogeneity has led to a hypothesis that a small subset of cells might be responsible for the initiation, progression and recurrence of tumors [1]. These cells have been called cancer stem cells (CSCs, also known as tumor-initiating cells) since they exhibit stem cell-like characteristics [1]. The first evidence of CSCs was demonstrated in acute myeloid leukemia [2]. Only a small fraction of primary leukemia cells was capable of initiating and sustaining leukemia when transplanted into mice [2,3]. Since then, CSCs have been isolated from many solid cancers, such as those of the breast, brain, prostate, colon and rectum, pancreas, and liver (reviewed in [4]). These cells also show strong capability to initiate tumors in vivo [5]. More importantly, CSCs exhibit resistance to conventional chemo- and radiotherapy, and are enriched in residual tumors after chemotherapy [5]. Many studies have demonstrated that CSCs are present in recurring tumors and distant metastases of various cancers, including those of the breast, pancreas, and colon [6,7]. Therefore, CSCs may be used as a potential target for therapeutic drug development to reduce cancer recurrence or metastasis and achieve prolonged survival of cancer patients [7]. Many new experimental agents, such as Notch and Hedgehog inhibitors, are being developed to inhibit CSCs [6,8]. Several lines of evidence have demonstrated that vitamin D plays an important role in the regulation of stem cells of the prostate and the skin [9-11]. Moreover, vitamin D is a well known inducer of the terminal differentiation of human myeloid leukemia cells into monocytes and macrophages [12], possibly via mechanisms of regulating leukemic cancer stem cells/progenitors. Recently, vitamin D and its analogs were shown to reduce the number of CSCs in breast cancer [13], further supporting their potential as therapeutic agents. In this review, we summarize recent findings on the CSC markers, CSC signaling pathways and the effects of vitamin D on the CSC signaling.
1.1 Identification of cancer stem cells
Isolation of CSCs from total cancer cell population is the first and most critical step to characterize CSCs [4,5]. Multiple markers that have been utilized to identify CSCs in various types of solid tumors [4] are summarized in Table 1. CD44 is a receptor for extracellular matrix components, including hyaluronan and osteopontin [14]. High CD44 protein levels have been used as a key characteristic of CSCs in solid tumors with epithelial origin, such as breast, colon, prostate and pancreas [14]. Expression of an epithelial cell adhesion molecule (EpCAM, also known as an epithelial specific antigen) was utilized as a cell surface marker in a combination with CD44 to further identify specific CSCs [4,15]. CD49f, also known as integrin α6, is a receptor for laminin, and its high expression has been a good indicator for CSCs in breast, colon and brain cancers [16]. A glycoprotein CD133, also known as Prominin 1 (PROM1), is expressed in stem cells from neural, epithelial, endothelial and hematopoietic tissues. A high expression of CD133 is a surface marker for CSCs of breast, brain, lung, colon, pancreas and liver cancers [4]. C-X-C chemokine receptor type 4 (CXCR4), a specific receptor for chemokine stromal cell-derived factor 1 (SDF-1), has been used as an additional marker in isolation of CD133-positive cancer cells to further enrich highly metastatic CSCs from breast, prostate and pancreatic cancers [4]. Aldehyde dehydrogenease-1 (ALDH-1) is an enzyme oxidizing cellular aldehydes, and its high activity has been a useful CSC marker for breast and pancreatic cancers [4,17]. CD24 is a heavily glycosylated adhesion molecule and the only known ligand for P-selectin. A low or no expression of CD24 in combination with a high expression of CD44 has been utilized as a CSC marker in breast and prostate cancers [4]. However, in pancreatic cancer, CD44-positive cells also expressing CD24 have been isolated as CSCs [4] indicating that CSCs differ depending on the types of cancer. Many studies looking for additional stem cell markers are in progress with the goal to isolate defined CSCs from different types of cancers [6].
Table 1.
Cancer stem cell markers in different cancers.
| Cancer Types | Cancer Stem Cell Markers | Associated Functions | Reference |
|---|---|---|---|
| Breast | CD44+/CD24−/low | Tumor Initiation | [78] |
| CD44+/CD24−/low/EpCAM+ | Tumor Initiation, Drug Resistance | [79] | |
| CD44+/CD49high/CD133+ | Tumor Initiation | [80] | |
| CD133+ | Tumor Initiation, Drug Resistance | [81] | |
| CD133+/CXCR4+ | Tumor Initiation, Tumor Metastasis | [82] | |
| ALDH-1+ | Tumor Initiation, Poor Prognosis | [83] | |
| Colorectal | CD133+ | Tumor Initiation | [84,85] |
| EpCAM+/CD44+/CD166+ | Tumor Initiation | [86] | |
| EpCAM+/CD44+/ALDH1+ | Tumor Initiation, Drug Resistance | [87] | |
| CD44+/ALDH-1+ | Tumor Initiation | [88] | |
| CD44+/CD133+/CD49f+ | Tumor Initiation | [89] | |
| Prostate | CD44+/α2β 1 high/CD133+ | Tumor Initiation | [90] |
| CD44+/CD24− | Tumor Initiation, Poor Prognosis | [91] | |
| Brain | CD133+ | Tumor Initiation | [92,93] |
| aIntegrin α6+ | Tumor Initiation | [94] | |
| Pancreatic | CD44+/CD24+/EpCAM+ | Tumor Initiation | [95] |
| CD133+/CXCR4+ | Tumor Initiation, Tumor Metastasis |
[96] | |
| ALDH-1+ | Tumor Initiation, Drug Resistance | [97,98] | |
| Lung | CD133+ | Tumor Initiation, Drug Resistance | [99,100] |
| ALDH-1+ | Tumor Initiation, Poor Prognosis | [101] | |
| Liver | CD133+ | Tumor Initiation | [102,103] |
| CD90+/CD44+ | Tumor Initiation, Tumor Metastasis |
[104] | |
| Head and Neck |
CD44+ | Tumor Initiation | [105] |
| ALDH-1+ | Tumor Initiation | [106,107] |
Integrin α6 is also known as CD49f.
1.2 Stem cell-associated pathways in cancer stem cells
The two critical features of normal stem cells–self-renewal and differentiation into phenotypically diverse cells–are also required for the maintenance of CSCs in human tumors [18]. The stem cell-associated signaling pathways, such as Notch, Hedgehog, Wnt and TGF-β, regulate self-renewal and differentiation of normal stem cells as well as CSCs [8,19]. The process of epithelial-mesenchymal transition (EMT), characterized by a loss of cellular polarity and cell-cell interaction and a gain of mesenchymal properties, has been closely associated with CSCs in solid tumors [20]. Aberrant activation of the stem cell-associated signaling pathways in cancer cells induces EMT and causes cancer cells to acquire phenotypes of CSCs [21]. Therefore, the stem cell-associated signaling pathways–Notch, Hedgehog, Wnt and TGF-β–have been considered as novel targets against CSCs in human tumors [8,21].
1.3 Notch signaling pathway
The evolutionally conserved Notch signaling pathway plays a key role in deciding cellular fate during embryogenesis and in maintenance of stem cells in adult tissues [22]. Four Notch receptors (Notch1, Notch2, Notch3 and Notch4) and five ligands (JAG1, JAG2, DLL1, DLL3 and DLL4) have been discovered in mammals [22]. Upon the interaction with ligands from adjacent cells, Notch receptors undergo a series of enzymatic cleavages by a disintegrin and metalloproteinase (ADAM) and γ-secretase to produce an active intracellular domain of Notch (NICD, also known as cleaved Notch) [22]. NICD translocates to the nucleus and regulates specific target genes such as c-Myc HES and HEY [22]. Aberrant activation of Notch signaling by elevated expression of Notch receptors and ligands has been shown in various human cancers [22]. Moreover, recent studies demonstrated that Notch signaling plays an important role in self-renewal and maintenance of CSCs in breast, lung, brain, pancreatic, and ovarian cancers [23-27]. Inhibition of Notch signaling by antibodies against the Notch receptors or by γ-secretase inhibitors decreased the number of CSCs and their tumorigenic potential in preclinical models of breast and brain cancers [28-31].
1.4 Hedgehog signaling pathway
The Hedgehog signaling pathway controls stem cell maintenance during embryonic development [32]. Upon the activation by ligands–Sonic (SHH), Desert (DHH) and Indian Hedgehog (IHH)–Hedgehog receptor (Patched 1, PTCH1) releases repression on Smoothened (SMO). Then, the activated SMO initiates a signaling cascade leading to the target gene regulation by the GLI family of transcription factors [32]. The high activity of Hedgehog signaling has been found in various human cancers [33]. Hedgehog inhibitors showed anti-tumor activity in clinical studies of advanced solid tumors, such as breast cancer, medulloblastoma and basal cell carcinoma [34-36]. Activated Hedgehog signaling has been reported in CSCs of many human tumors, including brain, breast and pancreatic cancers [37-39]. Hedgehog inhibitors, such as cyclopamine, suppressed CSCs in glioblastoma and pancreatic cancer [40,41].
1.5 Wnt signaling pathway
The Wnt signaling pathway determines the cell fate during embryogenesis and regulates tissue self-renewal in adults [42]. The binding of Wnt proteins to a receptor complex containing Frizzled (FZD)/low-density lipoprotein receptor related protein (LRP) initiates the canonical and non-canonical signaling cascades [42]. The activation of canonical Wnt signaling pathway leads to accumulation of β-catenin in the nucleus and subsequent transcriptional regulation of target genes [42,43]. The non-canonical Wnt signaling pathway is β-catenin independent and regulates movement and polarity of embryonic cells; however, little is known about its role in human cancer [43]. The critical role of canonical Wnt signaling in human tumorigenesis has been well-recognized in colorectal cancer with a majority of tumors harboring activating mutations in the Wnt signaling pathway [42]. In addition, canonical Wnt signaling seems to maintain CSCs by regulating their proliferation and self-renewal during intestinal and prostate tumor development [44-46]. A recent study demonstrated that an antibody targeting FZD receptors reduced tumor growth and the number of CSCs in breast and pancreatic cancer cells [47]. A phase 1 clinical study using this antibody in patients with solid tumors is ongoing [48].
1.6 TGF-β signaling pathway
The TGF-β signaling pathway is a complex pathway with 42 known TGF-β superfamily ligands, such as TGF-βs, activins, Nodal and bone morphogenetic proteins (BMPs) [49]. Depending on the ligand and its downstream signaling mediators, TGF-β signaling can be divided into two signaling cascades, TGF-β/Activin/Nodal and BMP, which activate downstream mediators SMAD2/3 and SMAD1/5/8, respectively [50]. TGF-β signaling regulates a variety of cellular processes including differentiation, proliferation, migration and cell death both in adult organisms and developing embryos [50]. In human tumors, TGF-β signaling has been shown to have either a tumor-suppressing or tumor-promoting function depending upon the cellular context and the type of ligand [50]. Recent studies have also demonstrated diverse effects of TGF-β signaling on CSCs [50]. TGF-β signaling via activated TGF-β/Activin strongly induces EMT in many cancer cells, promoting maintenance of CSCs and tumor metastasis [51]. TGF-β signaling via activated Activin/Nodal is required for self-renewal and tumorigenicity of CSCs in pancreatic cancer [52]. In contrast, BMP signaling antagonizes TGF-β-induced EMT in prostate cancer cells and represses their bone metastasis [53]. A BMP treatment strongly reduced the number of CSCs in breast cancer and inhibited bone metastasis in an animal model [54].
2 Regulation of stem cell signaling by vitamin D in solid tumors
Crosstalk between vitamin D and Notch signaling was first demonstrated in osteoblastic cells, where 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) cooperated with HES1, a downstream effector of Notch signaling, to induce osteopontin expression [55]. In contrast, 1α,25(OH)2D3 treatment significantly reduced NOTCH1, JAG1, JAG2 and DLL1 mRNA levels in prostate epithelial cells, indicating an inhibitory effect of vitamin D on Notch signaling [56]. We have also found that treatment of breast cancer cells with 1α,25(OH)2D3 or its analogs markedly decreased mRNA levels of the Notch ligands and resulted in the inhibition of Notch1 signaling. Moreover, the inhibition of Notch signaling by vitamin D analogs also resulted in the reduction of CSCs (unpublished data). However, 1α,25(OH)2D3 did not inhibit the Notch signaling in brain cancer cell lines and did not exhibit anti-proliferative effects in these cells [57]. These data suggest that effects of vitamin D on Notch signaling may differ based on tissue and cellular context.
Vitamin D3 or 3β-hydroxysteroid (pro-) vitamin D3 inhibits activation of the Hedgehog signaling by directly binding to SMO in zebrafish, yeast, and mouse fibroblast cells [58]. The mRNA levels of Hedgehog signaling molecules, Shh, Gli1 and Gli2, were increased in chemically induced epidermal tumors of mice with knocked-out vitamin D receptor (VDR) when compared to those in tumors of wild-type mice [59]. The expression of the Hedgehog molecules was inhibited by 1α,25(OH)2D3 in mouse skin cells in a VDR-dependent manner [60]. In basal cell carcinoma, vitamin D3 or 1α,25(OH)2D3 inhibited Hedgehog signaling by repressing GLI1 mRNA and exerted anti-proliferative effects in vitro and in vivo [61,62]. Vitamin D3 (cholecalciferol) also inhibited Hedgehog signaling by repressing GLI2 expression and tumor growth of renal carcinoma xenografts [63].
Vitamin D and its analogs inhibit Wnt signaling by several mechanisms in cancer cells [64]. In the presence of 1α,25(OH)2D3, VDR can directly bind to β-catenin, competing with a T cell transcription factor (TCF)-4 and repressing the β-catenin/TCF-4 transcriptional activity [65-67]. 1α,25(OH)2D3 suppresses Wnt signaling by inducing the expression of DKK-1, which antagonizes Wnt signaling by binding to LRP5/6 [68]. In colon cancer cells, 1α,25(OH)2D3 inhibited Wnt signaling by the induction of E-cadherin [65]. Treatment with 1α,25(OH)2D3 or its analogs reduced tumor load in Apcmin/+ mice, an animal model of intestinal cancer with dysregulated Wnt signaling, by inducing E-cadherin expression and reducing nuclear β-catenin level [69,70]. In contrast to its effects in cancer cells, VDR was required for the activation of Wnt signaling in normal keratinocytes to form the hair follicle [9]. However, treatment with a vitamin D analog inhibited β-catenin induced-hair follicle tumors in mice [71], suggesting that the effects of vitamin D on Wnt signaling may differ in cancer cells where aberrant activation of β-catenin is sustained.
Vitamin D and the TGF-β superfamily signaling interact in a cellular context-dependent manner both in normal and malignant cells [72]. A recent genome-wide study demonstrated that a large number of genomic sites can be co-occupied by VDR and SMAD3. 1α,25(OH)2D3 or its analog reduced the occupancy of SMAD3 on the target genes, blocking TGF-β1-mediated activation of hepatic stellate cells and the development of liver fibrosis [73]. 1α,25(OH)2D3 also inhibited TGF-β1-stimulated EMT and a pro-fibrotic phenotype of lung fibroblasts and epithelial cells [74]. In contrast, 1α,25(OH)2D3 or its analogs increased mRNA levels of BMP2 and BMP6, and activated the BMP signaling in normal and premalignant breast epithelial cells [75,76]. The expression of BMP6 mRNA was also significantly induced by 1α,25(OH)2D3 in prostatic epithelial and breast cancer cells [77].
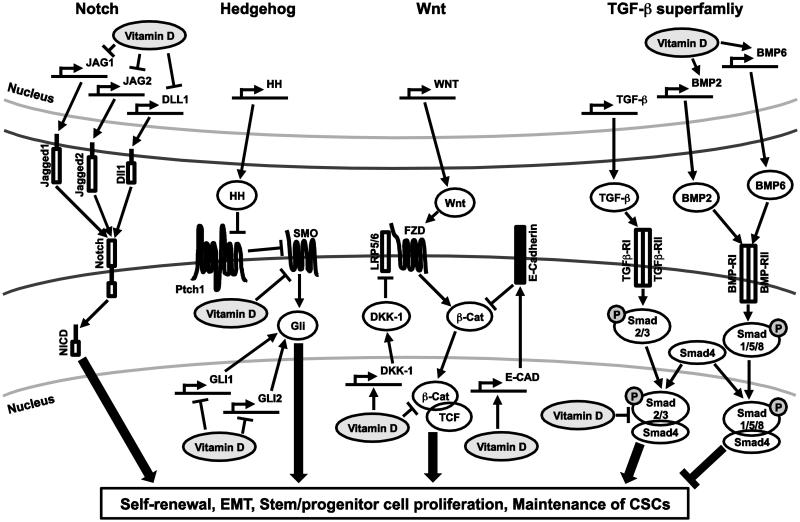
Vitamin D seems to have opposite effects on stem cell signaling between normal and malignant cells. In many cases, vitamin D and its analogs exert inhibitory effects on the cancer stem cell signaling pathways, which may be due to aberrant and highly activated status of these signaling pathways in cancer cells. The diverse effects of vitamin D on the stem cell-associated signaling pathways suggest that vitamin D may regulate and target CSCs. Some of the key vitamin D actions on stem cell signaling in cancer cells are summarized in Fig. 1.
Fig. 1.
A schematic diagram depicting actions of vitamin D on the Notch, Hedgehog, Wnt and TGF-β superfamily signaling pathways. Main components of the Notch, Hedgehog, Wnt and TGF-β superfamily and their regulation by vitamin D are presented. Detailed explanation and related references can be found in the text. Full names of the abbreviations shown in the diagram are listed; JAG1, Jagged1; JAG2, Jagged2; DLL1, Delta-like protein 1; NICD, Intracellular domain of Notch; HH, Hedgehog; Ptch1, Patched1; SMO, Smoothened; LRP5/6, Low-density lipoprotein receptor-related protein 5/6; FZD, Frizzled; β-Cat β-Catenin; DKK-1, Dickkopf-related protein 1;TCF, T cell factor; E-cad, E-cadherin; BMP2, Bone morphogenetic protein 2; BMP6, Bone morphogenetic protein 6; TGFβ-RI, TGF-β receptor 1; TGFβ-RII, TGF-β receptor 2; BMP-RI, BMP receptor 1; BMP-RII, BMP receptor 2.gr1
3 Conclusion
Because of the importance of Notch, Hedgehog, Wnt and TGF-β in the maintenance of CSCs in human tumors, these signaling pathways are attractive as potential targets for the development of new anti-cancer agents. Vitamin D and its analogs have inhibitory effects on cancer stem cell signaling in various types of human cancer cells and may be promising therapeutic/preventive agents against CSCs. However, the effects of vitamin D on the stem cell signaling pathways vary depending on cell types and cellular contexts. Considering that vitamin D can differentially regulate a wide range of genes in normal and malignant cells, further investigations are required to better understand its cellular context-dependent effects on stem cell signaling.
Highlights.
Provides a list of various markers of cancer stem cells (CSCs) from solid tumors.
Reviews roles of stem cell signaling, Notch, Hedgehog, Wnt and TGF-β, in CSCs.
Summarizes recent findings for the effects of Vitamin D on stem cell signaling.
Suggests vitamin D and its analogs as potential agents against CSCs.
Acknowledgement
The authors thank Dr. Pavel Kramata for his helpful suggestions. This work was supported in part by the NIH National Cancer Institute R01CA127645, the National Institute of Environmental Health Sciences Grant ES005022, and The Trustees Research Fellowship Program at Rutgers, The State University of New Jersey.
Glossary
- CSC
Cancer stem cell
- 1α,25(OH)2D3
1α 25-dihydroxyvitamin D3
- EpCAM
Epithelial cell adhesion molecule
- PROM1
Prominin 1
- CXCR4
C-X-C chemokine receptor type 4
- SDF1
Stromal cell-derived factor 1
- ALDH-1
Aldehyde dehydrogenease-1
- EMT
Epithelial-mesenchymal transition
- ADAM
A disintegrin and metalloproteinase
- NICD
Intracellular domain of Notch
- SHH
Sonic Hedgehog
- DHH
Desert Hedgehog
- IHH
Indian Hedgehog
- PTCH1
Patched 1
- SMO
Smoothened
- FZD
Frizzled receptor
- LRP
Low-density lipoprotein receptor-related protein
- BMP
Bone morphogenetic protein
- TCF4
T cell factor 4
- VDR
Vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- [1].Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea-a paradigm shift. Cancer Res. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. 1. [DOI] [PubMed] [Google Scholar]
- [2].Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. 1. [DOI] [PubMed] [Google Scholar]
- [3].Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. 1. [DOI] [PubMed] [Google Scholar]
- [4].Hermann PC, Bhaskar S, Cioffi M, Heeschen C. Cancer stem cells in solid tumors. Semin. Cancer Biol. 2010;20(2):77–84. doi: 10.1016/j.semcancer.2010.03.004. 1. [DOI] [PubMed] [Google Scholar]
- [5].Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. 1. [DOI] [PubMed] [Google Scholar]
- [6].Clevers H. The cancer stem cell: premises. promises and challenges, Nat. Med. 2011;17(3):313–319. doi: 10.1038/nm.2304. 1. [DOI] [PubMed] [Google Scholar]
- [7].Sampieri K, Fodde R. Cancer stem cells and metastasis. Semin. Cancer Biol. 2012;22(3):187–193. doi: 10.1016/j.semcancer.2012.03.002. 1. [DOI] [PubMed] [Google Scholar]
- [8].Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. 1. [DOI] [PubMed] [Google Scholar]
- [9].Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc. Natl. Acad. Sci. U. S. A. 2007;104(22):9428–9433. doi: 10.1073/pnas.0702884104. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luderer HF, Demay MB. The vitamin D receptor, the skin and stem cells. J. Steroid Biochem. Mol. Biol. 2010;121(1–2):314–316. doi: 10.1016/j.jsbmb.2010.01.015. 1. [DOI] [PubMed] [Google Scholar]
- [11].Maund SL, Barclay WW, Hover LD, Axanova LS, Sui G, Hipp JD, Fleet JC, Thorburn A, Cramer SD. Interleukin-1alpha mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer Res. 2011;71(15):5276–5286. doi: 10.1158/0008-5472.CAN-10-2160. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gocek E, Studzinski GP. Vitamin D and;1; differentiation in cancer. Crit. Rev. Clin. Lab. Sci. 2009;46(4):190–209. doi: 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, Liu F, Suh N. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol. Pharmacol. 2011;79(3):360–367. doi: 10.1124/mol.110.068403. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer. 2011;11(4):254–267. doi: 10.1038/nrc3023. 1. [DOI] [PubMed] [Google Scholar]
- [15].Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69(14):5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- [16].Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Cancer stem cell markers in common cancers - therapeutic implications. Trends Mol. Med. 2008;14(10):450–460. doi: 10.1016/j.molmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- [17].Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011;7(2):292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- [18].Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- [19].Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim. Biophys. Acta. 2013;1830(2):2481–2495. doi: 10.1016/j.bbagen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- [20].Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- [21].Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, Miele L. Targeting Notch to target cancer stem cells. Clin. Cancer Res. 2010;16(12):3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hassan KA, Wang L, Korkaya H, Chen G, Maillard I, Beer DG, Kalemkerian GP, Wicha MS. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin. Cancer Res. 2013;19(8):1972–1980. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70(2):709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307(1):26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [26].McAuliffe SM, Morgan SL, Wyant GA, Tran LT, Muto KW, Chen YS, Chin KT, Partridge JC, Poole BB, Cheng KH, Daggett J, Jr., Cullen K, Kantoff E, Hasselbatt K, Berkowitz J, Muto MG, Berkowitz RS, Aster JC, Matulonis UA, Dinulescu DM. Targeting Notch a key pathway for ovarian cancer stem cells sensitizes tumors to platinum therapy. Proc. Natl. Acad. Sci. U. S. A. 2012;109(43):E2939–E2948. doi: 10.1073/pnas.1206400109. Targeting Notch a key pathway for ovarian cancer stem cells sensitizes tumors to platinum therapy Proc. Natl Acad. Sci. U. S. A., 109, ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xing F, Okuda H, Watabe M, Kobayashi A, Pai SK, Liu W, Pandey PR, Fukuda K, Hirota S, Sugai T, Wakabayshi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Mo YY, Watabe K. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011;30(39):4075–4086. doi: 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kondratyev M, Kreso A, Hallett RM, Girgis-Gabardo A, Barcelon ME, Ilieva D, Ware C, Majumder PK, Hassell JA. Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of ERBB2 breast cancer. Oncogene. 2012;31(1):93–103. doi: 10.1038/onc.2011.212. [DOI] [PubMed] [Google Scholar]
- [29].Sharma A, Paranjape AN, Rangarajan A, Dighe RR. A monoclonal antibody against human Notch1 ligand-binding domain depletes subpopulation of putative breast cancer stem-like cells. Mol. Cancer Ther. 2012;11(1):77–86. doi: 10.1158/1535-7163.MCT-11-0508. [DOI] [PubMed] [Google Scholar]
- [30].Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66(15):7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- [31].Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28(1):5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013;14(7):416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- [33].Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29(4):469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- [34].LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, Graham RA, Zerivitz KL, Low JA, Von Hoff DD. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(8):2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, Sarantopoulos J, Mahalingam D, Shou Y, Moles MA, Yang L, Granvil C, Hurh E, Rose KL, Amakye DD, Dummer R, Mita AC. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20(7):1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- [36].Jimeno A, Weiss GJ, Miller WH, Jr., Gettinger S, Eigl BJ, Chang AL, Dunbar J, Devens S, Faia K, Skliris G, Kutok J, Lewis KD, Tibes R, Sharfman WH, Ross RW, Rudin CM. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clinical Cancer Research. 2013;19(10):2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, Gabrielson KL, Matsui W, Maitra A. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67(5):2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS ONE. 2011;6(11):e27306. doi: 10.1371/journal.pone.0027306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- [43].Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin. Cancer Res. 2010;16(12):3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- [44].Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19(6):683–697. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- [45].Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12(5):468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- [46].Shenoy AK, Fisher RC, Butterworth EA, Pi L, Chang LJ, Appelman HD, Chang M, Scott EW, Huang EH. Transition from colitis to cancer: high Wnt activity sustains the tumor-initiating potential of colon cancer stem cell precursors. Cancer Res. 2012;72(19):5091–5100. doi: 10.1158/0008-5472.CAN-12-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. U. S. A. 2012;109(29):11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].David LR, Smith C, Wang Min, Zhang Chun, Xu Lu, Chugh Rashmi, Tolcher Anthony, Goldman Jonathan, Dupont Jakob, Brachmann Raiiner K., Papadopoulos Kyriakos, Kapoun Ann M. Biomarker analysis in the first-in-human phase 1a study for vantictumab (OMP-18R5; anti-Frizzled) demonstrates pharmacodynamic (PD) modulation of the Wnt pathway in patients with advanced solid tumors. Mol. Cancer Ther. 2013;12(11 Suppl) Abstract B24. [Google Scholar]
- [49].Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3(1):7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- [50].Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat. Rev. Cancer. 2010;10(6):415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- [51].Drabsch Y, ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31(3–4):553–568. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- [52].Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC, Torres-Ruiz R, Garcia E, Hidalgo M, Cebrian DA, Heuchel R, Lohr M, Berger F, Bartenstein P, Aicher A, Heeschen C. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9(5):433–446. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- [53].Buijs JT, Rentsch CA, van der Horst G, van Overveld PG, Wetterwald A, Schwaninger R, Henriquez NV, Ten Dijke P, Borovecki F, Markwalder R, Thalmann GN, Papapoulos SE, Pelger RC, Vukicevic S, Cecchini MG, Lowik CW, van der Pluijm G. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J. Pathol. 2007;171(3):1047–1057. doi: 10.2353/ajpath.2007.070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Buijs JT, van der Horst G, van den Hoogen C, Cheung H, de Rooij B, Kroon J, Petersen M, van Overveld PG, Pelger RC, van der Pluijm G. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene. 2012;31(17):2164–2174. doi: 10.1038/onc.2011.400. [DOI] [PubMed] [Google Scholar]
- [55].Shen Q, Christakos S. The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol. Chem. 2005;280(49):40589–40598. doi: 10.1074/jbc.M504166200. [DOI] [PubMed] [Google Scholar]
- [56].Kovalenko PL, Zhang Z, Cui M, Clinton SK, Fleet JC. 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics. 2010;11:26. doi: 10.1186/1471-2164-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Reichrath S, Muller CS, Gleissner B, Pfreundschuh M, Vogt T, Reichrath J. Notch- and vitamin D signaling in 1,25(OH) 2D3-resistant glioblastoma multiforme (GBM) cell lines. J Steroid Biochem. Mol. Biol. 2010;121(1–2):420–424. doi: 10.1016/j.jsbmb.2010.02.028. [DOI] [PubMed] [Google Scholar]
- [58].Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-) vitamin D3 secretion. PLoS Biol. 2006;4(8):e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J. Invest. Dermatol. 2011;131(11):2289–2297. doi: 10.1038/jid.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bikle DD, Elalieh H, Welsh J, Oh D, Cleaver J, Teichert A. Protective role of vitamin D signaling in skin cancer formation. J. Steroid Biochem. Mol. Biol. 2013;136:271–279. doi: 10.1016/j.jsbmb.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Uhmann A, Niemann H, Lammering B, Henkel C, Hess I, Nitzki F, Fritsch A, Prufer N, Rosenberger A, Dullin C, Schraepler A, Reifenberger J, Schweyer S, Pietsch T, Strutz F, Schulz-Schaeffer W, Hahn H. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol. Cancer Ther. 2011;10(11):2179–2188. doi: 10.1158/1535-7163.MCT-11-0422. [DOI] [PubMed] [Google Scholar]
- [62].Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, So PL, Hebert J, Bikle D, Epstein EH., Jr. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Phila) 2011;4(5):744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dormoy V, Beraud C, Lindner V, Coquard C, Barthelmebs M, Brasse D, Jacqmin D, Lang H, Massfelder T. Vitamin D3 triggers antitumor activity through targeting hedgehog signaling in human renal cell carcinoma. Carcinogenesis. 2012;33(11):2084–2093. doi: 10.1093/carcin/bgs255. [DOI] [PubMed] [Google Scholar]
- [64].Larriba MJ, Gonzalez-Sancho JM, Barbachano A, Niell N, Ferrer-Mayorga G, Munoz A. Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers (Basel) 2013;5(4):1242–1260. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 2001;154(2):369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol. Cell. 2006;21(6):799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- [67].Jiang YJ, Teichert AE, Fong F, Oda Y, Bikle DD. 1alpha,25(OH) 2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the beta-catenin pathway. J Steroid Biochem. Mol. Biol. 2013;136:229–232. doi: 10.1016/j.jsbmb.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, Barbachano A, Lopez de Silanes I, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, Gonzalez-Sancho JM, Munoz A. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha 25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28(9):1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- [69].Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, Harris DM. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) Mouse. Cancer Res. 2002;62(3):741–746. [PubMed] [Google Scholar]
- [70].Xu H, Posner GH, Stevenson M, Campbell FC. Apc(MIN) modulation of vitamin D secosteroid growth control. Carcinogenesis. 2010;31(8):1434–1441. doi: 10.1093/carcin/bgq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pálmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE. 2008;3(1):e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat. Rev. Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- [73].Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, Lau SL, Atkins AR, Barish GD, Gunton JE, Liddle C, Downes M, Evans RM. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153(3):601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zugel U, Roman J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J. Steroid Biochem. Mol. Biol. 2010;118(3):142–150. doi: 10.1016/j.jsbmb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lee HJ, Wislocki A, Goodman C, Ji Y, Ge R, Maehr H, Uskokovic M, Reiss M, Suh N. A novel vitamin D derivative activates bone morphogenetic protein signaling in MCF10 breast epithelial cells. Mol. Pharmacol. 2006;69(6):1840–1848. doi: 10.1124/mol.105.022079. [DOI] [PubMed] [Google Scholar]
- [76].Lee HJ, Ji Y, Paul S, Maehr H, Uskokovic M, Suh N. Activation of bone morphogenetic protein signaling by a Gemini vitamin D3 analogue is mediated by Ras/protein kinase C alpha. Cancer Res. 2007;67(24):11840–11847. doi: 10.1158/0008-5472.CAN-07-1549. [DOI] [PubMed] [Google Scholar]
- [77].Peehl DM, Shinghal R, Nonn L, Seto E, Krishnan AV, Brooks JD, Feldman D. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem. Mol. Biol. 2004;92(3):131–141. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- [78].Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Meyer MJ, Fleming JM, Lin AF, Hussnain SA, Ginsburg E, Vonderhaar BK. CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer Res. 2010;70(11):4624–4633. doi: 10.1158/0008-5472.CAN-09-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10(1):R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, Lee EY, Chang KJ, Lee WH. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS ONE. 2009;4(12):e8377. doi: 10.1371/journal.pone.0008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- [85].Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- [86].Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, Clarke MF, Hoey T, Lewicki J, Gurney AL. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3(6):e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chu P, Clanton DJ, Snipas TS, Lee J, Mitchell E, Nguyen ML, Hare E, Peach RJ. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int. J. Cancer. 2009;124(6):1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- [89].Haraguchi N, Ishii H, Mimori K, Ohta K, Uemura M, Nishimura J, Hata T, Takemasa I, Mizushima T, Yamamoto H, Doki Y, Mori M. CD49f-positive cell population efficiently enriches colon cancer-initiating cells. Int. J. Oncol. 2013;43(2):425–430. doi: 10.3892/ijo.2013.1955. [DOI] [PubMed] [Google Scholar]
- [90].Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- [91].Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br. J. Cancer. 2008;98(4):756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- [93].Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- [94].Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- [96].Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [97].Jimeno A, Feldmann G, Suarez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, Garcia-Garcia E, Lopez-Rios F, Matsui W, Maitra A, Hidalgo M. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol. Cancer Ther. 2009;8(2):310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS ONE. 2011;6(6):e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl. Acad. Sci. U. S. A. 2009;106(38):16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- [101].Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009;7(3):330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- [103].Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int. J. Cancer. 2007;120(7):1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- [104].Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- [105].Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, Ku HH, Chiou SH, Lo WL. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009;385(3):307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- [107].Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32(9):1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]