Abstract
Hydrogen sulfide (H2S) is a novel endogenous gaseous signal transducer (gasotransmittor). Its emerging role in multiple facets of inter- and intra-cellular signaling as a metabolic, inflammatory, neuro and vascular modulator has been increasingly realized. Although H2S is known for its effects as an anti-hypertensive, anti-inflammatory and anti-oxidant molecule, the relevance of these effects in skeletal muscle biology during health and during metabolic syndromes is unclear. H2S has been implicated in vascular relaxation and vessel tone enhancement, which might lead to mitigation of vascular complications caused by the metabolic syndromes. Metabolic complications may also lead to mitochondrial remodeling by interfering with fusion and fission, therefore, leading to mitochondrial mitophagy and skeletal muscle myopathy. Mitochondrial protection by H2S enhancing treatments may mitigate deterioration of muscle function during metabolic syndromes. In addition, H2S might upregulate uncoupling proteins and might also cause browning of white fat, resulting in suppression of imbalanced cytokine signaling caused by abnormal fat accumulation. Likewise, as a source for H+ ions, it has the potential to augment anaerobic ATP synthesis. However, there is a need for studies to test these putative H2S benefits in different patho-physiological scenarios before its full-fledged usage as a therapeutic molecule. The present review highlights current knowledge with regard to exogenous and endogenous H2S roles in skeletal muscle biology, metabolism, exercise physiology and related metabolic disorders, such as diabetes and obesity, and also provides future directions.
Keywords: Hydrogen sulfide, skeletal muscle, ATP, mitochondria, diabetes, obesity
Hydrogen sulfide (H2S) is a novel endogenous gaseous signal transducer and its emerging role in multiple facets of inter- and intra-cellular signaling as a metabolic, inflammatory, neuro and vascular modulator has been increasingly realized. It is produced enzymatically by different prokaryotes and eukaryotes. Since detection of the endogenous H2S presence, the perception of H2S as a poisoning gas has been changed. While liver has been documented to generate majority of the endogenous H2S, kidney also produces decent levels of H2S [1]. Production of H2S in other organs is relatively very less [1]. In addition to organ specific differences in H2S production, species specific variations within H2S production were also reported. For example, in contrast to the mouse scenario, the ability of rat brain tissue to generate H2S is comparable to the rat liver tissue [2]. Discovery that H2S production in cardiovascular tissue and the role of H2S as a vasodilator and modulator of hypertension [3], has numerous implications in regulation of nutrient supply to different end organs. Recently, it was also observed that mouse colon tissue also makes significant levels of H2S [4]. These studies have highlighted broader distribution of H2S generating enzymes in different tissues and H2S function in multiple tissue homeostasis.
The total H2S pool in the body at any given time is dynamic and can come from diverse sources. For instance, gut bacteria were also reported to engender significant amount of H2S [5]. Furthermore, human diet that is rich in organosulfur compounds such as garlic, onions, leeks and chives have been reported to contribute to H2S pool and to exert significant influence on the metabolic state and hypertension [6]. Nonetheless, regulated H2S production at defined tissue milieu and sub-cellular spaces occurs through specific enzymes. Thus far, three main enzymatic contributors of endogenous H2S have been identified namely: cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3 mercaptopyruvate sulfurtransferase (3MST) enzymes [7]. These enzymes use sulfur containing amino acids cysteine and homocysteine as substrates to produce H2S in a single or multi-step process. The enzymes CBS and CSE have been demonstrated to localize in cytoplasm, and 3MST is localized mainly in mitochondria although small quantities of this enzyme may also be present in cytosol [8, 9]. Excellent reviews detailing molecular pathways of endogenous H2S production with necessary co-factors are available for readers and are not discussed in detail in the present review [10–14]. Here we will provide an overview of perceived and predicted functions of H2S in skeletal muscle biology, exercise physiology, bio-energetics and metabolic disorders, such as diabetes and obesity.
1.1 H2S role in skeletal muscle biology
Species specific differences were reported with regard to expression of CBS and CSE enzymes in skeletal muscles. Human skeletal muscles express significant amounts of CBS and CSE [15], whereas mouse skeletal muscles completely lack these enzymes (data in communication and [15]). A recent report suggested that all the three enzymes (CBS, CSE, 3-MST) were present in detectable levels in the rat skeletal muscles [16]. Nonetheless, their expression is very low when compared to that of the liver and kidney. Only human skeletal muscles express CBS and CSE enzymes that are comparable to the expression levels in liver in relative abundance. Given that skeletal muscles are the dominant organ, consequences of such contrasting CBS and CSE expression variation over cysteine and homocysteine metabolism and H2S signaling are currently unknown. Expression levels of 3MST were not determined in rodent skeletal muscles thus far. Our results (in communication) indicated that mouse skeletal muscles express non-detectable levels of this enzyme. As we only examined the presence of these enzymes in one particular mouse stain (C57) at present, there is a need for confirmation of these findings in other mouse stains before any further conclusions. Nevertheless, studies from ovine and bovine tissues also indicated that skeletal muscles express the least amount of 3MST when compared to that of the other tissues [17]. In contrast to mouse skeletal muscles, rat muscles were shown to express considerable levels of 3MST [18]. Contribution of these species specific H2S production differences in skeletal muscle biology and function are currently unknown.
Nonetheless, studies suggested that with age there is an increase in plasma homocysteine (Hcy), precursor for H2S, levels which is correlated with enhanced risk for decline in physical function [19, 20]. However, it is not clear whether such decline in physical function is because of changes in H2S levels as well. Due to complete lack of H2S producing enzymes in mouse skeletal muscles (data communicated), the author has proposed to utilize the mouse skeletal muscles as a unique place to unravel the biology of the H2S signaling and homocysteine abundance (hyperhomocysteinemia). As the homozygous CBS knockout mice give birth to healthy offsprings [21], it is more unlikely that CBS might have any determinant role during embryonic development. Interestingly, however, higher expression levels of CBS were observed in mouse limb buds of embryonic age E9.5 and E 10.5 [22], suggesting H2S might involve in tissue specification during the embryonic stage. Future studies are necessary to test if H2S plays any role in limb bud specification as H2S can also be produced by the other two enzymes (3MST and CSE). The possible causes for non-detectable levels of H2S producing enzymes in adult mouse muscles could be due to epigenetic modifications or inbred mouse strain differences, which need to be clarified further.
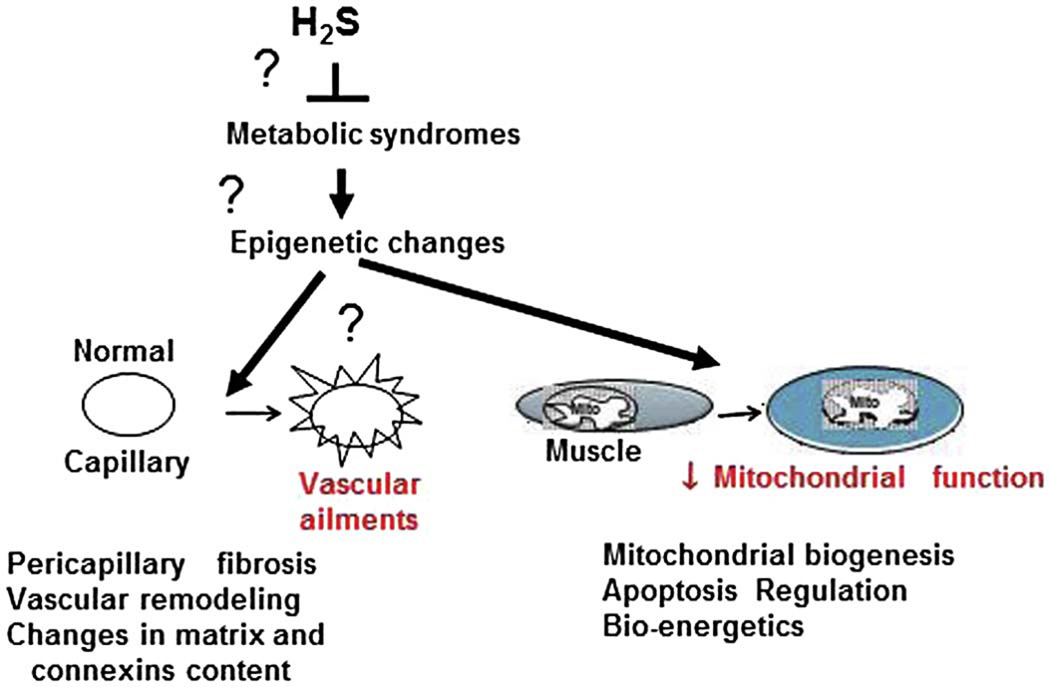
In lieu of paucity of the knowledge with regard to the H2S role in skeletal muscle biology, here we are proposing a hypothetical model where in H2S might exert therapeutic potential in skeletal muscle wasting/fibrosis, resulting from metabolic complications, such as diabetes and obesity. Although there is a need for direct evidence for its role in skeletal muscle function, studies from other tissues/organs do suggest such possibilities [3, 23]. Here we have hypothesized that H2S presence might reverse abnormal epigenetic changes caused by metabolic syndromes and produce beneficial effects on skeletal muscle vasculature and mitochondrial function (Fig.1). For instance, treatment of brain endothelial cells with H2S donors has been shown to alter quantities of DNA methyl transferases, which regulate gene expression at promoter level through CpG methylation [24], and thereby confers protection against oxidative damage. Moreover, epigenetic alterations associated with vascular pathologies have been documented [25, 26]. Given that metabolic syndromes induce vascular fibrosis and stiffening [27] and altered gap junctional content and communication at both micro and macro vascular level [28], it is plausible that presence of H2S might exert protective role through epigenetic changes and restore vascular function. Likewise, protective nature of H2S in mitochondrial integrity and function were also reported [29, 30]. All these features of H2S reverse muscle damage and ameliorate metabolic myopathy (Fig. 1).
Figure 1.
Hypothetical model for protective function of H2S in skeletal muscle myopathy/dysfunction induced by metabolic syndromes such as diabetes and obesity. H2S may reverse changes in epigenetic modifications induced by metabolic syndromes. Metabolic syndromes are perceived to cause vascular [73] and mitochondrial [74] dysfunction potentially through Epigenetic modifications. H2S by mitigating vascular dysfunction and by preventing mitochondrial damage might reverse deterioration of muscle function as well as alleviation of other symptoms associated with metabolic syndromes.
1.2 H2S and exercise capacity
The influence of acute H2S exposure on various biochemical parameters in human skeletal muscles during sub-maximal and maximal exercise was reported before [31–33]. The findings from these studies indicated that there was a shift in metabolism from aerobic to anaerobic side, as there was significant accumulation of lactate in the blood, especially after inhalation of 5 ppm H2S during maximal power output without significant change in heart rate and power output capacity [32]. Furthermore, inhalation of higher concentrations of H2S (10ppm) during sub-maximal exercise was reported to cause decline in oxygen uptake by the tissues without any significant change in arterial blood parameters, lactate concentrations, and aerobic and anaerobic metabolic enzyme activities [31]. These studies underscored the fascinating systemic tolerance to external H2S inhalation without compromising physical performance.
The apparent shift from aerobic metabolism to anaerobic metabolism was mainly owing to significant reduction in the citrate synthase enzyme levels, the regulator of first rate limiting step in citrate cycle during aerobic metabolism, in the skeletal muscles during maximal power output. There were also increased levels of both lactate and lactate dehydrogenase along with decreased levels of cytochrome C oxidase (CytoCOx) after sub-maximal exercise with concomitant H2S breathing, but these changes are non-significant. Interestingly, the H2S mediated inhibition of aerobic metabolism was observed only in men but not in women. Causes for such gender related differences in H2S impact on skeletal muscle metabolism are yet to be known. It is plausible that sex steroids and thyroid levels might act as confounding factors. Although there is a tendency for overdependence on anaerobic metabolism during exercise in the presence of H2S, long term adaptations of chronic H2S presence or absence, both in skeletal muscle metabolism, and in overall physical capacity such as hemoglobin and myoglobin levels, and mitochondrial density are not clear.
It was shown that mild mitochondrial uncoupling prolongs yeast lifespan, as it lowers ROS accumulation [34]. Moreover, eccentric exercise causes ROS accumulation [35] leading to mild muscle damage. Given that H2S can act as a ROS scavenger [36] and/or mild mitochondrial uncoupler, it is of interest to know whether chronic low doses of H2S exposure might prolong longevity and enhance benefits of exercise by limiting ROS-induced cellular damage to macro molecules.
1.3 H2S in mitochondrial energy production and function
In many prokaryotes and eukaryotes H2S can serve as a source for ATP synthesis [37]. As such, its role in mitochondrial energy production and its influence over different mitochondrial enzymes has been studied. A bell shaped dose dependent oxygen consumption rate (OCR) response, at low concentrations stimulation of OCR and at high concentrations inhibition of OCR, was observed after treating isolated rat mitochondria with H2S donors [29, 38]. Inhibition of cytochrome c oxidase activity and mitochondrial uncoupling were observed after in vitro treatment of human colon carcinoma cells or isolated enzyme system with H2S donors [38, 39]. Interestingly, there was statistically non-significant reduction in the total ATP production that is accompanied by significant reduction in OCR after the treatment [39]. Mechanisms of such compensatory ATP production in the H2S presence are not entirely known [39].
Recently, it was proposed that mitochondria could act as a source for carbon (organic substrates) and sulfide oxidation [40]. While sulfide oxidation provides additional supply of electrons through a sulfide oxidizing complex [consisting of sulfide quinone reductase (SQR), dioxygenase and sulfurtransferase], the electron flux from organic compound oxidation to quinone pool gets inhibited to keep the electron flux at the same level ( because of constant quinine pool) [40]. Hence the presence of H2S may lead to inhibition of organic compound oxidation and suppression of normal metabolism. Interestingly, when the electron flux from complex I was inhibited by the chemical means, maximal electron flow through the Complex III & IV and maximal OCR were observed in the presence of H2S donors, suggesting the usage of maximal compensatory electron donation from H2S when normal metabolism is inhibited [29]. These findings further imply that electron flux from H2S through SQR and complex I are in competition with each other (Fig. 2). Furthermore, it has been shown that silencing of SQR attenuates L-cysteine stimulated mitochondrial OCR, suggesting that SQR is indispensable for the electron transfer from H2S [29]. All these studies together imply that 1) SQR complex is the sole oxidizing entity and 2) H2S presence sustains ATP synthesis when normal organic metabolism is inhibited.
Figure 2.
H2S mediated ATP generation in mitochondria. In this hypothetical model, H2S not only supply electrons but also serves as a source for protons. The yet to be fully characterized protein complex may function to supply electrons to quinines and also contribute to proton gradient necessary for ATP generation as a byproduct during H2S metabolism in mitochondria. The hypothetical protein functional unit most likely involves SQR complex proteins as well [40]. Both cytoplasmic and mitochondrial pool of sulfur containing compounds (such as cysteine) contributes to the H2S production by locally residing enzymes. Both oxygen ( during hypoxic cases) or other compounds as in anaerobes [75] (during hibernation or ischemia) might accept final electrons. Presence of H2S inhibits organic compound oxidation.
Though SQR activities are present in detectable range in liver, kidney and heart tissues, no such noticeable activity was observed in brain tissue, hence the authors suggested that brain has low tolerance for sulfide poisoning, but the other organs exhibit tolerance and possibly preference to H2S oxidation over carbon oxidation [40]. Although not yet determined, presence of SQR activity in skeletal muscles would explain the earlier observation: H2S inhalation during acute exercise has no effect, as sulfide oxidation could serve as a potential source for alternative electron flux. Whether such electron flux from sulfide oxidation requires more oxygen consumption [40] awaits further investigation. Nonetheless, coupled respiration and ATP production was observed with chicken liver mitochondria, albeit at low doses of sulfide with reduced oxygen/sulfide ratio [41]. Based on these recent results [40–44], the author proposes the following model, where in SQR complex along with its associated protein entities (not completely defined) could also serve as a proton pump in addition to electron donation to coenzyme Q after sulfide oxidation (Fig. 2). If indeed such a possibility exists, then H2S can sustain alternative ATP synthesis at low doses in mammalian mitochondria. The future challenge then would be to deliver sustained low levels of H2S in the mitochondrial matrix without inhibiting CytoCOx function, as higher doses leads to uncoupling.
In addition to the ATP generating role played by H2S as mentioned above, other protective effects of H2S on mitochondrial integrity and function have been reported. Furthermore, H2S was proposed to confer protection of end organs against low oxygen and nutrient supply and its supplementation through chemical donors or enzymatic production was found to protect from ischemic injury in multiple organs including skeletal muscles [18, 30, 45–53]. The protective effects of H2S could be due to the following mechanisms: 1) H2S upregulated uncoupling proteins (UCPs) in mitochondria attenuated reactive oxygen species (ROS) production thereby prevented mitochondrial injury and consequent neuronal degeneration [54]. Given that uncoupling and ATP generation are mutually exclusive events and H2S has been implicated in both these processes, it is possible that other factors such as ROS levels, oxygen supply, nutrient supply, functional proteome and available H2S levels might have a determinant function over the final outcome and suggests dynamic nature of H2S dependent cellular energy modulation. 2) At very high concentrations H2S was proposed to inhibit cytochrome oxidase and metabolism leading to hypothermia and tissue preservation [55]. 3) Inhibition of apoptotic initiation by the H2S most likely through preservation of mitochondrial integrity [55, 56]. 4) Reducing ability of H2S was proposed to decrease oxygen utilization during mitochondrial respiration [38].
Together, all these studies imply that H2S could serve as an alternative route of energy production and suppresses ROS accumulation. These particular abilities of H2S could offer protection against low nutrient oxygen environments and might also generate enough ATP to withstand temporary adverse conditions. Consistent with this model, recent study also proposed that H2S can act as an oxygen sensor in hypoxic environments [57]. To conclude, H2S might orchestrate broader survival signals encompassing from neutralization of ROS species, production of ATP, preservation of mitochondrial function and heat generation to regulation of vascular responses, end organ nutrient supply and regeneration or protection during hypoxia. All these abilities elevate H2S as a tissue protective molecule especially in cases of crisis and most likely these phenomena also translates in skeletal muscle scenario
2. H2S and diabetes
Evidence for both pro-diabetogenic and anti-diabetogenic effects of H2S exists in the literature. Hydrogen sulfide was implicated in hypoinsulinemia and hyperglycemia as higher levels H2S and CSE expression correlated with reduced pancreatic islet insulin production in Zucker diabetic fatty rats when compared to the control Zucker fatty or lean rats [58]. Further, this study also indicated that the compound (DL-propargylglycine (PPG)) that inhibits H2S production was able to restore normal serum insulin levels [58]. Based on these findings, it was proposed that H2S mediated abnormal activation of KATP channels caused hyperpolarization of pancreatic islets and made them insensitive to hyperglycemia. In a subsequent study, it was also observed that H2S through p38 MAPK activates endoplasmic reticular stress pathways and causes apoptosis in insulin secreting β-cells [59]. Inhibition of CSE enzyme using pharmacological means resulted in alleviation of acute pancreatitis and lung injury in animal models of pancreatitis [60]. This study suggested that H2S might augment pancreatic injury and might diminish insulin output. The effects of H2S over KATP channels can be explained by its influence as a metabolic suppressor and mitochondrial uncoupler, as described above, and need to be investigated further for mechanistic insights.
In contrast to the above studies, high fructose diet-induced type II diabetes in rats was found to associate with the decline in serum H2S levels [61]. Interestingly, chronic feeding of raw garlic paste, a commonly used natural food spice containing H2S donor substances, could not only increase serum H2S levels but also correlated with improved insulin resistance, reduced triglyceride levels, and enhanced oxidative stress tolerance [61]. Though other studies also reported hypoglycemic effects of garlic extracts in different rat and rabbit models of diabetes, concomitant plasma/serum levels of H2S were not enumerated in these studies [62–68]. One study indicated no effect of garlic extracts on hyperglycemia [69]. Another recent report suggested that either systemic increase or decrease in H2S levels by pharmacological means causes reduction in insulin resistance [70], implying varied organ specific metabolic responses to different doses of H2S.
Future studies are necessary to clarify the above mentioned contradictory nature of H2S effects on blood glucose and insulin levels. Potential factors for consideration are 1) enhancement of H2S detection limits without compromising sensitivity and 2) enumeration of long-term effects of H2S with tandem usage of natural compounds, chemical H2S donors and endogenous enzymatic enhancers. Different genetic backgrounds and tissue specific responses also need to be evaluated. Notably, the H2S dose should also be carefully considered to distinguish between physiological and pathological responses.
3. H2S and obesity
Recently, endogenous CBS and CSE expression and H2S production were detected in adipose tissues [71]. Although, no significant amount of CBS enzyme have been detected in brown fat, levels of CBS in other adipose tissues (perirenal and epididymal fat) are substantial. Expression of CSE has been more versatile across different adipose tissues. Based on these observations, CSE could act as a main contributor for H2S production in adipose tissue [71]. The levels of H2S in adipose tissue are comparable to that of the major endogenous H2S producing organs [71]. Contribution of 3-MST for adipose tissue derived H2S and its expression in different kinds of adipose tissues are yet to be unraveled. Along with increase of mice age, adipose tissue was observed to express higher levels of CSE protein and also generated larger amounts of H2S [71]. As fat mass in any given individual is influenced by age, diet, physical activity and genetic background, determination of adipose derived H2S contribution to the total plasma H2S pool would serve to clarify its contribution to metabolic alterations associated with obesity.
As with diabetes, role of H2S and its association with obesity are not straightforward. In rodent diabetic models, H2S was found to inhibit glucose uptake by mature adipocytes in a dose dependent manner with or without insulin presence [71]. Importantly, fructose induced insulin resistance was also correlated with enhanced CSE expression and H2S production in epididymal fat tissue [71]. Given that fructose enhances de novo fat synthesis, contribution of fructose induced H2S in fat synthesis is not known. Interestingly, it was recently noted that pharmacological enhancement of H2S levels also reduced lipolysis [70]. Together these studies suggested that a combination of enhanced lipogenesis and reduced lipolysis are associated with increased H2S levels and rise in H2S levels may cause obesity syndrome.
In contrast to the H2S mediated negative effects observed in animal studies involving diabetic models, a recent human prospective study encompassing small group of participants showed that plasma H2S levels are negatively correlated with type II diabetes [72]. Furthermore, greater waist to hip ratio (higher in obese people) was independently associated with reduced plasma H2S levels after adjusting for systolic blood pressure, microvascular function, insulin sensitivity, glycemic control and lipid profile variables [72]. In recent studies, link between H2S effects and UCPs have been observed [39, 54]. Whether H2S induces UCPs in different tissues, especially in adipose tissue is currently unknown. Given that UCPs are important regulators of browning of white fat, it is most likely that the negative correlation between H2S levels and fat accumulation in humans may provide a future therapeutic intervention if H2S upregulates UCPs in adipose tissue. The contradictory nature of observed correlations between H2S and obesity syndrome in different species need to be reconciled further to better understand H2S mediated signaling in adipose tissues.
Conclusions
The salient points from our review with regard to the functions of H2S in skeletal muscle biology and metabolism are the following: 1) There are species specific and tissue specific variations with regard to expression of enzymatic endogenous H2S producers and levels of ambient H2S production. The significance of such variation is currently unknown. Though there have been significant improvements in detection of H2S quantities in tissues, there is a need for ultrasensitive, dynamic detection of de novo H2S production. 2) There are limited studies with regard to the role of H2S in skeletal muscle biology and disease. 3) Though, H2S has been perceived to serve as an alternative electron donor and modulates oxygen consumption, its role in mitochondrial function and ATP production is yet to be fully uncovered in different pathophysiological scenarios. 4) Many studies point to the protective function of H2S on mitochondrial integrity and tissue preservation especially during crisis situations. Yet there is a need for comprehensive delineation of mechanistic aspects of such protection. 5) The role of H2S in diabetes and obesity is rather controversial and need to be fully illustrated further.
Highlights.
Tissue and species specific differences in endogenous H2S generators are present.
H2S role in skeletal muscle biology and disease is not yet known.
H2S might function in anaerobic ATP generation and mitochondrial protection.
H2S role in metabolic diseases is controversial and needs further investigation.
Acknowledgements
A part of the review paper is supported by the NIH grants (HL-74185, NS-84823, and HL-108621) to Dr. Tyagi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bioliography
- 1.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxidants & redox signaling. 2011;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science (New York, NY) 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernard CE, Furne JK, Levitt MD, Farrugia G, Szurszewski JH. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. Journal of neurochemistry. 2008;106:1577–1585. doi: 10.1111/j.1471-4159.2008.05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci U S A. 2013;110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchini F, Vainio H. Allium vegetables and organosulfur compounds: do they help prevent cancer? Environmental health perspectives. 2001;109:893–902. doi: 10.1289/ehp.01109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 8.Nagahara N, Ito T, Kitamura H, Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochemistry and cell biology. 1998;110:243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 9.Kuo SM, Lea TC, Stipanuk MH. Developmental pattern, tissue distribution, and subcellular distribution of cysteine: alpha-ketoglutarate aminotransferase and 3-mercaptopyruvate sulfurtransferase activities in the rat. Biology of the neonate. 1983;43:23–32. doi: 10.1159/000241634. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 11.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? Faseb j. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 12.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiological reviews. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 13.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. 2013;14:15074–15091. doi: 10.3390/ijms140715074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen NC, Yang F, Capecci LM, Gu ZY, Schafer AI, Durante W, Yang XF, Wang H. Regulation of homocysteine metabolism and methylation in human and mouse tissues. Faseb Journal. 2010;24:2804–2817. doi: 10.1096/fj.09-143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du JT, Li W, Yang JY, Tang CS, Li Q, Jin HF. Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress. Chinese Med J-Peking. 2013;126:930–936. [PubMed] [Google Scholar]
- 17.Aminlari M, Gilanpour H, Taghavianpour H, Veseghi T. Comparative studies on the distribution of rhodanese and beta-mercaptopyruvate sulfurtransferase in different organs of sheep (Ovis aries) and cattle (Bos taurus) Comparative biochemistry and physiology C, Comparative pharmacology and toxicology. 1989;92:259–262. doi: 10.1016/0742-8413(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 18.Du JT, Li W, Yang JY, Tang CS, Li Q, Jin HF. Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress. Chinese medical journal. 2013;126:930–936. [PubMed] [Google Scholar]
- 19.Kado DM, Bucur A, Selhub J, Rowe JW, Seeman T. Homocysteine levels and decline in physical function: MacArthur studies of successful aging. American Journal of Medicine. 2002;113:537–542. doi: 10.1016/s0002-9343(02)01269-x. [DOI] [PubMed] [Google Scholar]
- 20.Veeranki S, Tyagi SC. Defective Homocysteine Metabolism: Potential Implications for Skeletal Muscle Malfunction. International journal of molecular sciences. 2013;14:15074–15091. doi: 10.3390/ijms140715074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice Deficient in Cystathionine Beta-Synthase - Animal-Models for Mild and Severe Homocyst(E)Inemia. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert K, Vialard F, Thiery E, Toyama K, Sinet PM, Janel N, London J. Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2003;51:363–371. doi: 10.1177/002215540305100311. [DOI] [PubMed] [Google Scholar]
- 23.Kundu S, Pushpakumar S, Khundmiri SJ, Sen U. Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling. Biochimica et biophysica acta. 2014;1843:2816–2826. doi: 10.1016/j.bbamcr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamat PK, Kalani A, Tyagi SC, Tyagi N. Hydrogen Sulfide Epigenetically Attenuates Homocysteine-Induced Mitochondrial Toxicity Mediated through NMDA Receptor in Mouse Brain Endothelial (bEnd3) Cells. Journal of cellular physiology. 2014 doi: 10.1002/jcp.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abi Khalil C. The emerging role of epigenetics in cardiovascular disease. Therapeutic advances in chronic disease. 2014;5:178–187. doi: 10.1177/2040622314529325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanan N, Pushpakumar SB, Givvimani S, Kundu S, Metreveli N, James D, Bratcher AP, Tyagi SC. Epigenetic regulation of aortic remodeling in hyperhomocysteinemia. Faseb j. 2014 doi: 10.1096/fj.14-250183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, Caldwell RB, Caldwell RW. Diabetes-induced vascular dysfunction involves arginase I. Am J Physiol Heart Circ Physiol. 2012;302:H159–H166. doi: 10.1152/ajpheart.00774.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright JA, Richards T, Becker DL. Connexins and diabetes. Cardiology research and practice. 2012;2012:496904. doi: 10.1155/2012/496904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British journal of pharmacology. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhambhani Y, Burnham R, Snydmiller G, MacLean I. Effects of 10-ppm hydrogen sulfide inhalation in exercising men and women. Cardiovascular, metabolic, and biochemical responses. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 1997;39:122–129. doi: 10.1097/00043764-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Bhambhani Y, Burnham R, Snydmiller G, MacLean I, Martin T. Effects of 5 ppm hydrogen sulfide inhalation on biochemical properties of skeletal muscle in exercising men and women. American Industrial Hygiene Association journal. 1996;57:464–468. doi: 10.1080/15428119691014819. [DOI] [PubMed] [Google Scholar]
- 33.Bhambhani Y, Singh M. Physiological-Effects of Hydrogen-Sulfide Inhalation during Exercise in Healthy-Men. Journal of applied physiology. 1991;71:1872–1877. doi: 10.1152/jappl.1991.71.5.1872. [DOI] [PubMed] [Google Scholar]
- 34.Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- 35.Silva LA, Bom KF, Tromm CB, Rosa GL, Mariano I, Pozzi BG, Tuon T, Stresck EL, Souza CT, Pinho RA. Effect of eccentric training on mitochondrial function and oxidative stress in the skeletal muscle of rats. Brazilian Journal of Medical and Biological Research. 2013;46:14–20. doi: 10.1590/1414-431X20121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS. Hydrogen Sulfide, the Next Potent Preventive and Therapeutic Agent in Aging and Age-Associated Diseases. Mol Cell Biol. 2013;33:1104–1113. doi: 10.1128/MCB.01215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd D. Hydrogen sulfide: clandestine microbial messenger? Trends in microbiology. 2006;14:456–462. doi: 10.1016/j.tim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Collman JP, Ghosh S, Dey A, Decreau RA. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc Natl Acad Sci U S A. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Bba-Gen Subjects. 2005;1725:201–212. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochimica et biophysica acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Yong R, Searcy DG. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 2001;129:129–137. doi: 10.1016/s1096-4959(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 42.Jackson MR, Melideo SL, Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry-Us. 2012;51:6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 43.Kabil O, Banerjee R. Redox Biochemistry of Hydrogen Sulfide. Journal of Biological Chemistry. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. Febs J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu YH, Lu M, Bian JS. Hydrogen sulfide and renal ischemia. Expert review of clinical pharmacology. 2011;4:49–61. doi: 10.1586/ecp.10.127. [DOI] [PubMed] [Google Scholar]
- 46.Skovgaard N, Gouliaev A, Aalling M, Simonsen U. The role of endogenous H2S in cardiovascular physiology. Curr Pharm Biotechnol. 2011;12:1385–1393. doi: 10.2174/138920111798280956. [DOI] [PubMed] [Google Scholar]
- 47.Luan HF, Zhao ZB, Zhao QH, Zhu P, Xiu MY, Ji Y. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 2012;45:898–905. doi: 10.1590/S0100-879X2012007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henderson PW, Singh SP, Weinstein AL, Nagineni V, Rafii DC, Kadouch D, Krijgh DD, Spector JA. Therapeutic metabolic inhibition: hydrogen sulfide significantly mitigates skeletal muscle ischemia reperfusion injury in vitro and in vivo. Plast Reconstr Surg. 2010;126:1890–1898. doi: 10.1097/PRS.0b013e3181f446bc. [DOI] [PubMed] [Google Scholar]
- 49.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxidants & redox signaling. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 50.Henderson PW, Jimenez N, Ruffino J, Sohn AM, Weinstein AL, Krijgh DD, Reiffel AJ, Spector JA. Therapeutic delivery of hydrogen sulfide for salvage of ischemic skeletal muscle after the onset of critical ischemia. J Vasc Surg. 2011;53:785–791. doi: 10.1016/j.jvs.2010.10.094. [DOI] [PubMed] [Google Scholar]
- 51.Li HH, Xu J, Wasserloos KJ, Li J, Tyurina YY, Kagan VE, Wang X, Chen AF, Liu ZQ, Stoyanovsky D, Pitt BR, Zhang LM. Cytoprotective effects of albumin, nitrosated or reduced, in cultured rat pulmonary vascular cells. American journal of physiology Lung cellular and molecular physiology. 2011;300:L526–L533. doi: 10.1152/ajplung.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ball CJ, Reiffel AJ, Chintalapani S, Kim M, Spector JA, King MR. Hydrogen sulfide reduces neutrophil recruitment in hind-limb ischemia-reperfusion injury in an L-selectin and ADAM-17-dependent manner. Plast Reconstr Surg. 2013;131:487–497. doi: 10.1097/PRS.0b013e31827c6e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villamaria CY, Fries CA, Spencer JR, Roth M, Davis MR. Hydrogen sulfide mitigates reperfusion injury in a porcine model of vascularized composite autotransplantation. Annals of plastic surgery. 2014;72:594–598. doi: 10.1097/SAP.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 54.Lu M, Zhao FF, Tang JJ, Su CJ, Fan Y, Ding JH, Bian JS, Hu G. The neuroprotection of hydrogen sulfide against MPTP-induced dopaminergic neuron degeneration involves uncoupling protein 2 rather than ATP-sensitive potassium channels. Antioxid Redox Signal. 2012;17:849–859. doi: 10.1089/ars.2011.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo W, Kan JT, Cheng ZY, Chen JF, Shen YQ, Xu J, Wu D, Zhu YZ. Hydrogen Sulfide as an Endogenous Modulator in Mitochondria and Mitochondria Dysfunction. Oxidative medicine and cellular longevity. 2012 doi: 10.1155/2012/878052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu N, Dong M, Ren J. Hydrogen sulfide alleviates cardiac contractile dysfunction in an Akt2-knockout murine model of insulin resistance: role of mitochondrial injury and apoptosis. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306:R761–R771. doi: 10.1152/ajpregu.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson KR. Hydrogen sulfide as an oxygen sensor. Clin Chem Lab Med. 2013;51:623–632. doi: 10.1515/cclm-2012-0551. [DOI] [PubMed] [Google Scholar]
- 58.Wu L, Yang W, Jia X, Yang G, Duridanova D, Cao K, Wang R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Laboratory investigation; a journal of technical methods and pathology. 2009;89:59–67. doi: 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- 59.Yang GD, Yang W, Wu LY, Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. Journal of Biological Chemistry. 2007;282:16567–16576. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- 60.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. Faseb j. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 61.Padiya R, Khatua TN, Bagul PK, Kuncha M, Banerjee SK. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr Metab. 2011;8 doi: 10.1186/1743-7075-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jalal R, Bagheri SM, Moghimi A, Rasuli MB. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J Clin Biochem Nutr. 2007;41:218–223. doi: 10.3164/jcbn.2007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Demerdash FM, Yousef MI, Abou El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Jain RC, Vyas CR. Garlic in Alloxan-Induced Diabetic Rabbits. American Journal of Clinical Nutrition. 1975;28:684–685. doi: 10.1093/ajcn/28.7.684. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasan K. Plant foods in the management of diabetes mellitus: Spices as beneficial antidiabetic food adjuncts. Int J Food Sci Nutr. 2005;56:399–414. doi: 10.1080/09637480500512872. [DOI] [PubMed] [Google Scholar]
- 66.Joharchi K, Jorjani M. The role of nitric oxide in diabetes-induced changes of morphine tolerance in rats. European journal of pharmacology. 2007;570:66–71. doi: 10.1016/j.ejphar.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 67.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 68.Masjedi F, Gol A, Dabiri S. Preventive Effect of Garlic (Allium sativum L.) on Serum Biochemical Factors and Histopathology of Pancreas and Liver in Streptozotocin-Induced Diabetic Rats. Iran J Pharm Res. 2013;12:325–338. [PMC free article] [PubMed] [Google Scholar]
- 69.Baluchnejadmojarad T, Roghani M. Garlic extract attenuates time-dependent changes in the reactivity of isolated aorta in streptozotocin-diabetic rats. Life Sci. 2003;73:2281–2289. doi: 10.1016/s0024-3205(03)00604-0. [DOI] [PubMed] [Google Scholar]
- 70.Geng B, Cai B, Liao F, Zheng Y, Zeng Q, Fan X, Gong Y, Yang J, Cui QH, Tang C, Xu GH. Increase or decrease hydrogen sulfide exert opposite lipolysis, but reduce global insulin resistance in high fatty diet induced obese mice. PloS one. 2013;8:e73892. doi: 10.1371/journal.pone.0073892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng XJ, Chen Y, Zhao J, Tang CS, Jiang ZS, Geng B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochemical and biophysical research communications. 2009;380:153–159. doi: 10.1016/j.bbrc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 72.Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 73.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54:2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- 74.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 75.Friedrich CG, Rother D, Bardischewsky F, Quentmeier A, Fischer J. Oxidation of reduced inorganic sulfur compounds by bacteria: emergence of a common mechanism? Applied and environmental microbiology. 2001;67:2873–2882. doi: 10.1128/AEM.67.7.2873-2882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]