Abstract
The vertebrate inner ear is a morphologically complex sensory organ comprised of two compartments, the dorsal vestibular apparatus and the ventral cochlear duct, required for motion and sound detection, respectively. Fgf10, in addition to Fgf3, is necessary for the earliest stage of otic placode induction, but continued expression of Fgf10 in the developing otic epithelium, including the prosensory domain and later in Kolliker’s organ, suggests additional roles for this gene during morphogenesis of the labyrinth. While loss of Fgf10 was implicated previously in semicircular canal agenesis, we show that Fgf10−/+ embryos also exhibit a reduction or absence of the posterior semicircular canal, revealing a dosage-sensitive requirement for FGF10 in vestibular development. In addition, we show that Fgf10−/− embryos have previously unappreciated defects of cochlear morphogenesis, including a somewhat shortened duct, and, surprisingly, a substantially narrower duct. The mutant cochlear epithelium lacks Reissner’s membrane and a large portion of the outer sulcus--two non-contiguous, non-sensory domains. Marker gene analyses revealed effects on Reissner’s membrane as early as E12.5–E13.5 and on the outer sulcus by E15.5, stages when Fgf10 is expressed in close proximity to Fgfr2b, but these effects were not accompanied by changes in epithelial cell proliferation or death. These data indicate a dual role for Fgf10 in cochlear development: to regulate outgrowth of the duct and subsequently as a bidirectional signal that sequentially specifies Reissner’s membrane and outer sulcus non-sensory domains. These findings may help to explain the hearing loss sometimes observed in LADD syndrome subjects with FGF10 mutations.
Keywords: FGF signaling, cochlea, Reissner’s membrane, outer sulcus
Introduction
The mammalian inner ear is a morphologically complex sensory organ with two functionally distinct compartments, the ventral auditory and dorsal vestibular divisions, which are responsible for the perception of sound and movement, respectively. The auditory compartment, the cochlea, derives from a ventral outgrowth of the otic vesicle epithelium, which undergoes progressive extension and, in mammals, coils as it matures. Vestibular structures derive from dorsal and lateral evaginations of the otic epithelium and are sculpted into the three semicircular canals used to detect angular acceleration, as well as two central pouches, the utricle and saccule, used to detect linear acceleration. The inner ear epithelium also has a dorsally projecting non-sensory appendage, the endolymphatic duct and sac, which maintains the unique ionic composition of the endolymph fluid in the inner ear lumen and is essential for normal sensory functions (Groves and Fekete, 2012; Wu and Kelley, 2012).
Gross morphogenesis of the inner ear epithelium is largely complete by E15.5 in the mouse (Morsli et al., 1998), at which time the cochlea has achieved 1.75 turns and remodeling of dorsal orthogonal epithelial pouches has resulted in formation of the three patent semicircular canals (see Fig. 1A). Differentiation of the epithelium into distinct sensory and non-sensory domains with their characteristic cell types occurs concomitantly with gross morphogenesis and is not complete until well after birth in mouse. Although many human sensorineural deafness and balance disorders are caused by genetic or environmental insults that affect the function of particular inner ear cell types and are not detectable radiologically, almost 40% of inner ear dysfunction may be accompanied by congenital malformation of the epithelium (Mafong et al., 2002; Wu et al., 2005) and the presence of malformations impacts treatment plans. Therefore, to advance both treatment and ultimately prevention, it is essential to elucidate the functions of signals controlling otic morphogenesis.
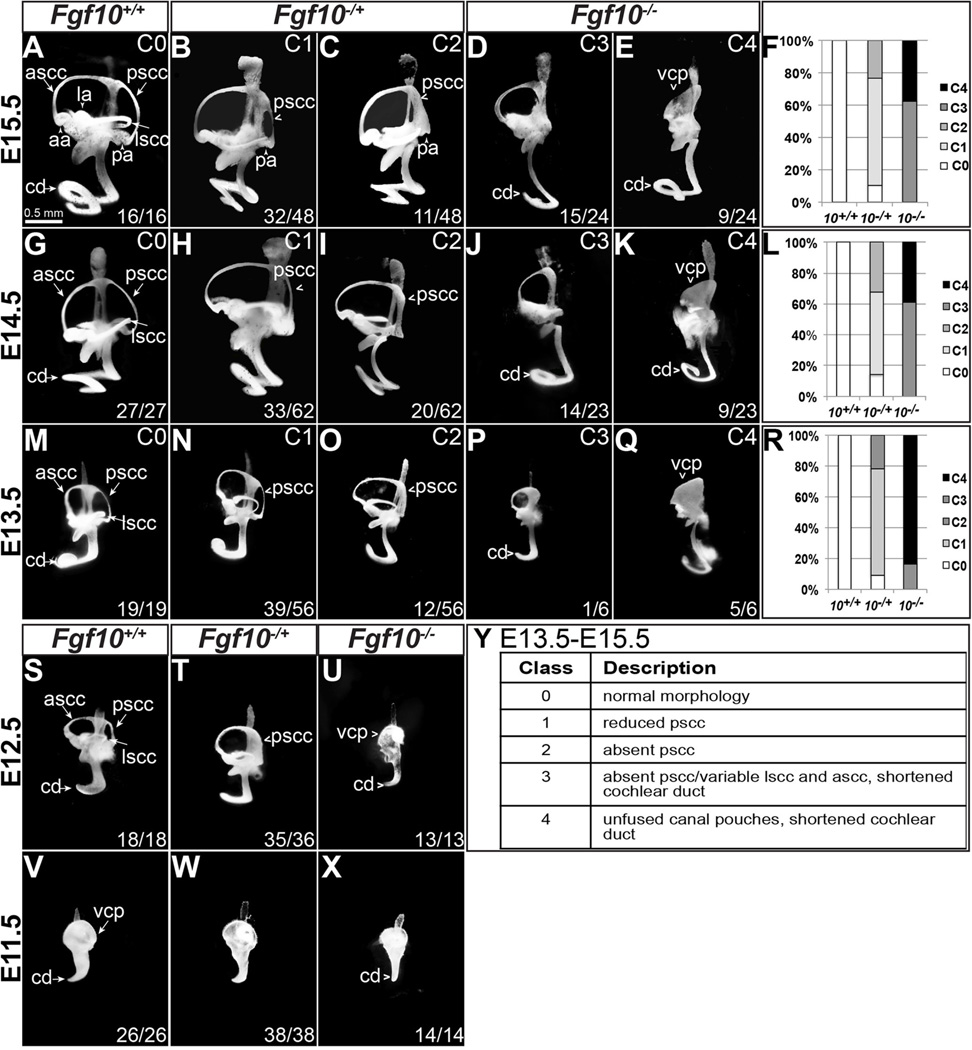
Figure 1. Vestibular morphogenesis is affected in both Fgf10 heterozygous and Fgf10 homozygous null mutants, whereas cochlear development is affected only in homozygotes.
(A–E, G–K, M–Q, S–X) Inner ear epithelia were filled with paint at the stages indicated to the left of each row. Genotypes are indicated at the top of each column. The phenotypic class designations for E13.5-E15.5 samples as described in the main text (C0–C4) are summarized in panel Y and indicated in the upper right portion of each panel. The percentage of each E13.5–E15.5 genotype falling into each phenotypic class is shown (F, L, R). Abbreviations: aa, anterior ampula; ascc, anterior semicircular canal; cd, cochlear duct; la, lateral ampula; lscc, lateral semicircular canal; pa, posterior ampula; pscc, posterior semicircular canal; vcp, vertical canal plate.
Genetic analyses show that FGF signaling is particularly important for inner ear morphogenesis. Multiple FGFs functioning from nearby tissues induce formation of the otic placode in posterior head ectoderm (Alvarez et al., 2003; Ladher et al., 2005; Wright and Mansour, 2003; Zelarayan et al., 2007). The otic placode then invaginates and closes, forming the spherical otic vesicle. Several FGFs, whether signaling from the nearby hindbrain (FGF3) or from within the otic epithelium itself (FGF3, FGF9 and FGF10) are required for subsequent morphogenesis of the dorsal otic vesicle. Mice that lack Fgf3 function fail with variable penetrance and expressivity to form an endolymphatic duct and the affected otic vesicles develop variably, with impacts on both vestibular and cochlear morphogenesis and function (Hatch et al., 2007; Mansour et al., 1993). The relative contributions of hindbrain and otic epithelial Fgf3 to these phenotypes have not been determined.
Mice lacking Fgf9, which is expressed in non-sensory domains of both the vestibular and cochlear epithelia, have severe abnormalities of vestibular morphogenesis. Semicircular canals are absent or rudimentary and these phenotypes are thought to arise from disrupted communication between the otic epithelium and the surrounding mesenchyme. The cochlear abnormalities of Fgf9 mutants are subtle. The cochlear epithelium appears relatively normal, but mesothelial cells adjacent to the epithelial component of Reissner’s membrane, which is normally a site of Fgf9 expression, fail to attach to the membrane and the adjacent scala vestibuli (a channel cleared of mesenchyme and filled with perilymph) is enlarged. This is consistent with a disruption of epithelial/mesenchymal communication (Pirvola et al., 2004).
Finally, mice lacking Fgf10, which is expressed in both vestibular and cochlear prosensory epithelial tissue as well as in the developing otic ganglion, show complete agenesis of the posterior sensory and non-sensory vestibular tissue (crista and posterior semicircular canal, respectively), and have milder deformations of the anterior and lateral cristae and their associated semicircular canals. The otic ganglion forms normally, but innervation of the vestibular system is disrupted, presumably due to the absence of targets (Ohuchi et al., 2005; Pauley et al., 2003). In contrast, no specific abnormalities of cochlear histogenesis or morphogenesis were described, although Pauley et al. (2003) noted a slight shortening of the cochlear duct. The individual contributions of otic ganglion vs. epithelial sources of Fgf10 are unknown.
As a prelude to studies dissecting the individual and combinatorial requirements for epithelial Fgf3 and Fgf10 in mouse otic morphogenesis, we observed that global Fgf10 heterozygous inner ears also presented with vestibular defects (a small or absent posterior semicircular canal). Furthermore, the Fgf10 homozygous null mutant cochlear duct was noticeably shorter and, most surprisingly, narrower than that of heterozygous or wild type littermates. Histologic and marker analyses revealed that while the cochlear sensory domain and two non-sensory domains, Kolliker’s organ (the inner sulcus) and the stria vascularis, appeared to develop normally, epithelial tissue in two other non-contiguous non-sensory domains was deleted; namely, all of Reissner’s membrane and a substantial portion of the outer sulcus. Analysis of molecular markers during development showed that the Reissner’s membrane defect preceded the outer sulcus defect, and that both could be correlated to loss of signaling from FGF10 to its major receptor, FGFR2b, at times when the ligand and receptor were in close proximity. However, we did not detect differences between controls and mutants with respect to proliferation or cell death. Taken together, these data demonstrate a previously unappreciated role for cochlear FGF10 as a bi-directional signal that promotes sequential specification of noncontiguous non-sensory domains of the cochlear epithelium. This is the first report of such a signal and our findings have implications for understanding the hearing loss of LADD syndrome individuals bearing mutations in FGF10.
Materials and methods
These studies complied with protocols approved by the University of Utah Institutional Animal Care and Use Committee.
Generation of Fgf10 null embryos
Generation and PCR genotyping of the Fgf10 null allele (Fgf10−, also known as Fgf10Δ2 and formally designated Fgf10tm1.1Sms; MGI:3526181) were described previously (Urness et al., 2010). Fgf10−/+ mice were maintained on a mixed genetic background comprised of C57Bl/6 and various 129 substrains. Fgf10−/+ adults were intercrossed and noon of the day on which a mating plug was observed was designated as E0.5.
Paintfilling of embryonic inner ears
E11.5–E15.5 embryos were fixed in Bodian’s solution, dehydrated in ethanol, cleared with methyl salicylate and the inner ear lumens filled with white latex paint as described (Kiernan, 2006; Morsli et al., 1998). Filled ears were roughly dissected and photographed under darkfield illumination using a QImaging Micropublisher digital camera mounted on a Zeiss Discovery V.12 microscope.
RNA in situ hybridization to paraffin sections
Heads of E12.5–E18.5 intercross embryos were fixed in modified Carnoy’s solution, embedded in paraffin (Paraplast X-tra) and 10 µm sections were dewaxed, blocked and hybridized with digoxigenin-labeled anti-sense RNA probes, which were detected with alkaline phosphatase conjugated anti-digoxigenin antibodies as described (Urness et al., 2008). Probes for Fgf10, Myo6, Lgr5, Fgfr1, Spry2, Spry1, Bmp4, Fgfr2, Fgf9, and Lnfg were generated by transcription of cDNA-containing plasmids. A list of the template plasmids and acknowledgements is found in Supplementary Table 1. The rest of the RNA probes were generated by transcription of a PCR-amplified, gene-specific 3’ UTR fragment containing a T7 promoter. The primer sequences can be found in Supplementary Table 2.
Immunofluorescence analysis of inner ear frozen sections
Whole E18.5 heads were bisected in the sagittal or transverse plane and fixed for 2 hours at room temperature in 4% paraformaldehyde prepared in phosphate buffered saline (PBS). Heads were cryoprotected and embedded in sucrose/gelatin as described (Hurley et al., 2003) with the following modifications: 5% sucrose infiltration overnight at 4°C, 15% sucrose infiltration overnight at 4°C, 15% sucrose/7.5% gelatin (Bloom 300, Sigma G2500) infiltration at 37°C overnight. Samples were cryosectioned at 10 µm thickness (6 µm for proliferation studies) in the sagittal plane, collected on SuperFrost Plus slides and stored at −20°C. Primary antibodies were diluted into PBS/5% normal serum of the secondary antibody species/0.2% Triton X-100 and applied at the following dilutions: mouse anti-p27[Kip1] (BD Biosciences #610241), 1:300; rat anti-CD44 (BD Biosciences #550538), 1:800; rabbit anti-MYO7A (Proteus Biosciences #25-6790), 1:800; goat anti-SOX2 (Santa Cruz Biotechnology #sc-17320), 1:200; rabbit anti-S100 (Millipore #07-476), 1:1000; rabbit anti-cleaved Caspase-3 (Cell Signaling 9661), 1:100; rabbit anti-phospho-Histone H3 (Millipore 06-570), 1:400. Permeabilization was enhanced by one hr incubation in PBS/1% deoxycholate/0.2% Triton X-100 at room temperature. Secondary antibodies were all from Invitrogen and diluted 1:350 into PBST/5% normal serum (Alexa Fluor® 594 goat anti-rat (A11007); Alexa Fluor® 488 goat anti-rabbit (A11034); and Alexa Fluor® 594 donkey anti-goat (A11058) Alexa Fluor® 488 donkey anti-goat (A11055); Alexa Fluor® 488 donkey anti-rabbit (A21206). DAPI was included in the mounting medium (Vectashield, Vector Labs) and fluorescent signal channels were overlaid using Photoshop CS4.
Analysis of cochlear duct proliferation
Pregnant dams (E13.5) were injected once with BrdU solution (50 µg/g body weight, Invitrogen 00-0103), harvested one hour later and fixed overnight in 4% PFA. Heads were infiltrated with sucrose/gelatin and 6 µm cryosections prepared as described above. DNA was denatured by incubating the slides in 1N HCl at 48° for 30 minutes, followed by neutralization in PBS. BrdU was detected as described above using a mouse monoclonal antibody, MoBu-1 (Invitrogen B35128) at 1:100 and Alexa Fluor® 594 goat anti-mouse (Invitrogen A11032). For analysis at E11.5, cryosections were prepared and phospho-Histone H3 was detected as described above. To differentiate between sensory and non-sensory duct domains, we co-stained the sections to detect SOX2 also as described above. E11.5 data came from 8 sections in each of 6 controls and 6 Fgf10 null mutants. E13.5 data came from 5 sections in each of 4 controls and 4 Fgf10 null mutants. Student’s t-test (unpaired, two-tailed; Prism 6.0) was used to compare the mean number of BrdU or phospho-Histone H3-positive cells per unit area in control and Fgf10−/− samples. SOX2-positive and SOX2-negative domains were considered separately.
Cochlear duct cross-sectional area analysis
For the E18.5 measurements of the three scalae, we photographed the cochlea in H&Estained sagittal sections of the head. Three sections spaced at 50 µm intervals near the center of each of 3 control and 3 Fgf10−/− inner ears were analyzed. The AxioVison measurement module (Zeiss) was used to measure the area of each the three scalae in the basal cross section and the three measurements for each sample were averaged. The average area of each scala from the 3 control and 3 mutant samples were compared using multiple t tests. Statistical significance was determined using the Holm-Sidak method (Prism 6.0 software). For E12.5 and E13.5 scala media area measurements, three contiguous sections were photographed from the cochlear base of 3 control and 3 Fgf10−/− inner ears sectioned coronally. For E15.5 scala media area measurements, three contiguous images were captured representing the most basal turn. Area measurements were captured and analyzed as described above.
Results
Fgf10 is required for both vestibular and cochlear morphogenesis
It is well known that Fgf10 is required for morphogenesis of the vestibular system. Specifically, Fgf10 null inner ears lack posterior sensory and non-sensory tissue (the cristae and semicircular canals, respectively), and have variable defects of anterior and lateral semicircular canal morphogenesis, or appear to have un-fused vertical and lateral canal pouches (Ohuchi et al., 2005; Pauley et al., 2003). The status of the cochlear duct is less clear, with one report of a somewhat shortened, but otherwise normal cochlea (Pauley et al., 2003) and another report of a cochlea of normal length (Ohuchi et al., 2005). To enable morphologic assessment of multiple inner ears of each Fgf10 genotype we intercrossed Fgf10−/+ animals, collected embryos at E15.5 when morphogenesis is virtually complete (Morsli et al., 1998), and paint-filled the inner ear epithelia. Visual inspection revealed five morphologic classes (Fig. 1A–E, Y). All 16 wild type ears were normal (Class 0, Fig. 1A). In contrast, only five of 48 heterozygous inner ears were normal, whereas 32 showed a reduced posterior semicircular canal (pscc; Class 1, Fig. 1B) and 11 virtually lacked the pscc, though the posterior ampulla (pa, housing the posterior crista) was present (Class 2, Fig. 1C). Homozygotes were even more severely affected and fell into two classes. As previously observed by other groups (Ohuchi et al., 2005; Pauley et al., 2003), all 24 Fgf10−/− inner ears lacked the posterior ampulla and canal. We also observed small and variable reductions in the lateral and anterior semicircular canals, but these were not quantified. Furthermore, all Fgf10−/− inner ears had a variably shortened cochlear duct (cd; ~0.75 to 1.5 turns; Fig. 1D, E). In 15 of 24 Fgf10−/− inner ears, the process of canal pouch fusion to make an anterior semicircular canal was complete (Class 3, Fig. 1D), whereas in the other 9 inner ears the remaining portion of the vertical canal pouch (vcp) failed to fuse and clear (Class 4, Fig. 1E). These results revealed dosage sensitive requirements for Fgf10 in vestibular morphogenesis and an unexpected requirement in cochlear morphogenesis (Fig. 1F).
To determine when the abnormalities seen in Fgf10−/+ and Fgf10−/− inner ears were first apparent, we paint-filled embryonic inner ears from Fgf10−/+ intercrosses at progressively earlier stages of development. All five previously defined classes (Fig. 1Y) were apparent at both E14.5 (Fig. 1G–K) and E13.5 (Fig. 1M–Q) in proportions roughly similar to those seen at E15.5 (Fig. 1F, L, R). Therefore the vestibular and cochlear abnormalities noted at E15.5 must have initiated before E13.5.
At E12.5, only three morphologic classes were clearly distinguishable and these correlated with genotype. At this stage there was variability across all genotypes with respect to the extent of canal pouch fusion plate clearing and this correlated with overall embryo size and developmental stage; i.e. younger embryos had uncleared fusion plates and older ones had cleared, so this parameter was not considered. All 18 E12.5 wild type inner ears were normal (Fig. 1S). Virtually all (35/36) heterozygotes showed a reduction in the posterior semicircular canal, but we could not clearly distinguish reduction from complete absence (Fig. 1T), and all 13 homozygotes had a reduced/absent posterior canal and a shortened cochlear duct (Fig. 1U). Therefore, Fgf10 is required for normal vestibular and cochlear development before E12.5.
At E11.5, a stage at which no embryonic inner ears showed canal pouch fusion plate clearing, only two morphologic classes were evident. All 26 wild type and all 38 heterozygous embryos had a normal morphology (Fig. 1V, W) and all 14 homozygotes appeared narrower than normal along the anterior-posterior axis and had a cochlear duct that had not initiated coiling (Fig. 1X). Therefore, Fgf10 heterozygosity has its first effect on vestibular morphogenesis between E11.5 and E12.5, whereas the complete absence of Fgf10 affects both vestibular and cochlear morphogenesis as early as E11.5. We did not attempt to paint-fill E10.5 intercross inner ears, but histologic studies of a limited number of samples did not reveal obvious differences between genotypes (Suppl. Fig. 1). Since the Fgf10 null vestibular phenotype has been well documented (Ohuchi et al., 2005; Pauley et al., 2003), but the cochlear phenotype is relatively unexplored, we focused subsequent studies on the cochlea.
Fgf10 is required for development of two discrete regions of non-sensory cochlear epithelial tissue
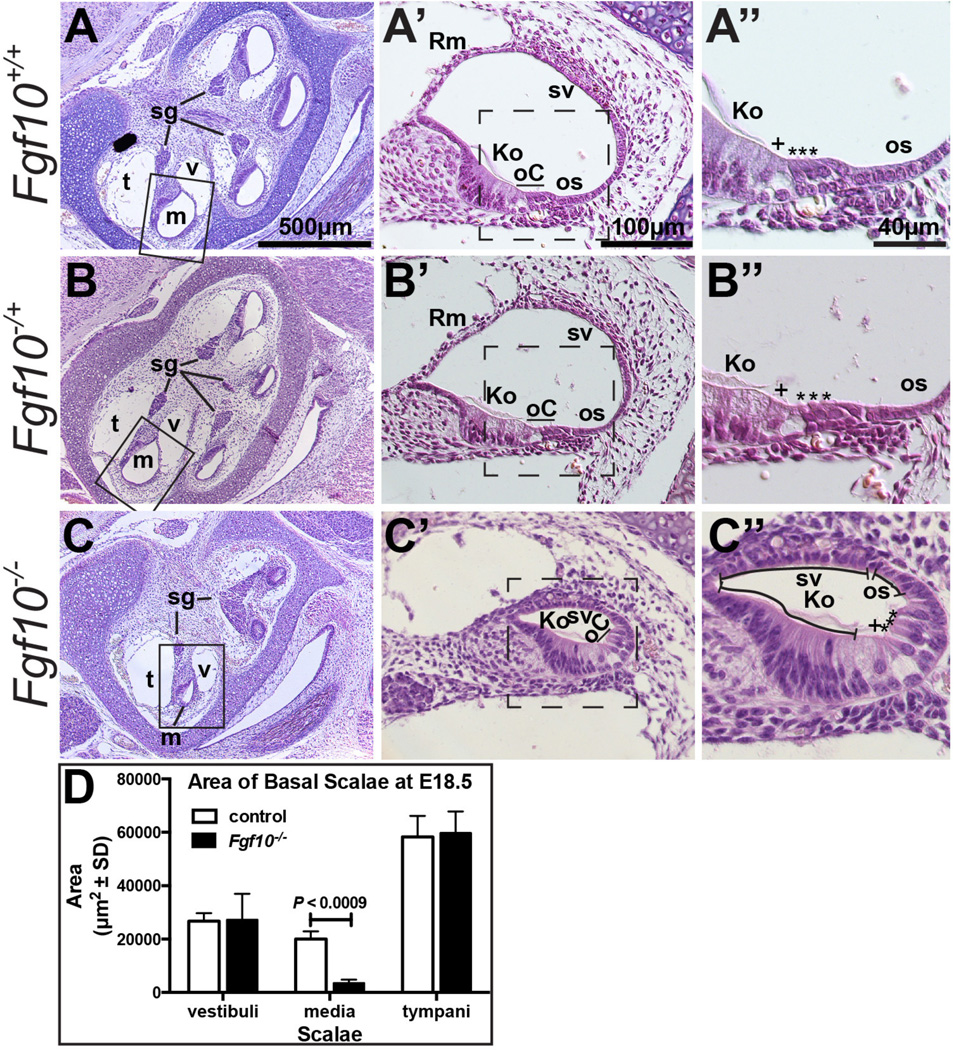
To determine whether the abnormal cochlear morphogenesis observed in Fgf10−/− inner ears was accompanied by any cellular changes, we compared hematoxylin and eosin-stained histologic sections of Fgf10−/− cochlear ducts with those from Fgf10−/+ and Fgf10+/+ embryos. As anticipated from the observations of E15.5 paint-filled inner ears, sagittal sections taken through E18.5 Fgf10+/+ and Fgf10−/+ cochleae revealed a normal auditory structure with four cross sections of the cochlear duct visible (Fig. 2A, B), whereas Fgf10−/− cochleae were shorter and less extensively coiled, with only three cross sections of the cochlear duct evident (Fig. 2C). We were surprised to find that the Fgf10 null cochlear duct (scala media, m) was not simply shorter, but also had a significantly reduced cross-sectional area. In the basal turn, the cross sectional area of the mutant scala media averaged only 17.6% of the corresponding wild type or heterozygous area (n = 3; P < 0.0009), whereas there was no significant difference between mutants and controls with respect to the cross sectional area of the basal scala tympani (t) or scala vestibuli (v) (Fig. 2D). Furthermore, compared to wild type and heterozygous cochlear ducts (Fig. 2A’, B’), Fgf10−/− cochlear ducts appeared to lack Reissner’s membrane (Rm) and much of the outer sulcus (os) (Fig. 2C’). The Fgf10−/− samples appeared to have a normally developed sensory organ of Corti (oC), as well as the non-sensory Kolliker’s organ/inner sulcus (Ko), stria vascularis (sv) and spiral ganglion (sg) (Fig. 2A’–C’). Higher magnification views confirmed the normal morphologic appearance and number of inner (+) and outer (*) hair cells, as well as a full complement of the organ of Corti supporting cells in all samples (Fig. 2A”–C”).
Figure 2. Fgf10 null cochlear ducts lack non-sensory domains and have a reduced cross-sectional area.
Hematoxylin and eosin-stained E18.5 cochlear duct cross sections at three magnifications. Boxes in A–C indicate the region magnified in A’–C’. Dashed boxes in A’–C’; indicate the region magnified in A”–C”. C” Morphologic structures remaining in Fgf10 mutants are indicated with lines. Genotypes are indicated to the left of each row. D. Graphical comparison of the cross sectional area of basal scalae (n = 3 controls and 3 mutants). Abbreviations: Ko, Kolliker’s organ; m, scala media (cochlear duct), oC, organ of Corti; os, outer sulcus; Rm, Reissner’s membrane; sg, spiral (cochlear) ganglion; sv, stria vascularis; t, scala tympani; v, scala vestibuli. Asterisks indicate outer hair cells, plus symbols indicate inner hair cells. Scale bars in A, A’, A’’ apply to all panels in the same column.
Molecular markers of Reissner’s membrane and the outer sulcus are missing or reduced in Fgf10 null cochleae
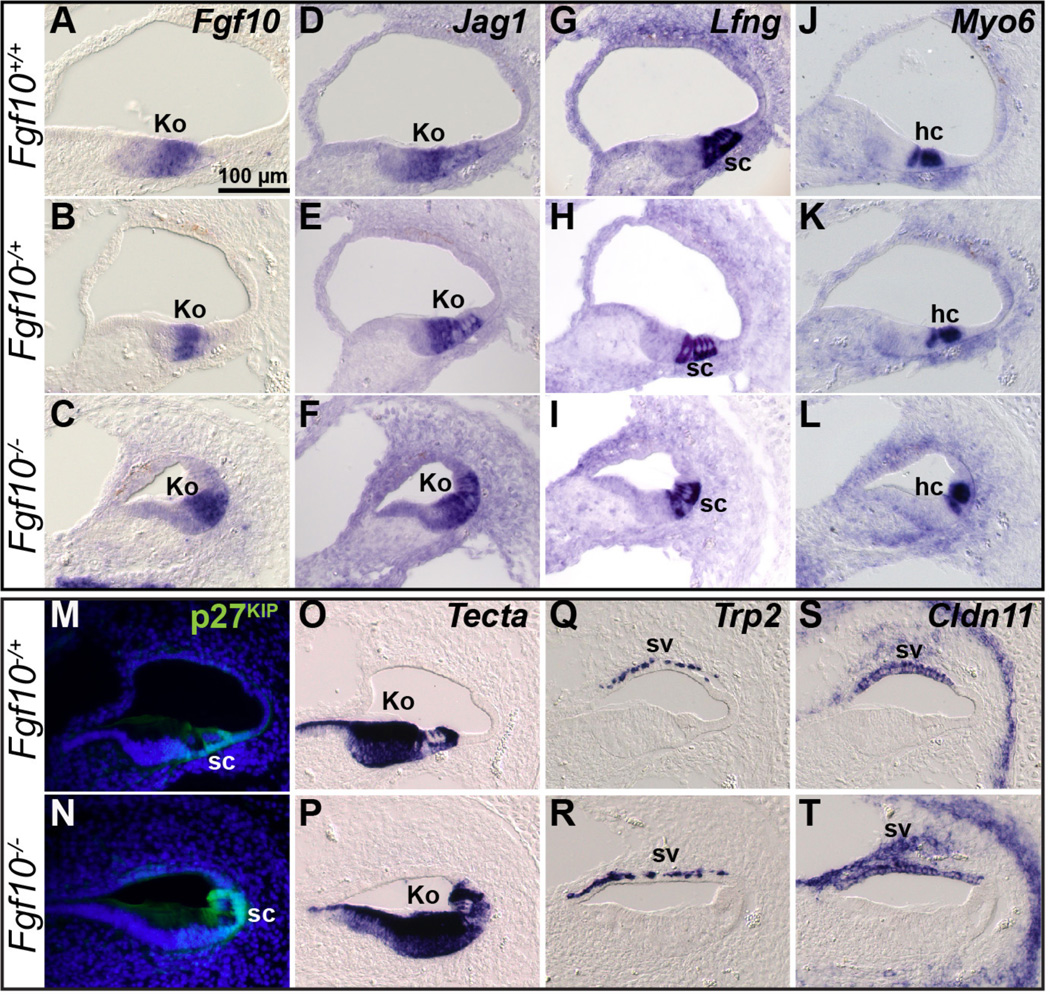
To better characterize the differences between Fgf10 genotypes at E18.5 we used RNA in situ hybridization or immunostaining of cochlear duct cross sections to detect expression of genes and proteins characteristic of the various cell types. Among these markers was Fgf10 itself, as the transcript produced by the mutant allele (a deletion of exon 2 that causes a frameshift) is stable (Urness et al., 2011). Consistent with the histologic study, no differences between genotypes were detected using markers of Kolliker’s organ (Fgf10, Jag1 and Tecta; Fig. 3A–F, O–P), hair cells (Myo6; Fig. 3J–L), organ of Corti supporting cells (Jag1, Lfng, p27Kip and Tecta; Fig. 3D–I, M–P), and the intermediate and basal cell layers of the stria vascularis (Trp2 and Cldn11, respectively; Fig. 3Q–T). Because we observed no differences in cochlear histology or marker gene expression between Fgf10+/+ and Fgf10+/− samples, we used these genotypes interchangeably as controls in subsequent analyses.
Figure 3. Markers of Kolliker’s organ, supporting cells, hair cells and the stria vascularis are unchanged in E18.5 Fgf10 null mutants.
In situ hybridization (A–L, O–T) or immunostaining analysis (M, N) of cochlear cross sections. Genotypes are indicated to the left of each row and probes are indicated at the upper right of the top panel in each series. Abbreviations: hc, hair cells; Ko, Kolliker’s organ; sc, supporting cells; sv, stria vascularis. Scale bar in A applies to all panels.
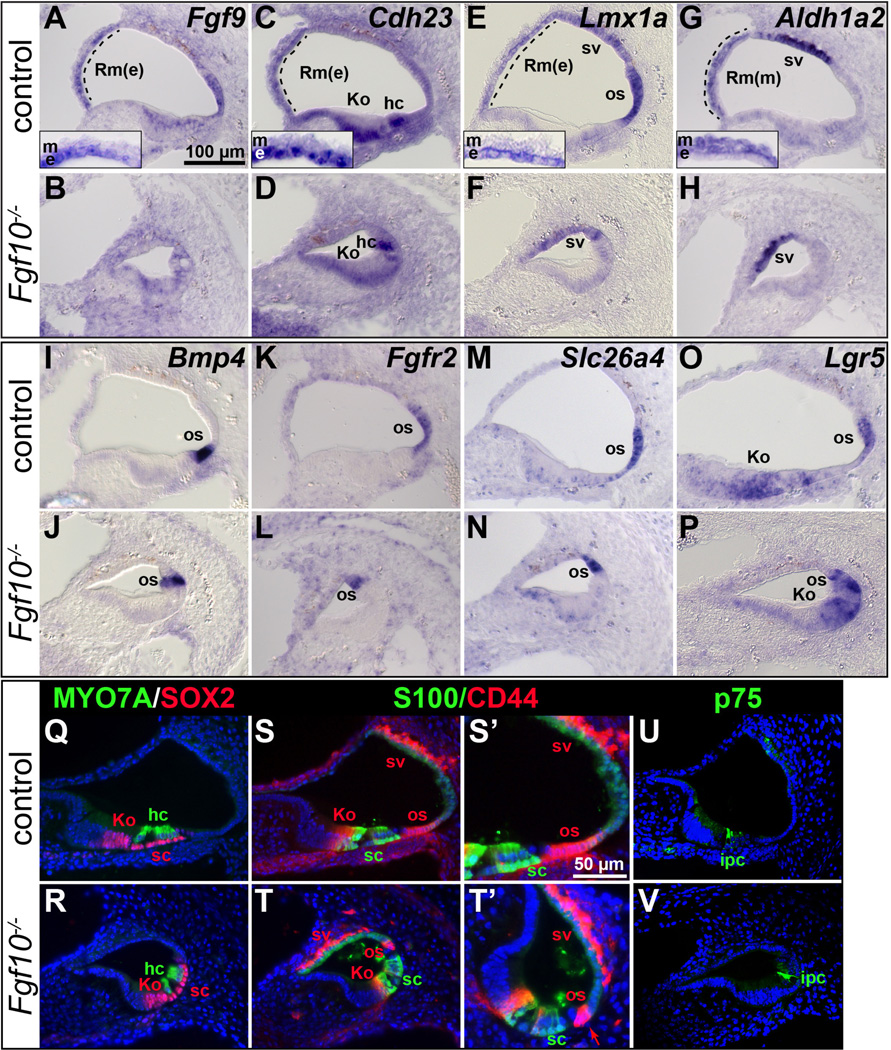
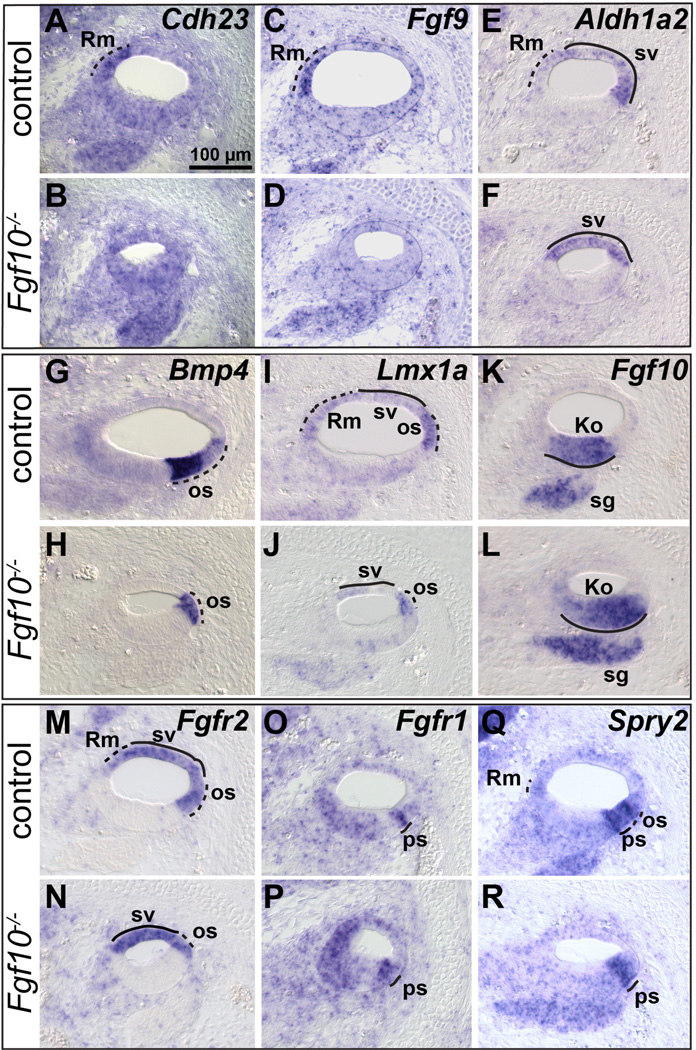
In contrast to the results with Kolliker’s organ, organ of Corti and stria vascularis markers, markers of Reissner’s membrane normally expressed in either the epithelial (e) or mesenchymal/mesothelial (m) layers (Fgf9, Cdh23 and Lmx1a, and Aldh1a2 (previously, Raldh2), respectively) were absent from Fgf10 null cochleae (Fig. 4A–H). In addition, markers of the outer sulcus (Lmx1a, Bmp4, Fgfr2, Slc26a4 and Lgr5) showed a reduced domain in these mutants (Fig. 4E, F, I–P). However, Lmx1a and Aldh1a2 expression in the stria vascularis was preserved (Fig. 4E–H) similarly to Trp2 and Cldn11 at E18.5 (Fig. 3Q–T), further suggesting that this tissue was unaffected by the absence of FGF10. Also, Cdh23 expression in Kolliker’s organ and hair cells (Figs. 4C, D), and Lgr5 expression in Kolliker’s organ (Figs. 4O, P) were retained in Fgf10 null cochleae, confirming that these cells developed normally.
Figure 4. Markers of Reissner’s membrane are absent and outer sulcus markers show a reduced domain in E18.5 Fgf10 null mutant cochleae.
In situ hybridization (A–P) and immunostaining (Q–V) analyses of basal cochlear duct cross sections. Genotypes are indicated to the left of each row and probes are indicated to the upper right of each pair of panels. Insets in A, C, E, G show magnifications of Reissner’s membrane. S’–T’ provide enlargements of S–T, with the arrow in T’ indicating the remnant Claudius cells in the mutant os. Abbreviations: Rm(e), Reissner’s membrane-epithelial layer; Rm(m), Reissner’s membrane-mesenchymal/mesothelial domain; os, outer sulcus. See Figure 2 or 3 legend for others. Scale bar in A applies to all panels except S’–T’. Scale bar in S’ applies to T’.
To further delineate the status of the Fgf10 null outer sulcus in relation to the organ of Corti, we used combinations of antibodies. MYO7A antibodies label hair cell soma and SOX2 antibodies label nuclei in the lateral half of Kolliker’s organ and all organ of Corti supporting cells laterally, including Hensen’s cells (Hume et al., 2007). Consistent with all previous markers, these two markers revealed no differences between Fgf10 genotypes (Fig. 4Q, R). CD44 antibodies label cell surfaces in lateral Kolliker’s organ, the outer pillar cell, Claudius cells in the outer sulcus, Reissner’s membrane and the stria vascularis (Hertzano et al., 2010), whereas S100A1 antibodies label the soma of the inner hair cell and all supporting cells except pillar cells (Sage et al., 2005) and p75NTR antibodies label the inner pillar cells (Mansour et al., 2013; Mueller et al., 2002). There were no differences between genotypes with respect to S100A1 or p75NTR staining (Fig. 4S–V, green), again showing that Fgf10 is not uniquely required for organ of Corti supporting cell development. However, CD44 staining differed markedly between genotypes. In Fgf10 null mutants, the Reissner’s membrane domain was absent, as was the lateral domain of the outer sulcus, with only a few of the most medial Claudius cells remaining (Fig. 4S–T, S’–T’ red). In contrast, outer pillar cell and stria vascularis staining were preserved. Taken together, the histologic and marker analyses performed at E18.5 show that Fgf10 null cochleae are missing two non-contiguous, non-sensory regions of the epithelium, namely, Reissner’s membrane and most of the outer sulcus.
The requirement for Fgf10 in Reissner’s membrane development precedes that in outer sulcus development
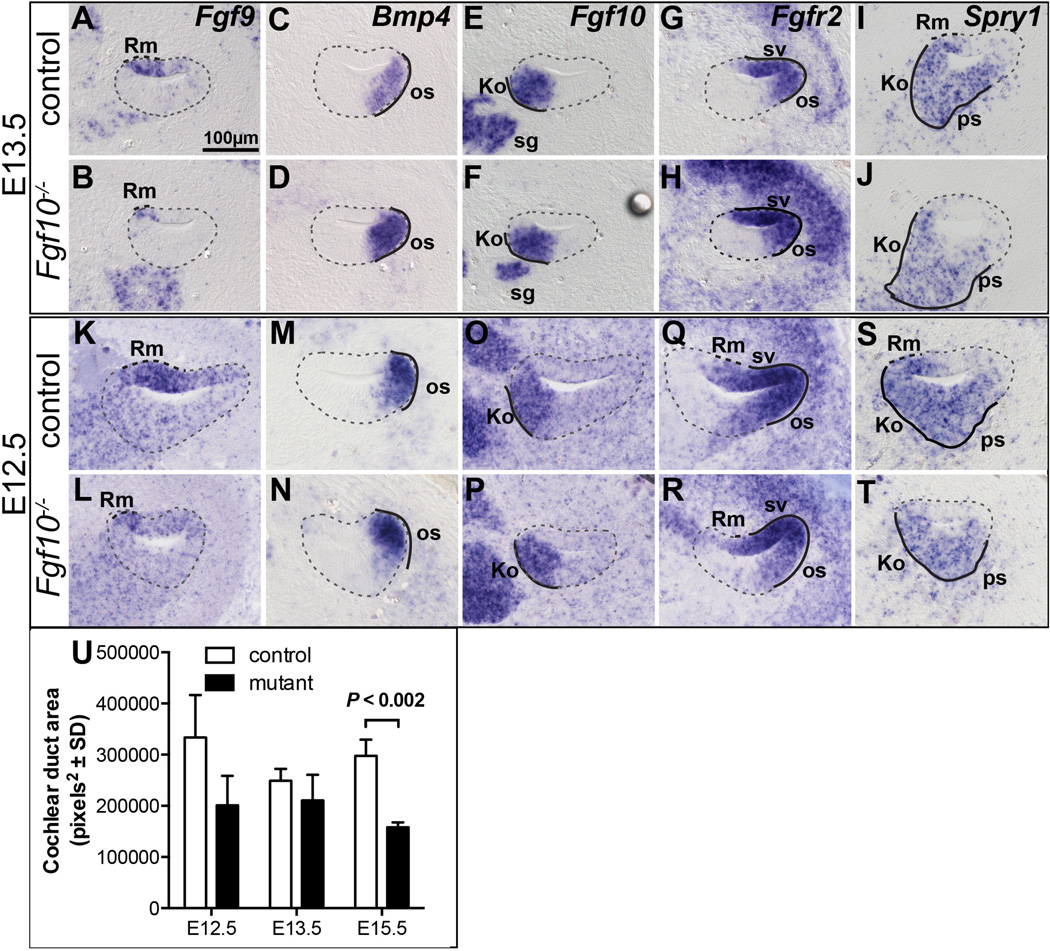
To determine the ontogeny of the cellular defects found in E18.5 Fgf10 null cochleae and their relation to FGF signaling disruptions, we examined the expression of genes for relevant cell type-specific markers, as well as FGF signaling components and signaling indicators at progressively younger stages. At E15.5, markers of the presumptive Reissner’s membrane, Cdh23 and Fgf9 (Pirvola et al., 2004; Wilson et al., 2001), were absent in Fgf10−/− samples (Fig. 5A–D). Aldh1a2 is a marker of both the presumptive Reissner’s membrane region and the stria vascularis (Burton et al., 2004; Romand et al., 2001; Romand et al., 2004), and only the former domain was missing from Fgf10 mutants (Fig. 5E, F). The outer sulcus markers, Bmp4 and Lmx1a (Koo et al., 2009; Morsli et al., 1998) were present in mutants, but had a reduced domain (Fig. 5G–J). At this stage and as noted by others (Pauley et al., 2003), Fgf10 transcripts were expressed in the prosensory domain of the cochlear epithelium and in the spiral ganglion. As expected, the stable transcripts produced from the Fgf10 exon 2 deletion allele had a similar distribution in the mutants (Fig. 5K, L). Fgf3, which is often redundant with Fgf10, is expressed just laterally of Fgf10 at the edge of the prosensory and outer sulcus domains, but we found no changes in its expression in Fgf10 mutants (Fig. S2A, B), suggesting that this region is intact.
Figure 5. Presumptive Reissner’s membrane markers are absent and presumptive outer sulcus markers show a reduced domain in E15.5 Fgf10 null mutant cochleae.
In situ hybridization analyses of basal cochlear duct cross sections are shown. Genotypes are indicated to the left of each row and probes are indicated to the upper right of each pair of panels. Dashed and solid lines indicate expression that is altered or unchanged, respectively, in mutants. Abbreviations: Ko, presumptive Kolliker’s organ; os, presumptive outer sulcus; ps, presumptive prosensory domain; Rm, presumptive Reissner’s membrane; sg, spiral ganglion; sv, stria vascularis. Scale bar in A applies to all panels.
FGF10 and FGF3 signal mainly through FGFR2b, which is the primary FGFR2 isoform expressed in otic epithelium (Pirvola et al., 2000). Therefore, we used a pan probe that detects all Fgfr2 transcripts, as a proxy for an Fgfr2b-specific probe. As expected (Hayashi et al., 2010; Pirvola et al., 2000), this probe labeled the entire non-sensory domain of the control otic epithelium, including the prospective Reissner’s membrane, stria vascularis and outer sulcus (Fig. 5M). The Fgfr2 domain was reduced in Fgf10 mutants and was still confined to the thin, non-sensory region (Fig. 5N). FGF10 can also signal through FGFR1b, which is co-expressed with FGFR1c in the otic epithelium (Mansour et al., 2013; Pirvola et al., 2002), and as expected (Hayashi et al., 2010; Pirvola et al., 2002), a pan probe for all Fgfr1 transcripts detected broad expression throughout the cochlear epithelium, with a focus just medial to the outer sulcus. This domain was still present in Fgf10 null mutants (Fig. 5O, P).
Spry2 is a transcriptional target of FGF signaling through the RAS/MAPK pathway (Minowada et al., 1999) and was detected as expected in a domain spanning the lateral prosensory and medial outer sulcus region (Shim et al., 2005) and also weakly in the prospective Reissner’s membrane. The Reissner’s membrane domain was absent from mutants and the outer sulcus domain was reduced (Fig. 5Q, R). Several other transcriptional targets of FGF signaling (Erm, Pea3, Spry1 and Dusp6) were found in the epithelium or surrounding mesenchyme, but none of these was affected in the Fgf10 mutants (Suppl. Fig. S2C-X). Together, these results suggest that the blocks to Reissner’s membrane and outer sulcus development in Fgf10 mutants occurred earlier than E15.5.
Examination of a subset of the Reissner’s membrane and outer sulcus markers in sections taken through the base of the cochlear duct at even earlier stages (E13.5 and E12.5) revealed stage-specific differences. As previously observed (Pirvola et al., 2004), the prospective Reissner’s membrane marker, Fgf9, had a broad expression domain encompassing most of the thin, non-sensory cochlear epithelium in E12.5 and E13.5 controls (Fig. 6A, K). This domain was substantially reduced, but not completely eliminated in stage-matched Fgf10 mutants (Fig. 6B, L). In contrast, the prospective outer sulcus marker, Bmp4, had the same expression domain in controls and mutants at both stages (Fig. 6C, D, M, N). At these stages and as previously noted by Pirvola et al. (2000), Fgf10 was expressed in the control prosensory regions (Fig. 6E, O) in a pattern almost exactly complementary to that of Fgfr2 in the non-sensory tissue (Fig. 6G, Q). As expected, the Fgf10 expression domain (reflecting perdurant exon 2 deletion transcripts) was unchanged in Fgf10 mutants (Fig. 6F, P), but at E12.5, the Fgfr2 domain in the roof of the mutant duct was reduced (Fig. 6H) relative to the control (Fig. 6G). No further change in Fgfr2 expression was observed at E13.5 (Fig. 6Q, R). The FGF signaling indicator, Spry1, did not overlap with Fgfr2, so presumably reveals signaling through FGFR1, which is widely expressed at these stages (Hayashi et al., 2010; Pirvola et al., 2002). The lateral extent of Spry1 expression was significantly reduced in Fgf10 mutants at both E13.5 (Fig. 6I, J) and E12.5 (Fig. 6S, T). Spry2, which marks the lateral prosensory and medial outer sulcus domains, was unchanged at this stage (Fig. S2U, V). Finally, Fgf9, Fgfr2 and Spry1 were unaffected at E11.5 (data not shown). Collectively, these data suggest that the specification of Reissner’s membrane occurs relatively early in cochlear development, at or before E12.5, while medial outer sulcus specification follows between E13.5 and E15.5.
Figure 6. The effects of FGF10 absence on Reissner’s membrane development precede those on outer sulcus development.
In situ hybridization analyses of basal cochlear duct cross sections at E13.5 (A–J) and E12.5 (K–T). Genotypes are indicated to the left of each row and probes are indicated to the upper right of the top panels. Dashed and solid black lines indicate expression that is altered or unchanged, respectively, in mutants. The cochlear duct is outlined with a dashed grey line. U. Graphical comparison of the cochlear duct area of the basal scala media at the developmental ages shown (n = 3 controls and 3 mutants). Abbreviations: Ko, Kolliker’s organ; Rm, presumptive Reissner’s membrane; os, outer sulcus; ps, prosensory region; sg, spiral ganglion; sv, stria vascularis. Scale bar in A applies to all panels.
To determine when the non-sensory tissue deletions affected the overall cochlear duct area, we compared control and mutant cochlear ducts at E12.5, E13.5 and E15.5. Although the mutant means were always less than control means, the difference did not reach significance until E15.5 (Fig. 6U).
Proliferation and death of cochlear epithelial cells are unaffected in Fgf10 null mutants
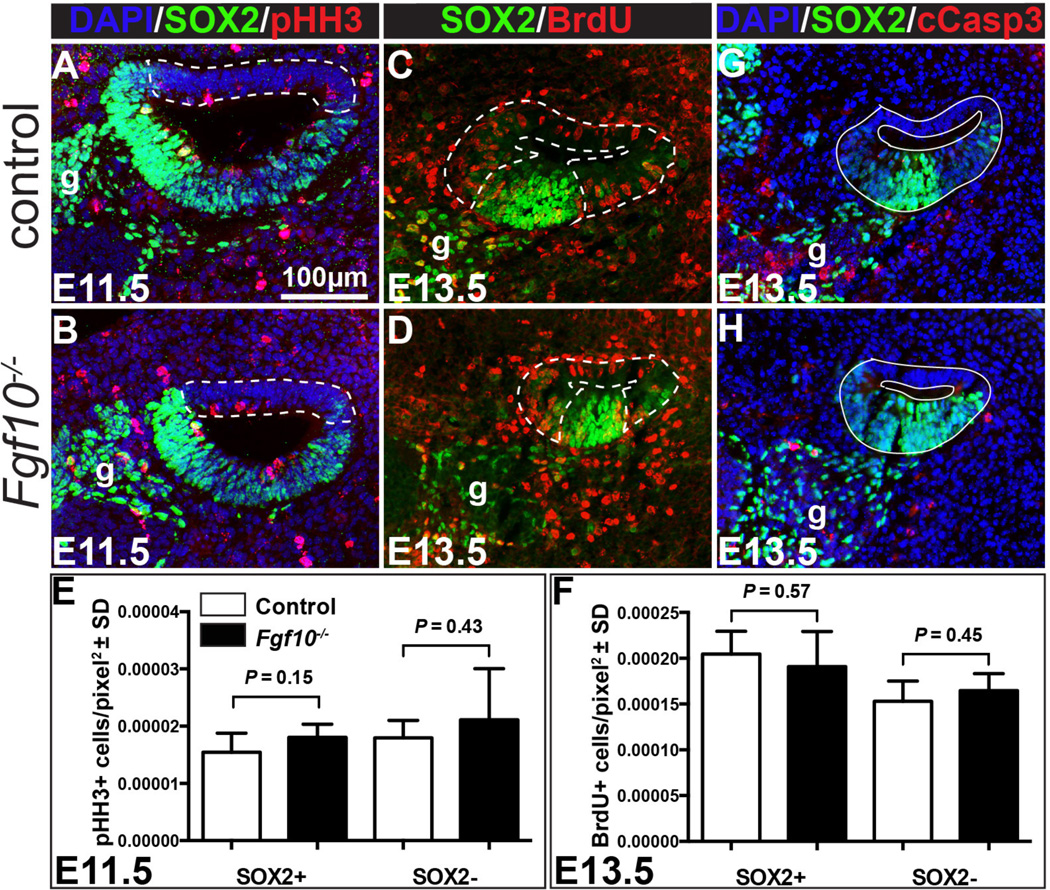
To determine whether Fgf10 is necessary for proliferation of the non-sensory epithelial regions at the stages when they are first developing, we labeled M-phase cells at E11.5 and Sphase cells at E13.5 with antibodies directed against phospho-Histone H3 and pulse-incorporated BrdU, respectively. Samples were co-stained with antibodies directed against SOX2 to distinguish prosensory (SOX2+) from non-sensory (SOX2-) domains (Fig. 7A–D). We determined the average number of proliferation marker-positive cells per unit area in each of the two epithelial domains, but no significant differences between genotypes were evident in either domain at either stage (Fig. 7E, F). A limited number of E12.5 and E14.5 BrdU-labeled samples was examined and these also showed no evidence of differences between genotypes (data not shown). Similarly, labeling of dying cells at E13.5 with antibodies directed against cleaved Caspase 3 failed to reveal differences between genotypes in either the SOX2+ or SOX2- domain (Fig. 7G, H; n = 3 each); however, the very small number of dying cells, in both control and mutant epithelia, precluded detailed quantitation. Thus, neither proliferation nor cell survival in the non-sensory regions appear to require Fgf10.
Figure 7. Neither cell proliferation nor cell survival is altered in Fgf10 null cochlear ducts.
Double labeling of control (A) and Fgf10 null (B) E11.5 cochlear duct cross sections with antibodies directed against phospho-Histone H3 (red) and SOX2 (green). Nuclei are counterstained with DAPI (blue). Double labeling of control (C) and Fgf10 null (D) E13.5 cochlear duct cross sections with antibodies directed against BrdU (red) and SOX2 (green). Dotted lines delineate the regions considered non-sensory (SOX2−). The remaining SOX2+ areas were considered prosensory. Graphical comparisons of the mean number of proliferating cells per pixel2 in SOX2+ (E) and SOX2− (F) regions of control (white bars) and Fgf10 null mutants (black bars). Error bars indicate standard deviation (SD). Double labeling of control (G) and Fgf10 null (H) E13.5 cochlear duct cross sections with antibodies directed against cleaved Caspase 3 (cCasp3; red) and SOX2 (green). Nuclei are counterstained with DAPI (blue). Abbreviation: g, ganglion. Scale bar in panel A applies to all images.
Discussion
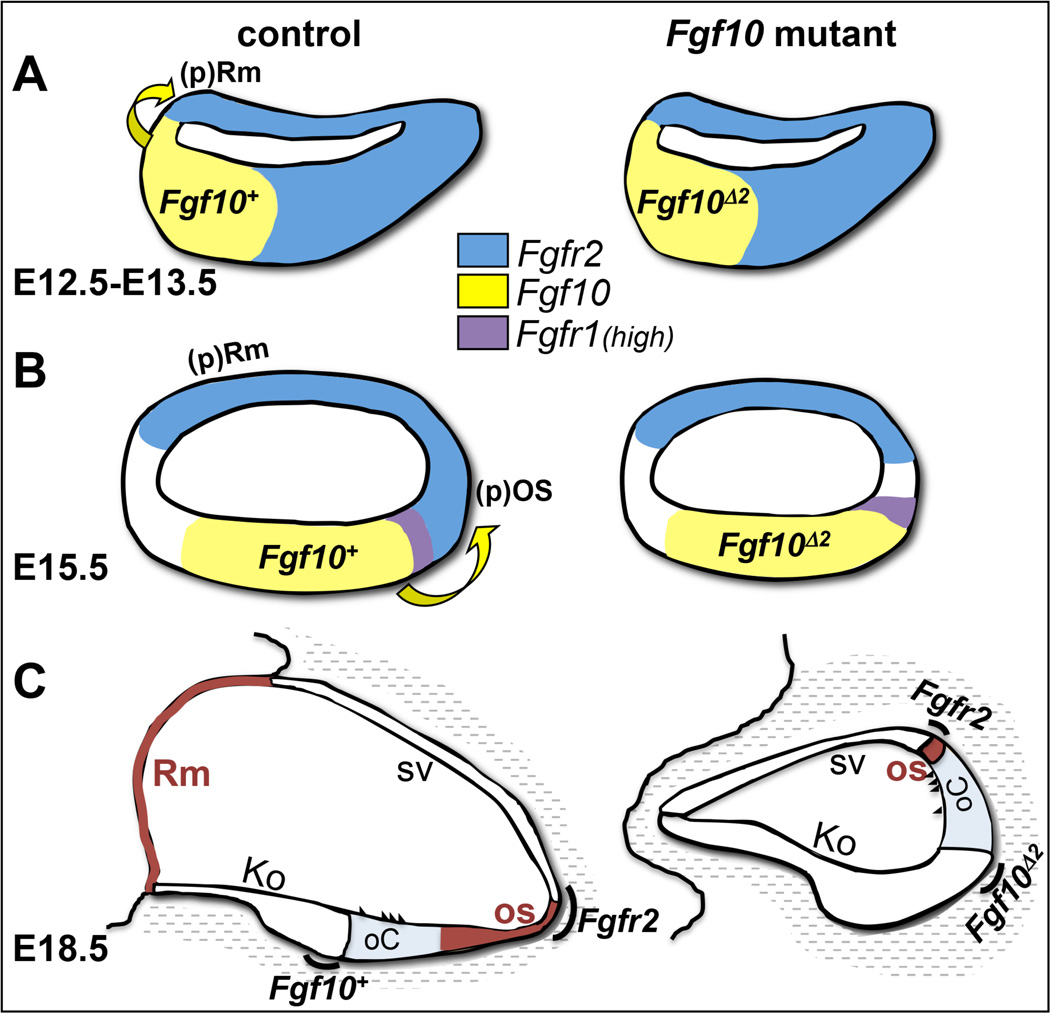
Fgf10 is expressed robustly in the developing inner ear epithelium and ganglion, and has known roles in vestibular morphogenesis. To determine its roles in cochlear development we studied global null mutants and found that in addition to the previously described defects of vestibular morphogenesis in homozygous null mutants (Ohuchi et al., 2005; Pauley et al., 2003), the heterozygotes showed a fully penetrant reduction or loss of the posterior semicircular canal, indicating that this region of the otic labyrinth is particularly sensitive to FGF10 dosage. We also found that while some Fgf10 null mutants had defects of semicircular canal formation similar to those described previously (Ohuchi et al., 2005; Pauley et al., 2003), others had a more severe defect in which canal fusion plates failed to clear. Most significantly, and the focus of this report, we found that homozygous null mutants had a previously undetected cochlear defect, namely loss or reduction of two non-contiguous non-sensory domains, Reissner’s membrane and the outer sulcus, respectively (Fig. 8). This was accompanied by a noticeable shortening of the cochlear duct.
Figure 8. Model depicting the effects of FGF10 absence on development of non-sensory cochlear domains.
(A) At E12.5–E13.5 FGF10 induces Reissner’s membrane development at the medial boundary of Fgf10/Fgfr2 expression. (B) By E15.5, FGF10 induces outer sulcus development at the lateral boundary of Fgf10/Fgfr2 expression. Expression domains of Fgf10 and receptor genes are color coded in A and B. (C) Failure of FGF10 signaling leads by E18.5 to a total loss of Reissner’s membrane and a significant reduction in the outer sulcus. Both affected domains are colored brown. Other morphologic domains are delineated and the now quite separated expression domains for Fgf10 and Fgfr2 are indicated with black arcs. There is lowlevel diffuse expression of Fgfr1 throughout much of the cochlear duct at all stages (not shown). Fgf10Δ2 refers to the stable exon 2-deleted transcript that does not encode functional FGF10, but perdures in the mutant. All other abbreviations have been defined previously.
These new cochlear data suggest an interesting parallel between the function of Fgf10 in the vestibular compartment and its action in the cochlea. Fgf10 is expressed in the developing semicircular canal prosensory domain (giving rise to the cristae) while Fgfr2b is expressed in the non-sensory canal epithelium. Loss of Fgf10 or chemical blockage of FGF signaling results in the non-sensory canal defects shown here and previously by others (Chang et al., 2004; Ohuchi et al., 2005; Pauley et al., 2003). Similarly, Fgf10 is expressed in a sensory-competent domain early in cochlear development, eventually becoming restricted in mice to lateral Kolliker’s organ, adjacent to the organ of Corti (Ohyama et al., 2010; Pujades et al., 2006; Sanchez-Guardado et al., 2013), and, as we have shown here, is required for specification of two non-sensory domains expressing Fgfr2b. Thus, in contrast to the epithelial-mesenchymal signaling paradigm apparent in other FGF10-dependent structures (Beenken and Mohammadi, 2009; Itoh and Ornitz, 2011; Turner and Grose, 2010), non-sensory development in the inner ear may depend upon intraepithelial paracrine signaling as posited by Pirvola et al. (2000).
When and how do the Fgf10 cochlear phenotypes arise? Analysis of numerous cell type specific markers showed that the two affected non-sensory tissues appear to initiate development at different times when Fgf10 and Fgfr2b are expressed in adjacent cochlear epithelial domains, with the medial Reissner’s membrane initiating first at E12.5–13.5, and the lateral outer sulcus domain initiating later at E15.5 (Fig. 8). The failure to generate these two non-sensory tissues did not appear to be compensated for by expansion of other tissues, and by E15.5, the mutant cochlear duct area was significantly less than that of controls. The lack of an effect on proliferation or cell death at the two boundaries of the Fgf10 and Fgfr2b expression domains suggests that both phenotypes are likely to result from defects of localized progenitor cell specification rather than a failure of proliferation or cell survival.
Fgf10 and Reissner’s membrane
The molecular mechanisms by which FGF10 induces Reissner’s membrane and outer sulcus development are not clear, but our data suggest some possibilities that could be tested in future studies. Reissner’s membrane spans the region between Kolliker’s organ and the stria vascularis. It consists of an inner, epithelial layer facing the endolymph of the scala media and an outer mesothelial layer facing the perilymph of the scala vestibuli. Its function is to help maintain the distinct ionic compositions of endolymph and perilymph (Kim et al., 2014; Kim and Marcus, 2011) as well as to propagate traveling waves that play a role in otoacoustic emissions (Reichenbach et al., 2012). The genetic networks required for Reissner’s membrane development are unexplored. Ours is the first report detailing the specific and complete loss Reissner’s membrane via mutation of a gene expressed locally within the cochlear epithelium. Given the dramatic reduction of Fgf9 found in the presumptive Reissner’s membrane of Fgf10 null mutants at E12.5–E13.5, it is tempting to speculate that Fgf9 functions downstream of Fgf10 in specification of Reissner’s membrane. However, Fgf9 null mutants retain Reissner’s membrane, albeit with detachment of the mesothelial and epithelial layers. In addition, Fgf9 mutants exhibit other dysmorphic features of the scala vestibuli (enlargement and irregular trabeculae) and they have a normally proportioned and elongated cochlea (Pirvola et al., 2004). None of these features are recapitulated in Fgf10 mutants. Thus, the down-regulation of Fgf9 in the Fgf10 null cochleae may be more correlative than causal, and low-level expression of Fgf9 at E12.5–13.5 in Fgf10 mutants may be sufficient to provide its normal function in signaling to the mesenchyme.
Aldh1a2, encoding a retinoic acid biosynthetic enzyme, is strongly expressed in the mesenchymal/mesothelial domain of Reissner’s membrane at E18.5 and was absent in Fgf10 mutants, but we did not observe any expression in or adjacent to the cochlear duct at E12.5–E13.5, when Reissner’s membrane is induced. Thus it is unlikely to play a role downstream of Fgf10 in its specification. Nevertheless, the later mesechymal/mesothelial expression of Aldh1a2 remains suggestive of a role in Reissner’s membrane development, possibly in conjunction with epithelial Fgf9. Addressing this issue will require analysis of appropriate conditional mutants.
Lmx1a, which encodes a LIM homeodomain-containing transcription factor and is expressed in the prospective and late embryonic epithelial Reissner’s membrane of controls, but was absent from Fgf10 null mutants, may be a more likely candidate as a downstream effector of FGF10 signaling in Reissner’s membrane specification. Lmx1a likely null mutants (dreher, mtl, bsd) have dysmorphic cochleae in which the basal sensory domain is merged with and resembles a neighboring vestibular sensory domain (Koo et al., 2009; Nichols et al., 2008; Steffes et al., 2012), suggesting improper sensory boundary formation. This phenotype is not seen in Fgf10 null mutants. However, Reissner’s membrane appears abnormal in Lmx1a mutants, with a possible reduction in the cross sectional area of the cochlear duct (Nichols et al., 2008), and further examination and comparison with Fgf10 null mutants is warranted. In addition, as in Fgf10 null mutants, the Lmx1a mutant cochleae are shortened (Koo et al., 2009; Nichols et al., 2008; Steffes et al., 2012). As Fgf10 transcripts are not affected in the Lmx1a cochlea (Nichols et al., 2008), but the Reissner’s membrane and outer sulcus domains of Lmx1a transcripts are absent from Fgf10 null mutants (this study), it is likely that Fgf10 is upstream of Lmx1a. Whether Lmx1a actually mediates FGF10 functions in the cochlear duct could only be tested by replacing its function in the Fgf10 null mutant.
Fgf10 and the outer sulcus
Similar to the role of Reissner’s membrane, the outer sulcus provides a crucial component of the non-sensory epithelial compartment of the cochlear duct. Cells in this domain actively reabsorb cations from the endolymph, providing the optimum homeostatic environment for mechanoelectrical transduction to occur in hair cells (Jagger and Forge, 2013; Kim and Marcus, 2011). As with Reissner’s membrane, we do not yet have a clear grasp on the regulatory pathways by which Fgf10 orchestrates outer sulcus development, but it is reasonable to suggest that Fgf10 may do so by regulating expression of Bmp4. Conditional mutagenesis of Alk3 and Alk6, encoding type I BMP receptors, revealed that BMP signaling, like Fgf10 signaling, is required for outer sulcus specification and normal cochlear length. The Alk mutants exhibit a lateral expansion of the cochlear Fgf10 expression domain in Kolliker’s organ at E13.5. Furthermore, exposure of cochlear organ cultures to BMP4 ligand induces markers of the outer sulcus and suppresses Fgf10 and other markers of Kolliker’s organ, suggesting that BMP signaling negatively regulates FGF signaling in the cochlear duct. (Ohyama et al., 2010). Our data suggest that FGF10 may be a positive regulator of Bmp4 expression, but since there is residual Bmp4 expression and residual outer sulcus tissue in Fgf10 mutants and other BMP ligands may also be present in either the epithelium or mesenchyme, it is not surprising that we did not observe an expansion of the Fgf10Δ2 expression domain in mutants. Further studies will be required to dissect the regulatory relationships between FGF and BMP signaling in cochlear duct patterning and morphogenesis. In addition, it will be interesting to learn whether removing the remaining FGFR2b ligand (FGF3) leads to complete deletion of the outer sulcus.
Fgf10 and cochlear length
Despite the obvious, but relatively mild, shortening of the cochlear duct noted in gross observations of Fgf10 null mutants, we did not detect proliferative differences that could account for this phenotype. Additional studies may be necessary to determine if the shortening is in fact caused by small effects on proliferation or instead by an alternative mechanism, such as a slowing of convergent extension (McKenzie et al., 2004; Yamamoto et al., 2009). Indeed, the cells in the residual Bmp4-positive outer sulcus region of the mutants appear taller than those of control cochleae. As reducing the dosage of Fgf3, which encodes a ligand with the same receptor activating profile as FGF10 (Zhang et al., 2006), leads to further shortening of the Fgf10 null cochlea (data not shown, manuscript in preparation), and Fgfr2b null mutants never develop a cochlea (Pirvola et al., 2000), we suspect that FGF10 together with FGF3-stimulated proliferation will prove to be one of the factors regulating cochlear length.
Implications for human hearing loss
Heterozygous mutations in FGF10 leading to reduced signaling activity are a cause of the very rare autosomal dominant LADD (lacrimo-auriculo-dento-digital) syndrome and its allelic variant, ASLG (aplasia of lacrimal and salivary glands) syndrome. LADD syndrome is also caused by heterozygous mutations in FGFR2 and FGFR3 that reduce signaling activity (Milunsky et al., 2006; Rohmann et al., 2006; Shams et al., 2007). Neither LADD nor ASLG subjects, nor mice that are heterozygous for Fgf10, Fgfr2 or Fgfr3 loss-of-function mutations have ever been reported with vestibular symptoms. In light of our findings that Fgf10−/+ mice have posterior semicircular canal defects, it would be interesting to image the inner ears of LADD subjects with known FGF10 mutations to determine the status of the posterior semicircular canal. Interestingly, there is a case report of a LADD syndrome subject with unilateral dysplastic posterior and lateral semicircular canals (Moses, 2013), and another with bilateral dysplasia of the semicircular canals (Azar et al., 2000), but no information about the responsible mutations is available. Given that only one semicircular canal is affected in mice, it is likely that the remainder of the vestibular system compensates, though it is possible that an abnormal vestibular phenotype might be revealed with specific testing.
55% of LADD syndrome subjects are reported with hearing loss (Milunsky et al., 2006), but the type (conductive, sensorineural or mixed) is not always specified and the mutation status is rarely known. Nevertheless, imaging of some of these individuals has revealed cochlear hypoplasia (Lemmerling et al., 1999; Meuschel-Wehner et al., 2002; Moses, 2013). We did not detect any morphologic or molecular aberrations in the cochlear ducts of Fgf10 heterozygotes and their hearing is normal (Mansour et al., 2013), but homozygotes had cochlear defects that would be expected to cause hearing loss if the other major consequences of Fgf10 homozygosity, such as the failure of lung development, could be bypassed. In particular, the failure of normal Reissner’s membrane and outer sulcus development, both of which are important in maintaining the endolymph homeostasis necessary for hearing, suggests that these tissues could be compromised to some extent even in LADD syndrome subjects without reported cochlear malformations and might contribute to the hearing loss reported in some individuals. Our inner ear findings in mice suggest that LADD and ASLG syndrome subjects should receive hearing and vestibular function evaluations and counseling as part of their clinical care.
In summary, our findings on the development of Fgf10 null inner ears provide new insight into the specification of cochlear non-sensory domains and suggest a basis for the hearing loss reported for some LADD syndrome subjects.
Supplementary Material
Highlights.
Fgf10 has a dosage-sensitive role in vestibular morphogenesis.
Fgf10 is required for cochlear morphogenesis.
Fgf10 null mutants lack Reissner’s membrane and are deficient in outer sulcus tissue.
FGF10 signals sequentially and bi-directionally to specify these non-sensory domains.
Acknowledgements
We are grateful to the numerous colleagues who supplied the DNA clones used to prepare probes for ISH; they are acknowledged in Supplementary Table 1. We thank Jerry Spangrude for rat anti-CD44, Tim Goodman for advice regarding BrdU detection, Shannon Odelberg for consultations regarding statistical analyses and Gary Schoenwolf for helpful comments on the manuscript. This work was supported by grants to S.L.M from the NIDCD/NIH (DC011819) and to T.O. from NIDCD/NIH (DC012085). These funding sources had no involvement in the study design, data collection, analysis or interpretation, or in the writing or choice of where to submit this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–6338. doi: 10.1242/dev.00881. [DOI] [PubMed] [Google Scholar]
- Azar T, Scott JA, Arnold JE, Robin NH. Epiglottic hypoplasia associated with lacrimo-auriculo-dental-digital syndrome. Ann Otol Rhinol Laryngol. 2000;109:779–781. doi: 10.1177/000348940010900814. [DOI] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Q, Cole LK, Mulheisen M, Chang W, Wu DK. The role of Pax2 in mouse inner ear development. Dev Biol. 2004;272:161–175. doi: 10.1016/j.ydbio.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development. 2004;131:4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EP, Noyes CA, Wang X, Wright TJ, Mansour SL. Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development. 2007;134:3615–3625. doi: 10.1242/dev.006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Younkins C, Bermingham-McDonogh O. Expression patterns of FGF receptors in the developing mammalian cochlea. Dev Dyn. 2010;239:1019–1026. doi: 10.1002/dvdy.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzano R, Puligilla C, Chan SL, Timothy C, Depireux DA, Ahmed Z, Wolf J, Eisenman DJ, Friedman TB, Riazuddin S, Kelley MW, Strome SE. CD44 is a marker for the outer pillar cells in the early postnatal mouse inner ear. J Assoc Res Otolaryngol. 2010;11:407–418. doi: 10.1007/s10162-010-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley PA, Clarke M, Crook JM, Wise AK, Shepherd RK. Cochlear immunochemistry--a new technique based on gelatin embedding. J Neurosci Methods. 2003;129:81–86. doi: 10.1016/s0165-0270(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger DJ, Forge A. The enigmatic root cell - emerging roles contributing to fluid homeostasis within the cochlear outer sulcus. Hear Res. 2013;303:1–11. doi: 10.1016/j.heares.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Kiernan AE. The paintfill method as a tool for analyzing the three-dimensional structure of the inner ear. Brain Res. 2006;1091:270–276. doi: 10.1016/j.brainres.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Kim KX, Sanneman JD, Kim HM, Harbidge DG, Xu J, Soleimani M, Wangemann P, Marcus DC. Slc26a7 chloride channel activity and localization in mouse Reissner's membrane epithelium. PLoS One. 2014;9:e97191. doi: 10.1371/journal.pone.0097191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Marcus DC. Regulation of sodium transport in the inner ear. Hear Res. 2011;280:21–29. doi: 10.1016/j.heares.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol. 2009;333:14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmerling MM, Vanzieleghem BD, Dhooge IJ, Van Cauwenberge PB, Kunnen MF. The Lacrimo-Auriculo-Dento-Digital (LADD) syndrome: temporal bone CT findings. J Comput Assist Tomogr. 1999;23:362–364. doi: 10.1097/00004728-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Mafong DD, Shin EJ, Lalwani AK. Use of laboratory evaluation and radiologic imaging in the diagnostic evaluation of children with sensorineural hearing loss. Laryngoscope. 2002;112:1–7. doi: 10.1097/00005537-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Goddard JM, Capecchi MR. Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development. 1993;117:13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Li C, Urness LD. Genetic rescue of Muenke syndrome model hearing loss reveals prolonged FGF-dependent plasticity in cochlear supporting cell fates. Genes Dev. 2013;27:2320–2331. doi: 10.1101/gad.228957.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–812. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- Meuschel-Wehner S, Klingebiel R, Werbs M. Inner ear dysplasia in sporadic lacrimoauriculo- dento-digital syndrome. A case report and review of the literature. ORL J Otorhinolaryngol Relat Spec. 2002;64:352–354. doi: 10.1159/000066077. [DOI] [PubMed] [Google Scholar]
- Milunsky JM, Zhao G, Maher TA, Colby R, Everman DB. LADD syndrome is caused by FGF10 mutations. Clin Genet. 2006;69:349–354. doi: 10.1111/j.1399-0004.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses JE. Lacrimo-auriculo-dento-digital syndrome with unilateral inner ear dysplasia and craniocervical osseous abnormalities: case report and review of literature. Clin Neuroradiol. 2013;23:221–224. doi: 10.1007/s00062-012-0170-1. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J Neurosci. 2002;22:9368–9377. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Yasue A, Ono K, Sasaoka S, Tomonari S, Takagi A, Itakura M, Moriyama K, Noji S, Nohno T. Identification of cis-element regulating expression of the mouse Fgf10 gene during inner ear development. Dev Dyn. 2005;233:177–187. doi: 10.1002/dvdy.20319. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Zhang X, Mantela J, Ornitz DM, Ylikoski J. Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev Biol. 2004;273:350–360. doi: 10.1016/j.ydbio.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Pujades C, Kamaid A, Alsina B, Giraldez F. BMP-signaling regulates the generation of hair-cells. Dev Biol. 2006;292:55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Reichenbach T, Stefanovic A, Nin F, Hudspeth AJ. Waves on Reissner's membrane: a mechanism for the propagation of otoacoustic emissions from the cochlea. Cell Rep. 2012;1:374–384. doi: 10.1016/j.celrep.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann E, Brunner HG, Kayserili H, Uyguner O, Nurnberg G, Lew ED, Dobbie A, Eswarakumar VP, Uzumcu A, Ulubil-Emeroglu M, Leroy JG, Li Y, Becker C, Lehnerdt K, Cremers CW, Yuksel-Apak M, Nurnberg P, Kubisch C, Schlessinger J, van Bokhoven H, Wollnik B. Mutations in different components of FGF signaling in LADD syndrome. Nat Genet. 2006;38:414–417. doi: 10.1038/ng1757. [DOI] [PubMed] [Google Scholar]
- Romand R, Albuisson E, Niederreither K, Fraulob V, Chambon P, Dolle P. Specific expression of the retinoic acid-synthesizing enzyme RALDH2 during mouse inner ear development. Mech Dev. 2001;106:185–189. doi: 10.1016/s0925-4773(01)00447-6. [DOI] [PubMed] [Google Scholar]
- Romand R, Niederreither K, Abu-Abed S, Petkovich M, Fraulob V, Hashino E, Dolle P. Complementary expression patterns of retinoid acid-synthesizing and - metabolizing enzymes in pre-natal mouse inner ear structures. Gene Expr Patterns. 2004;4:123–133. doi: 10.1016/j.modgep.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guardado LO, Puelles L, Hidalgo-Sanchez M. Fgf10 expression patterns in the developing chick inner ear. J Comp Neurol. 2013;521:1136–1164. doi: 10.1002/cne.23224. [DOI] [PubMed] [Google Scholar]
- Shams I, Rohmann E, Eswarakumar VP, Lew ED, Yuzawa S, Wollnik B, Schlessinger J, Lax I. Lacrimo-auriculo-dento-digital syndrome is caused by reduced activity of the fibroblast growth factor 10 (FGF10)-FGF receptor 2 signaling pathway. Mol Cell Biol. 2007;27:6903–6912. doi: 10.1128/MCB.00544-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Steffes G, Lorente-Canovas B, Pearson S, Brooker RH, Spiden S, Kiernan AE, Guenet JL, Steel KP. Mutanlallemand (mtl) and Belly Spot and Deafness (bsd) are two new mutations of Lmx1a causing severe cochlear and vestibular defects. PLoS One. 2012;7:e51065. doi: 10.1371/journal.pone.0051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Urness LD, Bleyl SB, Wright TJ, Moon AM, Mansour SL. Redundant and dosage sensitive requirements for Fgf3 and Fgf10 in cardiovascular development. Dev Biol. 2011;356:383–397. doi: 10.1016/j.ydbio.2011.05.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness LD, Li C, Wang X, Mansour SL. Expression of ERK signaling inhibitors Dusp6, Dusp7, and Dusp9 during mouse ear development. Dev Dyn. 2008;237:163–169. doi: 10.1002/dvdy.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness LD, Paxton CN, Wang X, Schoenwolf GC, Mansour SL. FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev Biol. 2010;340:595–604. doi: 10.1016/j.ydbio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Householder DB, Coppola V, Tessarollo L, Fritzsch B, Lee EC, Goss D, Carlson GA, Copeland NG, Jenkins NA. Mutations in Cdh23 cause nonsyndromic hearing loss in waltzer mice. Genomics. 2001;74:228–233. doi: 10.1006/geno.2001.6554. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chen YS, Chen PJ, Hsu CJ. Common clinical features of children with enlarged vestibular aqueduct and Mondini dysplasia. Laryngoscope. 2005;115:132–137. doi: 10.1097/01.mlg.0000150691.85387.3f. [DOI] [PubMed] [Google Scholar]
- Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4:a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Okano T, Ma X, Adelstein RS, Kelley MW. Myosin II regulates extension, growth and patterning in the mammalian cochlear duct. Development. 2009;136:1977–1986. doi: 10.1242/dev.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelarayan LC, Vendrell V, Alvarez Y, Domínguez-Frutos E, Theil T, Alonso MT, Maconochie M, Schimmang T. Differential requirements for FGF3, FGF8 and FGF10 during inner ear development. Developmental Biology. 2007;308:379–391. doi: 10.1016/j.ydbio.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.