Abstract
Background
The role of atherosclerosis in the progression of global left ventricular dysfunction and cardiovascular events has been well recognized. Left ventricular (LV) dyssynchrony is a measure of regional myocardial dysfunction. Our objective was to investigate the relationship of subclinical atherosclerosis with mechanical LV dyssynchrony in a population-based asymptomatic multi-ethnic cohort.
Methods and Results
Participants of the Multi-Ethnic Study of Atherosclerosis (MESA) at exam 5 were evaluated using 1.5T cardiac magnetic resonance (CMR) imaging, carotid ultrasound (n=2,062) for common carotid artery (CCA) and internal carotid artery (ICA) intima-media thickness (IMT), and cardiac computed tomography (n=2,039) for coronary artery calcium (CAC) assessment (Agatston method). Dyssynchrony indices were defined as the standard deviation of time to peak systolic circumferential strain (SD-TPS) and the difference between maximum and minimum (max-min) time to peak strain using harmonic phase imaging in 12 segments (3-slices × 4 segments). Multivariable regression analyses were performed to assess associations after adjusting for participant demographics, cardiovascular risk factors, LV mass, and ejection fraction. In multivariable analyses, SD-TPS was significantly related to measures of atherosclerosis, including CCA-IMT (8.7msec/mm change in IMT, p=0.020), ICA-IMT (19.2 msec/mm change in IMT, p<0.001), carotid plaque score (1.2 msec/unit change in score, p<0.001), and log transformed CAC+1 (0.66 msec/unit log-CAC+1, p=0.018). These findings were consistent with other parameter of LV dyssynchrony i.e. max-min.
Conclusion
In the MESA cohort, measures of atherosclerosis are associated with parameters of subclinical LV dyssynchrony in the absence of clinical coronary event and left-bundle-branch block.
Keywords: Left Ventricular Dyssynchrony, Carotid IMT, Coronary Calcium Score, Atherosclerosis
Introduction
Atherosclerosis is a major cause of cardiovascular morbidity and mortality and one of the primary causes of heart failure.[1] Identification of individuals with subclinical atherosclerosis at risk of cardiovascular events is essential in initiating primary preventive measures so as to delay the onset of global LV systolic and diastolic dysfunction and thereby heart failure.[2] Carotid artery intima-media thickness is an established marker of subclinical arterial injury and is associated with increased risk of cardiovascular events,[3, 4] coronary risk factors,[5] and serves as an atherosclerotic surrogate end-point for therapeutic interventions.[6, 7, 8] Furthermore, presence of carotid plaque either enhances the predictive power of carotid IMT [9] or is itself a better predictor of coronary events than carotid IMT.[10] Similarly, coronary artery calcium (CAC) measured by computed tomography (CT) represents a quantifiable marker of coronary atherosclerosis.[11, 12]
LV dyssynchrony in an asymptomatic population with narrow QRS (<120 msec) has emerged as a marker of regional myocardial dysfunction and is associated with increased risk of an adverse cardiovascular event.[13] Cardiac magnetic resonance (CMR) imaging allows accurate and detailed analyses of regional LV function using myocardial tagging.[14, 15] The Multi-ethnic Study of Atherosclerosis (MESA) offers a unique opportunity to assess the relationship between subclinical atherosclerosis and incipient alterations of regional myocardial function in a large epidemiologic cohort. Therefore, the main aim of this study is to investigate the association between subclinical atherosclerosis—defined by carotid IMTs, carotid plaque score, and CAC—and subclinical LV dyssynchrony independent of myocardial scar in participants with no prior history of a clinical coronary event.
Methods
Study Population
MESA is a prospective cohort study designed to investigate the subclinical characteristics and underlying mechanisms involving cardiovascular disease in asymptomatic individual.[16] A total of 6,814 individuals 45 to 84 years of age from different ethnic backgrounds (White, African-American, Hispanic, and Chinese-American) were enrolled. Individuals with cardiac symptoms or known cardiovascular disease were excluded during initial recruitment. During the longitudinal follow-up of the fifth examination from April 2010 to February 2012, 2,062 participants underwent tagged CMR for regional function and carotid ultrasound; 2,039 participants underwent CMR and cardiac computed tomography (CT) at six clinical sites (Johns Hopkins University, Baltimore, MD; University of Minnesota, Minneapolis, MN; Northwestern University, Chicago, IL; Wake Forest University, Winston Salem, NC; and UCLA, Los Angeles, CA; Columbia University, NY). Individuals with an interim coronary event (n=32) or left bundle-branch-block (LBBB) (n=6) were preemptively excluded from the statistical analyses. Institutional review boards at each participating site approved study protocol, and informed consent was obtained from each participant.
CMR studies
Tagged CMR images were acquired by whole body 1.5T scanners with ECG-triggered, segmented K-space fast spoiled gradient-echo pulse sequence during breath hold. After the standard protocol, 3 tagged short-axis slices (base to apex) were obtained using spatial modulation of magnetization encoding gradients. Detailed scanning parameters include the following: field of view 360 × 360 mm, slice thickness 10 mm, repetition time 5msec, echo time 2.5 msec, flip angle 10–12 degrees, phase encoding views per segment 9, temporal resolution 40–45 msec, tag spacing 7 mm.
LV mass and functional analyses were performed using CIM software (version 6.2, University of Auckland, New Zealand).
Late gadolinium enhancement (LGE) analyses
Contrast enhanced images were acquired 15 min after gadolinium contrast administration using inversion-recovery fast gradient-echo pulse sequences, in the same short-axis locations as the cine images. The LGE analysis was performed using Medis research software (Leiden University, Netherlands) using a semi-automated method; a visual selection step followed by manual adjustment of scar delineation. An experienced observer first assesses all short and long-axis LGE images to determine the presence or absence of LGE areas. For the image analysis, one set of epicardial and endocardial contours were manually traced to define specific myocardial regions, proceeding then by drawing a region of interest in the remote myocardium (defined as a region with no enhancement and normal wall motion) and obtaining separated sets of planimetered scars delineated by automatic software computations defined as those areas with signal (SI) intensity between 1 SD and 10 SD above remote myocardium SI. The planimetry output that best fits the observer's visual assessment of scar is chosen and manually corrected to remove regions of blood pool or epicardial fat and evident artifacts, as well as to include areas of microvascular obstruction.
Dyssynchrony analysis
Harmonic phase imaging (HARP, Diagnosoft, Palo Alto, CA) was used to analyze 3 short-axis slices (basal, mid-ventricular, and apex) and 4 regions (septum, anterior, posterior, and lateral) (Fig. 1). HARP is a standard method for assessment of myocardial strain.[15] Times from end-diastole to peak systolic circumferential strain was measured. Standard deviation of time to peak systolic strain (SD-TPS) and difference between the maximum and minimum time to peak systolic strain (max-min) in 12 regions (3 slices × 4 regions) were determined to be the measures of myocardial dyssynchrony. Rosen et al.[17] described these parameters as equivalent to the echocardiographic method described by Yu et al.[18]
Figure 1.

Sample representation of cardiac magnetic resonance study with myocardial tagging and analysis of circumferential strain curves using harmonic phase imaging. In the graph, the x-axis represents time (cine frame number) and the y-axis represents circumferential strain (%).
Carotid artery measurements
B-mode ultrasound longitudinal images of the right and left common, bifurcation, and internal carotid artery segments were obtained with a Logiq 700 ultrasound system using the M12L transducer (General Electric Medical Systems, CCA frequency 13 MHz). The video stream output was digitized at high resolution and frame rates using a Medical Digital Recording device (PACSGEAR, Pleasanton, CA) and converted into DICOM compatible digital records. Trained and certified sonographers from all 6 MESA sites performed all exams. MESA exam-5 ultrasound images were reviewed and interpreted at the MESA Air Carotid Ultrasound Reading Center (UW AIRP, Madison, WI). Digitized images were imported into syngo Ultrasound Workplace 3.5B reading stations loaded with Arterial Health Package software (Siemens Medical, Malvern, PA) for carotid IMT measurement and plaque scoring.
The distal CCA was defined as the distal 10mm of the vessel. The proximal ICA was defined as the initial 10mm of the ICA after the flow divider. CCA-IMT was defined as the intima-media thickness measured as the mean of the left and right far wall distal CCA wall thicknesses. Similarly, ICA-IMT was defined by the mean intima-media thickness of the left and right far wall proximal ICA. Carotid plaque burden was defined by the carotid plaque scored as the number of plaques (0-12) in the internal, bifurcation, and common segments of both carotid arteries.[19] Carotid plaque was defined as a discrete, focal wall thickening ≥1.5 mm or focal thickening at least 50% greater than the surrounding IMT.[20]
The intra-class correlation coefficient (ICC) for intra-reader reproducibility for mean CCA IMT was 0.99; ICC for inter-reader CCA IMT reproducibility was 0.95. For mean ICA, intra-reader reproducibility was between 0.98 and 0.99; inter-reader reproducibility was 0.93. For carotid plaque presence and score, intra-reader reproducibility was kappa=0.83 (95% confidence interval [CI] 0.70-0.96) and inter-reader reproducibility was kappa=0.89 (95% CI 0.72-1.00).
Coronary artery calcium score assessment
CAC score was obtained using either a cardiac-gated electron-beam computed tomography scanner or a multidetector CT system as previously described.[21] Mean CAC (Agatston) score[11] was used for all analyses after adjustment for known calcium concentrations in a phantom that was included in the field of view. Images were interpreted using an interactive scoring system at the MESA CT reading center (Los Angeles Biomedical Research Institute-UCLA, Torrance, CA).
Statistical analysis
Baseline characteristics were described as mean ± standard deviation or percentages, as appropriate. SD-TPS and max-min were regressed against common and internal carotid IMT, and plaque score as continuous variables. Participants with missing data in multivariable models were excluded. Multivariable linear regression analysis was performed as follows: model 1, unadjusted; model 2, adjusted for age, gender, ethnicity, and heart rate; model 3, model 2 with further adjustment for traditional cardiovascular risk factors such as diabetic status, hypertension status, antihypertensive medication, total cholesterol, HDL-cholesterol, body mass index, smoking status (never, former, current), education level. For model 4, in addition to covariables in model 3, statistical adjustment was performed for LV end-diastolic mass, ejection fraction as measures of LV structure and global function. Further sub-analyses in 1244 participants with LGE analyses was performed with further adjustment for the presence or absence of myocardial scar based on LGE analyses.
The Shapiro-Wilk test for normality revealed a positively skewed coronary calcium score (p<0.001). Therefore, for CAC analyses, (1) we treated natural logarithm (log) of (CAC+1) as a continuous variable and (2) categorized CAC into 3 groups (bottom 33% and 2 upper tertiles) to account for the fact that 667 participants (∼33%) of participants had CAC of zero. The cut off for Agatston CAC score in each of the 1st, 2nd, & 3rd tertiles were 0, ≤131.8, and >131.8, respectively. The log(CAC+1) transformation better normalized the CAC distribution. Multivariable regression analyses for log(CAC+1) using hierarchical models were performed as detailed above. Analysis of variance with Bonferroni's correction was done to determine the difference between the means of SD-TPS among the groups categorized based on tertiles of CAC distribution.
For comparative analyses to determine the best atherosclerotic predictor of dyssynchrony using SD-TPS, the CCA-IMT, carotid plaque score, and log(CAC+1) were included in the same model, with further adjustment for covariables as in model 3. All statistical analyses were performed using STATA 12.0 (StataCorp, College Station, Texas, USA). All reported p-values are 2-tailed and values < 0.05 were considered as significant. The authors had full access to the data and take full responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
A total of 2,062 participants (mean age 69 years, women 54.2%) were analyzed for carotid plaque score after excluding participants with prior history of myocardial infarction and presence of left bundle branch block on 12-lead ECG; 2,015 individuals for CCA-IMT and 1,333 participants for ICA-IMT were analyzed; 2,039 participants were evaluated for correlation between CAC and LV dyssynchrony. Subset characteristics of the study population were similar to that of the MESA cohort. Mean QRS duration of study participants was 95.14 msec with mean ejection fraction of 62%. Detailed participant characteristics are described in Table 1.
Table 1. Participant demographics and characteristics.
| Parameters | (n=2062) |
|---|---|
| Age, years | 69.10 ± 9.20 |
| Gender- female, n (%) | 1117 (54.2) |
| Race, n (%) | |
| White | 843 (40.9) |
| Chinese-American | 283 (13.7) |
| African-American | 529 (25.7) |
| Hispanic | 407 (19.7) |
| Body mass index, kg/m2 | 28.01 ± 5.16 |
| Body surface area, m2 | 1.83 ± 0.22 |
| Risk factors | |
| Diabetes mellitus, n (%) | |
| Impaired fasting glucose | 446 (21.6) |
| Untreated | 26 (1.3) |
| Treated | 340 (16.5) |
| Heart rate, bpm | 61.44 ± 9.49 |
| Systolic blood pressure, mm Hg | 123.15 ± 19.82 |
| Diastolic blood pressure, mm Hg | 68.30 ± 9.85 |
| Antihypertensive medication, % | 1118 (54.22) |
| Total cholesterol, mg/dL | 184.13 ± 36.81 |
| LDL cholesterol, mg/dL | 106.08 ± 32.37 |
| HDL cholesterol, mg/dL | 56.19 ± 16.74 |
| Glomerular filtration rate, ml/min/1.73m2 | 80.73 ± 19.93 |
| Cigarette smoking, % | |
| Never smoked | 1045 (50.67) |
| Former smoker | 888 (43.06) |
| Current smoker | 129 (6.27) |
| EKG parameters | |
| LBBB, number of participants, n | 6 |
| QRS duration, msec | 95.14 ± 15.58 |
| Global parameters | |
| LV ejection fraction, % | 62.08 ± 7.25 |
| LV mass-indexed to BSA, gm/m2 | 65.79 ± 13.41 |
| LV end-diastolic volume, mL | 118.36 ± 31.05 |
| LV end-systolic volume, mL | 45.57 ± 17.71 |
HDL, high-density lipoprotein, LBBB, left-bundle-branch block, LV, left ventricular.
Mean ± SD (median, inter-quartile range) for SD-TPS was 92.45 ± 29.56msec (91.51, 72.28 – 112.34msec) and for max-min was 287.04 ± 90.79 msec (280.0, 210.0- 350.0msec).
Carotid IMT and plaque score
Mean CCA-IMT was 0.85 ± 0.19 mm (25-75th percentile, 0.72-0.95 mm); 368 individuals had mean CCA-IMT >1.0 mm. Mean ICA-IMT was 0.71 ± 0.26 (25-75th percentile, 0.54-0.78 mm); 1,374 individuals had presence of plaque on carotid ultrasound examination. Mean plaque score was 2.15 ± 2.33 (median 1.0, 25th-75th percentile 0-4).
Relationship of common carotid and internal carotid artery IMT with LV dyssynchrony
In multivariable regression analysis, CCA-IMT was significantly associated with SD-TPS (β-coefficient 8.71 msec/mm change in IMT, p=0.020) and max-min (β-coefficient 32.53 msec/mm IMT, p=0.005) (Table 2).
Table 2. Relationship of carotid artery (common and internal) intima-media thickness and carotid plaque score with parameters of left ventricular dyssynchrony.
| CCA-IMT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model-1 (2015) | Model-2 (1989) | Model-3 (1965) | Model-4 (1954) | |||||
| B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | |
| SD-TPS | 18.62 (11.91 - 25.32) | <0.001 | 12.95 (5.71 - 20.19) | <0.001 | 9.26 (1.84 - 16.68) | 0.014 | 8.71 (1.37 - 16.04) | 0.020 |
| Max-min | 57.05 (36.48 - 77.63) | <0.001 | 43.96 (21.62 - 66.3) | <0.001 | 33.18 (10.28 - 56.09) | 0.005 | 32.53 (9.82 - 55.24) | 0.005 |
| ICA-IMT | ||||||||
| Model-1 (1333) | Model-2 (1317) | Model-3 (1302) | Model-4 (1295) | |||||
| B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | |
| SD-TPS | 19.36 (13.49 - 25.23) | <0.001 | 18.41 (12.43 - 24.38) | <0.001 | 17.62 (11.52 - 23.73) | <0.001 | 19.23 (13.24 - 25.22) | <0.001 |
| Max-min | 47.62 (29.35 - 65.89) | <0.001 | 46.21 (27.51 - 64.9) | <0.001 | 44.73 (25.63 - 63.83) | <0.001 | 49.67 (30.9 - 68.45) | <0.001 |
| Carotid plaque score | ||||||||
| Model-1 (2062) | Model-2 (2036) | Model-3 (2011) | Model-4 (2000) | |||||
| B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | B-coefficient (95% CI) | p-value | |
| SD-TPS | 2.2 (1.66 - 2.74) | <0.001 | 1.59 (1.02 - 2.16) | <0.001 | 1.22 (0.634 - 1.8) | <0.001 | 1.24 (0.66 - 1.82) | <0.001 |
| Max-min | 6.18 (4.53 - 7.83) | <0.001 | 4.72 (2.97 - 6.48) | <0.001 | 3.78 (1.97 - 5.59) | <0.001 | 3.85 (2.06 - 5.64) | <0.001 |
CCA, common carotid artery, ICA, Internal carotid artery, IMT, intima-media thickness. Unit of β-coefficient, msec/mm change in IMT. CI, confidence interval. SD-TPS, standard deviation of time to peak systolic circumferential strain, Max-min, Difference between the earliest and latest time to peak segmental systolic circumferential strain. Model 1, unadjusted; Model 2, adjusted for age, gender, ethnicity, and heart rate; Model 3, model 2 plus diabetic status, hypertension status, antihypertensive medication, total cholesterol, HDL-cholesterol, body mass index, smoking status (never, former, current), education level; Model 4: model 3 plus end-diastolic LV mass and LV ejection fraction.
Similarly, in the multivariable regression model, ICA-IMT was also related to parameters of LV dyssynchrony; SD-TPS (β-coefficient 19.23 msec/mm change in IMT, p<0.001) and max-min (β-coefficient 49.67 msec/mm, p<0.001).
Relationship of carotid artery plaque score and LV dyssynchrony parameters
Using multivariable regression, plaque score as a continuous variable was significantly associated with SD-TPS (β-coefficient 1.24 msec/unit change in plaque score, p<0.001) and max-min (β-coefficient 3.85 msec/unit change in plaque score, p<0.001).
Coronary artery calcium score and LV dyssynchrony
Mean CAC was 256 ± 539 (median 35, 25th-75th percentile 0-247). 667 (32.7 33%) participants had CAC of zero.
Unadjusted log(CAC+1) was significantly related to SD-TPS (β-coefficient 1.489msec/unit log(CAC+1), p<0.001) and max-min (β-coefficient 3.882msec/unit log(CAC+1), p<0.001). The relationship persisted for SD-TPS (β-coefficient 0.664msec/unit log(CAC+1), p=0.018) and max-min (β-coefficient 2.040msec/unit change in log(CAC+1), p=0.019) after adjustment for traditional and non-traditional cardiovascular risk factors including LV mass and ejection fraction (Table-3). In multivariable analyses, ICA-IMT, carotid artery plaque score, and coronary artery calcium score were not related to LV ejection fraction (data not shown).
Table 3.
Relationship of phantom adjusted coronary calcium score with parameters of left ventricular dyssynchrony.
| Parameters | Model-1 (n=2039) | Model-2 (n=2013) | Model-3 (n=1986) | Model-4 (n=1975) | ||||
|---|---|---|---|---|---|---|---|---|
| β-coefficient (95% CI) | p-value | β-coefficient (95% CI) | p-value | β-coefficient (95% CI) | p-value | β-coefficient (95% CI) | p-value | |
| SD-TPS | 1.489 (1.011 - 1.968) | <0.001 | 0.894 (0.358 - 1.431) | 0.001 | 0.571 (0.013 - 1.130) | 0.045 | 0.664 (0.113 - 1.215) | 0.018 |
| Max-min | 3.882 (2.407 - 5.358) | <0.001 | 2.528 (0.867 - 4.189) | 0.003 | 1.751 (0.021 - 3.481) | 0.047 | 2.040 (0.330 - 3.749) | 0.019 |
SD-TPS, standard deviation of time to peak systolic circumferential strain, Max-min, Difference between the earliest and latest time to peak segmental systolic circumferential strain. For adjustment parameter, please refer Table-2.
Participants categorized in tertiles based on incremental CAC score showed worsening dyssynchrony with mean SD-TPS of 87.84 ± 29.98msec (n=667), 92.10 ± 29.28msec (n=686), and 96.67 ± 29.46msec (n=686) in 1st, 2nd, & 3rd tertiles, respectively. ANOVA test with Bonferroni's correction revealed a statistically significant difference of mean SD-TPS between the tertile groups (Fig.-2).
Figure 2.
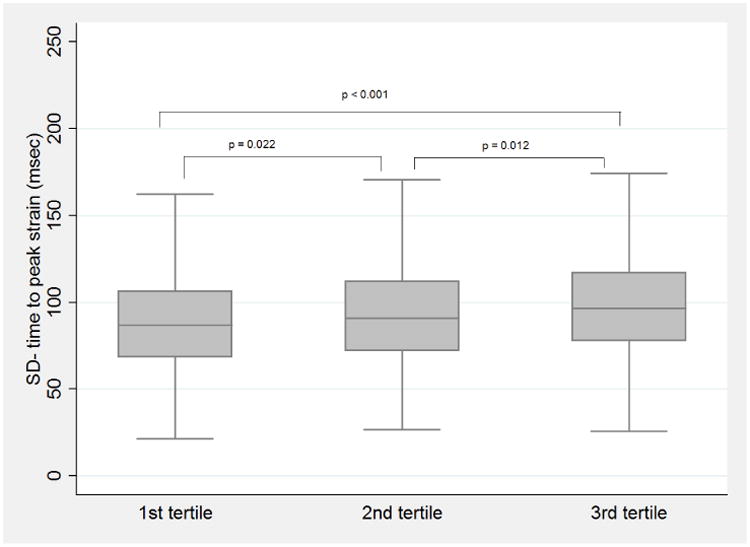
Box plot representing distribution of SD-time to peak systolic strain categorized by tertiles of coronary artery calcium score.
P-values denote the statistical significance of difference between SD-TPS mean among the groups using analysis of variance test with Bonferroni's correction.
Effect of myocardial scar based on LGE analyses
In multivariable regression analyses with further adjustment for presence of myocardial scar based on LGE analyses revealed improved relationship between CCA-IMT and LV dyssynchrony (β-coefficient 9.25 msec/mm change in IMT, p=0.014), carotid plaque score – LV dyssynchrony (β-coefficient 1.29 msec/unit change in plaque score, p<0.001) and coronary calcium score (β-coefficient 0.674msec/unit log(CAC+1), p=0.017). (Table 4)
Table 4. Table representing multivariable regression analyses to assess correlation between atherosclerotic markers and LV dyssynchrony, adjusted for presence of myocardial scar on delayed enhanced MRI.
| Parameters | B-coefficient (95% CI) | p-value |
|---|---|---|
| CCA-IMT | ||
| SD-TPS | 9.25 (1.85 - 16.65) | 0.014 |
| Max-min | 34.18 (11.28 - 57.08) | 0.003 |
| ICA-IMT | ||
| SD-TPS | 19.50 (13.51 - 25.49) | <0.001 |
| Max-min | 50.59 (31.79 - 69.39) | <0.001 |
| Carotid plaque score | ||
| SD-TPS | 1.29 (0.71 - 1.86) | <0.001 |
| Max-min | 3.98 (2.18 - 5.76) | <0.001 |
| Coronary calcium score | ||
| SD-TPS | 0.67 (0.12 - 1.23) | 0.017 |
| Max-min | 2.10 (0.38 - 3.83) | 0.017 |
CCA, common carotid artery, ICA, Internal carotid artery, IMT, intima-media thickness. Unit of β-coefficient, msec/mm change in IMT. CI, confidence interval. SD-TPS, standard deviation of time to peak systolic circumferential strain, Max-min, Difference between the earliest and latest time to peak segmental systolic circumferential strain. Model adjusted for age, gender, ethnicity, and heart rate, diabetic status, hypertension status, antihypertensive medication, total cholesterol, HDL-cholesterol, body mass index, smoking status (never, former, current), education level, end-diastolic LV mass, LV ejection fraction and presence of myocardial scar on LGE.
Participants with absence of coronary artery calcium and myocardial scar on LGE (calcium score=0, no scar) had lowest values of SD-TPS (n=415, mean±SD 87.27 ±29.14msec). In contrast, higher values of LV dyssynchrony were noted in participants with calcium score >0 and no scar (n=738, 93.27 ± 27.65msec, p<0.01) while participants with presence of coronary calcium and myocardial scar had highest values of SD-TPS (n=87, 94.38 ± 32.46msec, p<0.01 vs. Calcium score=0 and no scar group).
Comparative analysis of atherosclerosis parameters and association with dyssynchrony
A multivariable linear regression analyses with variable selection model including all three atherosclerosis parameters predicting LV dyssynchrony revealed that carotid plaque score was better correlated with SD-TPS (β-coefficient 1.255 msec/unit change in plaque score, p<0.001, R2 0.145). CCA-IMT (β-coefficient 4.176 msec/mm change in IMT, p=0.294, R2 0.138) and CAC score (β-coefficient 0.287 msec/unit log(CAC+1), p=0.345, R2 0.143) had attenuation of statistical significance in the model.
Discussion
This study demonstrates a relationship between subclinical atherosclerosis and impaired regional LV function expressed as dyssynchronous myocardial contraction in a multi-ethnic population of asymptomatic individuals. The CCA-IMT, ICA-IMT, carotid plaque score as surrogate markers of coronary atherosclerosis were consistently related to the extent of LV dyssynchrony independent of the presence of myocardial scar. Similarly, coronary calcium score as a measure of atherosclerotic burden in the coronary arterial system was associated with parameters of mechanical dyssynchrony. These findings illustrate a potential role of early atherosclerosis in inflicting subclinical myocardial damage in the absence of a discrete clinical event. To our knowledge, these findings are the first to exhibit the correlation of subclinical atherosclerosis with LV dyssynchrony as a distinct parameter of regional myocardial impairment using CMR in a population-based cohort—way before the occurrence of global LV dysfunction.
The natural history of ischemic heart failure associated with global enlargement and dysfunction focuses on patients who develop heart failure mediated by clinical coronary events triggering structural alterations and compensatory mechanisms that lead to LV dilation and remodeling. LV dyssynchrony-related impairment in cardiac function has been primarily considered in relevance to HF due to reduced ejection fraction, based on the potential for therapeutic intervention (e.g., cardiac resynchronization therapy). We found that alterations in the synchronization of LV myocardial contraction precede impairment in global LV function. Therefore, our results may have significant implications in regards to preventive efforts and management of ischemia-related LV dysfunction.
The mechanisms of atherosclerosis-induced dyssynchrony are complex. Carotid IMT represents early atherosclerotic changes in the vascular system and is considered as a surrogate end-point of atherosclerosis in epidemiology-based studies,[6, 22, 23] or clinical studies gauging efficacy of therapeutic interventions.[4, 8, 24] Therefore, IMT reflects an increased burden of atherosclerosis with greater incidence of epicardial vessel CAD and possible small vessel microembolization secondary to plaque rupture. In hypertensive patients without coronary artery disease, Takiuchi et al.[25] demonstrated an inverse relationship of carotid IMT with coronary flow reserve linked to early phase atherosclerosis. Furthermore, the presence of early subclinical atherosclerosis—even in the absence of stenotic CAD—is associated with increased resistance in smaller microvessels[26, 27], reduced coronary flow reserve[25, 28, 29], and endothelial dysfunction, causing repeated myocardial stunning from recurrent ischemia, thereby altering local myocardial contractility.[30] Using CMR perfusion imaging in the MESA study, Rosen et al. illustrated that reduced myocardial perfusion is accompanied by worsening of LV dyssynchrony.[17] LV dyssynchrony is also related to myocardial remodeling that occurs in response to cardiovascular risk burden[31, 32] as a common denominator for atherosclerosis as well as myocardial deformation.
Fernandes et al.[33] reported that, with greater carotid IMT, there is a decrease in segmental myocardial strain and diastolic function as a marker of incipient regional myocardial dysfunction that precedes global reduction of ejection fraction and symptoms in the heart failure continuum. In a similar MESA subset, Edvardsen et al. demonstrated a decrease in segmental myocardial strain with an increase in CAC.[34] Our study significantly extends this paradigm by demonstrating a distinctive mechanism of myocardial functional impairment.
Aging, LV mass and regional myocardial dysfunction
Since age and LV end-diastolic mass are directly related to subclinical LV dyssynchrony,[17] we performed supplementary analyses to control for the influence of aging and increased LV mass as potential confounders to the atherosclerosis-LV dyssynchrony relationship. Additional analyses (Tables 2 & 3) showed that even though age and LV mass affect dyssynchrony, the associations of IMT and CAC with LV dyssynchrony persist despite statistical control for age (model 2) and LV mass (model 4). The participants with LBBB were also excluded from the analyses to account for LBBB as a potential confounder in the induction of LV dyssynchrony. Moreover, the subclinical atherosclerosis-dyssynchrony relationship did not alter after adjusting for hypertension and other established cardiovascular risk factors contributing towards incipient regional dysfunction.[35, 36]
Methodological considerations
Our study demonstrates the relationship of carotid IMT with LV dyssynchrony as a marker of regional myocardial dysfunction in more than 2000 individuals with no previous history of clinical coronary event or left-bundle-branch block using tagged CMR, which is considered as a standard for assessment of regional LV function. Hence, the prevalence of risk factors and severity of carotid IMT may not reflect that found in the general population, and extrapolation of our study results must be considered in the context of the study design and the population examined. Moreover, the basic mechanisms involving myocardial dyssynchrony and atherosclerosis are complex and are beyond the scope of this paper. Finally, since our study is cross-sectional in nature, causal temporality cannot be determined.
In summary, our study demonstrates that subclinical atherosclerosis determined by carotid IMT, carotid plaque score, and coronary artery calcium score in the absence of clinical cardiovascular event are significantly associated with parameters of left ventricular dyssynchrony as a marker of subclinical regional myocardial dysfunction. These findings in a population based asymptomatic cohort provide important insights into the mechanisms and pathways of atherosclerosis-related incipient LV dysfunction and heart failure.
Supplementary Material
Highlights.
Our study demonstrate that in a multi-ethnic population free of clinical cardiovascular disease, subclinical atherosclerotic markers such as carotid intima-media thickness, carotid plaque score, and coronary calcium score are related to left ventricular dyssynchrony as a measure of regional myocardial dysfunction, independent of presence of myocardial scar and left bundle branch block.
These findings are the first to exhibit the correlation of subclinical atherosclerosis with LV dyssynchrony as a distinct parameter of regional myocardial impairment using cardiac magnetic resonance imaging in a population-based cohort—way before the occurrence of global LV dysfunction.
Of the atherosclerotic parameters, carotid plaque score was better correlated with subclinical left ventricular dyssynchrony.
These findings illustrate a potential role of early atherosclerosis in inflicting subclinical myocardial damage in the absence of a discrete clinical event and provide important insights into the mechanisms and pathways of atherosclerosis-related incipient LV dysfunction and heart failure.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Bayer HealthCare provided Magnevist contrast agent for the MESA CMR study.
Funding sources: This research was supported by contracts N01-HC-95159 through N01-HC95168 from the National Heart, Lung, and Blood Institute. This publication was developed under a STAR research assistance agreement, No.RD831697 (MESA Air), awarded by the U.S Environmental Protection Agency. The EPA has not formally reviewed this publication. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Abbreviations
- LV
Left ventricular
- CMR
Cardiovascular magnetic resonance
- CCA
Common carotid artery
- ICA
Internal carotid artery
- IMT
Carotid intima-media thickness
- CAC
Coronary artery calcium
- CT
Computed tomography
- HF
Heart failure
- LBBB
Left bundle-branch block
- SD
Standard deviation
- SD-TPS
SD time to peak strain
- Max-min
Maximum to minimum time to peak strain
- HDL
High-density lipoprotein
- eGFR
estimated glomerular filtration rate
Footnotes
Disclosures: Dr. Ravi K. Sharma is a recipient of a postdoctoral research grant by Unijules Life Sciences Ltd., India. Other authors have no conflict of interest to report.
Clinical trials info: MESA; http://www.mesa-nhlbi.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. The New England journal of medicine. 1992;327:685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 3.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Annals of internal medicine. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. The New England journal of medicine. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 5.Baldassarre D, Amato M, Pustina L, et al. Measurement of carotid artery intima-media thickness in dyslipidemic patients increases the power of traditional risk factors to predict cardiovascular events. Atherosclerosis. 2007;191:403–8. doi: 10.1016/j.atherosclerosis.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.de Groot E, van Leuven SI, Duivenvoorden R, et al. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nature clinical practice Cardiovascular medicine. 2008;5:280–8. doi: 10.1038/ncpcardio1163. [DOI] [PubMed] [Google Scholar]
- 7.Mortsell D, Malmqvist K, Held C, et al. Irbesartan reduces common carotid artery intima-media thickness in hypertensive patients when compared with atenolol: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) study. Journal of internal medicine. 2007;261:472–9. doi: 10.1111/j.1365-2796.2007.01775.x. [DOI] [PubMed] [Google Scholar]
- 8.Terpstra WF, May JF, Smit AJ, et al. Effects of amlodipine and lisinopril on intima-media thickness in previously untreated, elderly hypertensive patients (the ELVERA trial) Journal of hypertension. 2004;22:1309–16. doi: 10.1097/01.hjh.0000125412.50839.b5. [DOI] [PubMed] [Google Scholar]
- 9.Xie W, Liang L, Zhao L, et al. Combination of carotid intima-media thickness and plaque for better predicting risk of ischaemic cardiovascular events. Heart (British Cardiac Society) 2011;97:1326–31. doi: 10.1136/hrt.2011.223032. [DOI] [PubMed] [Google Scholar]
- 10.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–33. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 12.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Gustavo V, Ambale-Venkatesh B, et al. Left ventricular dyssynchrony in asymptomatic women predicts adverse cardiovascular outcome: multi ethnicity study of atherosclerosis (MESA) Journal of the American College of Cardiology. 2014;63 doi: 10.1161/JAHA.114.000975. published Online First: 4 August 2014. [DOI] [Google Scholar]
- 14.Gerber BL, Garot J, Bluemke DA, et al. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083–9. doi: 10.1161/01.cir.0000027818.15792.1e. [DOI] [PubMed] [Google Scholar]
- 15.Shehata ML, Cheng S, Osman NF, et al. Myocardial tissue tagging with cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2009;11:55. doi: 10.1186/1532-429X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Rosen BD, Fernandes VR, Nasir K, et al. Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:859–66. doi: 10.1161/CIRCULATIONAHA.108.787408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu CM, Zhang Q, Fung JW, et al. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. Journal of the American College of Cardiology. 2005;45:677–84. doi: 10.1016/j.jacc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Hollander M, Bots ML, Del Sol AI, et al. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105:2872–7. doi: 10.1161/01.cir.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 20.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89-90. [DOI] [PubMed] [Google Scholar]
- 21.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 22.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Journal of the American Heart Association. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz MW, von Kegler S, Steinmetz H, et al. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke; a journal of cerebral circulation. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 24.Geng DF, Deng J, Jin DM, et al. Effect of cilostazol on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;220:177–83. doi: 10.1016/j.atherosclerosis.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 25.Takiuchi S, Rakugi H, Fujii H, et al. Carotid intima-media thickness is correlated with impairment of coronary flow reserve in hypertensive patients without coronary artery disease. Hypertension research : official journal of the Japanese Society of Hypertension. 2003;26:945–51. doi: 10.1291/hypres.26.945. [DOI] [PubMed] [Google Scholar]
- 26.Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159–60. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 27.Danad I, Raijmakers PG, Kamali P, et al. Carotid artery intima-media thickness, but not coronary artery calcium, predicts coronary vascular resistance in patients evaluated for coronary artery disease. European heart journal cardiovascular Imaging. 2012;13:317–23. doi: 10.1093/ehjci/jes038. [DOI] [PubMed] [Google Scholar]
- 28.Raitakari OT, Toikka JO, Laine H, et al. Reduced myocardial flow reserve relates to increased carotid intima-media thickness in healthy young men. Atherosclerosis. 2001;156:469–75. doi: 10.1016/s0021-9150(00)00689-4. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Jerosch-Herold M, Jacobs DR, Jr, et al. Coronary artery calcification and myocardial perfusion in asymptomatic adults: the MESA (Multi-Ethnic Study of Atherosclerosis) Journal of the American College of Cardiology. 2006;48:1018–26. doi: 10.1016/j.jacc.2006.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen BD, Lima JA, Nasir K, et al. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the multi-ethnic study of atherosclerosis. Circulation. 2006;114:289–97. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
- 31.Bauer M, Cheng S, Unno K, et al. Regional cardiac dysfunction and dyssynchrony in a murine model of afterload stress. PloS one. 2013;8:e59915. doi: 10.1371/journal.pone.0059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Chettiveettil D, Jones F, et al. Left ventricular dyssynchrony in hypertensive patients without congestive heart failure. Clinical cardiology. 2008;31:597–601. doi: 10.1002/clc.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandes VR, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) Journal of the American College of Cardiology. 2006;47:2420–8. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 34.Edvardsen T, Detrano R, Rosen BD, et al. Coronary artery atherosclerosis is related to reduced regional left ventricular function in individuals without history of clinical cardiovascular disease: the Multiethnic Study of Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:206–11. doi: 10.1161/01.ATV.0000194077.23234.ae. [DOI] [PubMed] [Google Scholar]
- 35.Rosen BD, Edvardsen T, Lai S, et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 36.Rosen BD, Saad MF, Shea S, et al. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. Journal of the American College of Cardiology. 2006;47:1150–8. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


