Abstract
Rationale
Illicit use of MDMA (3,4-methylenedioxymethamphetamine; Ecstasy) may cause a mild or severe form of the serotonin syndrome. The syndrome intensity is not just influenced by drug doses but also by environmental factors.
Objectives
Warm environmental temperatures and physical activity are features of raves. The purpose of this study was to assess how these two factors can potentially intensify the syndrome.
Methods
Rats were administered MDMA at doses of 0.3, 1 or 3 mg/kg, and examined in the absence or presence of warm temperature and physical activity. The syndrome intensity was estimated by visual scoring for behavioral syndrome and also instrumentally measuring changes in symptoms of the syndrome.
Results
Our results showed that MDMA at 3 mg/kg, but not 0.3 or 1 mg/kg, caused a mild serotonin syndrome in rats. Each environmental factor alone moderately intensified the syndrome. When the two factors were combined, the intensification became more severe than each factor alone highlighting a synergistic effect. This intensification was blocked by the 5-HT2A receptor antagonist M100907, competitive NMDA receptor antagonist CGS19755, autonomic ganglionic blocker hexamethonium, and the benzodiazepine-GABAA receptor agonist midazolam, but not by the 5-HT1A receptor antagonist WAY100635 or nicotinic receptor antagonist methyllycaconitine.
Conclusions
Our data suggest that, in the absence of environmental factors, the MDMA-induced syndrome is mainly mediated through the serotonergic transmission (5HT-dependent mechanism), and therefore, is relatively mild. Warm temperature and physical activity facilitate serotonergic and other neural systems such as glutamatergic and autonomic transmissions, resulting in intensification of the syndrome (non-5HT mechanisms).
Keywords: MDMA, serotonin syndrome, neurotoxicity, 5-HT1A receptors, 5-HT2A receptors, hyperthermia, microdialysis and EEG
Although MDMA (3,4-methylenedioxymethamphetamine) is perceived as a safe recreational drug, some users develop illnesses or die (Parrott, 2002; Ben-Abraham et al., 2003; Bahora et al., 2009; Parrott et al., 2011). Symptoms vary widely but include clonus, hyperthermia, seizure and agitation (collectively called serotonin syndrome). Neurochemically, the drug causes excessive serotonin (5-hydroxytryptamine; 5HT) release widespread within the brain (Baumann et al., 2008; Ma et al., 2013). As a result, all 5HT receptor subtypes are simultaneously activated to a great extent, presumably upsetting a neural balance between inhibitory and excitatory networks, and disturbing many aspects of the body and mind such as mood, autonomic function and somatic neuromuscular activities (Mason et al., 2000; Rang et al., 2008).
In animal studies, however, MDMA fails to cause the serotonin syndrome unless high dosages are used (Malberg and Seiden, 1998; Ma et al., 2013). Furthermore, there are debates over whether animals represent suitable models to study the adverse effects that MDMA has on people who attend raves and rave-like parties (Green et al., 2012; Parrott, 2012a; Doblin et al., 2014). Recently, the difference between animal and human data has been reconciled by animal tests carried out under distinct environments to mimic such rave scenarios (Turner and Parrott, 2000; Parrott, 2012b). Several environmental factors have been investigated, including warm ambient temperatures, physical activity, loud music, lights and crowded situations (Malberg and Seiden, 1998; Fantegrossi et al., 2003; Iannone et al., 2006; Stanley et al., 2007; Von Huben et al., 2007; Gilpin et al., 2011). Despite many efforts, the environment explored in laboratory tests appears to have very moderate effects on MDMA toxicity (Green et al., 2004b; Shortall et al., 2013) unless a huge dose, (e.g., 20-40 mg/kg) was used for animals (Gordon et al., 1991; Malberg and Seiden, 1998). In contrast, the rave environment for humans could seriously potentiate the toxic effect of MDMA at only 1-3 tablets (roughly equivalent to 1-3 mg/kg) (Ben-Abraham et al., 2003; Halpern et al., 2011; Davies et al., 2014).
Thus, there are at least three barriers responsible for the disengagement of an animal model from the study of a health risk in humans. Firstly, there is a dosage disparity between animals and humans. In earlier investigations, dosages used for animals were as high as 20-40 mg/kg (Gordon et al., 1991; Malberg and Seiden, 1998). Although dosages have been greatly reduced in recent studies, the dose most appropriate for conducting a basic animal test is still an open question (Baumann et al., 2009; Concheiro et al., 2014). Secondly, environments for MDMA human users are more complex than for animals. Animal data available in the literature are almost exclusively obtained in the presence of a single environmental factor, such as warmer temperatures or physical activity alone (Gordon et al., 1991; Malberg and Seiden, 1998; Gilpin et al., 2011). As a result, single factor studies lead to a prevailing conclusion that environment plays a relatively minor role in MDMA toxicity, compared to drug dosages. However, the actual role of environment is likely underestimated, because human users encounter a combination of multiple environmental factors, and thus animal research has not looked at the synergic effects of these multiple environmental factors. Thirdly, there is a clear gap between basic research and clinical practice. For instance, although excessive 5HT is the cause of the syndrome as demonstrated by fundamental research studies, drugs typically used for treating MDMA toxicity in patients are non-serotonergic (Armenian et al., 2013; Davies et al., 2014). How non-serotonergic mechanisms are involved in the pathways mediating serotonin syndrome is not understood and requires elucidation.
In the present work, three sets of experiments were conducted. The first study explored whether human-relevant dosages (0.3-3 mg/kg) could produce excessive 5HT, behavioral responses or even a syndrome in laboratory rats. Dosages of 0.3 mg/kg, 1 mg/kg and 3 mg/kg are equivalent to low-to-moderate human recreational doses (Green et al., 2003; Green et al., 2004a; Baumann et al., 2007; Concheiro et al., 2014). In the second study, we tested how warm temperature and physical activity, combined or independently, influenced the intensity of MDMA-elicited syndrome and lastly, we conducted pharmacologic intervention tests by which serotonergic and non-serotonergic mechanisms were explored.
Materials and Methods
Animals
Adult male Sprague–Dawley rats weighing 250-275 g were purchased from Charles River Laboratories (Raleigh, NC, USA) and group-housed (two to three per cage) in a temperature (22 ±1 °C)- and humidity (40-70%)-controlled facility on a 12-h light/dark cycle (lights on from 07:00-19:00). Food and water were available at all times. Animals were used for experiments when their body weight had reached 300-350 g. All animal use procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care & Use Committee (IACUC) at Florida Atlantic University. Animals were euthanized immediately upon completion of a test.
Drugs
Racemic MDMA [(±)-3,4-methylenedioxymethamphetamine hydrochloride] and M100907 were kindly provided by the National Institute of Drug Abuse (Baltimore, MD, USA). Ketanserin tartrate and methyllycaconitine citrate were purchased from TOCRIS (Ellisville, MO, USA). Hexamethonium bromide, midazolam hydrochloride, and WAY100,635 maleate were purchased from Sigma (St. Louis, MO, USA). Drug dosages (mg/kg) were expressed as the weight of the salt and injected at a constant volume of 1 mL/kg (dissolved in 0.9% NaCl). MDMA and ketanserin were injected intraperitoneally, and other drugs were administered subcutaneously.
Behavioral syndrome test
Behavioral syndrome was scored according to an established method described in our previous work (Ma et al., 2008). Scoring was performed 30 - 60 min after MDMA or vehicle administration. Each rat was rated twice at time blocks of 30–45 min and 45–60 min. Changes in behavioral activity, including forepaw treading, hind-limb abduction, salivation and penile erection were visually identified and assessed at 4 levels ranging from 0 (no effect) to 1 (mild), 2 (moderate) and 3 (severe).
Force-plate actimeter test
Methods of calibration and details of data acquisition have been described elsewhere (Fowler et al., 2001). Briefly, the force-plate actimeter purchased from BASi Corp (Model FPA-I; West Lafayette, IN, USA) consists of a force-sensitive plate at a resolution of 200 Hz, a sound-attenuation chamber, a computerized data-acquisition board and analysis system software (FPA 1.10.01). Prior to each trial, an animal was first placed in the apparatus for a 30-min acclimatization period. Then, the animal was taken out for drug treatment and immediately put back for a 30 min tremor recording session. Between each test, the plate was thoroughly cleaned with paper towels followed by a deodorant treatment (70% ethanol, 1% acetic acid and then water) to remove animal waste (i.e., feces, urine, saliva and furs) and odor. Trace data of movements were automatically stored on the hard drive for offline analysis. Changes in muscular contraction force were revealed through power spectral analysis and expressed as a percent of body weight (% BW; force variation independent of body weight). The unit for changes in the power force was arbitrary.
Protocols for examination of two environmental factors
Interaction of MDMA with warm temperature and physical activity was examined in three measures as follows:
1) Absence of the factor
Tests were conducted in freely behaving rats at ambient temperature of 22 °C (± 1 °C).
2) Presence of a single factor
a) Warm ambient temperature. Temperature-controlled chambers were set to and maintained at 32 °C (±1 °C). Temperature of 32 °C was chosen because it is slightly higher than the thermoneutral zone [29.5-30.5 °C; (Romanovsky et al., 2002)]. Animals were placed in the chamber for acclimatization to the warm temperature at least 2 h before starting experiments. b) Physical stimulation. Physical stimulation as a factor was tested by treadmill activity at a running speed of 2 meters/min. The treadmill apparatus was equipped with a food container and bottled water for animals to access freely. The treadmill belt was covered with a bottomless Plexiglas cage (length 50 cm × width 16 cm × height 30 cm) to prevent animals from escaping or falling off. The rear end of the cage extended over the edge of the belt to create an approximate 3-mm opening that allowed hind-limbs but not the whole body falling out from the belt if animals stopped walking. When walking on the treadmill, animals attempted to avoid falling, so they constantly moved forward toward the other end. This design was intended to keep animals walking without the use of electric shock. Control animals were also placed on the treadmill, but with the belt disengaged from the motor. Animals were exposed to the treadmill only once, and the test duration was 30 min.
3) Combination of two factors
A treadmill for physical stimulation was placed in the temperature-controlled chamber set at 32 °C. Animals were placed in the treadmill cage at least 2 h before starting experiments. The treadmill motor was turned on immediately after injection of MDMA or vehicle and lasted for 30 min. Animals remained in the treadmill cage at 32 °C throughout all the experiments. The design was used to collect animal data including the behavioral syndrome scoring, 5HT microdialysis, EEG and Tcor. Since the force-plate actimeter, EPM or DLT apparatus cannot be placed in the chamber or treadmill, tests regarding the environmental effect on neuromuscular activity or anxiety-like behavior were not conducted (see details below).
Electroencephalogram (EEG)
a)Data recording
Rats weighing 300 to 350 g were anesthetized with a combination of xylazine (4 mg/kg IP) and ketamine (80 mg/kg IP). Two stainless steel screws were anchored on the skull over the frontal cortex (AP +2 mm relative to the bregma, ML ±2 mm relative to the midline) for positive and reference electrodes. A third screw was implanted on the skull over the parietal cortex (AP +2 mm relative to the lambda, ML ±2 mm) for the negative electrode. The positive and negative electrodes were transversely arranged across two hemispheres. Two wires were surgically inserted into deep neck muscles serving as the electromyogram (EMG) electrodes. After surgery, the rats were housed individually and allowed to recover for one week. The day before EEG/EMG recording, animals were placed in a recording chamber overnight for habituation. Recording began on the following day with a 1 h baseline measurement and then 2 h post-MDMA recordings. Digital data were filtered (low-pass filter set to 70 Hz and high-pass filter to 0.3 Hz) and continuously sampled by a computer running Chart 7 software (ADInstruments, Milford, U.S.A).
b) Data analysis
Values of baseline and drug responses were determined off-line. Given that MDMA administration is associated with wakefulness, only active waking EEG waves were used as a baseline for comparing drug-induced responses. Criteria on waking waves were based on EMG potentials as described in literature (Jacobs and Donoghue, 1991). The spectral power density (μV2/Hz) for particular frequency bands (delta 0.5-4 Hz, theta 4-8 Hz, alpha 8-12 Hz and beta 12-30 Hz) was calculated at 0.25 Hz resolution performed using the Chart 7 software with Fast Fourier Transform (FFT). For data comparison, EEG frequency domain was transformed to the time domain. In order to minimize the chance of error, each 30-s epoch time domain (point) was the mean of triplicate samples consisting of the start, middle and end of each 30 min time period. To obtain overall evaluation, the power spectral density at each time point was computerized as percent (%) changes from the baseline values.
Elevated plus-maze test (EPM)
The EPM apparatus included two open arms (50 × 10 cm) with 0.5 cm high edges to prevent rats from falling off and also two closed arms of the same dimensions with walls 40 cm high. Four arms were connected by a central square (10 × 10 cm) as the neutral zone, and thus the maze formed a plus sign. It was elevated 70 cm above the floor. The illumination level was approximately 20 lux (UDT Instruments Model 370, San Diego, USA). Animals were placed in the neutral zone, facing one of the open arms. Recording was not started unless the entire body was entered into one of the closed arms. Numbers of entries, time, and distance in individual zones were recorded for 5 min by a video-tracking system (Viewpoint, Montreal, Canada). At the end of each test, the data was expressed as a percentage of total numbers of arm entries, duration and distance.
Dark-light transition test (DLT)
The DLT apparatus was an 80 cm × 40 cm box divided equally into ‘light’ and ‘dark’ sides by a Plexiglas insert. The ‘light side’ of the arena was illuminated by a compact fluorescent light (~100 lux). In each trial, a rat was first placed in the light arena, and recording was started as soon as it entered the dark side. Data were collected for a total of 5 min. During this span, the numbers of light–dark transitions, the total time spent in each transition, and the distances each rat traveled in the light compartment were analyzed.
Neurochemical assay
Animals weighing 300-350 g were anesthetized with a combination of xylazine (4 mg/kg IP) and ketamine (80 mg/kg IP) and placed in a stereotaxic device with the flat skull in a fixed position. Guide cannulas for microdialysis were surgically implanted, and animals were allowed to recover for at least one week. Microdialysis was performed with I-shaped probe constructed from 26-gauge stainless steel tubing and glass silica. The dialysis tubing was hollow nitrocellulose fiber (200 μm i.d., 13,000 MW cut-off; Spectrum Medical Industries, Los Angeles, CA, USA). The length of the exchange surface of dialysis membrane was 1.0 mm for the preoptic area/anterior hypothalamus (POA) and 2.5 mm for the prefrontal cortex (PFC). The evening before an experiment, aseptic dialysis probes were inserted through the guide cannulae. Dialysis probe shafts were measured, and the length adjusted to the brain targets using collars prepared from 21-gauge stainless steel tubing. The target coordinates for the tip of the probe were in the PFC, AP +3.7 mm relative to the bregma, ML 0.7 mm relative to the midline and DV 5.5 mm relative to the dura, in the POA, AP -1.3 mm, ML 0.9 mm, and DV 9.0 mm. Rats were then placed in the test chamber and attached to a fluid swivel that allowed animals to move freely. Food and water were available ad libitum. The dialysis probes were perfused overnight with an artificial cerebrospinal fluid (aCSF; 140 mM NaCl, 3.0 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 0.25 mM NaH2PO4, and 1.0 mM Na2HPO4). Samples were collected every 15 min and analyzed by high-performance liquid chromatography (HPLC) with electrochemical detection (HPLC-EC; HTEC-500, Eicom, Japan) in conjugation with an autoinjector (SIL-10Avp, Shimadzu, Maryland, USA). 5HT was separated through a mobile phase (0.1 M phosphate buffer at pH 6.0, 500 mg/L 1-decanesulfonic acid, 50 mg/L EDTA, and 1.0% methanol) at a rate of 0.50 ml/min. The detection limit for 5-HT was approximately 0.05 pg per sample based on a signal-to-noise ratio of 3:1. The mean of 4 consecutive measurements was obtained as a baseline, immediately before drug or vehicle injection. Changes in the 5HT overflow were expressed as a fold increase above baseline. Data were expressed either as line or bar graphs. Line graphs indicate the time course of the 5HT overflow both before and after injection, while bar graphs show mean (± s.e.m) of the 5HT overflow between 0 - 45 min after injection.
Core temperature (Tcor) measurements
Changes in Tcor were used to estimate the adverse effect relevant to autonomic activity induced by MDMA. Measurements and data analysis procedures were similar to our previous work (Ma et al., 2008) with minor modifications. Rats were allowed to acclimatize to the temperature-controlled measurement chamber for at least a 2 h period prior to obtaining body temperature measurements. A 7-mm flexible thermoprobe was inserted into the rectum and Tcor displayed on a digital monitor. To minimize physical disturbance, the thermoprobe extension cord was secured on rat tail and thus Tcor was continuously recorded at 5 min intervals throughout the experiment. Four consecutive measurements, immediately before drug or vehicle injection, were averaged as baseline value. Changes in Tcor from the baseline were used in data analysis. Data were expressed either as line or bar graphs. Line graphs revealed the time course of Tcor responses, while bar graphs showed mean changes (± s.e.m) between 0 - 45 min.
Statistical analysis
Data were expressed as mean ± s.e.m. Statistical analysis was performed by ANOVA test. If significant interactions of drug treatment × time courses were found, further statistical analysis was carried out using the post-hoc Scheffe test in determining the significance of respective time points. When appropriate, student t-test was used to assess the difference between saline and drug treatment. The level for statistical significance was set at 0.05.
Results
Experiment 1: Absence of environmental factors
All animals used for this set of experiments were freely behaving at a normal temperature of 22 °C.
Experiment 1a, Behavioral syndrome tests
Forepaw treading, hind-limb abduction, salivation and penile erection were not observed using a 4-level evaluation scale, and behavioral scores were at zero in response to MDMA doses of 0.3-3 mg/kg (N = 4 of each dose).
Experiment 1b, Neuromuscular hyperactivity tests
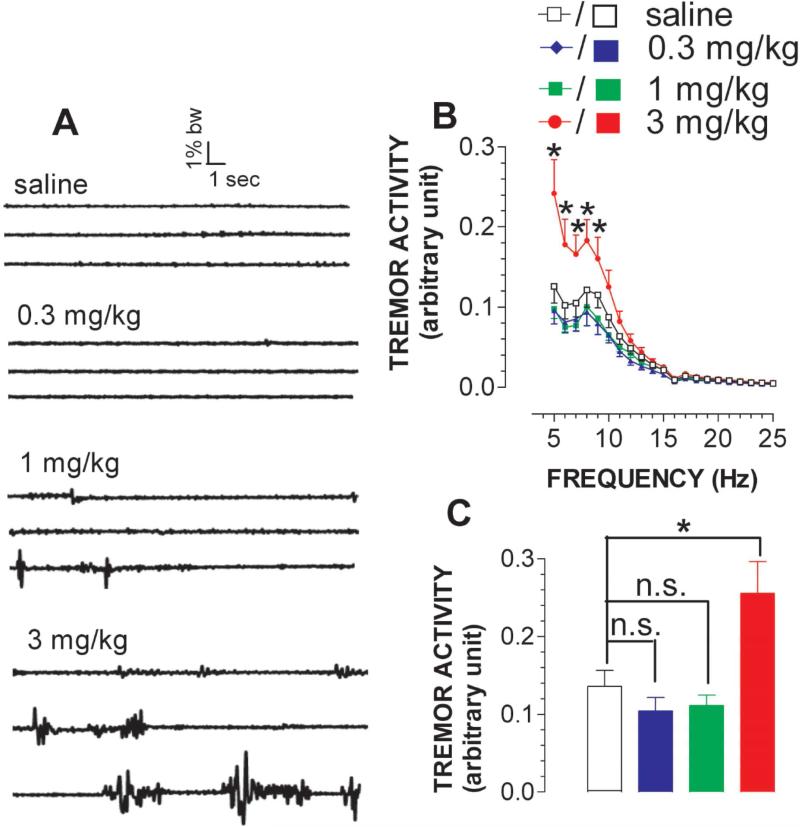
As shown in fig. 1A, tremor-like neuromuscular hyperactivity was detected at 3 mg/kg MDMA, but not 0.3 mg/kg or 1 mg/kg. The effect was quantified by neuromuscular contraction force (% body weight; arbitrary unit) and then the frequency range for the occurrence of tremor-like activity was determined. As shown in fig. 1B, tremor-like activity occurred mainly at frequencies of 5-10 Hz [FFrequency(20,560) = 133.428, P < 0.0001]. For a better comparison, the hyperactivity between 5-10 Hz was averaged and shown in fig. 1C. Statistical analysis revealed significant differences between doses [FDose(3,28) = 5.048, P = 0.0064].
Figure 1.
Effect of MDMA on tremor-like activity. Animals were moving freely in a test chamber at temperature of 22 °C. A, Example of 3 continuous neuromuscular-related activity traces recorded at the beginning of each 30-min time frame in response to saline (top), 0.3 mg/kg (top middle), 1 mg/kg (bottom middle) and 3 mg/kg (bottom); Note that there was a total of 90 sweeps (20 s/sweep) for 30 min. Time bar, 1 s; force bar, 1% body weight (BW). B, Representative analysis of the force power across tremor frequency between 5-25 Hz (data summarized from the sweeps #88, #89 and #90). For the sake of clarification, data are expressed as the frequency resolution of 0.5 Hz (N = 8). C, Overall effect between 0-30 min. MDMA at 3 mg/kg but not 0.3 or 1 mg/kg caused tremor-like neuromuscular hyperactivity. *P < 0.05 vs. control.
Experiment 1c, EEG test
Compared to saline, injection of 3 mg/kg MDMA caused a reduction in EEG amplitude (fig. 2A-B). The reductive response was confirmed by power density analysis, showing differences in power density between 0.5-30 Hz (fig. 2C). For data comparison, the power density over the entire frequency range was averaged and expressed in fig. 2D top and the density over the alpha-band between 8-12 Hz was depicted in fig. 2D bottom. A similar responsive pattern of dose-dependent effects was obtained for delta, theta and beta bands (data not shown). Statistical analysis indicates that 3 mg/kg, but not 0.3 mg/kg or 1 mg/kg MDMA, caused a significant reduction in the overall EEG frequency activity (paired t-test; P =0.0005) and alpha activity (P =0.0007). In addition to the dose effect, a time-dependent effect was determined at 30 min intervals as shown in fig. 2E. Other bands (delta, theta and beta) also had a similar pattern of amplitude reduction in a time-dependent manner (data not shown).
Figure 2.
Effect of MDMA on EEG activity. Animals were moving freely in a test chamber at temperature of 22 °C. A, Example of EEG traces represents basal activity (left panel) and vehicle injection (right panel). Time bar, 1 s; voltage bar, 0.25 mV. B, Example of traces represents basal activity (left panel) and 3 mg/kg MDMA injection (right panel). C, Representative analysis of power density in the frequency range between 0.5-30 Hz. Power density (μV2/Hz) is expressed as mean ± s.e.m (N = 6) on the y-axis plotted against EEG frequency (0.5 – 30 Hz) on the x-axis. Frequency bands (delta, alpha, theta and beta) are indicated by respective horizontal bars. D, Overall effect on EEG between 0 and 120 min. Injection of 3 mg/kg MDMA, but not 0.3 or 1 mg/kg, caused a reduction of power density in EEG activity over entire frequency (0.5 – 30 Hz, top) and individual bands [for instance, α-band (8 - 12 Hz, bottom)]. *P<0.05 vs. baseline, paired t-test. E, Time course of power density changes in α-band. Data are normalized to percentage (%) of baseline (± s.e.m; N = 6). *P<0.05 and **P<0.01 vs. saline control at respective time points. ANOVA followed by Scheffe test.
Experiment 1d, Anxiety-like behavioral tests
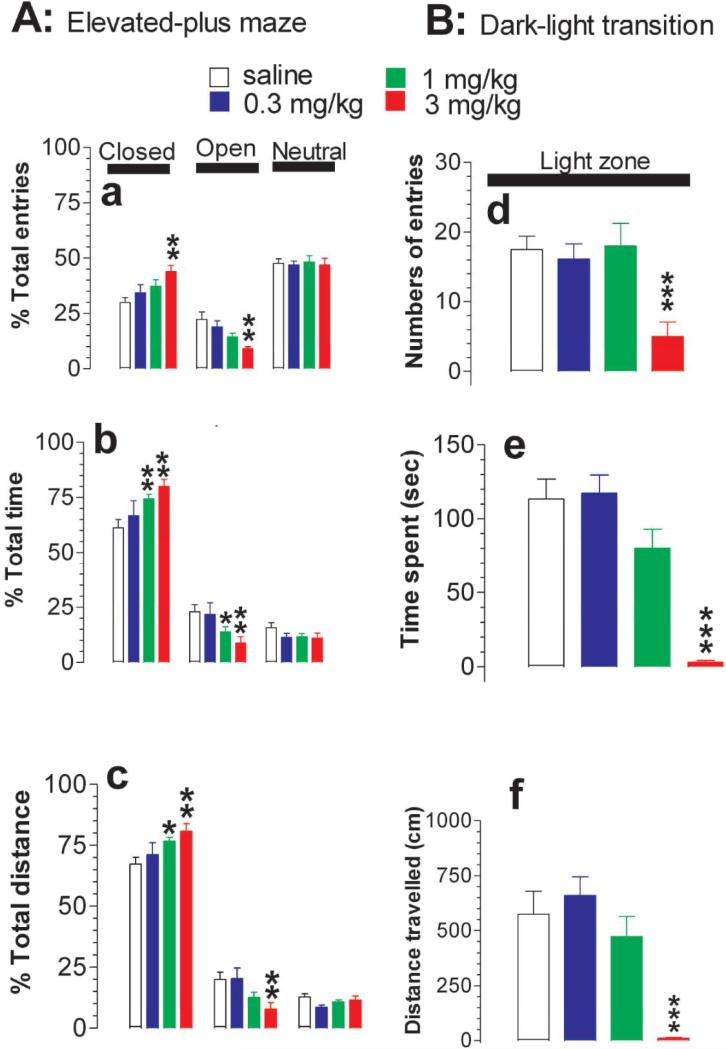
Anxiety-like behavior was determined using elevated-plus maze (EPM; fig. 3A) and the dark-light transition test (DLT; fig. 3B). Compared to the saline treatment, MDMA induced a dose-dependent increase in the percentage of entries into the closed EPM arms (F(3,28) = 3.886, P = 0.0193) while a decrease into the open EPM arms (F(3,28) = 5.858, P = 0.0031; fig. 3A-a). Of these doses, 3 mg/kg MDMA induced a statistically significant effect on the numbers of open and closed arm entries. Likewise, MDMA significantly increased the percentage of time spent in the closed arms (F(3,28) = 3.622, P = 0.0239) and decreased the time in the open arms (F(3,28) = 3.443, P = 0.0301; fig. 3A-b). The minimum dose that induced a statistically significant effect was at 1 mg/kg. The percentage of distance traveled was also increased in the closed arms (F(3,28) = 3.126, P = 0.0415) and decreased in the open arms (F(3,28) = 3.667, P = 0.024; fig. 3A-c). The minimum dose inducing a statistically significant effect on distance was at 1 mg/kg in the closed arms and at 3 mg/kg in the open arms.
Figure 3.
Effect of MDMA on anxiety-like behavior. Tests were conducted at ambient temperature of 22 °C. A, Elevated-plus maze (EPM) assay. Data are mean ± s.e.m (N = 8). MDMA produced an increase in the percentage of entries into (a), time spent in [b] and distance traveled in the closed arms (c) while the respective response was reduced in the open arms; B, Dark-light transition (DLT) assay. Data are mean ± s.e.m (N = 8). Likewise, MDMA evoked a decrease in numbers of entries into (d), time spent in (e) and distance traveled in the light arena (f). N = 8. *P < 0.05; **P<0.01; ***P<0.001 vs. control; unpaired student t- test.
The DLT test also revealed this anxiety-like behavior in response to human relevant doses. As shown in fig. 3B, 3 mg/kg but not 0.3 or 1 mg/kg MDMA significantly decreased the number of reentries (F(3,28) = 6.544, P = 0.0017; fig. 3B-d), the time spent (F(3,28) = 22.495, P < 0.0001; fig. 3B-e) and the distance traveled in the light zone (F(3,28) = 12.512, P < 0.0001; fig. 3B-f).
Experiment 1e, Extracellular 5HT measurement
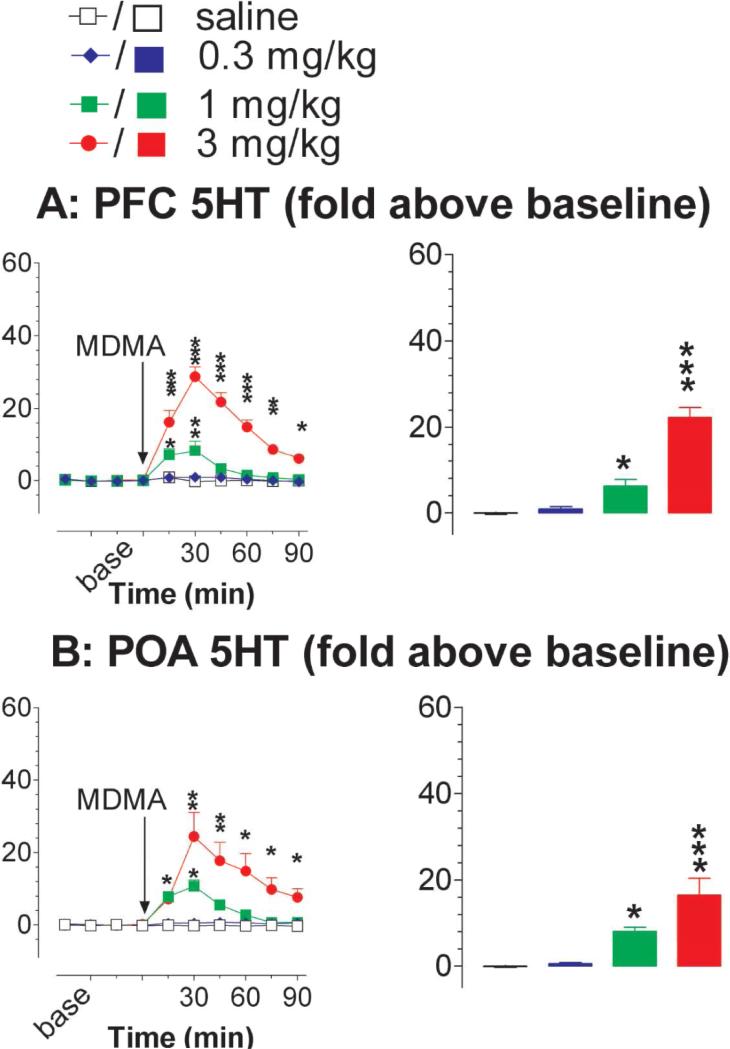
Changes in extracellular 5HT in the prefrontal cortex (PFC) was determined using an in vivo microdialysis technique. As shown in fig. 4A, MDMA injection caused a dose- and time-dependent increase in 5HT [FDose(3,23) = 41.829, P < 0.0001; FTime(5,115) = 20.968, P < 0.0001; FDose×Time(15,115) = 10.685, P < 0.0001]. Specifically, MDMA at 0.3 mg/kg had no effect. As the dose increased to 1 mg/kg, there was a transient (<30 min) but marked elevation in extracellular 5HT with the peak increase at 5 to 8-fold above the baseline. Remarkably, 3 mg/kg MDMA caused a prolonged and marked increase in the 5HT overflow with the peak level at 20 to 30-fold above the baseline.
Figure 4.
Effect of MDMA on 5HT overflow in the brain. Animals were moving freely in a test chamber at temperature of 22 °C. A, 5HT overflow in the prefrontal cortex (PFC). Data are mean ± s.e.m. (N = 5-8). The time course of 5HT overflow is depicted in the line graph (left panel) and the mean overflow during the time period of 0-45 min is shown in the bar graph (right panel). B, 5HT overflow in the preoptic area (POA) of the anterior hypothalamus. Data are mean ± SEM (N = 6-7). Similarly, there was a dose- and time-dependent increase in the hypothalamic 5HT in response to MDMA injection (left panel). The bar graph shows the mean overflow during the time period of 0 - 45 min (right panel). *P<0.05, **P<0.01 and ***P<0.001 vs. saline control.
To determine whether the excessive increase likely occurs at multiple sites within the brain, additional microdialysis tests were conducted in the preoptic area/anterior hypothalamus (POA), far from the PFC. Similarly, 5HT overflow was also dose- and time-dependently elevated by MDMA at human-relevant doses [fig. 4B; FDose(3,21) = 11.957, P < 0.0001; FTime(5,105) = 15.857, P < 0.0001; FDose×Time(15,105) = 7.957, P < 0.0001]. Specifically, 0.3 mg/kg had no effect on 5HT; 1 mg/kg and 3 mg/kg caused an elevation in the 5HT by ~8-fold (P < 0.5) and 20-to 30-fold (P < 0.001), respectively, above the baseline.
Experiment 1f, Body-core temperature (Tcor) measurement
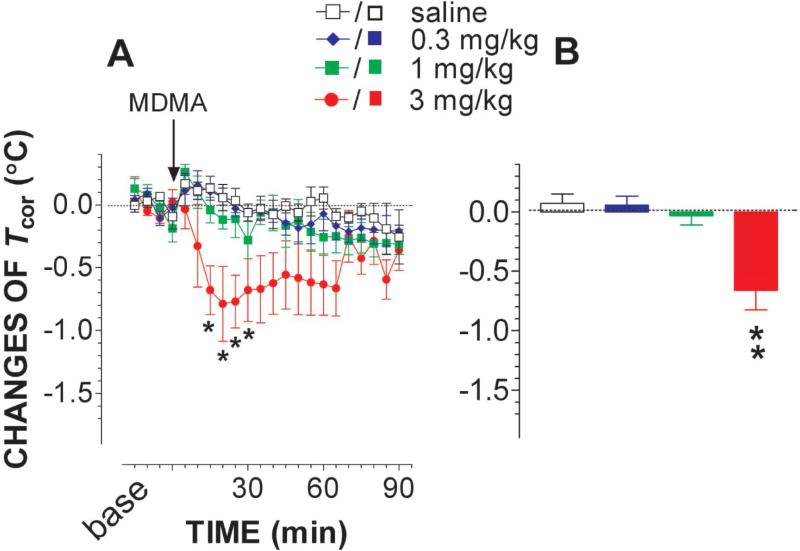
Fig. 5A shows a time-dependent effect of MDMA on changes in Tcor while fig. 5B depicts the dose-dependent effect. MDMA at 0.3 mg/kg or 1 mg/kg had no significant effect on Tcor. As the dose increased to 3 mg/kg, Tcor was significantly reduced [FDose(1,7) = 8.859, P = 0.0206; FTime(17,118) = 2.018, P = 0.0151; FDose×Time(17,118) = 2.105, P = 0.00107].
Figure 5.
Effect on body-core temperature (Tcor) of MDMA. Animals were moving freely in a test chamber at temperature of 22 °C. Data are mean ± s.e.m (N = 4-7). Time course of changes in Tcor is depicted in the line graph (A) and mean change during the time period of 0 - 45 min is displayed in the bar graph (B). Injection of 3 mg/kg MDMA, but not 0.3 or 1 mg/kg, caused significant reduction in Tcor. *P<0.05 and **P<0.01 vs. saline control.
Experiment 2: Presence of environmental factors
Experiments below were conducted in a temperature-controlled chamber of 22 or 32 °C. Environmental effect on neuromuscular activity or anxiety-like behavior was not performed because the force-plate actimeter, EPM or DLT apparatus cannot be placed in the chamber or treadmill. To reduce the numbers of animals used and also the amount of workloads, we just elected to move forward focusing on 3 mg/kg since only this dosage was effective in our instrumental tests abovementioned.
Experiment 2a, Environmental effect on behavioral syndrome
First, behavioral tests were carried out in a temperature-controlled chamber of 32 °C. Injection of MDMA at 3 mg/kg did not cause changes in behavioral scores (N = 4). Next, animals were placed on a treadmill to test effects of physical activity on behavioral scores. Injection of 3 mg/kg MDMA still had no effect on the scores (N = 4). Lastly, we tested whether the combination of physical activity and warm temperature had effects on behavioral syndrome. There were no differences in forepaw treading, hind-limb abduction, salivation or penile erection (N = 4).
Experiment 2b, Environmental effect on basal Tcor
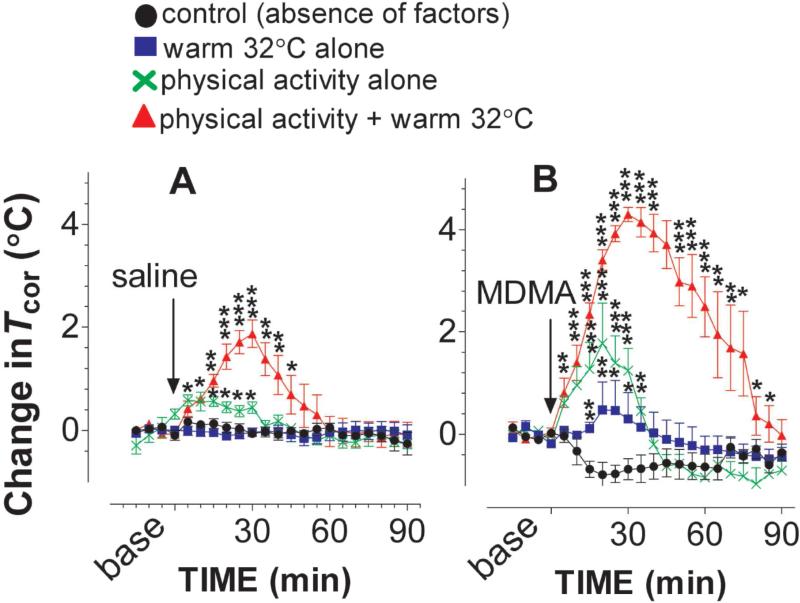
Basal Tcor was 38.6 ±0.1 (n = 20) while chamber temperature was set at a 22 °C. Next, the chamber temperature was increased to 32 °C. After habituation for 2h, the basal Tcor at the same animals was 38.8 ±0.1. There was no significant difference (paired t =0.621, P =0.5417) as ambient temperature increased from 22 to 32 °C. Saline injection had no effect on basal Tcor (fig. 6A). Next, animals were placed on a treadmill to test how physical activity had an impact on basal Tcor. As shown in fig. 6A, basal Tcor was increased by 0.8 −1 °C. Compared to the freely-behaving group (absence of the factors), the increase was significant [F(1,8) =10.129, P =0.0129]. Lastly; basal Tcor was measured while in the presence of both physical activity and warm temperature. Compared to each factor alone, the combination of these two factors caused a significant increase in basal Tcor [fig. 6A; F(3,18) =5.268, P =0.0087]. The rank order of intensification potency was warm temperature +physical activity >>physical activity alone > warm temperature alone.
Figure 6.
Environmental factors altered the Tcor response to MDMA. Animals were placed on a treadmill at 22 or 32 °C. The treadmill motor power was turned on during a time period of 0-30 min (see details in Methods section). Data are mean ± s.e.m (N = 4-8). A, Environmental factors exert a characteristic influence on basal Tcor with the rank order of a multifactorial combination > physical stimulation alone > warm temperature alone. B, The effect of MDMA on Tcor was potentiated by the environmental factors with the rank order of a multifactorial combination > physical stimulation alone > warm temperature alone. *P<0.05, **P<0.01, ***P<0.001 vs. freely behaving rats at 22 °C.
Experiment 2c, Environmental effect on the Tcor response to MDMA
As shown in fig. 6B, there was a reduction in Tcor following 3 mg/kg MDMA administered into rats under the standard condition (i.e., absence of factors), consistent with the previous results. When the test was carried out under the influence of warm temperature, 3 mg/kg MDMA no longer caused Tcor reduction. Instead, there was an increase in Tcor. This change was significant, compared to the Tcor response in the absence of environmental factors [F(1,8) =8.487, P =0.0195]. Next; a treadmill was used to test whether physical activity alone altered the MDMA-induced reduction in Tcor. As expected, there was an increase in Tcor while rats administered with 3 mg/kg were followed by treadmill exercise. The effect was significantly different from the Tcor response in the absence of environmental factors [F(1,10) =20.545, P =0.0011]. Lastly, we tested how the combination of two factors altered the effect of MDMA on Tcor. As shown in fig. 6B, the combination significantly intensified the MDMA-induced hyperthermic response, compared to the Tcor response in the absence of environmental factors [F(1,9) =204.844, P =0.0001] or each factor alone [F(3,18) =47.003, P <0.0001]. The maximum elevation was nearly 4 °C above baseline.
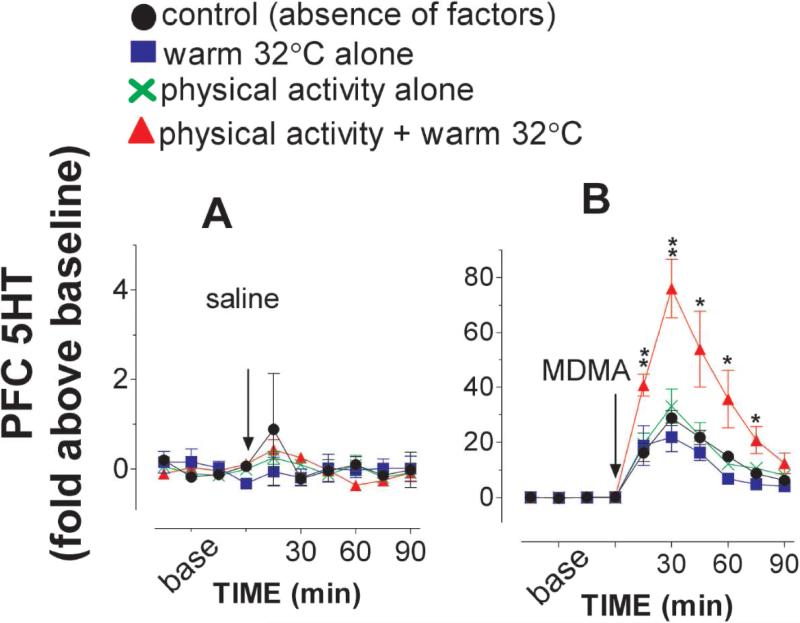
Experiment 2d, Environmental effect on 5HT overflow
The PFC 5HT was used as a reference for determining how environmental factors altered the 5HT response to drug administration. As shown in fig. 7A, basal 5HT was not significantly altered by these factors, neither alone nor in combination [F(3,24) =0.017, P =0.9969]. The 5HT response to 3 mg/kg MDMA was not altered by either warm ambient temperature [fig. 7B, F(1,13) = 0.396, P = 0.5398] or physical activity alone [F(1,13) =0.19, P =0.6699]. Interestingly, the two factors combination significantly potentiated the MDMA-elicited increase in the 5HT [F(1,14) = 6.759, P = 0.021].
Figure 7.
Environmental factors altered the 5HT response to MDMA. Animals were placed on a treadmill at 22 or 32 °C and treadmill motor power was turned on during a time period of 0-30 min (see details in Methods section). Data are mean ± s.e.m (N = 4-8). A, Environmental factors had no effect on basal 5HT overflow. B, The effect of MDMA was not potentiated by physical stimulation or warm temperature alone. In contrast, a multifactorial combination potentiated MDMA-induced increases in the 5HT overflow. *P<0.05, **P<0.01vs. freely behaving rats at 22 °C.
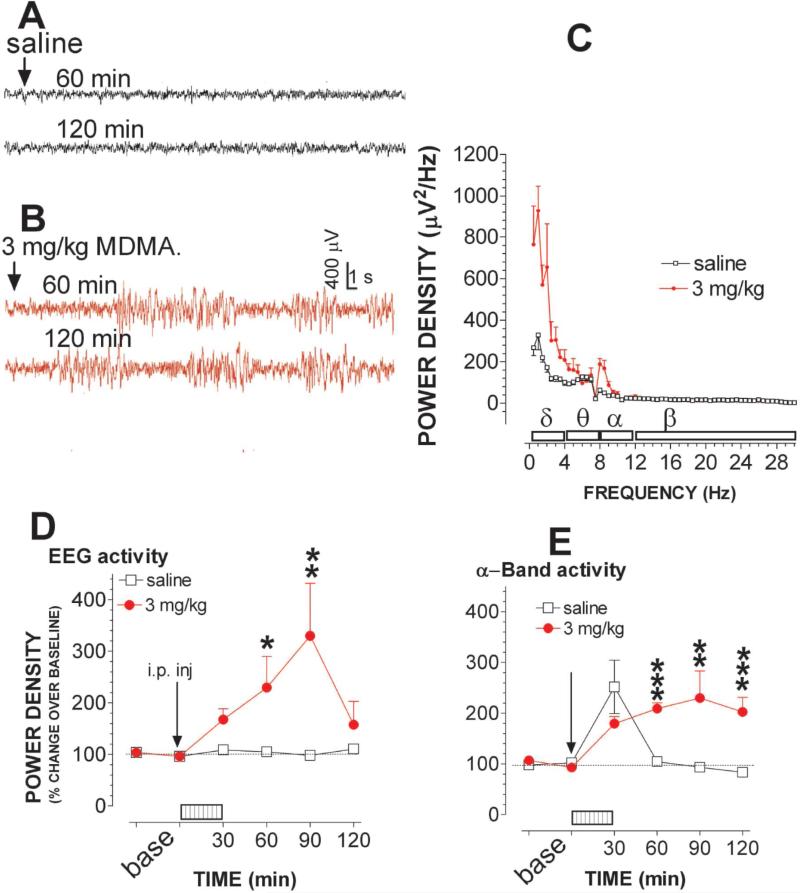
Experiment 2e: Environmental effect on the EEG activity
In this study, the role of the two factors combined was examined because the previous study showed that the combination, and not a single factor alone, could consistently alter the effect of MDMA on Tcor and 5HT. Fig. 8A shows the raw EEG traces (20 s) in the time frame of 60 min (top) and 120 min (bottom) after animals were injected with saline followed by a 30-min physical stimulation at warm temperature of 32 °C. Although the two factors combined had no apparent effect on brain activity in the control group, MDMA was no longer to cause the reduction in EEG activity as shown in the earlier work (see fig. 2B). Instead, in the present incidence, MDMA administration induced much higher amplitude and slow frequency bursts (fig. 8B), similar to previously described seizures induced by epileptogenic agents (Nishida et al., 2007). Fig. 8C shows the power density in the frequency range of 0.5-30 Hz in response to saline and 3 mg/kg MDMA. To minimize individual variables or differences, the density changes were normalized as the percentage changes relative to the respective baseline. Percentage changes in power density were time-dependently increased by measuring the entire frequency range between 0.5-30 Hz [fig. 8D; F(1,8) = 11.75, P = 0.009], or by measuring a specific frequency such as alpha-waves [fig. 8E; F(1,8) = 17.864, P < 0.0001] or delta-waves [data not shown; F(1,8) = 42.148, P < 0.0001].
Figure 8.
Environmental factors altered the EEG response to MDMA. Animals were placed on a treadmill at 32 °C. The treadmill motor power was turned on during a time period of 0-30 min (see details in Methods section). A-B, Example of EEG traces represents EEG activity in the time frame of 60 min (top) and 120 min (bottom) after saline (A) or 3 mg/kg MDMA injection (B). Each tracer is a 30-s epoch. Time bar, 1 s; voltage bar, 0.4 mV. C, Representative analysis of power density, 60 min after MDMA injection. Data are mean ± s.e.m (N = 4 – 6; μV2/Hz). Horizontal bars indicate the frequency range of delta (0.5 - 4 Hz), theta (4-8 Hz), alpha (8 - 12 Hz) and beta-waves (12-30 Hz). D, Time-dependent effects on EEG activity. Data are expressed as percentage of basal level (N = 4 - 6). The combination potentiated MDMA-elicited changes in EEG activity over entire frequency (0.5 – 30 Hz). E, Time-dependent effects on α–band EEG activity (8 - 12 Hz) *P<0.05, **P<0.01 and ***P<0.001 vs. control animals at respective time points.
Experiment 3: Pharmacologic intervention tests
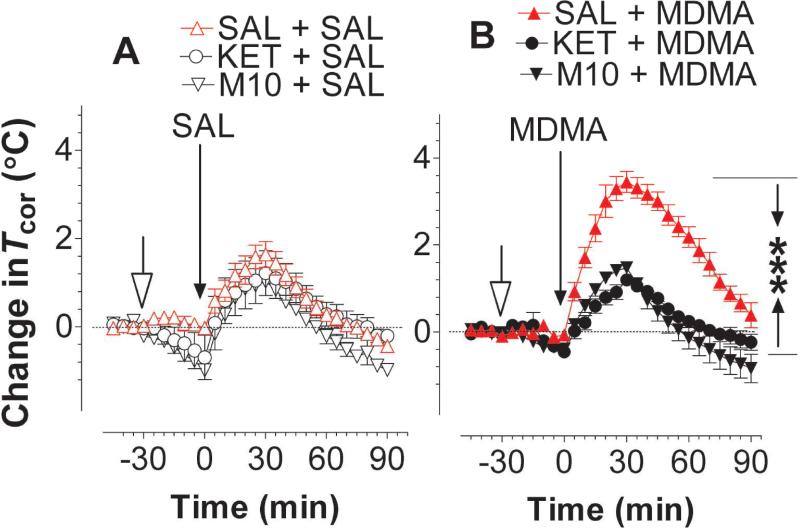
Experiment 3a: Intervention at 5HT2ARs
Animals were placed on a treadmill at a warm temperature of 32 °C and received an injection of M100907 (2 mg/kg), ketanserin (5 mg/kg), or saline (1 ml/kg). Thirty minutes later, rats received a second injection with vehicle or 3 mg/kg MDMA and followed by physical activity on the treadmill for 30 min. After terminating the physical activity episode, animals still remained on the same treadmill at 32 °C for 60 min. As shown in fig. 9A, physical activity on a treadmill at 32 °C caused an increase in basal Tcor elevation in all three groups. There was no difference between rats pretreated with saline, M100907 or ketanserin [F(2,12) = 0.32, P = 0.7323]. Fig. 9B shows the prevention of hyperthermia at 5HT2ARs with M100907 or ketanserin. Specifically, MDMA alone at 3 mg/kg (SAL+MDMA group) caused hyperthermia. Pretreatment with M100907 or ketanserin significantly blocked the MDMA-induced hyperthermia (fig. 9B; P < 0.001 vs. SAL+MDMA).
Figure 9.
Pharmacologic intervention of hyperthermia induced by MDMA. Animals were placed on a treadmill at 32 °C. The treadmill motor power was turned on during a time period of 0-30 min (see details in Methods section). M100907 or ketanserin (indicated by the open-headed arrow) was administered 30 min prior to 3 mg/kg MDMA. Data are mean ± s.e.m (N = 4-7). SAL (saline; 1 ml/kg); M10 (M100907; 2 mg/kg) or KET (ketanserin; 5 mg/kg); MDMA (3 mg/kg). A, There was no difference in Tcor among animals injected with SAL, M10 or KET while animals had physical activity at 32 °C. B, MDMA contingent with physical activity at 32 °C caused hyperthermia. This effect was blocked by pretreatment of M10 or KET. ***P < 0.001; SAL+MDMA vs. M10+MDMA or KET+MDMA group.
Experiment 3b: Intervention at other receptor sites
5-HT1A, NMDA, ganglionic nACh, and central nACh receptors were blocked with WAY100635, MK-801 and CGS19755, hexamethonium, and methyllycaconitine respectively, whereas, midazolam was used as a benzodiazepine-GABAA receptor agonist. Injection of a receptor ligand alone at the dose provided in table 1 followed by physical activity at 32 °C for 30 min caused only a small increase in Tcor (data not shown, but the levels similar to those showed in fig. 9A). As elucidated in table 1, the MDMA-induced-hyperthermia was blocked by pretreatment of CGS19755, hexamethonium, and midazolam, not by WAY100635, MK801, or methyllycaconitine.
Table 1.
Pharmacologic intervention on hyperthermia induced by MDMA
| Dosage (mg/kg) | Potential targets | Maximum Tcor changes from baseline | |
|---|---|---|---|
| saline | - | - | 3.45 ±0.24 (5) |
| WAY100635 | 1 | 5HT1AR | 3.32 ±0.20 (4) |
| MK-801 | 0.1 | NMDA-R | 3.40 ±0.35 (4) |
| CGS19755 | 5 | NMDA-R | 0.83 ±0.35 (5)*** |
| hexamethonium | 20 | Ganglionic nAChR | 1.05 ±0.10 (6)* |
| methyllycaconitine | 10 | Central nAChR | 3.20 ±0.15 (4) |
| midazolam | 2 | GABAA-benzodiazepine-R | 1.84 ±0.13 (4)* |
Data are mean ±S.E.M. and parentheses indicate numbers of animals in the group. Drug injection for intervention at specific receptor sites took place 30 min before MDMA administration. Drug dosages were expressed as mg/kg indicated in the second column. Dosage of MDMA was 3 mg/kg followed by 30-min physical activity on a treadmill at warm temperature of 32 °C.
P <0.05
P <0.01
P <0.001 vs. saline+MDMA examined by unpaired Student t-test.
Discussion
Our study made three contributions to understanding the relationship between MDMA and serotonin syndrome. Firstly, we showed that the effects of MDMA at a low dosage (i.e., <1 mg/kg) were tolerable, although there was an excessive extracellular 5HT in the brain (<10-fold above baseline). When the dosage was increased to 3 mg/kg, it caused numerous mild symptoms of the serotonin syndrome and this was associated with excessive 5HT overflow approximately 20-30 folds greater than the baseline in the brain. Thus, results of our data provide a dosage range for MDMA suitable for animal research. Secondly, we showed that, in the absence of environmental factors, 3 mg/kg MDMA in rats caused only a mild syndrome. The synergistic effects of multiple environmental factors (but not each alone) intensified the syndrome from mild to severe. The results are consistent with investigations in humans (Curran, 2000; Parrott, 2002, 2012b) and support the hypothesis that the situations in which the drugs are taken are probably more critical than dosages in deciding the outcomes associated with MDMA administration at low to moderate dosages. Thirdly, we showed that the intensified syndrome was blocked by the 5HT2AR blocker M100907, competitive NMDA receptor blocker CG19755, the ganglionic blocker hexamethonium and benzodiazepine-GABAA receptor agonist midazolam. This suggests that, in addition to serotonergic mechanism, non-serotonergic mechanisms are involved in the syndrome intensification, consistent with previous studies with other 5HT-promoting drugs (Tao et al., 2014).
There has been a great debate for decades about an appropriate MDMA dosage used for basic animal research relevant to humans (Turner and Parrott, 2000; Baumann et al., 2007; Green et al., 2012). In this regard, previous investigations focused mainly on the difference in MDMA metabolism between humans and rodents (Baumann et al., 2009; Concheiro et al., 2014). Since an immediate risk of MDMA abuse is a serotonin syndrome, an alternative way to clarify the debate is to determine a minimum dosage for MDMA that causes a mild syndrome in animals. This is possible because the mild syndrome is elicited in rats when extracellular 5HT exceeds 10-fold above baseline (Ma et al., 2008; Zhang et al., 2009; Tao et al., 2014). However, this relationship between excessive 5HT and the development of the syndrome in rats is established through 5-hydroxytryptophan (5HTP) combined with monoamine oxidase inhibitors (MAOI), prototype drugs for inducing the serotonergic behavioral syndrome such as forepaw treading, hind-limb abduction, salivation and penile erection (Jacobs and Klemfuss, 1975).
Thus in the first set of experiments, we attempted to address two issues. The first was to establish a dosage range in which MDMA could cause a mild syndrome in rats, and the second was to determine how much extracellular 5HT was elicited during this syndrome. However, major challenges to investigate serotonin syndrome are determining what constitutes a mild syndrome [see references reviewed by (Isbister and Buckley, 2005; Nelson et al., 2007)] and this requires the use of innovative technologies. We found that MDMA at the human-relevant doses (i.e., 0.3-3 mg/kg) failed to alter behavioral scores that have been historically used in animals since 1960's (Jacobs and Klemfuss, 1975; Ma et al., 2008). However, it should be kept in mind that this behavioral approach was developed, almost exclusively, from 5HTP+MAOI studies. An immediate question is: why the behavioral syndrome could be evoked by 5HTP+MAOI, but not MDMA? An explanation for this difference may be ascribed to distinct groups of neurons and neural pathways affected by two drugs. For example, 5HT elicited by 5HTP+MAOI is derived from both serotonergic and non-serotonergic neurons that contain aromatic-L-amino-acid decarboxylases for the 5HT synthesis from 5HTP [e.g., dopaminergic; (Arai et al., 1995)]. As a result, in addition to activating serotonergic postsynaptic neurons, excessive 5HT evoked by 5HTP+MAOI also activates additional postsynaptic neurons, which may contain 5HT receptors but normally have synaptic connections with dopaminergic neurons. Given the motor function of the dopaminergic pathway, it is therefore not surprising to observe a strong behavioral response to 5HTP+MAOI injection. In contrast, 5HT elicited by MDMA is derived exclusively from serotonergic neurons, and thereby exerts effects only on serotonergic postsynaptic pathways. Toward this end, the absence of behavioral scores caused by MDMA is not necessarily indicative of free from a syndrome, but suggests that behavioral signs in MDMA-induced syndrome may not be identical to those caused by 5HTP+MAOI. Consistent with this hypothesis, behavioral signs caused by inhibitors of serotonin selective uptake (SSRIs) combined with MAOI were very moderate in comparison with the syndrome caused by 5HTP+MAOI in drug-naïve rats (Izumi et al., 2007; Tao et al., 2014). Thus, although behavioral signs and scores are considered to be classic criteria for identifying a syndrome, they are unlikely applicable to the syndrome induced by MDMA, particularly at this low-to-moderate dosage. In addition, the syndrome induced by this dosage level may not be easily identified with the naked-eye assay and may therefore be considered subclinical. Instrumental measurements used in the present study provided high sensitive testing on the relationship between excessive 5HT and onset of the syndrome induced by MDMA. In rats, like humans, symptoms of the syndrome are expressed as a triad of neuromuscular, psychological and autonomic abnormalities (Sternbach, 1991; Boyer and Shannon, 2005; Isbister and Buckley, 2005). In order to obtain a concrete conclusion, all the three aspects were instrumentally evaluated in this study. For instance, to determine whether neuromuscular activity was altered by MDMA, we utilized the force-plate actimeter which was previously known to detect neuromuscular tremors that might occur in the syndrome induced by 5HTP+MAOI or SSRI+MAOI (Tao et al., 2014). Moreover, to determine whether MDMA could cause changes in the psychological status, animals were examined with EEG, EPM and DLT. By measuring Tcor, we determined whether MDMA could alter autonomic functions in the syndrome (Ma et al., 2008).
We found that 0.3 mg/kg MDMA had no effect on any aspect of the instrumental tests, including force-plate actimeter, EEG, EPM, DLT or Tcor. This is not surprising since MDMA at this dosage level had no significant effect on 5HT overflow in the brain (see fig. 4). We noticed that 1 mg/kg MDMA caused significant increases in 5HT concentrations, as high as 7-10 folds above baseline. This level of extracellular 5HT is considered excessive in contrast to the therapeutic effect of the selective serotonin reuptake inhibitors (SSRIs), which usually cause only a 1-5 folds increase (Rutter and Auerbach, 1993; Fuller, 1994). Despite this, 1 mg/kg MDMA still had no effect on any instrumental measurements. A possible explanation is that rats may tolerate excessive 5HT brain concentrations consistent with a report from a 5HTP+MAOI study (Zhang et al., 2009). Another possibility is that the time course of the 5HT increase evoked by 1 mg/kg MDMA was too short (less than 45 min) to impose adverse effects. In contrast, 3 mg/kg MDMA caused a long-lasting increase in 5HT as high as 20-30 folds above baseline in the PFC and POA. The present study also showed that the remarkable elevation likely took place simultaneously in many areas across the brain as documented by others and believed to be the driver for the serotonin syndrome (Baumann et al., 2008; Starr et al., 2008). This is not unexpected since MDMA is a substrate for 5HT transporters that are widely distributed in the brain. The fact that MDMA at 3 mg/kg in the rat exerted significant impacts on all instrumental measures, showing a tremor-like neuromuscular hyperactivity (fig. 1), reduction in EEG activity (fig. 2), increases in anxiety-like behavior (fig. 3), and changes in autonomic activity such as hypothermia (fig. 5) highlighted the value of this model. In summary, although a low dose (<1 mg/kg) already causes excessive 5HT, this excess is likely tolerable in the rat brain. However, the dosage range from a tolerable to adverse effect is very small. We demonstrate that excessive 5HT is no longer tolerable in the rat brain when the dose increased to 3 mg/kg.
In the second set of experiments, we tested how two environmental factors, each alone or in combination, have the potential to intensify the syndrome. Unfortunately, EPM, DLT or the force-plate actimeter could not be utilized for this part of investigation since those instruments were too big to be placed in the environmental chamber. Evidence suggests that Tcor measurement is an appropriate approach to estimate interactions between MDMA and environment (Farfel and Seiden, 1995; Malberg and Seiden, 1998; Ma et al., 2008). This is because changes in Tcor reflect a state of the autonomic function in the syndrome. A reduction in Tcor is believed to be a protective action of the autonomic response against neural injury (Farfel and Seiden, 1995) and thereby the syndrome is relatively mild (Ma et al., 2008). This neuroprotective action is dysfunctional when Tcor is elevated. Moreover, Tcor elevation could exert an adverse effect on neurons and thereby the syndrome intensity is aggravated. In rats, reduction in Tcor by 1 °C from baseline is generally defined as hypothermia while elevation by 1 °C as hyperthermia (Ma et al., 2008). Additionally, hyperthermia is subdivided into two levels: low hyperthermia (between 1 and 2 °C above baseline) and high hyperthermia (greater than 2 °C above baseline). While low hyperthermia causes few adverse consequences, high hyperthermia is malignant and life-threatening (Rusyniak and Sprague, 2005). Therefore, hypothermia and high hyperthermia are considered to be a valuable marker for mild and severe syndrome, respectively (Ma et al., 2008).
In the present study, we found that, under normal conditions (absence of environmental factors), 3 mg/kg caused only hypothermia (fig. 5-6), implicating a mild syndrome. While in the presence of one environmental factor alone (i.e., warm temperature or physical activity), we found that 3 mg/kg caused only normothermia or low hyperthermia, suggesting that the syndrome intensity was moderate, but not severe (Ma et al., 2008). The results are consistent with previous investigations demonstrating that a single environmental factor is unlikely able to significantly intensify the syndrome induced by MDMA at a human-relevant dose (Green et al., 2005; Banks et al., 2007; Kiyatkin et al. 2014).
We found that combinations of two factors exerted a great effect on basal Tcor (fig. 6A), and also evoked a 4 °C increase in Tcor following 3 mg/kg MDMA (fig. 6B). Interestingly, this hyperthermic response is similar to that caused by a high dosage [for instance, 20 mg/kg (Malberg and Seiden, 1998)], implying that these combined environmental factors have the same impact on MDMA toxicity as the high dosage, or drug overdose. This observation is consistent with clinical experiences that environmental factors are likely responsible for wide variations of the toxic response to the recreational use of MDMA, ranging from mild to serious illness (Parrott, 2002; Ben-Abraham et al., 2003). Methodologically, the findings that the syndrome can be experimentally intensified provides an appropriate laboratory model, which may be used for testing whether or how MDMA at a human-relevant dose causes serotonergic injury in the human brain (Parrott, 2012a; Doblin et al., 2014).
Previous studies suggest that a reduction in EEG amplitude is associated with vigilance and wakefulness (Berridge and Morris, 2000). While we did not measure the vigilance or wakefulness in this study, we did find that EEG amplitude was reduced (fig. 2) when the MDMA dosage was increased to 3 mg/kg. A similar reduction has been reported at a high dosage (Ma et al., 2013). Concerning excessive 5HT in the brain, the EEG reduction may be associated with not only vigilance and wakefulness but also changes in brain function. Interestingly, MDMA no longer caused the reduction in EEG activity while in the presence of combined environmental factors. Instead, there was an elevation in EEG activity (fig.8). Since increases in EEG amplitude likely reflect drowsiness and drug intoxication (Lukas et al., 1989; Darbari et al., 2005; Niemarkt et al., 2008), the results of our EEG test support that brain function was likely further compromised by the presence of combinations of two environmental factors.
It could be argued that 5HT is the molecule link between environment and the syndrome intensification. However, warm temperature or physical activity alone did not have any effect on basal 5HT or 5HT elicited by MDMA (fig. 7B), consistent with previous studies (O'Shea et al., 2005). There are other reports demonstrating that 5HT concentrations was increased while environmental temperatures as a single factor were used for the studies (Stanley et al., 2007; Feduccia et al., 2011). The inconsistency may be attributed to numerous differences between experimental designs, particularly drug dosages used in other studies. Interestingly, combinations of two factors significantly elevated 5HT by 60-80 folds above baseline, which were almost tripled from the previous level (i.e., 20-30 fold) while in the absence of environmental factors. The findings support the hypothesis that combinations of two environmental factors, but not each factor alone, could promote the increase in 5HT induced by MDMA at a low-to-moderate dosage, contributing to syndrome intensification.
A question may be raised as to whether there is a direct relationship between increasing concentrations of 5HT and the severity of the syndrome. We have shown in previous studies that, although 5HT was increased to such a high level caused by 5HT-promoting drugs, including MDMA, the syndrome intensity was not always severe (Krishnamoorthy et al., 2010; Ma et al., 2013). To reconcile the existing data, we have developed the hypothesis by which a discrete role of excessive 5HT in the syndrome has been defined (Tao et al., 2014). Specifically, development of a syndrome consists of two phases: syndrome initiation followed by intensification. While initiation is exclusively attributed to excessive 5HT over 10-fold above baseline (serotonergic mechanism), the syndrome intensity is determined mainly by how extensive the involvement of non-5HT neurotransmissions are (non-serotonergic mechanisms). This hypothesis can well explain the influences of underlying environmental factors for intensifying the MDMA-induced syndrome. It is known that MDMA can elicit an increase in many kinds of neurotransmitters in the brain (Nair and Gudelsky, 2006; Baumann et al., 2008; Starr et al., 2012; Anneken et al., 2013). In the absence of environmental factors, however, the responses of other kinds of neurotransmitters to MDMA are too small to have an impact on the syndrome. It is conceivable that combining two environmental factors can promote a further elevation of both 5HT and non-5HT neurotransmissions and likely upregulate functional activity of postsynaptic receptors that influence neural balance between inhibitor and excitatory network. As a result, in addition to 5HT-engaged pathways, non-5HT pathways may also be activated. The findings that the intensified syndrome was blocked by pretreatment of the competitive NMDA receptor antagonist CGS19755, benzodiazepine-GABAA receptor agonist midazolam, and ganglionic (nAChR) blocker hexamethonium (fig. 9 and table 1) clearly support this hypothesis. It further shows that, in addition to the previously known involvement of serotonergic, dopaminergic and adrenergic neurotransmissions (Bexis and Docherty, 2005; Shioda et al., 2008), glutamatergic, GABAergic and cholinergic neurotransmissions may also be involved in the severe syndrome.
In conclusion, an understanding of the relationship between multifactorial environment and MDMA toxicity will likely to answer a long-standing medical question of why recreational abuse of MDMA causes negligible symptoms of the syndrome in some users (Bahora et al., 2009) but life-threatening in others (Ben-Abraham et al., 2003).
Acknowledgements
This study was supported by the NIH grant (R15DA029863), the FAU university program for undergraduate research and the Ross University School of Veterinary Medicine seed grant. We would like to thank the National Institute on Drug Abuse (Rockville, MD) for providing (±)3,4-methylenedioxymethamphetamine (±MDMA) and M100907 to this work. Authors wish to acknowledge Mary Rudacille for her skillful technical assistance.
Footnotes
Conflict of interest: Authors do not have any conflict of interest to report.
References
- Anneken JH, Cunningham JI, Collins SA, Yamamoto BK, Gudelsky GA. MDMA increases glutamate release and reduces parvalbumin-positive GABAergic cells in the dorsal hippocampus of the rat: role of cyclooxygenase. J Neuroimmune Pharmacol. 2013;8:58–65. doi: 10.1007/s11481-012-9420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Nagatsu T, Nagatsu I. Exogenous L-5-hydroxytryptophan is decarboxylated in neurons of the substantia nigra pars compacta and locus coeruleus of the rat. Brain Res. 1995;669:145–149. doi: 10.1016/0006-8993(94)01259-k. [DOI] [PubMed] [Google Scholar]
- Armenian P, Mamantov TM, Tsutaoka BT, Gerona RR, Silman EF, Wu AH, Olson KR. Multiple MDMA (Ecstasy) Overdoses at a Rave Event: A Case Series. J Intensive Care Med. 2013;28:252–258. doi: 10.1177/0885066612445982. [DOI] [PubMed] [Google Scholar]
- Bahora M, Sterk CE, Elifson KW. Understanding recreational ecstasy use in the United States: a qualitative inquiry. Int J Drug Policy. 2009;20:62–69. doi: 10.1016/j.drugpo.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007;35:1840–1845. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience. 2008;152:773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (±)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med. 2003;10:309–313. doi: 10.1097/00063110-200312000-00013. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Morris MF. Amphetamine-induced activation of forebrain EEG is prevented by noradrenergic beta-receptor blockade in the halothane-anesthetized rat. Psychopharmacology (Berl) 2000;148:307–313. doi: 10.1007/s002130050055. [DOI] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Role of α2A-adrenoceptors in the effects of MDMA on body temperature in the mouse. Br J Pharmacol. 2005;146:1–6. doi: 10.1038/sj.bjp.0706320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- Concheiro M, Baumann MH, Scheidweiler KB, Rothman RB, Marrone GF, Huestis MA. Nonlinear Pharmacokinetics of (±)3,4-methylenedioxymethamphetamine (MDMA) and its Pharmacodynamic Consequences in the Rat. Drug Metab Dispos. 2014;42:119–125. doi: 10.1124/dmd.113.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV. Is MDMA (‘Ecstasy’) neurotoxic in humans? An overview of evidence and of methodological problems in research. Neuropsychobiology. 2000;42:34–41. doi: 10.1159/000026668. [DOI] [PubMed] [Google Scholar]
- Darbari FP, Melvin JJ, Piatt JH, Jr., Adirim TA, Kothare SV. Intrathecal baclofen overdose followed by withdrawal: clinical and EEG features. Pediatr Neurol. 2005;33:373–377. doi: 10.1016/j.pediatrneurol.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung. 2014;43:117–119. doi: 10.1016/j.hrtlng.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Doblin R, Greer G, Holland J, Jerome L, Mithoefer MC, Sessa B. A reconsideration and response to Parrott AC (2013) “Human psychobiology of MDMA or ‘Ecstasy’: an overview of 25 years of empirical research”. Hum Psychopharmacol. 2014;29:105–108. doi: 10.1002/hup.2389. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Seiden LS. Role of hypothermia in the mechanism of protection against serotonergic toxicity. I. Experiments using 3,4-methylenedioxymethamphetamine, dizocilpine, CGS 19755 and NBQX. J Pharmacol Exp Ther. 1995;272:860–867. [PubMed] [Google Scholar]
- Feduccia AA, Kongovi N, Duvauchelle CL. Heat increases MDMA-enhanced NAcc 5-HT and body temperature, but not MDMA self-administration. Eur Neuropsychopharmacol. 2011;20:884–894. doi: 10.1016/j.euroneuro.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- Fuller RW. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 1994;55:163–167. doi: 10.1016/0024-3205(94)00876-0. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Wright MJ, Jr., Dickinson G, Vandewater SA, Price JU, Taffe MA. Influences of activity wheel access on the body temperature response to MDMA and methamphetamine. Pharmacol Biochem Behav. 2011;99:295–300. doi: 10.1016/j.pbb.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O'Callaghan JP, Miller DB. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav. 1991;38:339–344. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Colado MI. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur J Pharmacol. 2004a;500:3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KC. Lost in translation: preclinical studies on 3,4-methylenedioxymethamphetamine provide information on mechanisms of action, but do not allow accurate prediction of adverse events in humans. Br J Pharmacol. 2012;166:1523–1536. doi: 10.1111/j.1476-5381.2011.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA ('ecstasy') on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol. 2005;146:306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Sanchez V, O'Shea E, Saadat KS, Elliott JM, Colado MI. Effect of ambient temperature and a prior neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA) on the hyperthermic response of rats to a single or repeated (‘binge’ ingestion) low dose of MDMA. Psychopharmacology (Berl) 2004b;173:264–269. doi: 10.1007/s00213-003-1725-2. [DOI] [PubMed] [Google Scholar]
- Halpern P, Moskovich J, Avrahami B, Bentur Y, Soffer D, Peleg K. Morbidity associated with MDMA (ecstasy) abuse: a survey of emergency department admissions. Hum Exp Toxicol. 2011;30:259–266. doi: 10.1177/0960327110370984. [DOI] [PubMed] [Google Scholar]
- Iannone M, Bulotta S, Paolino D, Zito MC, Gratteri S, Costanzo FS, Rotiroti D. Electrocortical effects of MDMA are potentiated by acoustic stimulation in rats. BMC Neurosci. 2006;7:13. doi: 10.1186/1471-2202-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol. 2005;28:205–214. doi: 10.1097/01.wnf.0000177642.89888.85. [DOI] [PubMed] [Google Scholar]
- Izumi T, Iwamoto N, Kitaichi Y, Kato A, Inoue T, Koyama T. Effects of co-administration of antidepressants and monoamine oxidase inhibitors on 5-HT-related behavior in rats. Eur J Pharmacol. 2007;565:105–112. doi: 10.1016/j.ejphar.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Klemfuss H. Brain stem and spinal cord mediation of a serotonergic behavioral syndrome. Brain Res. 1975;100:450–457. doi: 10.1016/0006-8993(75)90500-4. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Ma Z, Zhang G, Wei J, Auerbach SB, Tao R. Involvement of 5-HT2A receptors in the serotonin (5-HT) syndrome caused by excessive 5-HT efflux in rat brain. Basic Clin Pharmacol Toxicol. 2010;107:830–841. doi: 10.1111/j.1742-7843.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Critical role of peripheral vasoconstriction in fatal brain hyperthermia induced by MDMA (Ecstasy) under conditions that mimic human drug use. J Neurosci. 2014;34:7754–7762. doi: 10.1523/JNEUROSCI.0506-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Woods BT, Mello NK, Teoh SK. Topographic distribution of EEG alpha activity during ethanol-induced intoxication in women. J Stud Alcohol. 1989;50:176–185. doi: 10.15288/jsa.1989.50.176. [DOI] [PubMed] [Google Scholar]
- Ma Z, Rudacille M, Prentice HM, Tao R. Characterization of electroencephalographic and biochemical responses at 5-HT promoting drug-induced onset of serotonin syndrome in rats. J Neurochem. 2013;125:774–789. doi: 10.1111/jnc.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zhang G, Jenney C, Krishnamoorthy S, Tao R. Characterization of serotonintoxicity syndrome (toxidrome) elicited by 5-hydroxy-l-tryptophan in clorgylinepretreated rats. Eur J Pharmacol. 2008;588:198–206. doi: 10.1016/j.ejphar.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2000;79:201–209. doi: 10.1097/00005792-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gudelsky GA. 3,4-Methylenedioxymethamphetamine enhances the release of acetylcholine in the prefrontal cortex and dorsal hippocampus of the rat. Psychopharmacology (Berl) 2006;184:182–189. doi: 10.1007/s00213-005-0271-5. [DOI] [PubMed] [Google Scholar]
- Nelson LS, Erdman AR, Booze LL, Cobaugh DJ, Chyka PA, Woolf AD, Scharman EJ, Wax PM, Manoguerra AS, Christianson G, Caravati EM, Troutman WG. Selective serotonin reuptake inhibitor poisoning: An evidence-based consensus guideline for outof-hospital management. Clin Toxicol (Phila) 2007;45:315–332. doi: 10.1080/15563650701285289. [DOI] [PubMed] [Google Scholar]
- Niemarkt HJ, Halbertsma FJ, Andriessen P, Bambang Oetomo S. Amplitude-integrated electroencephalographic changes in a newborn induced by overdose of morphine and corrected with naloxone. Acta Paediatr. 2008;97:132–134. doi: 10.1111/j.1651-2227.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- Nishida N, Huang ZL, Mikuni N, Miura Y, Urade Y, Hashimoto N. Deep brain stimulation of the posterior hypothalamus activates the histaminergic system to exert antiepileptic effect in rat pentylenetetrazol model. Exp Neurol. 2007;205:132–144. doi: 10.1016/j.expneurol.2007.01.021. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Escobedo I, Orio L, Sanchez V, Navarro M, Green AR, Colado MI. Elevation of ambient room temperature has differential effects on MDMA-induced 5-HT and dopamine release in striatum and nucleus accumbens of rats. Neuropsychopharmacology. 2005;30:1312–1323. doi: 10.1038/sj.npp.1300673. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Recreational Ecstasy/MDMA, the serotonin syndrome, and serotonergic neurotoxicity. Pharmacol Biochem Behav. 2002;71:837–844. doi: 10.1016/s0091-3057(01)00711-0. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA and 5-HT neurotoxicity: the empirical evidence for its adverse effects in humans - no need for translation. Br J Pharmacol. 2012a;166:1518–1520. doi: 10.1111/j.1476-5381.2012.01941.x. discussion 1521-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. MDMA and temperature: a review of the thermal effects of 'Ecstasy' in humans. Drug Alcohol Depend. 2012b;121:1–9. doi: 10.1016/j.drugalcdep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Gibbs A, Scholey AB, King R, Owens K, Swann P, Ogden E, Stough C. MDMA and methamphetamine: some paradoxical negative and positive mood changes in an acute dose laboratory study. Psychopharmacology (Berl) 2011;215:527–536. doi: 10.1007/s00213-011-2184-9. [DOI] [PubMed] [Google Scholar]
- Rang ST, Field J, Irving C. Serotonin toxicity caused by an interaction between fentanyl and paroxetine. Can J Anaesth. 2008;55:521–525. doi: 10.1007/BF03016672. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North Am. 2005;89:1277–1296. doi: 10.1016/j.mcna.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Rutter JJ, Auerbach SB. Acute uptake inhibition increases extracellular serotonin in the rat forebrain. J Pharmacol Exp Ther. 1993;265:1319–1324. [PubMed] [Google Scholar]
- Shioda K, Nisijima K, Yoshino T, Kuboshima K, Iwamura T, Yui K, Kato S. Risperidone attenuates and reverses hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology. 2008;29:1030–1036. doi: 10.1016/j.neuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Green AR, Swift KM, Fone KC, King MV. Differential effects of cathinone compounds and MDMA on body temperature in the rat, and pharmacological characterization of mephedrone-induced hypothermia. Br J Pharmacol. 2013;168:966–977. doi: 10.1111/j.1476-5381.2012.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley N, Salem A, Irvine RJ. The effects of co-administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) or para-methoxyamphetamine and moclobemide at elevated ambient temperatures on striatal 5-HT, body temperature and behavior in rats. Neuroscience. 2007;146:321–329. doi: 10.1016/j.neuroscience.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Starr MA, Page ME, Waterhouse BD. MDMA (3,4-methylenedioxymethamphetamine)-mediated distortion of somatosensory signal transmission and neurotransmitter efflux in the ventral posterior medial thalamus. J Pharmacol Exp Ther. 2008;327:20–31. doi: 10.1124/jpet.108.139337. [DOI] [PubMed] [Google Scholar]
- Starr MA, Page ME, Waterhouse BD. Effects of repeated 3,4-methylenedioxymethamphetamine administration on neurotransmitter efflux and sensory-evoked discharge in the ventral posterior medial thalamus. J Pharmacol Exp Ther. 2012;340:73–82. doi: 10.1124/jpet.111.185728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- Tao R, Rudacille M, Zhang G, Ma Z. Changes in Intensity of Serotonin Syndrome caused by Adverse Interaction between Monoamine Oxidase Inhibitors and Serotonin Reuptake Blockers. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.49. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JJ, Parrott AC. ‘Is MDMA a human neurotoxin?’: diverse views from the discussants. Neuropsychobiology. 2000;42:42–48. doi: 10.1159/000026669. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (±)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology. 2007;32:673–681. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Krishnamoorthy S, Ma Z, Vukovich NP, Huang X, Tao R. Assessment of 5-hydroxytryptamine efflux in rat brain during a mild, moderate and severe serotonintoxicity syndrome. Eur J Pharmacol. 2009;615:66–75. doi: 10.1016/j.ejphar.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]