Abstract
Purpose
The heterogeneous nature of myelodysplastic syndromes (MDS) complicates therapeutic decision making, particularly for newly diagnosed disease. Factors impacting the treatment plan in this early period of disease course are poorly defined. This study determines whether therapeutic choices for newly diagnosed MDS are associated with location of treatment (community or academic), prognostic risk category, and patient age.
Methods
The Adults in Minnesota with Myelodysplastic Syndromes (AIMMS) database was utilized in this statewide, prospective population-based study conducted by the University of Minnesota (UMN), Mayo Clinic, and Minnesota Department of Health. Adult (age 20+ years) cases of MDS newly diagnosed starting in April 2010 were invited to participate. This analysis includes patients enrolled during the first study year with one-year follow-up data. Treatment choices (supportive, active, and transplant) were stratified by the international prognostic scoring system (IPSS) and the revised-IPSS (IPSS-R), then separated into groups by location of care and age (<65 or 65+ years). Academic-based care was any contact with the UMN and Mayo Clinic; community-based care was all other clinical sites.
Results
Stratification by IPSS and IPSS-R showed supportive care decreased and active care increased with advancing risk categories (p <0.0001). Comparing treatment setting, community-based care had 77% supportive and 23% active treatment; academic-based care was 36% supportive, 41% active, and 23% transplant (p <0.0001). By age groups, patients <65 years with intermediate, high, or very high risk disease by IPSS-R received 97% active care/transplant, compared to only 52% of patients age 65+.
Conclusions
Younger patients and those treated at academic centers had a more aggressive treatment approach. Whether these treatment differences convey improved disease control and mortality, and therefore should be extended more frequently to older and community-based patients, is the subject of ongoing prospective study.
Keywords: myelodysplastic syndromes, hematologic malignancies, drug therapy, bone marrow transplantation
1. Introduction
Myelodysplastic syndromes (MDS) are a spectrum of bone marrow disorders with ineffective hematopoiesis from abnormal cellular differentiation and dysplasia resulting in peripheral cytopenias and a varying propensity for leukemic transformation [1]. Initial case series in the 1970's described a syndrome of “preleukemia,” although over the last four decades the spectrum of MDS are increasingly recognized as malignancies independent of the association with acute myelogenous leukemia [2,3]. Considerable disease heterogeneity in presentation, pathology, cytogenetics, prognosis, and ultimately treatment choice is characteristic of MDS. To address this disease disparity, an updated World Health Organization (WHO) system was introduced in 2008 to better define pathologic diagnosis. Risk stratification has also improved with introduction of the international prognostic scoring system (IPSS) in 1997 for use at initial diagnosis, followed by the revised IPSS (IPSS-R) and WHO prognostic scoring system (WPSS), both validated for risk stratification throughout disease course [4-8].
As MDS disease identification and risk stratification have improved, advances in treatment options have also developed, changing therapeutic decision-making for clinicians. Previously limited to the widely divergent options of supportive care with growth factors and blood transfusions or aggressive intervention with cytotoxic chemotherapy and bone marrow transplantation, the biologic agents decitabine, azacitidine, and lenalidomide have become available in the past decade to potentially alter disease course and, in the case of azacitidine, provide a definitive survival advantage [9-12]. The selection of a treatment strategy adapted to individual patient and disease determinants, and the timing for initiating or changing that strategy, is therefore a complex process.
Several reports have provided retrospective data on how the various treatment options are being utilized in clinical practice [13-16]; however, studies with well-defined patient populations and disease characteristics in relation to treatment strategies are not available. To better characterize therapeutic choices in newly diagnosed MDS, we report the practice patterns captured during the first year of MDS diagnosis for patients enrolled in a Minnesota population-based study. We highlight a comparison of treatment in community and academic centers, stratified by IPSS and IPSS-R prognostic risk scores.
2. Methods
2.1. Case accrual
Adults in Minnesota with MDS (AIMMS) is a statewide prospective population-based study conducted by the University of Minnesota (UMN), Mayo Clinic, and Minnesota Department of Health. In April 2010 the Minnesota Cancer Surveillance System (MCSS) began rapid case identification of all newly diagnosed adult cases (ages 20+ years) of MDS. Following physician approval, patients were contacted for invitation to enroll. An extensive questionnaire was completed by each participant at study entrance to gather retrospective epidemiologic data for comparison with a control cohort (data not included in this analysis).
2.2. Data collection
Following enrollment, central medical review was completed starting from MDS diagnosis and consisted of independent pathology review of bone marrow and peripheral blood samples by two hematopathologists, along with independent cytogenetic interpretation by a cytogeneticist. Reports were then integrated, with discrepancies in WHO classification requiring collaboration for a unifying subtype according to the 2008 revised criteria [4]. Cases without diagnostic verification were excluded.
The pathologic review was coupled with oncologist chart review assigning prognostic risk scores initially with IPSS and subsequently with IPSS-R in addition [5,6]. Scores were calculated independently for each patient, and lack of necessary cytogenetic and hematologic data to calculate risk scores resulted in case exclusion. Treatment exposures and responses were abstracted, including relevant exposures prior to formal diagnosis when available. All enrolled patients with prospective one year follow-up were included in this analysis. Per the AIMMS study design, enrollment of new cases will continue through December 2014, with annual clinical review of all patient cases planned for three years following the initial enrollment.
2.3. Treatment categories
Treatment exposures were classified as supportive, active, or transplant. Supportive care was defined as observation, growth factors, and/or transfusions. Active care included azacitidine, decitabine, lenalidomide, or induction-type chemotherapy. Transplant classification required the procedure performed within the designated study period. Patients were placed into a single treatment category based on the most aggressive treatment; for example, patients receiving erythropoietin and azacitidine qualified as active treatment, and patients receiving chemotherapy followed by transplant were categorized as transplant.
2.4. Definitions
Academic centers were defined as the UMN and Mayo Clinic, with all other sites designated as community based practices. Academic-based care was defined as any patient care at an academic center since diagnosis; community-based care required no contact with an academic center. Transfusion dependence was defined as having received any blood products. Demographic variables included sex and age at diagnosis (<65 years and 65+ years). All statistical analyses were conducted using SAS (Version 9.3, Cary, NC). Contingency table methods were used for comparison of categorical data. All reported p-values are two-sided.
3. Results
3.1. Case inclusion
This analysis was completed at the two-year mark of the AIMMS study and included data collected from June 2010 to June 2012. A total of 227 patients were enrolled to the AIMMS study at that point. Of those enrolled, 51 patients did not yet have one year follow-up data available, 24 patients were yet to have undergone central review, and 4 patients were excluded for lack of cytogenetics necessary to calculate risk scores, resulting in a study group of 148 patients.
3.2. Overall characteristics
The median patient age at enrollment was 73 years (range of 25-86), and patients aged 65 or older accounted for 73%. Males comprised 69% of patients. Transfusion dependence was prevalent in 40% of the population for red blood cells and 16% for platelets. WHO classification subtypes, IPSS and IPSS-R score distribution, and treatment strategies are detailed in Table 1.
Table 1.
Patient Demographics
| Variable | No. | % |
|---|---|---|
| N | 148 | |
| Age, y | ||
| Median | 73 | |
| Range | 25-86 | |
| <65 | 40 | 27 |
| 65+ | 108 | 73 |
| Sex | ||
| Male | 102 | 69 |
| Female | 46 | 31 |
| De novo MDS | 127 | 86 |
| Treatment-related MDS | 21 | 14 |
| Median presenting blood counts | ||
| Hemoglobin (g/dL) | 9.3 | |
| Platelet (×103/mm3) | 103 | |
| Neutrophil (×103/mm3) | 1.85 | |
| Transfusion dependencea | ||
| PRBC | 59 | 40 |
| Platelet | 23 | 16 |
| 2008 WHO classification | ||
| RCUD | 3 | 2 |
| RARS | 24 | 16 |
| RCMD | 38 | 26 |
| RAEB-1 | 21 | 14 |
| RAEB-2 | 30 | 20 |
| MDS-U | 7 | 5 |
| MDS with del(5q) | 4 | 3 |
| IPSS | ||
| Low | 46 | 31 |
| Int-1 | 41 | 28 |
| Int-2 | 53 | 36 |
| High | 8 | 5 |
| IPSS-R | ||
| Very low | 23 | 16 |
| Low | 39 | 26 |
| Intermediate | 25 | 17 |
| High | 27 | 18 |
| Very high | 34 | 23 |
| Treatmentb | ||
| Supportive | ||
| Observation | 61 | 41 |
| Growth factor | 23 | 16 |
| Total | 84 | 57 |
| Active | ||
| Azacitidine | 23 | 16 |
| Decitabine | 9 | 6 |
| Lenalidomide | 10 | 7 |
| Induction-type chemotherapy | 5 | 3 |
| Total | 47 | 32 |
| Transplant | 17 | 11 |
Defined as having received any PRBC or platelets.
Patients placed in only one treatment category based on most aggressive therapy.
Abbreviations: RCUD, refractory cytopenia with unilineage dysplasia; RARS, refractory anemia with ringed sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RAEB-1, refractory anemia with excess blasts-1; RAEB-2, refractory anemia with excess blasts-2; MDS-U, myelodysplastic syndrome unclassified.
Treatment strategy for each patient was identified. Supportive care was utilized in 57% of patients, with the majority having disease observation alone. Active care was utilized for 32% of patients, with azacitidine the most commonly used chemotherapeutic agent, followed by decitabine and lenalidomide. Induction-type chemotherapy was received by 3% of patients and the rate of transplantation was 11%.
3.3. Treatment by location of care
We analyzed the distribution of patients by IPSS and IPSS-R risk groups with respect to treatment strategy and location of care (academic or community). For the overall patient population, as risk categories advanced, the frequency of supportive care decreased while utilization of active and transplant strategies increased (Table 2). These differences in treatment choices by risk category had p-values of <0.0001 for both IPSS and IPSS-R.
Table 2.
Treatment Strategy by IPSS-R Risk Group
| IPSS-R Risk Group | ||||||
|---|---|---|---|---|---|---|
| Treatment Strategy | Very Low | Low | Intermediate | High | Very High | Total |
| All Patients | ||||||
| Supportive | 22 (96%) | 32 (82%) | 10 (40%) | 11 (41%) | 9 (26%) | 84 (57%) |
| Active | 0 | 6 (15%) | 9 (36%) | 14 (52%) | 18 (53%) | 47 (32%) |
| Transplant | 1 (4%) | 1 (3%) | 6 (24%) | 2 (7%) | 7 (21%) | 17 (11%) |
| Community-based carea | ||||||
| Supportive | 17 (100%) | 23 (92%) | 7 (54%) | 6 (55%) | 4 (50%) | 57 (77%) |
| Active | 0 | 2 (8%) | 6 (46%) | 5 (45%) | 4 (50%) | 17 (23%) |
| Transplant | 0 | 0 | 0 | 0 | 0 | 0 |
| Academic-based careb | ||||||
| Supportive | 5 (83%) | 9 (64%) | 3 (25%) | 5 (31%) | 5 (19%) | 27 (36%) |
| Active | 0 | 4 (29%) | 3 (25%) | 9 (56%) | 14 (54%) | 30 (41%) |
| Transplant | 1 (17%) | 1 (7%) | 6 (50%) | 2 (13%) | 7 (27%) | 17 (23%) |
| p-valuec | n/c | 0.07 | 0.02 | 0.43 | 0.13 | <0.0001 |
No contact with an academic center.
Any contact with an academic center.
Comparing community and academic treatment strategies for each risk group and total
Abbreviations: IPSS-R, revised international prognostic scoring system.
Comparison of treatment strategy by either academic or community setting resulted in groups of 74 patients (50%) receiving academic-based care and 74 patients (50%) with community-based care. Supportive care predominated for patients treated in community settings at 77%, while 23% received active care. Academic-based care had a significantly lower rate of supportive care at 36%, while 41% had active treatment and 23% had transplants (p-value <0.0001). Bone marrow transplantation, limited to academic centers, had a sequential increase in rate by IPSS risk categories, with Int-2 disease accounting for the highest transplant frequency (65%, 11/17). By IPSS-R, intermediate disease (35%, 6/17) and very high risk disease (42%, 7/17) accounted for the highest transplant frequencies.
Stratification by IPSS score showed both treatment settings had less supportive and more active care with advancing risk group, consistent with the trend for the overall patient population. Community-based care ranged from almost entirely supportive for low risk disease (97%) to a slight majority of active care for Int-2 disease (53%). In contrast, academic-based care had an earlier shift to predominantly active care, with 47% of Int-1 disease receiving this strategy. All patients with high risk disease had academic-based care, 88% of which received active treatment or transplant. Including all risk categories, treatment strategy by setting of care had a p-value of 0.03.
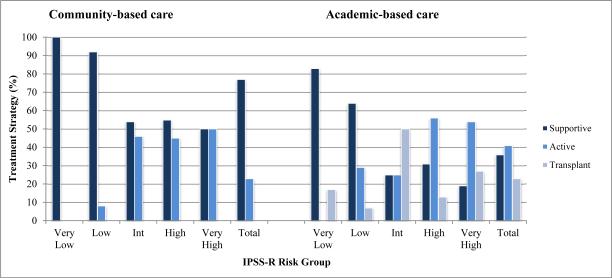
IPSS-R based stratification (Table 2; Figure 1) also revealed a significant difference in treatment strategy by setting of care (p-value 0.007). A higher percentage of supportive care was utilized in community-based care compared to the academic setting across all risk groups: 100 to 83% for very low, 92 to 64% for low, 54 to 25% for intermediate, 55 to 31% for high, and 50 to 19% for very high risk disease. Unlike the analysis by IPSS, active care never became the predominant treatment strategy for any risk group in the community setting. For academic-based care, a mostly supportive treatment strategy was supplanted by active/transplant strategies starting with the intermediate risk group.
Figure 1.
Treatment Strategy by IPSS-R Risk Group, Community and Academic Settings
3.4. Referral patterns
Diagnosis by location included 112 at community practices, the remaining 36 cases diagnosed at academic centers. Of the 112 community diagnoses, 38 patients (34%) were subsequently referred to an academic center. Referral rates increased with higher IPSS and IPSS-R risk categories, with 13% low risk, 24% Int-1, 54% Int-2, and 100% high risk referred by IPSS and 11% very low, 14% low, 32% intermediate, 48% high, and 67% very high referred by IPSS-R.
3.5. Treatment by age group
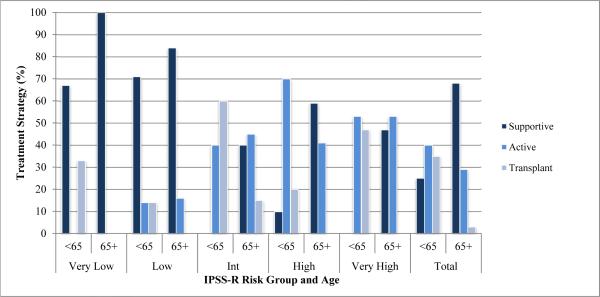
Comparison of treatment by age groups <65 and 65+ years showed younger patients received more aggressive therapy across all IPSS and IPSS-R risk groups, with a p-value of <0.0001 for overall treatment choices between the two age groups (Figure 2). Supportive care was utilized more frequently for patients 65+ at 68%, for patients <65 at 25%. Younger patients in higher risk groups received active therapy or transplant at rates of 100% for IPSS Int-2/high and 97% for IPSS-R intermediate/high/very high, while their elderly counterparts in the older 65+ age group received such strategies at rates of only 51% and 52%, respectively. The majority of transplants occurred in younger patients, with 14/17 (82%) in the <65 age group.
Figure 2.
Treatment Strategy by IPSS-R and Age Group
3.6. Outcomes
At one year follow-up 39 deaths had occurred, a mortality rate of 26%. Community-based patients accounted for 17 deaths and academic-based patients for 22 deaths. By treatment exposure, 65% (n=11/17) of the community-based deaths received supportive care. Of the academic based deaths, 59% (n=13/22) received active care, 36% (n=8/22) received supportive care, and one transplant death occurred.
4. Discussion
4.1. Primary comparison
Evaluation of MDS practice patterns during the first year of diagnosis revealed a number of insights. For the principal analysis comparing treatment at academic to community centers, academic-based care resulted in less supportive and more active treatment. This trend was consistent across all IPSS and IPSS-R risk categories. The shift from a predominantly supportive strategy to a predominantly active strategy occurred for academic-based patients with the IPSS Int-1 and IPSS-R intermediate risk groups; for community-based patients, not until the IPSS Int-2 risk group did the majority of patients receive active care. This transition towards active care never occurred for IPSS-R in the community (Figure 1). Earlier use of active care suggests a more aggressive treatment approach in the academic setting. This is to our knowledge the first study documenting this expected relationship.
The observed association between treatment strategy and treatment location may have occurred for several different reasons. As the active strategy was composed mainly of azacitidine, decitabine, and lenalidomide (with the remaining few percent being induction-type chemotherapy), level of experience with these newer agents may vary by practitioner location. However, the studies leading to FDA approval of azacitidine in 2004 [11,17,18] are now more than 10 years known, and approval of decitabine and lenalidomide [9,19-21] followed only shortly later, arguing against a difference in drug familiarity contributing to more active care. Rather, the emphasis on supportive care in previous guidelines [22,23], especially for lower-risk disease patients, may continue to disproportionately influence practitioners in non-academic settings.
Referral patterns likely had the most significant effect on treatment choice. Younger patients or those having higher risk disease characteristics within an individual risk category (i.e. “higher risk” Int-1) considered more appropriate for intensive therapy may have been referred preferentially to academic centers. Indeed, the median age of patients referred from community to academic centers was 66, compared to a median age of 73 in the overall population. Age was a strong predictor of treatment strategy across all risk groups; consequently, referral of younger patients to academic centers may have resulted in more active care or transplants because they were younger and presumed better able to tolerate such treatment, rather than the academic environment itself leading to more aggressive care.
4.2. Secondary observations
That younger patients (those <65 years) were treated more aggressively makes intuitive sense but deserves examination (Figure 2). MDS is a disease of the elderly; thus, more aggressive treatment for younger patients will limit the disease modifying effects of these treatment options to a small number, as evidenced by only 27% of patients in our study populating the <65 years age group. While this correlation of more intensive therapy in younger patients was also reported in several previous population-based studies [16,24], whether older patients can tolerate and benefit from a similarly intensive strategy has been investigated for both azacitidine and bone marrow transplantation, with studies concluding that age need not be a limiting factor for these treatments [25-28]. To what extent the treatment paradigm should be shifted from a preference for active and transplant therapy in younger patients to such an approach for all patients regardless of age is an area of continued interest and study.
For the overall patient population, less supportive and more active care strategies with increasing risk score is consistent with current guidelines recommending treatment decisions based on stratification of patients into risk categories [29-31]. With the median survival of MDS patients ranging widely, from 8.8 years for IPSS-R very low risk disease to 0.8 years for very high risk disease [6], it is reassuring that these essential risk-adapted treatment strategies are being utilized in clinical practice.
The range of treatment choices for active therapy in our study was limited to azacitidine, decitabine, lenalidomide, and induction-type chemotherapy. This is significantly consolidated compared to previous treatment reviews which documented use of androgens, immunosuppressive agents, hydroxyurea, and thalidomide [15,16]. Although various studies have evaluated the effectiveness of these agents [32-36], their use has declined with the growing evidence for improved disease modification with the hypomethylators and lenalidomide [37]. These differences may reflect a geographical divide, with two European studies showing higher utilization of alternative agents [15,16] compared to a 2008 US-based treatment strategy study [13] and the data presented here.
Allogeneic hematopoietic stem cell transplantation, the only potential cure for MDS, had a rate of 11% in our population. Numerous retrospective studies have evaluated disease and transplant-related factors for MDS transplants including age of recipient, pre-transplant disease burden, conditioning regimen intensity, and donor characteristics [38-41], yet the concern for high morbidity and mortality of this therapy combined with the generally older age of MDS patients has resulted in low utilization of this treatment strategy despite available data highlighting adequate tolerance in an older patient population [28]. The three main practice pattern studies to date reported transplant rates of only 2-5%, and all had a duration since diagnosis >1yr [13,15,16]. Our study was limited to one year follow-up since diagnosis, potentially underestimating the difference in transplant rates as more patients in our study may have been transplanted with continued observation past one year. As transplant is generally considered underutilized, a rate of 11% can be interpreted as appropriate rather than overly aggressive, the product of a relatively small statewide population (5.42 million) with access to two in-state bone marrow transplant programs [42].
Patients who received transplants were mostly higher risk—by IPSS 76% had Int-2/high risk disease and by IPSS-R 88% were categorized as intermediate/high/very high. This is consistent with existing data that support reserving transplants to these higher risk groups [43-45]. The very low and low risk categories (by IPSS-R) each had one transplant recipient, strategies that per current accepted practice may be considered inappropriately aggressive.
We included risk-stratification data for the well-established IPSS and also the more recently published IPSS-R, as the improved prognostic accuracy of the IPSS-R will likely result in this scoring system becoming the standard of care [46]. A general upstaging of risk scores can be appreciated when moving from IPSS to IPSS-R, evidenced by only 5% of patients placed into the IPSS high risk category while 41% were placed into the IPSS-R high/very high risk categories. Additionally, the IPSS-R intermediate risk category accounted for 17% of patients, in contrast to 64% of patients in the IPSS Int-1/Int-2 categories. Fewer patients in the intermediate risk categories may assist in treatment decisions, as placement on either end of the scoring spectrum (low or high risk) clarifies the most appropriate course of action.
4.3. Strengths and weaknesses
The rigorous central review process was a strength of our study, providing a high degree of diagnostic certainty through pathologic evaluation. Additionally, independent calculation of risk scores and verification of treatment strategy by direct chart review effectively eliminated the recall bias that may accompany a survey-based approach to collection of treatment data as relied upon by several previously published studies [13-15]. The case accrual process worked to minimize selection bias by not limiting data collection to specified physicians, as in the US-based study, or to patients presenting for care within a narrowly-defined period (7 days, in the French study) [13,16]. Selection bias may still be introduced in this study, as participation was voluntary and an estimated 50% of identified cases were enrolled, though the rolling nature of case accrual may lead to a higher percentage at a later date. As patients were excluded whose date of death preceded collection of all necessary preliminary data, this data set may bias towards lower risk disease.
Outcomes data, with the exception of deaths at one year follow-up, is not included in this study. As extended follow-up data becomes available, potential mortality differences based on location of care and treatment decisions will be investigated and detailed in later reports.
4.4. Cohort representativeness
The study population here is generally representative of typical MDS patients. The median age of 73 years is slightly younger than the 76 years reported in the SEER database from 2001-2003 [47]. Secondary MDS usually accounts for 10% of cases and in this cohort represents 14%. Median presenting blood counts (Hgb 9.3 g/dL, platelets 101 × 103/mm3, ANC 1.8 × 103/mm3) are nearly identical to data reported by Sekeres et al [13]. The patient population used to formulate the original IPSS had a distribution of 71% lower risk (IPSS low/Int-1) and 29% higher risk (IPSS Int-2/high) [5]; this population trended higher risk with 59% lower risk and 41% higher risk.
Conclusions
This prospective, population based study provides a well-defined patient cohort based on central review of pathologic and clinical data, allowing the observed correlations between IPSS/IPSS-R score, practice setting, and patient age in relation to treatment choices. Whether these differences in practice patterns within the first year of diagnosis impact subsequent treatment decisions, translate into improved disease control, and decrease mortality requires continued prospective analysis and will be detailed in future reports, as the larger AIMMS study continues to enroll new patients and gather follow-up data on existing participants.
Highlights.
Academic-based care was more aggressive compared to community-based care
Younger patients (<65 years) received more aggressive care than those 65+ years
Treatment strategies reflected disease activity
Choice of chemotherapy for MDS has consolidated around newer agents
Acknowledgments
This work was supported by NIH R01 CA142714 and K05 CA157439. The funding sources had no role in the design and conduction of this study, nor in the collection, analysis, and interpretation of the data. The funding sources had no role in the preparation, review, or approval of this manuscript.
An earlier version of this manuscript was presented in poster format at the American Society of Hematology annual meeting and exposition, December 2013 in New Orleans, Louisiana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest (financial, activity, relationships, or affiliations) to disclose related to the subject of this manuscript.
Authorship Contribution Statement
Daniel F. Pease, MD: meets authorship criteria through substantial contributions to conception and design, acquisition of data, and analysis/interpretation of data; drafting the article and revising it critically for important intellectual content; and final approval of the version to be published.
Julie A. Ross, PhD: meets authorship criteria through substantial contributions to analysis and interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published.
Jenny N. Poynter, PhD: meets authorship criteria through substantial contributions to analysis and interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published.
Phuong L. Nguyen, MD: meets authorship criteria through substantial contributions to analysis and interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published.
Betsy Hirsch, PhD: meets authorship criteria through substantial contributions to analysis and interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published.
Adina Cioc, MD: meets authorship criteria through substantial contributions to analysis and interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published.
Michelle A. Roesler, BS: meets authorship criteria through substantial contributions to acquisition of data; revising the article critically for important intellectual content; and final approval of the version to be published.
Erica D. Warlick, MD: meets authorship criteria through substantial contributions to conception and design, acquisition of data, and analysis/interpretation of data; drafting the article and revising it critically for important intellectual content; and final approval of the version to be published.
References
- 1.Catenacci DV, Schiller GJ. Myelodysplastic syndromes: a comprehensive review. Blood Rev. 2005;19(6):301–319. doi: 10.1016/j.blre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Fisher WB, Armentrout SA, Weisman R, et al. “Preleukemia:” a myelodysplastic syndrome often terminating in acute leukemia. Arch Intern Med. 1973;132(2):226–232. doi: 10.1001/archinte.132.2.226. [DOI] [PubMed] [Google Scholar]
- 3.Love N, Sekeres MA. Hematologic oncology update: Research to practice [CD] 2010.
- 4.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 6.Greenberg P, Tuechler H, Schanz J, et al. Revised international prognostic scoring system (IPSS-R) for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 8.Malcovati L, Della Porta MG, Strupp C. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based prognostic scoring system (WPSS). Haematologica. 2011;96(10):1433–1440. doi: 10.3324/haematol.2011.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 10.Joeckel TE, Lubbert M. Clinical results with the DNA hypomethylating agent 5-aza-2’-deoxycytidine (decitabine) in patients with myelodysplastic syndromes: an update. Semin Hematol. 2012;49(4):330–341. doi: 10.1053/j.seminhematol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 12.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. J Natl Cancer Inst. 200100(21):1542–1551. doi: 10.1093/jnci/djn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekeres MA. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J Natl Compr Canc Netw. 2011;9(1):57–63. doi: 10.6004/jnccn.2011.0006. [DOI] [PubMed] [Google Scholar]
- 15.Gatterman N, Kundgen A, Kellermann L, et al. Diagnosis and therapy of myelodysplastic syndromes in Germany: a retrospective multicenter analysis. Onkologie. 2012;35:350–356. doi: 10.1159/000338939. [DOI] [PubMed] [Google Scholar]
- 16.Kelaidi C, Stamatoullas A, Beyne-Rauzy O, et al. Daily practice management of myelodysplastic syndromes in France: data from 907 patients in a one-week cross-sectional study by the Groupe Francophone des Myelodysplasies. Haematologica. 2010;95(6):892–899. doi: 10.3324/haematol.2009.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman LR, Holland JF, Ellison RR. Low dose continuous infusion azacytidine is an effective therapy for patients with myelodysplastic syndromes, a study of Cancer and Leukemia Group B. J Cancer Res Clin Oncol. 1990;116(suppl):816. [Google Scholar]
- 18.Silverman LR, Holland JF, Demakos EP, et al. Azacitidine (Aza C) in myelodysplastic syndromes (MDS), CALGB studies 8421 and 8921. Ann Hematol. 1994;68:A12. [Google Scholar]
- 19.Saba H, Rosenfeld C, Issa JP, et al. First report of the phase III North American trial of decitabine in advanced myelodysplastic syndrome (MDS). Blood. 2004;104:23a. [Google Scholar]
- 20.Saba HI, Lubbert M, Wijermans PW. Response rates of phase 2 and phase 3 trials of decitabine (DAC) in patients with myelodysplastic syndromes (MDS). Blood. 2005;106:706a. [Google Scholar]
- 21.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 22.Alessandrino EP, Amadori S, Barosi G, et al. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica. 2002;87(12):1286–1306. [PubMed] [Google Scholar]
- 23.Bowen D, Culligan D, Jowitt S. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br Journal Haematol. 2003;120(2):187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 24.Gattermann N, Kundgen A, Kellermann L, Zeffel M, Paessens B, Germing U. The impact of age on the diagnosis and therapy of myelodysplastic syndromes: results from a retrospective multicenter analysis in Germany. Eur J Haematol. 20191(6):473–482. doi: 10.1111/ejh.12196. [DOI] [PubMed] [Google Scholar]
- 25.Seymour JF, Fenaux P, Silverman LR, et al. Effects of azacitidine compared with conventional care regimens in elderly (≥75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2010;73(3):218–227. doi: 10.1016/j.critrevonc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xicoy B, Jimenez MJ, Garcia O, et al. Results of treatment with azacitidine in patients aged ≥75 years included in the Spanish Registry of Myelodysplastic Syndromes. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.834532. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405–411. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 28.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg PL, Attar E, Bennett JM, et al. Myelodysplastic syndromes: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11(7):838–874. doi: 10.6004/jnccn.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Germing U, Kobbe G, Haas R, et al. Myelodysplastic syndromes: diagnosis, prognosis, and treatment. Dtsch Arztebl Int. 2013;110(46):783–790. doi: 10.3238/arztebl.2013.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malcovati L, Hellstrom-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943–2964. doi: 10.1182/blood-2013-03-492884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raza A, Meyer P, Dutt D, et al. Thalidomide produces transfusion independence in long-standing refractory anemias in patients with myelodysplastic syndromes. Blood. 2001;98(4):958–965. doi: 10.1182/blood.v98.4.958. [DOI] [PubMed] [Google Scholar]
- 33.Zorat F, Shetty V, Dutt D, et al. The clinical and biological effects of thalidomide in patients with myelodysplastic syndromes. Br J Haematol. 2001;115(4):881–894. doi: 10.1046/j.1365-2141.2001.03204.x. [DOI] [PubMed] [Google Scholar]
- 34.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26(15):2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olnes MJ, Sloand EM. Targeting immune dysregulation in myelodysplastic syndromes. JAMA. 2011;305(8):814–819. doi: 10.1001/jama.2011.194. [DOI] [PubMed] [Google Scholar]
- 36.Kobaba R, Kanamaru A, Takemoto Y, Kohsaki M, Kakishita E, Nagai K. Androgen in the treatment of refractory anemia. Int J Hematol. 1991;54(2):103–107. [PubMed] [Google Scholar]
- 37.Zeidan AM, Linhares Y, Gore SD. Current therapy of myelodysplastic syndromes. Blood Rev. 2013;27(5):243–259. doi: 10.1016/j.blre.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warlick E, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15(1):30–38. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling in myelodysplastic syndromes. Blood. 2006;108(3):836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 40.Martino R, de Wreede L, Fiocco M, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone Marrow Transplant. 2013;48(6):761–770. doi: 10.1038/bmt.2012.236. [DOI] [PubMed] [Google Scholar]
- 41.Kroger N, Zabelina T, de Wreede L, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia. 2013;27(3):604–609. doi: 10.1038/leu.2012.210. [DOI] [PubMed] [Google Scholar]
- 42.Minnesota. State & county quickfacts [January 15, 2014];US Census Bureau Web site. http://quickfacts.census.gov/qfd/states/27000.html. Published January 6, 2014.
- 43.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 44.Kuendgen A, Strupp C, Aivado M, et al. Myelodysplastic syndromes in patients younger than age 50. J Clin Oncol. 2006;24(34):5358–5365. doi: 10.1200/JCO.2006.07.5598. [DOI] [PubMed] [Google Scholar]
- 45.Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31(21):2662–2670. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bejar R. Prognostic models in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2013;2013:504–510. doi: 10.1182/asheducation-2013.1.504. [DOI] [PubMed] [Google Scholar]
- 47.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: Incidence and survival in the United States. Cancer. 2007;109(8):1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]