Abstract
There is emerging interest in understanding the role of progesterone receptors (PRs) in breast cancer. The aim of this study was to investigate the proliferative effect of progestins and antiprogestins depending on the relative expression of the A (PRA) and B (PRB) isoforms of PR. In mifepristone (MFP)-resistant murine carcinomas antiprogestin responsiveness was restored by re-expressing PRA using demethylating agents and histone deacetylase inhibitors. Consistently, in two human breast cancer xenograft models, one manipulated to overexpress PRA or PRB (IBH-6 cells), and the other expressing only PRA (T47D-YA) or PRB (T47D-YB), MFP selectively inhibited the growth of PRA-overexpressing tumors and stimulated IBH-6-PRB xenograft growth. Furthermore, in cells with high or equimolar PRA/PRB ratios, which are stimulated to proliferate in vitro by progestins, and are inhibited by MFP, MPA increased the interaction between PR and the coactivator AIB1, and MFP favored the interaction between PR and the corepressor SMRT. In a PRB-dominant context in which MFP stimulates and MPA inhibits cell proliferation, the opposite interactions were observed. Chromatin immunoprecipitation assays in T47D cells in the presence of MPA or MFP confirmed the interactions between PR and the coregulators at the CCND1 and MYC promoters. SMRT downregulation by siRNA abolished the inhibitory effect of MFP on MYC expression and cell proliferation. Our results indicate that antiprogestins are therapeutic tools that selectively inhibit PRA-overexpressing tumors by increasing the SMRT/AIB1 balance at the CCND1 and MYC promoters.
Introduction
There is compelling clinical evidence 1,2 and experimental models 3–5 suggesting that the progesterone receptor (PR) has a role in breast cancer development and growth. Two PR isoforms have been described, PRB and PRA, that are transcribed from a single gene 6, and each of these isoforms may exert unique functions 7. PRA and PRB adopt distinct conformations upon ligand binding, which suggests that different coregulators may interact with each isoform [reviewed in 8]. The corepressor silencing mediator for retinoid and thyroid hormone receptors (SMRT) preferentially interacts with the antagonist-bound PRA, and the coactivators steroid receptor coactivator 1 (SRC-1) and 2 (SRC-2) have a higher affinity for PRB 9. Less information is available regarding SRC-3 (AIB1), an oncogene associated with endocrine resistance 10–13 and PR action in the mammary gland 14.
Antiprogestins inhibit breast cancer growth in several experimental models [reviewed in 15]. Using medroxyprogesterone acetate (MPA)-induced mammary carcinomas, we have demonstrated that only tumors expressing high PRA levels regress with antiprogestin treatment 16,17. On this basis, we hypothesized that antiprogestins, together with conventional endocrine therapy, could be a valid therapeutic approach for patients with breast carcinomas that express higher levels of PRA than PRB.
Of the several available antiprogestins, Mifepristone (MFP; RU486) binds to PR with high affinity. The receptor-bound complex binds to DNA and can display agonistic activity in cells stimulated by cAMP/PKA pathway activators, but this occurs in a PRB tissue- and species-specific manner 18. At higher concentrations, MFP may also exert antiglucocorticoid effects 19. Aglepristone (Agle) is an antiprogestin approved for veterinary use that binds PR with high affinity and glucocorticoid receptor (GR) with lower affinity 20. Proellex (CDB 4124) is a new antiprogestin with diminished antiglucocorticoid activity 21. Although some of these antiprogestins have been used in cancer models 22, none have been tested for differential effectiveness against PRA compared with PRB in breast tumors.
The main goal of this study was to evaluate a) whether antiprogestin responsiveness in breast cancer is determined by the PRA/PRB expression ratio and b) to investigate the role of the corepressor SMRT and the coactivator AIB1 in mediating antiprogestin-induced effects.
MATERIALS AND METHODS
Reagents
5-aza-2′-deoxycytidine’ (5azadC), 17β-estradiol (E2), MFP and trichostatin A (TSA) were purchased from Sigma-Aldrich (St Louis, MO). Proellex was obtained from Repros Therapeutics (The Woodlands, TX). MPA was obtained from Craveri (Buenos Aires, Argentina), and Agle (Alizine®; Virbac, Carros, France) is commercially available.
Animals
Two-month-old virgin female BALB/c mice (IBYME Animal Facility), nude mice (nu/nu, University of La Plata, Buenos Aires) and NOD/LtSz-scid/IL-2Rgamma null mice (The Jackson Laboratory, Bar Harbor, ME and bred at IBYME) were used. Animal care and manipulation were in agreement with the NIH Guide for the Care and Use of Laboratory Animals.
Murine mammary carcinomas
Mammary carcinomas from the MPA-induced breast cancer model classified according to their MFP responsiveness as responsive, acquired resistant or constitutively resistant tumors were used. C4-HI and C4-2-HI tumors originated from the C4-HD tumor; 59-HI and 59-2-HI tumors originated from the 59-HD tumor 5. C4-HI and 59-2-HI tumors regress with MFP treatment, and C4-2-HI and 59-HI are constitutive MFP-resistant tumor variants. The acquired MFP-resistant variants C4-HIR and 59-2-HIR were generated from C4-HI and 59-2-HI tumors, respectively 17. MFP and Proellex were freshly prepared from ethanol solutions and administered subcutaneously (sc) at 10 mg/kg/day or were administered within silastic pellets (6 mg) implanted sc 23; Agle was administered once per week (1.5 mg/50 μl; oil). 5azadC (intraperitoneal, ip, 1 mg/kg) and TSA (sc, 1 mg/kg) were administered every other day.
Cell lines
IBH-6 cells, derived from a human breast cancer, express hormone receptors and grow in nude mice 24,25. The cells were transfected with human pSG5-PRB, pSG5-PRA or the empty vector (pSG5) and a plasmid encoding the neomycin resistance gene (pIRES-N1) 26 and subsequently cultured with 400 mg/ml G418 (Invitrogen Life Technologies, Carlsbad, CA). IBH-6 cells are hypodiploid (V. Fabris, C Lanari and Isabel Luthy, unpublished data). Cells were validated through karyotype analysis. T47D cells were validated in 2011 and reacquired from ATCC in 2013. T47D cells were similarly transfected with human PRB or empty vector as described above. T47D-YA and T47D-YB cells were cultured as previously described 27.
Xenograft studies
T47D (5×106), genetically modified IBH-6 (106) or T47D-YA/B (5×106) cells were inoculated orthotopically into nu/nu (IBH/6) or NOD/LtSz-scid/IL-2Rgamma null female mice. One week prior to T47D or T47D-YA/B inoculation, E2 silastic pellets containing 0.5 mg E2 were sc implanted 23.
In vitro studies
Primary cultures, 3H-thymidine uptake [cited in 5] and cell counting assays 28 were performed as previously described. In all studies, 10 nM MPA or MFP was used.
Immunoprecipitation (IP), western blots (WB), immunofluorescence (IF) and immunohistochemistry (IHC)
All assays were performed as previously described 28.
Antibodies
PR (SC-538 or SC-7208), ERK (SC-94), CCND1 (SC-753), MYC (SC-764), AIB1 (SC-25742) and SMRT (SC-20778) were obtained from Santa Cruz Biotech (Santa Cruz, CA); Ab-7 (PR) and Ab-6 (PRB) were obtained from Thermo Fisher (Fremont, CA), and PR (clone PgR 1294), CK and Ki67 were obtained from Dako (Carpinteria, CA). AIB1 (611105) was obtained from BD Biosciences (San José, CA); pSer294PR (Ab61785) was obtained from Abcam (Cambridge, MA), and pSer162PRB was kindly provided by D. Edwards (BCM, Houston).
Image quantification
Stained cells were analyzed using a Nikon Eclipse E800 Laser Confocal Microscope. Nuclear co-localization was estimated by a Pearson’s correlation coefficient (Rr) as previously described 29. Co-localizations of endogenous proteins were confirmed using Proximity Ligation Assays (PLA) 30, following the manufacturer’s instructions (Duolink II Orange Starter Kit 92102, Olink Bioscience, Uppsala, Sweden). Briefly, after incubations, cells were fixed in cold 70% ethanol during 30 min at −20°C, washed in PBS, blocked, and incubated with both primary antibodies ON. Secondary antibodies conjugated with oligonucleotides (PLA probe MINUS and PLA probe PLUS) were added to the reaction and incubated during 1 h at 37°C. Only if the two proteins are in close proximity, ligation of oligonucleotides will occur. Detection of protein interaction was done after amplification of the oligonucleotides previously ligated, incubation with a fluorescently labeled probe and mounting the slides in a medium with DAPI. Nuclear proteins interaction was evaluated under confocal microscopy. In IHC studies, stained nuclei were counted in 10 high-power fields (HPFs) of each section at a 1000X magnification, and the data are expressed as the mean ± SEM of the percentage of positive events in relation to the total cell number.
qPCR and chromatin immunoprecipitation (ChIP) assays
qPCR
Specific oligos for human PRB were designed using the Primer-BLAST program (NCBI) and those for PR were already used by others 31; both are presented in Supplementary Table 1 together with MYC, CCND1, SMRT and GAPDH primers. The assays were performed as described 28.
ChIP assays
After treatment, the cells were fixed and processed using the HighCell# ChIP kit (Diagenode, Denville, NJ) as previously described 29. The oligo sequences used in this study are presented in Supplementary Table 1.
siRNA SMRT or PRB experiments
T47D cells were transfected with siRNA SMRT (SC-36514; Santa Cruz Biotech) or siRNA Control (SC-37007; Santa Cruz Biotech) using Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer’s protocol. At 48 h post-transfection, the cells were incubated for 20 min (qPCR), 60 min (IF), or 48 h (3H-thymidine uptake) with MPA or MFP. Similar transfections were performed with siRNA PRB (Supplementary Table 1; Life Technologies). The cells were counted after 5 days of treatment.
Statistical analyses
ANOVA and Tukey multiple post t tests were used to evaluate the differences of means across multiple samples. The slopes of the tumor growth curves were compared using an ANOVA followed by parallelism analysis. In vivo experiments were performed at least twice and biochemical and IHC assays three times.
RESULTS
The effect of different antiprogestins on the growth of mammary carcinomas with different PRA/PRB ratios
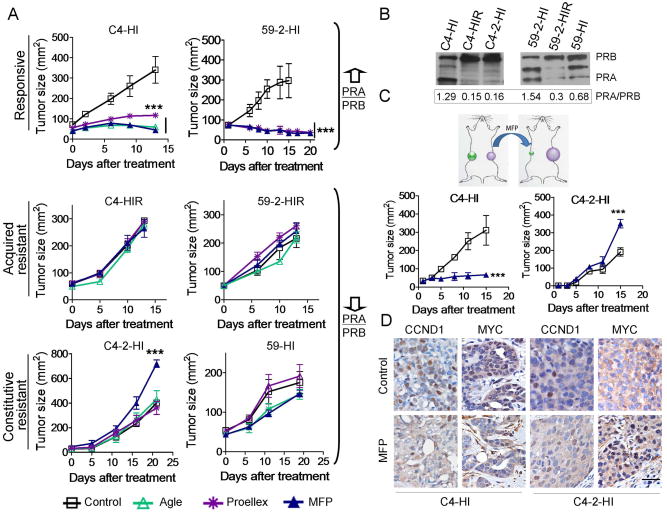
We have previously shown that MFP inhibits the growth of PRA-overexpressing mammary carcinomas using an MPA-induced breast cancer model 15,32. Resistance was characterized relative to the MFP effect. Now, we have extended that study to evaluate the effect of other antiprogestins such as Agle and Proellex in addition to MFP, using tumors with different PRA/PRB ratio and different MFP responsiveness including MFP responsive, acquired resistant or constitutive resistant variants 17 (Figure 1A). A representative WB illustrating the differential expression of PR isoforms in responsive or resistant tumors is shown in Figure 1B. The results demonstrated that the tested antiprogestins inhibited MFP-responsive tumor growth (Figure 1A). None of the compounds inhibited the growth of MFP-resistant tumors, and only MFP stimulated the growth of the constitutively resistant C4-2-HI tumor. C4-2-HI and C4-HI tumors simultaneously transplanted into the left and right flanks, respectively, of the same mouse retained their differential responses to MFP (Figure 1C), supporting the selective therapeutic effect of antiprogestins. We next studied the expression of two PR-regulated proteins involved in cell cycle turnover, CCND1 33 and MYC 34. In C4-HI tumors, MFP induced a 3.7 fold decrease in nuclear CCND1 staining (control: 29.7 ± 2.5 % and MFP: 8.0 ± 1%; p<0.01) and a 9.5 fold decrease in MYC staining (control: 19.4 ± 1.9 % and MFP: 2.0 ± 0.4 %; p<0.001), which showed cytosolic localization. A 2.4 fold increase in nuclear MYC staining (control: 10.4 ± 0.7 % and MFP: 25.2 ± 2.3%; p<0.05) was observed in C4-2-HI tumors treated with MFP (Figure 1D). The characteristics of C4-HI tumors regressing under MFP treatment have been already described 17. No changes in morphology were observed in C4-2-HI tumors.
Figure 1. Antiprogestins selectively inhibit the growth of mammary carcinomas with high PRA/PRB ratios.
A, Growth curves of mammary carcinomas from the MPA-induced breast cancer model classified according to their MFP responsiveness as responsive, acquired resistant or constitutively resistant tumors. Agle and Proellex inhibited the growth of tumors that expressed higher levels of PRA than PRB. No inhibitory effects were observed in the MFP-resistant variants with higher levels of PRB than PRA. Only MFP stimulated the growth of the C4-2-HI tumors. Drugs were administered sc in doses of 10 mg/kg/day. In the case of 59-2-HI tumors as treatment began when animals had a size near 100 mm2, MFP or Proellex were administered within silastic pellets (6 mg). A representative curve of at least other two is shown (n=5/group). B, A representative WB showing the different PR ratios observed in the tumors shown in (A). C, Ten mice were implanted simultaneously with C4-HI and C4-2-HI tumors in opposite flanks. When C4-2-HI tumors reached approximately 25 mm2, MFP pellets (6 mg) or control silastic pellets were sc implanted into the mice. MFP inhibited C4-HI and stimulated C4-2-HI tumor growth in the same mouse. The arrows indicate treatment initiation. D, CCND1 and MYC expression in tumors from the experiment shown in C, treated for 72 h with MFP. Nuclear MYC and CCND1 staining decreased in C4-HI tumors, while an increase in MYC nuclear staining was observed in C4-2-HI tumors. Hematoxylin was used for nuclear staining. **, p<0.01 and ***, p<0.001 experimental vs. control group. Scale bar = 50 μm.
Antiprogestin responsiveness and PRA expression are recovered using an HDAC inhibitor and a demethylating agent
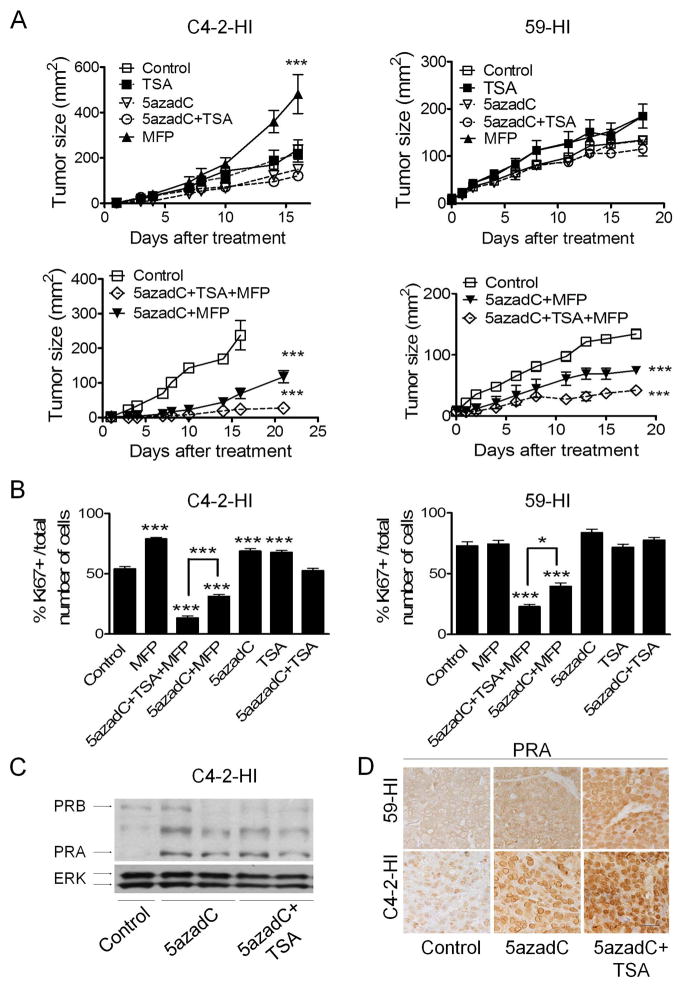
In constitutive MFP-resistant tumors, we have previously demonstrated that treatment with the demethylating agent 5azadC in vivo induced PRA re-expression, partially restoring tumor responsiveness to MFP 32. In this study, we further established that the addition of an HDAC inhibitor, TSA, improved the inhibitory effect of MFP when combined with 5azadC in two different constitutively resistant tumors (Figure 2A). When co-treated with TSA and 5azadC, the tumors treated with Proellex or Agle responded similar to MFP (Supplementary Figure 1). A decrease in cell proliferation was observed in all growth-inhibited tumors (Figure 2B and Supplementary Figure 2) and as expected, 5azadC and TSA treatment increased the nuclear PRA expression (Figures 2C and 2D) without increasing the levels of PRB (WB, Figure 2C and IHC data using the Ab-6 PRB antibody, data not shown).
Figure 2. DNMT and HDAC inhibitors induce PRA re-expression and restore MFP responsiveness in constitutively MFP-resistant tumors.
A, Growth curves. Animals (n=5/group) with palpable C4-2-HI and 59-HI tumors were treated with vehicle, MFP, 5azadC or TSA, each individually and in all possible combinations. The tumor sizes of one of three representative experiments are shown. The maximal therapeutic effect was obtained with the 5azadC+TSA+MFP combination treatment. Representative images are shown in Supplementary Figure 2A. B, Quantification of Ki67 expression; representative IHC images are shown in Supplementary Figure 2B. The percentage of Ki67 positive cells in relation to the total number of cells is plotted. Tumors treated with 5azadC+TSA+MFP had the lowest Ki67 values. C, Western blots of C4-2-HI tumor extracts confirmed PRA re-expression after 5azadC and TSA treatment. ERK served as a loading control. D, IHC studies using the C-19 antibody showed an increase in PRA nuclear expression in tumors treated with 5azadC and TSA. This antibody stains mainly PRA of mouse tissues in IHC studies (cited in 17; p<0.05). *, p<0.05 and ***, p<0.001 experimental vs. control group. Scale bar = 50 μm.
The increase in PRA levels in a human breast cancer cell line drives antiprogestin responsiveness
Next, we utilized a human xenograft model that expressed higher levels of PRB than PRA 25. PRA, PRB or empty vectors were transfected to these cells to modify the PRA/PRB ratio. The growth rate of the IBH-6-PRA tumors was inhibited by MFP (Supplementary Figure 3A) and Proellex (data not shown) treatment; however, MFP stimulated the growth of the control IBH-6-pSG5 and the IBH-6-PRB tumors. IBH-6-PRA/B xenografts maintained the expression of the PR isoforms (Supplementary Figure 3B). The exogenous addition of PRB did not improve the response that was obtained with the endogenous higher levels of PRB than PRA observed in control animals. MFP decreased CCND1 (3.4 fold) and MYC (2 fold) expression only in IBH-6-PRA tumors (Supplementary Figures 3C and D). A clone with high PRA expression levels was selected and further tested in vivo. Tumor growth was suppressed for more than two months in MFP-treated mice (Supplementary Figure 3E), and a decrease in CCND1 (2 fold) and MYC (6.7 fold) expression was observed in nuclear extracts from MFP-treated tumors (Supplementary Figure 3F).
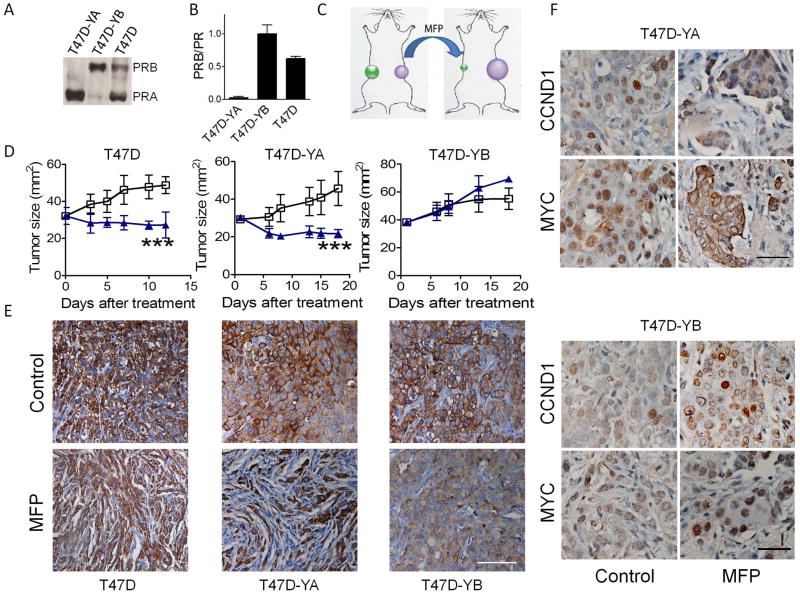
A chimeric xenograft assay using T47D cells confirms that PRA determines responsiveness to antiprogestins
We next used T47D-YA (expressing only PRA), T47D-YB (expressing only PRB) and T47D cells, which expressed high levels of both PR isoforms. The levels of PR isoform expression were confirmed using Western blots (Figure 3A) or qPCR assays (Figure 3B). T47D–YA and T47D–YB cells were inoculated into the right and left flanks, respectively, of the same E2-treated immunocompromised mice (Figure 3C). Interestingly, T47D and T47D-YA were growth inhibited after MFP treatment, but T47D-YB tumors continued growing (Figure 3D). Fewer epithelial cells (cytokeratin positive) were observed in the MFP-treated T47D or T47D-YA xenografts (Figure 3E). In contrast, no significant change in tumor morphology was observed after MFP treatment in T47D-YB tumors (Figure 3E). MFP induced a 2.4 fold decrease in nuclear CCND1 staining (control: 33.5 ± 1.3 % and MFP: 13.6 ± 3.3 %; p<0.01) and a 3 fold decrease in nuclear MYC staining (control: 46.9 ± 4.6 % and MFP 15.5 ± 1.3 %; p<0.001) in T47D-YA tumors but induced a 2.7 fold increase MYC staining (control: 3.7 ± 0.7 % and MFP: 10.1 ± 1.4 %; p<0.01) in T47D-YB tumors (Figure 3F).
Figure 3. Antiprogestins inhibit the growth of human T47D xenografts expressing high levels of PRA.
A, Western blots for PRA or PRB in T47D, T47D-YA and T47D-YB cell lysates. B, Relation between PRB/total PR mRNA determined by qPCR. In T47D-YB cells the ratio between PRB and total PRB is considered as 1. C, Schematic of the experimental protocol for the T47D-YA and –YB experiments. D, Growth curves. T47D cells were inoculated into NOD/LtSz-scid/IL-2Rgamma null female mice (n=6/group) bearing a 0.5 mg E2 pellet. T47D-YA and –YB cells were simultaneously inoculated into the right and left flanks, respectively, of the same mouse. When the tumors reached 30-40 mm2, MFP (10 mg/kg/day) or vehicle treatments were initiated. Only T47D and T47D-YA tumors regressed after 17 days of treatment. E, CK staining illustrating the remodeling induced by MFP in T47D and T47D-YA xenografts; scale bar = 100 μm. F, Seventy-two hours after treatment initiation, 2 mice in each group were euthanized, and the tumors were processed for CCND1 and MYC immunostaining. T47D-YA tumors showed nuclear CCND1 and MYC expression, whereas a decrease in nuclear staining was observed in MFP-treated tumors. No decrease in nuclear CCND1 and MYC staining was observed in MFP-treated T47D-YB tumors. Hematoxylin was used for nuclear staining. *, p<0.05; **, p<0.01; ***, p<0.001 experimental vs. control group. Scale bar = 50 μm.
PR colocalizes with the coactivator AIB1 or the corepressor SMRT
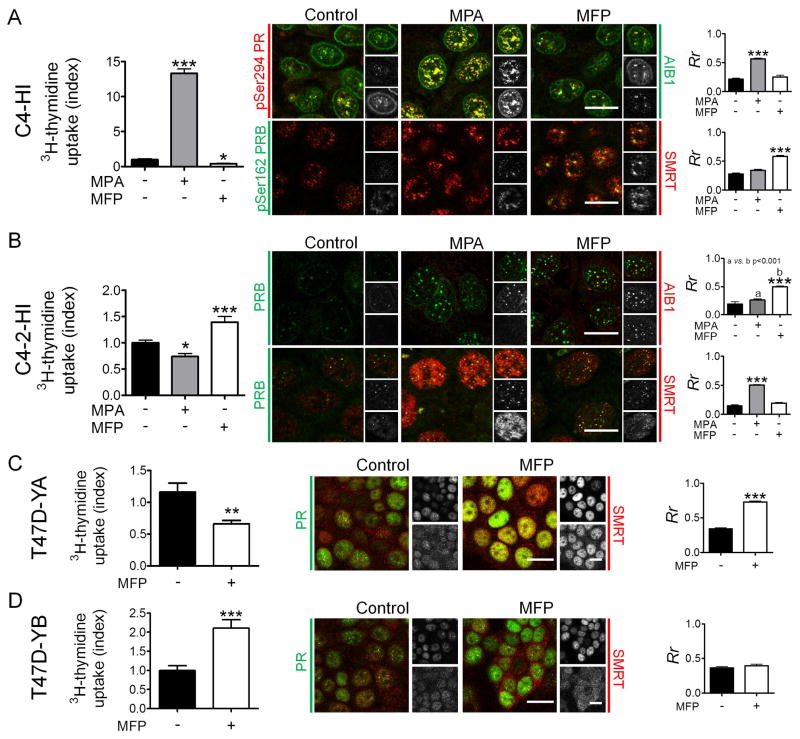
To better understand the mechanisms associated with the PR-mediated effects on tumor growth, we focused on the PR/AIB1 and PR/SMRT interactions. We first used C4-HI primary cultures, which proliferate in response to MPA and are inhibited by MFP (Figure 4A, left), and C4-2-HI cells, which have the opposite response (Figure 4B, left), and the opposite PR isoform ratio (Figure 1B).
Figure 4. Co-localization of PR with SMRT or AIB1 and the proliferative effects of MPA or MFP.
A, Left: MPA stimulates and MFP inhibits 3H-thymidine uptake in C4-HI cells. Middle: C4-HI cells were incubated for 30 min with MPA (10 nM) or MFP (10 nM), and confocal immunofluorescence images were captured using a Nikon Eclipse E800 Laser Confocal Microscope. Right: The nuclear co-localization of the proteins was estimated using a Pearson’s correlation coefficient. IF studies were performed with the available combinations of mouse monoclonal and rabbit antibodies (Vector Laboratories, Burlingame, CA), and the label color refers to the particular secondary antibody (Texas red, red; FITC, green). Black and white images at the right of each picture represent the separate green and red channels for each merged image. At the top, the merged image, at the middle the pPR/PR stains and at the bottom AIB1/SMRT stains. MPA increased the co-localization of pPR and AIB1, and MFP increased the co-localization with SMRT. Similar studies were performed in C4-2-HI (B) T47D-YA (C) and T47D-YB (D). Increased PR/SMRT colocalization was observed in MPA-treated C4-2-HI cells, which are growth inhibited by MPA, and in MFP- treated T47D/YA cells, which are both growth inhibited by MFP. An increase in PR/AIB1 co-localization was observed in MFP-treated C4-2-HI cells which are stimulated to proliferate with MFP.*, p<0.05; **p<0.01; ***, p<0.001 experimental vs. control group. Scale bar = 15 μm.
In C4-HI cells, pSer294PR/AIB1 nuclear co-localization increased after MPA treatment (Figure 4A, right), and pSer162PRB co-localization with SMRT increased in MFP-treated cells (Figure 4A, right), which also showed an increase in SMRT expression (p<0.05).
In C4-2-HI cells, PRB/AIB1 nuclear co-localization was observed in MFP-treated cells and PRB/SMRT nuclear co-localization only after MPA treatment (Figure 4B, right). In these cells, MPA increased SMRT expression (p<0.001). These opposite results between C4-HI and C4-2-HI cells are in accordance to their different MPA/MFP proliferative responsiveness. T47D-YA and -YB were also used to evaluate the effects of MFP on PR/SMRT interactions. In 3H-thymidine uptake assays, MFP inhibited T47D–YA cells but stimulated –YB cells. As shown in Figures 4C and 4D, MFP increased nuclear SMRT expression and PR/SMRT co-localization in T47D-YA cells and not in the T47D-YB cells.
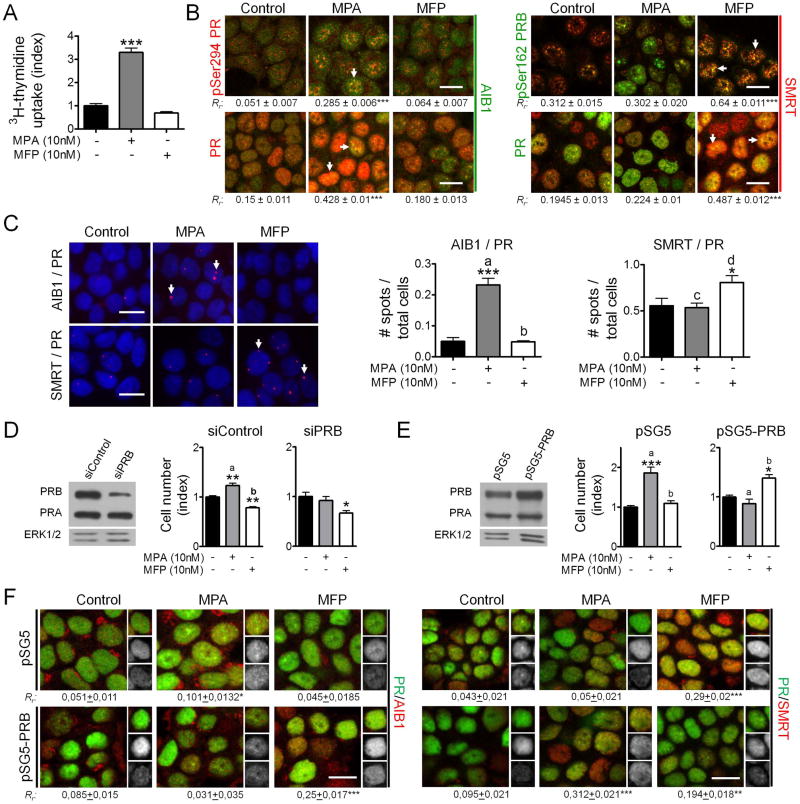
In T47D cells, MPA increased and MFP decreased 3H-thymidine uptake (Figure 5A), which correlated with previous cell counting experiments 28. While MPA increased the co-localization of PR and pSer294PR with AIB1, MFP increased the co-localization of PR and pSer162PRB with SMRT (Figure 5B). Physical interactions between proteins were further confirmed through Duolink assays (Figure 5C). The advantage of this technique is that identifies individual interactions between two proteins in their native form and in their natural compartment 35.
Figure 5. Changing the PR isoform ratio in T47D cells reverts the proliferative responses to MPA/MFP and the PR and AIB1/SMRT co-localization pattern.
A, Left: MPA (10 nM) stimulates and MFP (10 nM) inhibits 3H-thymidine uptake in T47D cells. B, Cells were incubated for 30 min with MPA or MFP, and confocal immunofluorescence images of the pPR or PR and AIB1 or SMRT staining were captured using a Nikon Eclipse E800 Laser Confocal Microscope. The nuclear co-localization of the proteins was estimated using a Pearson’s correlation coefficient. IF studies were performed with available combinations of mouse monoclonal and rabbit antibodies (Vector Laboratories), and the label color refers to the particular secondary antibody (Texas red, red; FITC, green). MPA increased the co-localization of PR or pPR and AIB1, and MFP increased the co-localization with SMRT. C, Duolink assays were performed in cells incubated as described above. Pink spots represent interactions between both proteins. Increased AIB1/PR interactions were observed in MPA-treated cells, and increased PR/SMRT interactions in MFP-treated cells. D, Left: Western blot showing PR expression in T47D cells transfected with siRNA scrambled (siControl) or siRNA PRB (siPRB); Right: Cell counting after 5 days of incubation with MPA or MFP in the presence of 2% steroid stripped FCS. MPA did not increase cell proliferation of siPRB-transfected cells while MFP exerted a similar inhibitory effect in both cases. E, Left: Western blot showing PR expression in empty vector (pSG5) or in PRB (pSG5-PRB)-transfected T47D cells; Right: Cell counting experiments of the cells in E left. MPA did not increase cell proliferation whereas MFP increased the number of PRB-transfected cells after 5 days of incubation. F, PRB-transfected or control cells were incubated with MPA or MFP for 15 min and the co-localization of PR with AIB1 or SMRT was evaluated as explained in B. A change in the pattern of co-localization was observed in PRB transfected cells. *, p<0.05; **, p<0.01; ***, p<0.001 experimental vs. control group; a vs. b, p<0.001 and c vs. d, p<0.05. Scale bar = 15 μm.
In summary, in all cases in which MFP inhibited cell proliferation, SMRT co-localized with PR (C4-HI, T47D-YA and T47D cells). In C4-2-HI cells it was MPA that induced inhibition of cell proliferation and PRB/SMRT co-localization.
PRB overexpression in T47D cells reverts the effects of MPA and MFP on proliferation and interferes with the interaction between PR and AIB1/SMRT
To further test the hypothesis that the PRA/PRB ratio drives MPA/MFP responsiveness, we overexpressed PRB, or we decreased PRB expression using specific siRNA. PRA-downregulation experiments are not feasible since the entire PRA gene sequence is included in the PRB gene.
Blunting PRB expression in T47D cells, thus increasing the PRA/PRB ratio, did not induce any significant change in the MFP responsiveness (Figure 5D), since the proliferation of these cells is already inhibited by MFP. However, a change in MFP proliferative responsiveness was observed when PRB overexpression decreased the PRA/PRB ratio (Figure 5E). This was accompanied by a change in the pattern of co-localization of PR and AIB1/SMRT: in MFP-treated cells, PR co-localized with AIB1 instead of SMRT (Figure 5F). Interestingly MPA did not stimulate cells in which PRB was silenced nor in cells with higher levels of PRB than PRA.
These experiments prove that it is the ratio of both isoforms rather than the total amount of PRA what drives antiprogestin responsiveness.
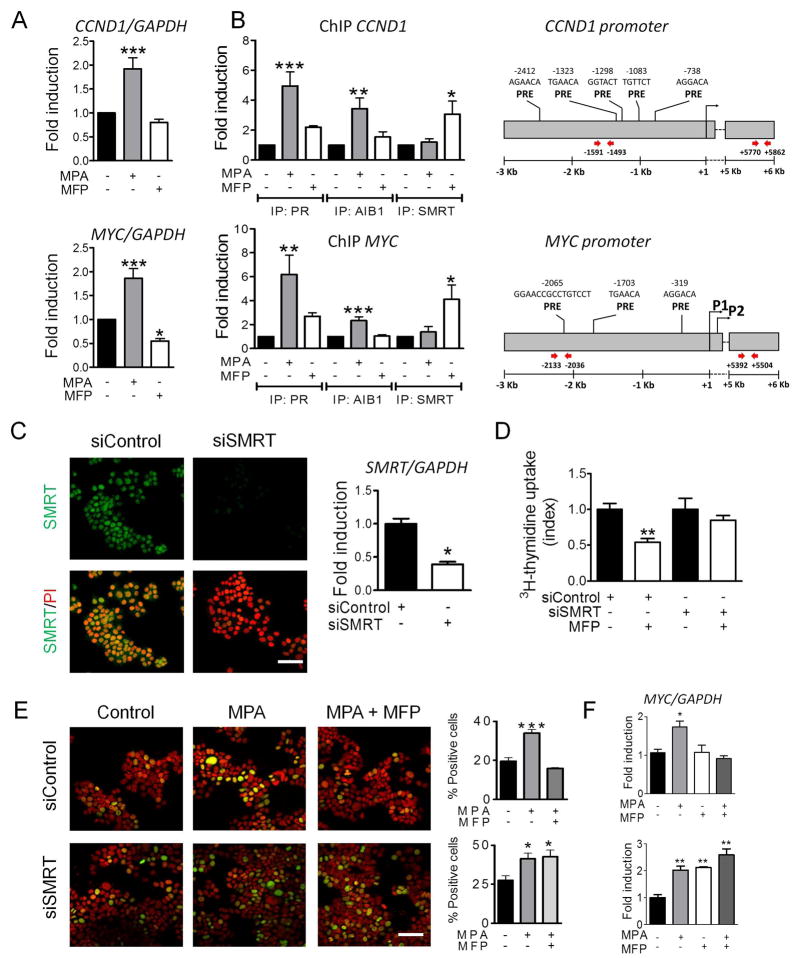
PR interacts with AIB1/SMRT at the CCND1 and MYC promoters
Subsequently, we examined the interactions of PR/AIB1 and PR/SMRT on the CCND1 and MYC promoters after incubating T47D cells with MPA or MFP respectively. We selected the promoter sites (Figure 6B) that we have already shown an increase in PR binding after MPA treatment (Supplementary Table 1) 29. Only MPA increased CCND1 and MYC mRNA expression levels (Figure 6A) and the recruitment of AIB1 and PR to the CCND1 and MYC promoters. MFP induced the recruitment of SMRT and PR to the same promoter sites (Figure 6B). No increase in PR, SMRT or AIB1 binding was observed when control primers of a region without PRE sites of both promoters were used (5/6 kb region; Figure 6B right and Supplementary Figure 4A). To corroborate the participation of both PR isoforms, T47D-YA or T47D-YB were similarly treated with MFP. Both PR isoforms were recruited by MFP at the CCND1 and MYC promoters, however only SMRT was recruited on T47D-YA cells and only the coactivator AIB1 on T47D-YB cells (Supplementary Figure 4B and 4C).
Figure 6. MPA and MFP activate PR, which interacts with AIB1 or SMRT at the CCND1 and MYC promoters to increase or repress gene transcription, respectively.
T47D cells treated with 10 nM MPA or MFP (30 min) were subjected to qPCR (A) and ChIP (B) assays. The presence of AIB1 or SMRT at the CCND1 and MYC promoters was evaluated in a region known to be bound by PR (right; Supplementary Table 1) 29. MPA increased the recruitment of AIB1 and PR at the MYC and CCND1 promoters, whereas MFP induced SMRT recruitment. No recruitment of PR, AIB1 or SMRT was observed using control primers (Supplementary Figure 4A). C, siRNA SMRT (mix of three siRNAs) or control scrambled siRNA were transfected into T47D cells. After 48 h, SMRT expression was evaluated by IF (left; FITC) or qPCR (right). Propidium iodide (PI; red) was used for nuclear counterstaining. Scale bar = 50 μm. D, 3H-thymidine uptake assay. Cells transfected with SMRT or control siRNAs were incubated with MFP for 48 h and 3H-thymidine for the last 18 h. The proliferation index was calculated as the cpm observed in MFP-treated cells as compared with their respective control groups. E, IF assays. MYC expression in siRNA SMRT-transfected or control cells incubated with MPA (10 nM) or MPA+MFP (10 nM) for 60 min. The percentage of MYC+ (FITC) cells in relation to the total number of cells was quantitated (PI stained cells). Scale bar = 50 μm. F, qPCR. MYC mRNA expression in siRNA SMRT-transfected or control cells incubated with MPA (10 nM) and/or MFP (10 nM) for 20 min. MFP was unable to decrease cell proliferation or MYC expression in siRNA SMRT-transfected cells. *, p<0.05; **, p<0.01; ***, p<0.001 experimental vs. control group.
SMRT knockdown reverses the MFP-induced inhibitory effect observed on cell proliferation and MYC expression
SMRT expression was reduced in T47D cells, transfected with a siRNA SMRT, compared with control cells (IF and qPCR; Figure 6C). MFP was unable to decrease 3H-thymidine uptake in siRNA SMRT-transfected cells (Figure 6D). Moreover, siRNA SMRT rescued the decreased MYC expression induced by MFP on MPA-treated cells as determined by IF (Figure 6E) and qPCR (Figure 6F) assays.
Collectively, these results indicate that in the presence of MFP, under conditions in which PRA expression is comparatively similar or higher than PRB, the interaction between PR and SMRT is favored, which results in CCND1 and MYC downregulation. (Supplementary Figure 5). In a PRB prevailing context, MFP is unable to recruit SMRT, and the stimulatory effect of MFP dominates.
DISCUSSION
In this study, we present evidence that the breast cancer cell response to antiprogestin treatment is highly dependent on the PR isoform ratio. The absolute levels of PR isoform in reproductive tissues vary as a consequence of developmental and hormonal status and their differential expression may be critical for the appropriate cellular responses to progesterone. It has been shown in PRA or PRB knockout mouse models that the PR isoforms have different roles in vivo. PRB mediates the proliferative effects of progesterone in the mammary gland, whereas PRA is more important in maintaining ovarian and uterine functions 7,36. In breast cancer cells, although some genes are regulated by progesterone through both PR isoforms, most genes are uniquely regulated through one or the other isoform, predominantly through PRB 37,38.
Although the mechanisms driving PR-mediated tumor growth are not clearly understood, there is a general consensus that upon progesterone exposure, PRB exerts a transcriptional effect stronger than that of PRA, whereas PRA exerts a repressive effect on numerous hormone receptors/transcription factors 4,39. The dual action of MFP, which can act as a PR agonist on PRB in a cAMP/PKA-driven context, have previously been described 18,40. More recently, using a new PRA/PRB bi-inducible breast cancer cell line, MFP was shown to exhibit stronger antiproliferative effects in PRA- compared with PRB-overexpressing MDA-MB-231 cells growing in vitro 41. However, no other studies have addressed the differential effects of antiprogestins on mammary carcinomas with different PR isoform ratios, except for those previously described by our group 16,17. Only in a few studies have the PR isoforms been evaluated in breast cancer samples 42–45, and increased PRA/PRB ratios were reported in those with the worse prognosis 44,45. We propose that MFP may be a therapeutic option for this group of patients.
In mice, the PRA promoter contains a higher density of CpG islands than the PRB promoter, qualifying for mechanisms of gene silencing by promoter methylation. We have previously shown that MFP-unresponsive mammary carcinomas exhibited high PRB/PRA ratios and that the re-expression of PRA by a demethylating agent partially rescued antiprogestin responsiveness 32. Here, we utilized a combination of DNMT and HDAC inhibitors to further improve PRA re-expression and, consequently, the response to MFP. Thus, it is possible that this approach may be beneficial for patients with triple negative tumors, in which a combination of DNMT and HDAC inhibitors could reprogram cells to express hormone receptors and sensitize the tumors to endocrine therapy. In fact, ERα can be re-expressed in MDA-MB-231 cells after treatment with combinations of chromatin-acting agents 46 or through other epigenetic manipulations 47, thereby sensitizing the cells to endocrine treatment.
To extend our previous findings, we used human cell lines engineered to express different PR isoform ratios. MFP selectively inhibited tumors with high PRA expression and did not affect or stimulate tumors that overexpressed PRB. Moreover, even T47D cells grown in the presence of estrogens 48 were inhibited by MFP, suggesting that MFP-activated PRA may also inhibit estrogen-mediated tumor growth.
There is evidence that MFP may exert antitumor effects through mechanisms unrelated to PR such as via direct cytotoxicity 49, or mechanisms related to the immune microenvironment 50. It is clear from the chimeric in vivo results presented here using immunocompetent (C4-HI vs. C4-2-HI) and immunocompromised mice (T47D-YA vs. T47D-YB) that the inhibitory effects of antiprogestins were observed only in tumors showing high levels of PRA, thus ruling out a significant role for systemic- or immune-mediated tumor involution induced by MFP. Inhibitory effects of antiprogestins have been reported by El Etreby et al. in MCF-7 xenografts, and a similar inhibition was observed with MFP, Onapristone (ZK98299) and Tamoxifen 51. Liang et al. 52 reported that MFP abolishes the stimulatory effect of MPA on T47D and BT474 xenografts. However, the PR isoform ratio was not evaluated in those two studies.
Previously, we have shown that Lonaprisan (ZK230211) and Onapristone have growth inhibitory effects similar to MFP 17,53. Here, we extended these studies by testing the effects of the new antiprogestins Proellex and Aglepristone to show that they were also inhibitory in MFP-responsive tumors. In addition, these compounds were unable to inhibit MFP-resistant tumors, and none of them stimulated tumor growth.
Although the mechanisms driving these selective effects are unknown, conformational changes favoring the recruitment of SMRT to a repressive complex in a PRA context have been proposed 9. Using in vitro reporter genes, it has also been shown that the SMRT expression level modulates the antagonistic activity of MFP 54. Interestingly, unlike MFP, MPA induced the association of SMRT with PR in C4-2-HI and in T47D cells transfected with PRB that express a high PRB/PRA ratio, which resulted in no increase or even a decrease in cell proliferation. Regardless of the ligand involved, an increase in the SMRT/PR interaction was associated with tumor growth inhibition. Although there are data suggesting that SMRT and AIB1 may cooperate to induce PR and CCND1 transcription 55, in this experimental context, the opposing interactions prevailed. It is interesting to highlight the fact that the use of a PRB-specific pSer162 antibody confirms that pPRB is also present and interacting with SMRT in PRA-overexpressing cells or in T47D cells treated with MFP. The opposing effects that MPA and MFP exerted on C4-HI and C4-2-HI cell proliferation, suggests that the PR isoform ratio may be also involved in progestin responsiveness. Although in this study we focused in antiprogestin responsiveness there is no doubt that the role of PR isoform ratio on progestin responsiveness deserves further consideration.
In summary, we have demonstrated that PRA overexpression dictates antiprogestin responsiveness in different in vivo breast cancer models. Antiprogestins induced tumor regression by increasing the association between activated PR and SMRT and by preventing an association with AIB1 at the MYC and CCND1 promoters. Conversely, in tumors with low PRA/PRB ratios, MFP induced the association of PR with the coactivator AIB1, while MPA favored the interaction of PR with SMRT, resulting in stimulatory or inhibitory effects, respectively. We propose that breast cancer patients with PR+ carcinomas expressing high PRA/PRB ratios may benefit from treatment with a combination of antiprogestins and the standard endocrine treatment.
Supplementary Material
Novelty and impact statement.
In this study we provide a mechanistic evidence to support that the responsiveness of breast cancer to antiprogestin treatment is determined by the ratio of expression of PRA and PRB isoforms. Our results suggest a personalized use of antiprogestins for breast cancer treatment.
Acknowledgments
Financial Support
This work was supported by ANAPCYT (PICT2007/932 and 939 and PICT2012/1091 and 1244), CONICET (PIP2010/692) and Fundación Sales. Dr. Molinolo is supported by the Intramural Research Program, NIDCR, NIH. VW, MR, GS, MM and MLP are fellows of CONICET; MAG is a technician at CONICET and SG, PR, VN and CL are members of the Research Career, CONICET.
We are very grateful to Dr. D. Edwards (BCM, Houston) for the pPR antibodies, to Dr. K. Horwitz for kindly sharing the PRA and PRB plasmids and the T47D-YA and T47D-YB cells and to Dr. I. Luthy (IBYME) for providing the IBH-6 cells. Repros Pharmaceutical supplied the CDB compounds, and Laboratorios Craveri provided Medrosterona. We thank Mr. Bruno Luna for excellent technical assistance.
References
- 1.Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 3.Aupperlee MD, Smith KT, Kariagina A, et al. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;146(8):3577–88. doi: 10.1210/en.2005-0346. [DOI] [PubMed] [Google Scholar]
- 4.Lange CA, Sartorius CA, Abdel-Hafiz H, et al. Progesterone receptor action: translating studies in breast cancer models to clinical insights. Adv Exp Med Biol. 2008;630:94–111. [PubMed] [Google Scholar]
- 5.Lanari C, Lamb CA, Fabris VT, et al. The MPA mouse breast cancer model: evidence for a role of progesterone receptors in breast cancer. Endocr Relat Cancer. 2009;16(2):333–50. doi: 10.1677/ERC-08-0244. [DOI] [PubMed] [Google Scholar]
- 6.Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313. [PubMed] [Google Scholar]
- 7.Mulac-Jericevic B, Lydon JP, DeMayo FJ, et al. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100(17):9744–9. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarpin KM, Graham JD, Mote PA, et al. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal. 2009;7:e009. doi: 10.1621/nrs.07009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giangrande PH, Kimbrel EA, Edwards DP, et al. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20(9):3102–15. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Arzayus MI, Font dM, Yuan J, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6(3):263–74. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Osborne CK, Bardou V, Hopp TA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95(5):353–61. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 12.O’Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69(21):8217–22. doi: 10.1158/0008-5472.CAN-09-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarado CV, Micenmacher S, Ruiz GM, et al. RAC3 overexpression is a transforming and proliferative signal that contributes to tumoral development. Medicina (B Aires) 2011;71(1):33–8. [PubMed] [Google Scholar]
- 14.Madauss KP, Grygielko ET, Deng SJ, et al. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol. 2007;21(5):1066–81. doi: 10.1210/me.2006-0524. [DOI] [PubMed] [Google Scholar]
- 15.Lanari C, Wargon V, Rojas P, et al. Antiprogestins in breast cancer treatment: are we ready? Endocr Relat Cancer. 2012;19(3):R35–R50. doi: 10.1530/ERC-11-0378. [DOI] [PubMed] [Google Scholar]
- 16.Helguero LA, Viegas M, Asaithamby A, et al. Progesterone receptor expression in medroxyprogesterone acetate-induced murine mammary carcinomas and response to endocrine treatment. Breast Cancer Res Treat. 2003;79(3):379–90. doi: 10.1023/a:1024029826248. [DOI] [PubMed] [Google Scholar]
- 17.Wargon V, Helguero LA, Bolado J, et al. Reversal of antiprogestin resistance and progesterone receptor isoform ratio in acquired resistant mammary carcinomas. Breast Cancer Res Treat. 2009;116(3):449–60. doi: 10.1007/s10549-008-0150-y. [DOI] [PubMed] [Google Scholar]
- 18.Beck CA, Weigel NL, Moyer ML, et al. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci U S A. 1993;90(10):4441–5. doi: 10.1073/pnas.90.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaillard RC, Riondel A, Muller AF, et al. RU 486: a steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci U S A. 1984;81(12):3879–82. doi: 10.1073/pnas.81.12.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polisca A, Scotti L, Orlandi R, et al. Aglepristone (RU534) administration to non-pregnant bitches in the mid-luteal phase induces early luteal regression. Theriogenology. 2010;74(4):672–81. doi: 10.1016/j.theriogenology.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Attardi BJ, Burgenson J, Hild SA, et al. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol Cell Endocrinol. 2002;188(1–2):111–23. doi: 10.1016/s0303-7207(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 22.Wiehle R, Lantvit D, Yamada T, et al. CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer Prev Res (Phila) 2011;4(3):414–24. doi: 10.1158/1940-6207.CAPR-10-0244. [DOI] [PubMed] [Google Scholar]
- 23.Sahores A, Luque GM, Wargon V, et al. Novel, low cost, highly effective, handmade steroid pellets for experimental studies. PLoS ONE. 2013;8(5):e64049. doi: 10.1371/journal.pone.0064049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez SM, Mladovan A, Garbovesky C, et al. Three novel hormone-responsive cell lines derived from primary human breast carcinomas: functional characterization. J Cell Physiol. 2004;199(3):460–9. doi: 10.1002/jcp.10466. [DOI] [PubMed] [Google Scholar]
- 25.Bruzzone A, Vanzulli SI, Soldati R, et al. Novel human breast cancer cell lines IBH-4, IBH-6, and IBH-7 growing in nude mice. J Cell Physiol. 2009;219(2):477–84. doi: 10.1002/jcp.21694. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs S, Jitrapakdee S, Wallace JC. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem Biophys Res Commun. 1998;252(2):368–72. doi: 10.1006/bbrc.1998.9646. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen BM, Richer JK, Schittone SA, et al. New Human Breast Cancer Cells to Study Progesterone Receptor Isoform Ratio Effects and Ligand-independent Gene Regulation. J Biol Chem. 2002;277(31):27793–800. doi: 10.1074/jbc.M202584200. [DOI] [PubMed] [Google Scholar]
- 28.Cerliani JP, Guillardoy T, Giulianelli S, et al. Interaction between FGFR-2, STAT5, and Progesterone Receptors in Breast Cancer. Cancer Res. 2011;71(10):3720–31. doi: 10.1158/0008-5472.CAN-10-3074. [DOI] [PubMed] [Google Scholar]
- 29.Giulianelli S, Vaque JP, Soldati R, et al. Estrogen Receptor Alpha Mediates Progestin-Induced Mammary Tumor Growth by Interacting with Progesterone Receptors at the Cyclin D1/MYC Promoters. Cancer Res. 2012;72(9):2416–27. doi: 10.1158/0008-5472.CAN-11-3290. [DOI] [PubMed] [Google Scholar]
- 30.Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 31.Das K, Leong DT, Gupta A, et al. Positive association between nuclear Runx2 and oestrogen-progesterone receptor gene expression characterises a biological subtype of breast cancer. Eur J Cancer. 2009;45(13):2239–48. doi: 10.1016/j.ejca.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wargon V, Fernandez SV, Goin M, et al. Hypermethylation of the progesterone receptor A in constitutive antiprogestin-resistant mouse mammary carcinomas. Breast Cancer Res Treat. 2011;126(2):319–32. doi: 10.1007/s10549-010-0908-x. [DOI] [PubMed] [Google Scholar]
- 33.Musgrove EA, Sutherland RL. Effects of the progestin antagonist RU 486 on T-47D breast cancer cell cycle kinetics and cell cycle regulatory genes. Biochem Biophys Res Commun. 1993;195(3):1184–90. doi: 10.1006/bbrc.1993.2169. [DOI] [PubMed] [Google Scholar]
- 34.Musgrove EA, Lee CS, Sutherland RL. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol Cell Biol. 1991;11(10):5032–43. doi: 10.1128/mcb.11.10.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson I, Bahram F, Li X, Gualandi L, Koch S, Jarvius M, Söderberg O, Anisimov A, Kholavá I, Pytowski B, Baldwin M, Ylä-Herttuala S, et al. VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 2010;29:1377–88. doi: 10.1038/emboj.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conneely OM, Jericevic BM, Lydon JP. Progesterone receptors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8(2):205–14. doi: 10.1023/a:1025952924864. [DOI] [PubMed] [Google Scholar]
- 37.Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278(41):39261–4. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- 38.Richer JK, Jacobsen BM, Manning NG, et al. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–18. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 39.McGowan EM, Russell AJ, Boonyaratanakornkit V, et al. Progestins reinitiate cell cycle progression in antiestrogen-arrested breast cancer cells through the B-isoform of progesterone receptor. Cancer Res. 2007;67(18):8942–51. doi: 10.1158/0008-5472.CAN-07-1255. [DOI] [PubMed] [Google Scholar]
- 40.Sartorius CA, Tung L, Takimoto GS, et al. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993;268(13):9262–6. [PubMed] [Google Scholar]
- 41.Khan JA, Bellance C, Guiochon-Mantel A, et al. Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line. PLoS ONE. 2012;7(9):e45993. doi: 10.1371/journal.pone.0045993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham JD, Yeates C, Balleine RL, et al. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995;55(21):5063–8. [PubMed] [Google Scholar]
- 43.Mote PA, Graham JD, Clarke CL. Progesterone receptor isoforms in normal and malignant breast. Ernst Schering Found Symp Proc. 2007;(1):77–107. [PubMed] [Google Scholar]
- 44.Bamberger AM, Milde-Langosch K, Schulte HM, et al. Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res. 2000;54(1):32–7. doi: 10.1159/000063434. [DOI] [PubMed] [Google Scholar]
- 45.Hopp TA, Weiss HL, Hilsenbeck SG, et al. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;10(8):2751–60. doi: 10.1158/1078-0432.ccr-03-0141. [DOI] [PubMed] [Google Scholar]
- 46.Fan J, Yin WJ, Lu JS, et al. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;134(8):883–90. doi: 10.1007/s00432-008-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farias EF, Petrie K, Leibovitch B, et al. Interference with Sin3 function induces epigenetic reprogramming and differentiation in breast cancer cells. Proc Natl Acad Sci U S A. 2010;107(26):11811–6. doi: 10.1073/pnas.1006737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sartorius CA, Shen T, Horwitz KB. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat. 2003;79(3):287–99. doi: 10.1023/a:1024031731269. [DOI] [PubMed] [Google Scholar]
- 49.Tieszen CR, Goyeneche AA, Brandhagen BN, et al. Antiprogestin mifepristone inhibits the growth of cancer cells of reproductive and non-reproductive origin regardless of progesterone receptor expression. BMC Cancer. 2011;11:207. doi: 10.1186/1471-2407-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Check JH, Dix E, Cohen R, et al. Efficacy of the progesterone receptor antagonist mifepristone for palliative therapy of patients with a variety of advanced cancer types. Anticancer Res. 2010;30(2):623–8. [PubMed] [Google Scholar]
- 51.el Etreby MF, Liang Y. Effect of antiprogestins and tamoxifen on growth inhibition of MCF-7 human breast cancer cells in nude mice. Breast Cancer Res Treat. 1998;49(2):109–17. doi: 10.1023/a:1006098910000. [DOI] [PubMed] [Google Scholar]
- 52.Liang Y, Besch-Williford C, Brekken RA, et al. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating antitumor therapeutics. Cancer Res. 2007;67(20):9929–36. doi: 10.1158/0008-5472.CAN-07-1103. [DOI] [PubMed] [Google Scholar]
- 53.Montecchia MF, Lamb C, Molinolo AA, et al. Progesterone receptor involvement in independent tumor growth in MPA-induced murine mammary adenocarcinomas. J Steroid Biochem Mol Biol. 1999;68(1–2):11–21. doi: 10.1016/s0960-0760(98)00166-6. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z, Auboeuf D, Wong J, et al. Coactivator/corepressor ratios modulate PR-mediated transcription by the selective receptor modulator RU486. Proc Natl Acad Sci U S A. 2002;99(12):7940–4. doi: 10.1073/pnas.122225699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karmakar S, Gao T, Pace MC, et al. Cooperative activation of cyclin D1 and progesterone receptor gene expression by the SRC-3 coactivator and SMRT corepressor. Mol Endocrinol. 2010;24(6):1187–202. doi: 10.1210/me.2009-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.