Abstract
Rationale
Nicotine dependence (ND) is a heterogeneous phenotype with complex genetic influences. The use of intermediate ND phenotypes may clarify genetic influences and reveal specific etiological pathways. Prior work has found that the four Primary Dependence Motives (PDM) subscales (Automaticity, Craving, Loss of Control, and Tolerance) of the Wisconsin Inventory of Smoking Motives (WISDM) represent heavy, pervasive smoking, which is a core feature of nicotine dependence, making these motives strong candidates as intermediate phenotypes.
Objective
This study examines the WISDM PDM as a novel intermediate phenotype of nicotine dependence.
Methods
The study used data from 734 European Americans who smoked at least 5 cigs/day [M=16.2 (SD=9.5) cigs/day], completed a phenotypic assessment, and provided a sample of DNA. Based on prior evidence of the role of genetic variation in the NCAM1-TTC12-ANKK1-DRD2 region on chromosome 11q23 in smoking behavior, associations among 12 region loci with nicotine dependence and PDM phenotypes were examined using haplotype and individual loci approaches. In addition, mediational analysis tested the indirect pathway from genetic variation to smoking motives to nicotine dependence.
Results
NCAM1-TTC12-ANKK1-DRD2 region loci and haplotypes were significantly associated with the motive of Automaticity and, further, Automaticity significantly mediated associations among NCAM1-TTC12-ANKK1-DRD2 cluster variants and nicotine dependence.
Conclusions
These results suggest that motives related to automaticity are a viable intermediate phenotype for understanding genetic contributions to nicotine dependence. Further, NCAM1-TTC12-ANKK1-DRD2 variants may increase the likelihood that a person will become dependent via a highly automatic smoking ritual that can be elicited with little awareness.
Keywords: haplotype, SNP, dopamine, nicotine, endophenotype
INTRODUCTION
Genetic factors influence the risk of initiating smoking and becoming dependent on nicotine (Goldman et al. 2005; MacKillop et al. 2010) and clarifying these influences may hold the key to further reductions in the chronic tobacco use disease burden. The genetic influences on nicotine dependence (ND) appear to be complex, with given genetic variants potentially related only to particular features of the multifaceted phenotype (Baker et al. 2009; Pergadia et al. 2006). Increased knowledge of the genetic influences on various manifestations of ND could clarify mechanisms of dependence and inform tobacco prevention and intervention efforts by revealing who is at elevated risk for dependence ((NCI) 2009).
Although a large number of genetic loci potentially influencing ND have been considered, the NCAM1-TTC12-ANKK1-DRD2 gene cluster is one that has been replicated in large samples using both genome wide and candidate approaches. Specifically, the dopamine receptor 2 (DRD2), the ankyrin repeat and kinase domain containing 1 (ANKK1), tetratricopeptide repeat domain 12 (TTC12), and neural cell adhesion molecule 1 (NCAM1) gene-cluster on chromosome 11q23 have been associated with ND in several studies (Bergen et al. 2009; Ducci et al. 2011; Gelernter et al. 2007; Gelernter et al. 2006; Laucht et al. 2008; Morley et al. 2006; Saccone et al. 2007). DRD2 and related variants became a focus of interest due to dopamine’s critical role in nicotine pharmacodynamics (Benowitz 2010) and addictive processes more broadly (Volkow et al. 2009). For DRD2, many studies have focused on a polymorphism known as Taq1A (rs1800497), which was subsequently shown to map in the neighboring ANKK1 (Neville et al. 2004). Although meta-analyses support a role for Taq1A in risk for smoking behavior (Li et al. 2004; Munafò et al. 2004), its specific functional role remains unknown and there is not a clear susceptibility locus in this region. Further, although data strongly support a role for DRD2 in influencing dopamine transmission (Usiello et al. 2000; Volkow et al. 2009), very little remains known about the specific functional molecular pathway of the NCAM1-TTC12-ANKK1-DRD2 gene cluster and, despite the historical focus on DRD2, the susceptibility locus within this region may not be dopamine-related. For example, the protein encoded by ANKK1 is one of a family of proteins involved in signal transduction pathways (Neville et al. 2004), yet, interestingly, studies have not detected ANKK1 in the brain. A notable study by Mota et al (2012) suggested that polymorphisms within the NCAM1-TTC12-ANKK1-DRD2 gene region serve as a clustered regulatory functional unit that is maintained across evolution in order to preserve phenotypic integrity. Thus, studies have frequently tested multiple individual loci within this gene cluster or used haplotype approaches to capture the linkage disequilibrium (LD) across the region. In a region such as this, with high LD and no clear putative risk allele, combining individual loci and haplotype-analyses provides an opportunity to narrow the location of a susceptibility locus by shedding light on both the role of specific individual variants and common combinations of variants (Gelernter et al. 2006). This strategy permits identifying whether individual loci or combinatorial patterns are the most relevant unit of analysis for observed associations.
In addition, studies supporting a role for NCAM1-TTC12-ANKK1-DRD2 gene cluster variants on ND have largely not taken into account the heterogeneity of the ND phenotype in order to examine specific etiological pathways. The complex and multidimensional nature of ND argues for relating genetic variants of interest with viable intermediate phenotypes, or intermediate measures that fall along the pathway between causal genetic variation and clinical outcome ((NCI) 2009; Baker et al. 2009). Intermediate phenotypes represent mechanistic traits that that may be more proximal to causal genetic variants and may permit distillation of the ND phenotype, and potentially more specific gene mapping, by producing a more homogeneous group of smokers who share a particular genetically-mediated vulnerability to ND ((NCI) 2009; Goldman and Ducci 2007; MacKillop and Munafò 2013). Notably, intermediate phenotypes are not necessarily “endophenotypes,” which refer to genetically-influenced processes that are putatively etiologically relevant, but are required to meet a number of characteristics, including being distinct from the clinical phenotype (Gottesman and Gould 2003; MacKillop and Munafò 2013). With regard to possible intermediate phenotypes, studies show that four subscales of the Wisconsin Inventory of Smoking Motives (WISDM-68), Automaticity, Craving, Loss of Control, and Tolerance have especially strong relationships with important dependence criteria (Piasecki et al. 2010; Piper et al. 2008) and, thus, have been dubbed the Primary Dependence Motives (PDM). Although the PDM are linked both empirically and theoretically with ND, they are also account for orthogonal variance to other features of ND such as withdrawal severity and relapse likelihood, suggesting that the PDM may associate with particular ND clinical features and be related to a particular ND risk pathway (NCI, 2009). Prior work has linked neuronal cholinergic receptor (CHRNA5-A3-B4) gene cluster haplotypes with the PDM subscales in early onset smokers (Baker et al. 2009), suggesting that a subset of smokers who start smoking in their teens and develop a profile of heavy, pervasive smoking, as reflected by the PDM, may follow a unique etiological pathway to ND. While this initial evidence supports PDM motivational profiles as a promising intermediate phenotype of ND, clearly more studies are needed to examine associations with PDM and other biologically-implicated genetic regions.
In addition, further work is needed to reveal the mechanisms underlying associations among smoking-related gene variants, smoking motives, and ND, including examining a potential mechanistic role for PDM in the pathway linking genetic risk and dependence. For example, genetic influences on ND may exert their influence via indirect pathways through motivational profiles. In this case, rather than a direct relationship among gene variants and ND, the motivational intermediate phenotype could serve as a mediator along an etiological pathway that explains the association between the two. Alternatively, genetic variants may show association with both ND and smoking motives without evidence of an indirect effect. Such associations would reflect a relationship between genetic influences and an individual’s motivational profile for smoking, but one that is independent of associations with ND. This would suggest an alternative manifestation of the clinical phenotype that is not a mechanism of risk per se. In this way, studies that employ formal mediation analyses using viable intermediate phenotypes, such as PDM, can clarify established genotype–ND relationships and explicate the mechanisms by which genetic variation exerts influence on clinical dependence phenotypes.
Current Study
While evidence is suggestive that motivational profiles may be promising intermediate phenotypes related to particular features of ND, no studies have directly examined associations among WISDM PDM and variants within the NCAM1-TTC12-ANKK1-DRD2 gene cluster, a well-replicated and biologically implicated region in smoking behavior. Thus, in the present study, our goal was to examine WISDM motivational profiles as a novel intermediate phenotype for ND in a European American (EA) sample using multiple strategies. We hypothesized that the WISDM PDM subscales would be associated with variation in the NCAM1-TTC12-ANKK1-DRD2 gene cluster and would be significant mediators along the gene to clinical ND phenotype pathway. Given the high LD and lack of clear risk allele in the region, association was initially approached using haplotype analyses. Significant haplotype tests were followed up with individual loci analyses in order to determine if associations were due to particular loci or primarily a function of membership within a larger combination of variants. We then used formal mediation analyses to evaluate mechanistic relationships and test whether PDM subscales were significant mediators of the genotype-ND relationship, which would suggest that the observed genotype-clinical phenotype relationship was attributable to the effect of the genotype on the intermediate PDM phenotype.
METHODS
Sample Description
Participants were 734 individuals, who self-reported as EA and smoked at least five cigarettes per day and were recruited through local advertizing as part of a larger study of behavioral economics and smoking (MacKillop et al. 2012). These individuals were 60% male (n=440) and, on average, were 30.1 (SD=12.4) years of age, had 13.2 (SD=2.2) years of education, smoked 16.2 (SD=9.5; Range 1–80) cigarettes each day, were 14.9 (SD=3.8) years old at their first cigarette, and had a median of 1 prior quit attempt.
Phenotypic Measures
Fagerström Test of Nicotine Dependence (FTND; Heatherton et al. 1991)
The FTND is a well-validated six-item measure of ND severity.
WISDM PDM
The 68-item WISDM-68 (Piper et al. 2004) was used to assess the primary dependence subphenotypes of heavy, pervasive smoking. The items within the four individual subscales that constitute the PDM, Automaticity (e.g. “I often smoke without thinking about it.”), Craving (e.g. “I frequently crave cigarettes.”), Loss of Control (LOC) (e.g. “Cigarettes control me.”), and Tolerance (e.g. “I can only go a couple hours between cigarettes.”), were averaged to create an index score for each PDM. As expected, the smoking motives were significantly correlated with each other and FTND (all p’s < .001; Table 1).
Table 1.
Means, standard deviations, and bivariate correlations for smoking phenotypes.
| Mean | SD | Median | Correlations among smoking measures (r)
|
||||
|---|---|---|---|---|---|---|---|
| FTND | Automaticity | Craving | Locus of Control | ||||
| FTND | 4.0 | 2.6 | 4.0 | - | |||
| WISDM-Automaticity | 3.9 | 1.7 | 4.0 | 0.55 | - | ||
| WISDM-Craving | 4.2 | 1.6 | 4.3 | 0.58 | 0.69 | - | |
| WISDM-Locus of Control | 3.7 | 1.7 | 3.5 | 0.59 | 0.67 | 0.77 | - |
| WISDM-Tolerance | 4.1 | 1.7 | 4.2 | 0.77 | 0.67 | 0.72 | 0.73 |
Note. FTND= Fagerström Test of Nicotine Dependence (range 0–10; 229 individuals had scores in the low range (0–2); 194 in the low moderate range (3–4), 246 in the moderate range (5–7), and 65 in the severe range 8+); WISDM-68 = Wisconsin Inventory of Smoking Motives (range for each subscale is 1–7); all correlations significant at p <.001.
Marker Information and Haplotype Derivation
Genotyping and SNP selection
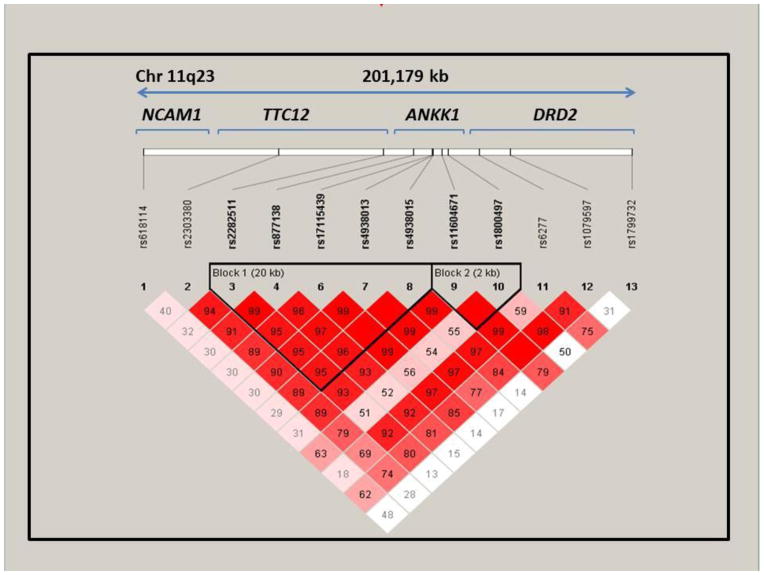
13 markers were selected across the NCAM1-TTC12-ANKK1-DRD2 candidate gene region using HapMap to determine the tag SNPs required to capture >80% of the variance within the gene cluster. These tag SNPs were augmented with loci that had been implicated in prior studies of this region and nicotine dependence (e.g. from Gelernter et al. 2006). Ethanol precipitation was used to extract DNA from collected saliva samples. Samples were genotyped using a MassEXTEND Sequenom assay based on the annealing of an oligonucleotide primer adjacent to the SNP of interest. The assay was performed in multiplex with 20 reactions in a single well; 20% of all samples were randomly run in duplicate resulting in a genotyping error rate of 0.02%. Primer sequences are available upon request. Genotypes were determined by investigators blinded to phenotypic data. Table 2 describes the prevalence of genotypes and alleles and HWE p-values for each marker. One marker (rs4938012) was excluded from subsequent analyses due to HW failure and greater than 15% missing genotypes. Haplotype derivation. In order to (a) maximize the amount of information provided by the multiple markers, and (b) more fully characterize correlated markers within the region, we also utilized all of the available polymorphic data to identify haplotype blocks (i.e., the combinations of SNP/InDel markers that are statistically associated). Haploview was used to visualize and define haplotype blocks in the region (Barrett 2009; Barrett et al. 2005). LD was defined at 95% confidence of non-random association of alleles at two or more loci (Gabriel et al. 2002). Two haplotype blocks were observed (Figure 1): Block 1 was based on rs2282511 (TTC12), rs877183 (ANKK1), rs17115439 (ANKK1), rs4938013 (ANKK1), and rs4938015 (ANKK1) and Block 2 was based on rs11604671 (ANKK1) and rs1800497 (ANKK1).
Table 2.
Genotype and minor allele frequencies of NCAM1-TTC12-ANKK1-DRD2 region loci.
| Polymorphism | Location | Genotypes N (%) | Allele Frequency | HWE p-value | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| NCAM1 | |||||||
| rs618114 | Intron | GG | GA / AG | AA | G | A | 0.36 |
| Frequency | 254 (36.2) | 327 (46.6) | 120 (17.1) | 0.57 | 0.39 | ||
| TTC12 | |||||||
| rs2303380 | Intron | GG | GA / AG | AA | G | A | 0.61 |
| Frequency | 110 (15.3) | 334 (46.5) | 274 (38.2) | 0.38 | 0.60 | ||
| rs2282511 | Unknown | CC | CA / AC | AA | C | A | 0.15 |
| Frequency | 315 (44.2) | 304 (42.7) | 93 (13.1) | 0.64 | 0.33 | ||
| ANKK1 | |||||||
| rs877138 | Unknown | GG | GA / AG | AA | G | A | |
| Frequency | 94 (13.2) | 311 (43.6) | 309 (43.3) | 0.34 | 0.63 | 0.26 | |
| rs4938012 | Intron | GG | GA / AG | AA | G | A | 5.3 E-39 |
| Frequency | 0 (0.0) | 377 (61.3) | 238 (38.7) | 0.27 | 0.58 | ||
| rs17115439 | Synonymous | TT | TC / CT | CC | T | C | |
| Frequency | 94 (13.1) | 311 (43.4) | 311 (43.4) | 0.34 | 0.64 | 0.24 | |
| rs4938013 | Synonymous | CC | CA / AC | AA | C | A | |
| Frequency | 325 (44.8) | 312 (43.0) | 89 (12.3) | 0.66 | 0.33 | 0.29 | |
| rs4938015 | Intron | TT | TC / CT | CC | T | C | 0.21 |
| Frequency | 95 (13.3) | 310 (43.4) | 310 (43.4) | 0.34 | 0.63 | ||
| rs11604671 | Missense | GG | GA / AG | AA | G | A | 0.67 |
| Frequency | 189 (26.5) | 363 (50.8) | 162 (22.7) | 0.50 | 0.47 | ||
| rs1800497 | Missense | TT | TC / CT | CC | T | C | 0.91 |
| Frequency | 36 (5.0) | 248 (34.5) | 434 (60.4) | 0.22 | 0.76 | ||
| DRD2 | |||||||
| rs6277 | Synonymous | TT | TC / CT | CC | T | C | 0.51 |
| Frequency | 206 (28.5) | 351 (48.5) | 166 (23.0) | 0.52 | 0.47 | ||
| rs1079597 | Intron | GG | GA / AG | AA | G | A | 0.05 |
| Frequency | 490 (68.2) | 197 (27.4) | 32 (4.5) | 0.80 | 0.18 | ||
| rs1799732 | Upstream | Del/Del | C.Del/Del.C | CC | Del | C | 0.22 |
| Frequency | 0 (0.0) | 78 (11.0) | 634 (89.0) | 0.05 | 0.92 | ||
Note. Table shows the genotypes and frequencies for each marker (in order of chromosomal location). All loci are single nucleotide polymorphisms except for rs1799732 which is an InDel polymorphism. rs4938012 was dropped from further analyses due to Hardy Weinberg Equilibrium (HWE) failure. Functional classification determined using UCSC genome browser (https://genome.ucsc.edu/cgi-bin/hgGateway). Missing N(%). rs618114 33(4.5); rs2303380 16(2.2); rs2282511 22(3.0); rs877138 20(2.7); rs4938012 119(16.2); rs17115439 18(2.5); rs4938013 8(1.1); rs4938015 19(2.6); rs11604671 20(2.7); rs1800497 16(2.2); rs6277 11(1.5); rs1079597 15(2.0); rs1799732 22(3.0).
Figure 1.
Marker-to-marker D′ values for the NCAM1-TTC12 -ANKK1-DRD2 region polymorphisms. D′ varies between 0 and 1 describes the extent of linkage disequilibrium, a measure of interdependency between genetic loci. A value of 0 for D′ suggests that the examined polymorphisms are independent of one another, while a value of 1 suggests that the polymorphisms provide redundant information. Numbers in the boxes are shown as Haploview output as whole numbers, but reflect D′ correlations that do not exceed 1 (e.g., 91 = .91); an empty box with no numerical value represents D′ of 1.
Haplotypes were then confirmed and extracted using PHASE [Version 2.1; (Stephens and Donnelly 2003; Stephens and Scheet 2005; Stephens et al. 2001), requiring that the probability of a haplotype be greater than or equal to 0.80 (Oroszi et al. 2009). PHASE haplotypes were used to construct diplotypes (i.e., combination of haplotypes across the pair of homologous chromosomes) that were used in the regression analyses. Table 3 describes the frequencies of measured haplotypes as determined by PHASE (Stephens et al. 2001). Because of the limited and inconsistent literature indicating a putative risk allele at each of our loci of interest, haplotype, and thus diplotype, scores were created using a model based on haplotype dosage (Lu et al. 2006; Pajewski et al. 2011). We assumed an additive effect for the presence of each of the identified haplotypes; consequently, an individual may possess 0, 1, or 2 copies of each haplotype observed. This scoring scheme was utilized for every haplotype that was more frequent than .20 in the study sample. Haplotypes present at less than .20 were excluded from further analysis.
Table 3.
NCAM1-TTC12-ANKK1-DRD2 region haplotypes and frequencies.
|
Block 1
|
Population Frequency (S.E.) | N (%) Carrying 0, 1, or 2 Copies | ||||||
|---|---|---|---|---|---|---|---|---|
| rs2282511 | rs877183 | rs17115439 | rs4938013 | rs4938015 | 0 | 1 | 2 | |
| C | A | C | C | C | 0.64 (0.00) | 111 (15.1) | 312 (42.5) | 311 (42.4) |
| A | G | T | A | T | 0.33 (0.00) | 345 (47.0) | 87 (41.1) | 87 (11.9) |
|
Block 2
|
||||||||
| rs11604671 | rs1800497 | |||||||
|
| ||||||||
| G | T | 0.22 (0.00) | 450 (61.3) | 248 (33.8) | 36 (4.9) | |||
| G | C | 0.29 (0.00) | 378 (51.5) | 289 (39.4) | 67 (9.1) | |||
| A | C | 0.48 (0.00) | 209 (28.5) | 363 (49.5) | 162 (22.1) | |||
Note: Table shows haplotype blocks and estimated population frequencies for haplotypes extracted from participants at a probability of at least 80% using PHASE. Proportions do not sum to 100 to allow for the accurate depiction of missingness in the data.
Statistical Analyses & Analysis Plan
Analyses were executed in PLINK v1.07 (Purcell http://pngu.mgh.harvard.edu/purcell/plink/; Purcell et al. 2007) and SPSS 19.0 (IBM Released 2010.). As this study was an exploratory arm of a parent policy study (MacKillop et al., 2012), no a priori power analysis was conducted. However, using Quanto (Gauderman 2002), we determined that the study had adequate power for associations reflecting 1% (β = .78) and excellent power for associations reflecting ≥2% (β≥.97). FTND and PDM were initially examined for outliers (using standard scores, criterion Z=3.29) and for distribution normality and no violations were observed (Tabachnick and Fidell 2001) and bivariate correlations examined the relationships among the smoking phenotypes. These data are reported in Table 2. Linear regressions were used to test the main effects of genetic variation on smoking phenotypes, using the observed haplotypes as the primary tests and following up with test of the individual loci. Separate models were run for each smoking measure. In addition, because there is not an agreed upon putative “risk” haplotype for this gene region, separate regression models were run for each possible observed haplotype code. In the individual loci tests, an additive model was assumed, where an individual may possess 0, 1, or 2 copies of each minor allele observed. To control for type-1 error inflation, we applied a family-wise false discovery rate (FDR) correction to genotype/haplotype-phenotype association tests (Benjamini and Hochberg 1995). Finally, where there was a significant association with the genetic variant and a PDM subscale, selected mediation examining the extent to which the genetic effect (genotype or haplotype) (IV) exerts its influence on FTND (DV) through the PDM (mediator) was tested via the products of the coefficients method in SPSS 19.0 (Preacher and Hayes, 2008). Because the assumption of normality of the sampling distribution of total indirect effects is questionable, bias corrected 95% confidence intervals of the indirect effect were also estimated using bootstrapping methods (Preacher and Hayes 2008). Importantly, testing of indirect effects was not conditional on the presence of a statistically significant genotype/haplotype-FTND association (i.e., the A→C path) because it is not necessary for the test and several scenarios can give rise to a nonsignificant A→C relationship (for a review, see Mackinnon and Fairchild 2009).
RESULTS
Main Effects of Genetic Variation on Smoking Phenotypes
The results of linear regression models testing association among the two haplotype blocks and smoking phenotypes (FTND and PDM) are presented in Table 4. After FDR correction, the CACCC haplotype (Block 1) was significantly associated with lower Automaticity (R2 = .01, p = .01). Further, the AGTAT haplotype (Block 1) was significantly associated with higher Automaticity (R2 = .01, p = .004). None of the block 2 haplotypes were associated significantly with any of the smoking phenotypes.
Table 4.
Unstandardized estimates from models predicting FTND and WISDM subscales from NCAM1-TTC12 -ANKK1-DRD2 region haplotypes.
| WISDM subscales
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FTND | Automaticity | Craving | Loss of Control | Tolerance | ||||||
| β | SE | β | SE | β | SE | β | SE | β | SE | |
| Haplotype Block 1 | ||||||||||
| CACCC | −0.20 | 0.13 | −0.21* | 0.09 | −0.07 | 0.08 | −0.05 | 0.09 | −0.14 | 0.09 |
| AGTAT | 0.26 | 0.14 | 0.27** | 0.09 | 0.07 | 0.09 | 0.10 | 0.09 | 0.15 | 0.09 |
| Haplotype Block 2 | ||||||||||
| GT | 0.24 | 0.16 | 0.21 | 0.11 | 0.13 | 0.10 | 0.09 | 0.11 | 0.17 | 0.12 |
| GC | 0.06 | 0.15 | 0.00 | 0.10 | −0.03 | 0.09 | 0.09 | 0.10 | 0.05 | 0.10 |
| AC | −0.26 | 0.13 | −0.14 | 0.09 | −0.11 | 0.09 | −0.15 | 0.09 | −0.15 | 0.09 |
Note. Direction of the regression coefficient represents the additive effect of each haplotype or extra minor allele (individual loci tests).
FTND= Fagerström Test of Nicotine Dependence; WISDM = Wisconsin Inventory of Smoking Motives; ANKK1 = Ankyrin repeat and kinase domain containing 1 gene; DRD2 = Dopamine D2 receptor gene; NCAM1 = Neural cell adhesion molecule 1 gene; TTC12 = Tetratricopeptide Repeat Domain 12 gene. Haplotypes: Block 1 = rs2282511 (TTC12), rs877183 (ANKK1), rs17115439 (ANKK1), rs4938013 (ANKK1), and rs4938015 (ANKK1); Block 2 = rs11604671 (ANKK1) and rs1800497 (ANKK1).
p<.05,
p<.01.
Haplotype models were followed up with linear regression models testing association among the individual loci, including the five loci that did not fall into haplotype blocks, which are presented in Table 5. The direction of the regression coefficient represents the effect of each extra minor allele (i.e. a positive regression coefficient means that the minor allele increases risk/phenotype mean). After FDR correction, seven loci were significantly associated with variation in Automaticity, including loci from TTC12 (rs2303380 and rs2282511), ANKK1 (rs877138, rs17115439, rs4938013, and rs4938015), and DRD2 (rs1079597) (p’s range from .004 to .017). Each of the significantly associated individual loci accounted for 1% of the variance in the phenotype (R2’s range from .006 to .012).
Table 5.
Unstandardized estimates from models predicting FTND and WISDM subscales from NCAM1-TTC12 -ANKK1-DRD2 region individual loci.
| WISDM Subscales
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FTND | Automaticity | Craving | Loss of Control | Tolerance | ||||||||
|
| ||||||||||||
| β | SE | β | SE | β | SE | β | SE | β | SE | |||
| Polymorphism | Haplotype | Gene | ||||||||||
| rs618114 | None | NCAM1 | 0.00 | 0.14 | 0.09 | 0.09 | 0.10 | 0.08 | 0.06 | 0.09 | 0.04 | 0.09 |
| rs2303380 | None | TTC12 | 0.18 | 0.14 | 0.24* | 0.09 | 0.06 | 0.09 | 0.06 | 0.09 | 0.13 | 0.09 |
| rs2282511 | Block 1 | TTC12 | 0.23 | 0.14 | 0.22* | 0.09 | 0.03 | 0.09 | 0.06 | 0.09 | 0.13 | 0.09 |
| rs877138 | Block 1 | ANKK1 | 0.24 | 0.14 | 0.24* | 0.09 | 0.06 | 0.09 | 0.07 | 0.09 | 0.17‡ | 0.09 |
| rs17115439 | Block 1 | ANKK1 | 0.23 | 0.14 | 0.26* | 0.09 | 0.07 | 0.09 | 0.09 | 0.09 | 0.15‡ | 0.09 |
| rs4938013 | Block 1 | ANKK1 | 0.27 | 0.14 | 0.26* | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.18† | 0.09 |
| rs4938015 | Block 1 | ANKK1 | 0.27 | 0.14 | 0.27* | 0.09 | 0.10 | 0.09 | 0.11 | 0.09 | 0.20† | 0.09 |
| rs11604671 | Block 2 | ANKK1 | −0.19 | 0.14 | −0.15 | 0.09 | −0.09 | 0.09 | −0.16 | 0.09 | −0.16 | 0.09 |
| rs1800497 | Block 2 | ANKK1 | 0.24 | 0.16 | 0.22 | 0.11 | 0.15 | 0.10 | 0.10 | 0.11 | 0.18 | 0.11 |
| rs6277 | None | DRD2 | 0.19 | 0.13 | 0.17 | 0.09 | 0.09 | 0.08 | 0.17 | 0.09 | 0.17 | 0.09 |
| rs1079597 | None | DRD2 | 0.22 | 0.17 | 0.26* | 0.11 | 0.14 | 0.10 | 0.20 | 0.11 | 0.19 | 0.11 |
| rs1799732 | None | DRD2 | −0.20 | 0.31 | −0.23 | 0.20 | −0.12 | 0.19 | 0.05 | 0.21 | −0.09 | 0.20 |
Note. Direction of the regression coefficient represents the additive effect of each extra minor allele. FTND= Fagerström Test of Nicotine Dependence; WISDM = Wisconsin Inventory of Smoking Motives; ANKK1 = Ankyrin repeat and kinase domain containing 1 gene; DRD2 = Dopamine D2 receptor gene; NCAM1 = Neural cell adhesion molecule 1 gene; TTC12 = Tetratricopeptide Repeat Domain 12 gene. Haplotypes: Block 1 = rs2282511 (TTC12), rs877183 (ANKK1), rs17115439 (ANKK1), rs4938013 (ANKK1), and rs4938015 (ANKK1); Block 2 = rs11604671 (ANKK1) and rs1800497 (ANKK1).
p<.05.
Indirect Effects of Genetic Variation through Smoking Motives
The indirect effects for all selected mediation tests are provided in Table 6. With regard to haplotypes, there was a significant indirect effect in the direction of reduced risk of the CACCC haplotype on FTND through Automaticity (p = .02). For the AGTAT haplotype, there was a significant indirect effect in the direction of increased risk of this haplotype on FTND through Automaticity (p = .002). Similarly, each of the 7 individual loci that were significantly associated with Automaticity exerted a significant indirect effect on FTND through Automaticity via increasing Automaticity (p’s range from .002–.01).
Table 6.
Tests of the indirect effects of the genotypes/haplotypes associated with automaticity motives in relation to nicotine dependence.
| Genetic Variant | β | 95% BC CI | Z | p |
|---|---|---|---|---|
| Polymorphism | ||||
| rs2303380 | .19 | .05–.34 | 2.59 | <.01 |
| rs2282511 | .18 | .04–.34 | 2.34 | .02 |
| rs877138 | .19 | .05–.33 | 2.53 | <.01 |
| rs17115439 | .21 | .07–.36 | 2.78 | <.01 |
| rs4938013 | .21 | .07–.36 | 2.77 | <.01 |
| rs4938015 | .22 | .08–.37 | 2.88 | <.01 |
| rs1079597 | .21 | .03–.40 | 2.31 | .02 |
| Haplotype | ||||
| CACCC | −.18 | −.31–−.04 | −2.41 | .02 |
| AGTAT | .22 | .08–.37 | 2.88 | <.01 |
Note. BC CI = Bias Corrected Confidence Intervals.
CONCLUSIONS
Our data provide further support for the association among smoking and common genetic variation within the NCAM1-TTC12-ANKK1-DRD2 gene-cluster and indicate that this region is likely to be involved, in part, in a specific motivational pathway leading to ND. After FDR correction, two haplotype blocks were observed in the region and two haplotypes within the larger block were associated with Automaticity in our EA sample. The risk-associated AGTAT haplotype spanning Block 1 showed a significant relationship with higher Automaticity. In addition, its converse haplotype CACCC, also of Block 1, was a protective haplotype significantly associated with Automaticity. Follow up tests indicated that the five SNPs that comprised this block were individually associated with Automaticity (rs2282511 (TTC12), rs877183 (ANKK1), rs17115439 (ANKK1), rs4938013 (ANKK1), and rs4938015 (ANKK1) suggesting that this region represents a correlated set of risk loci. In addition, two additional SNPs that did not fall into either haplotype block showed association with Automaticity (rs2303380 [TTC12] and rs1079597 [DRD2]). Thus, genetic relationships across the region were present with the intermediate phenotype of Automaticity as a motive for smoking. Our approach of combining haplotype and individual loci analyses in a single study provides an opportunity to narrow the location of a susceptibility locus and to shed light on the role of specific individual variants, while simultaneously accounting for genetic background.
Mediation analyses supported a significant indirect effect via Automaticity of associations of both haplotype and individual SNP variation in the NCAM1-TTC12-ANKK1-DRD2 gene cluster and ND. The finding of a significant indirect effect via the Automaticity motive is suggestive that, rather than automatic smoking motives being an alternative manifestation of the clinical ND phenotype, Automaticity serves as a mechanism along the pathway from genetic risk to clinical dependence. Thus, NCAM1-TTC12-ANKK1-DRD2 variants may increase the likelihood that a person will become dependent via ritualized, automatic smoking that can be elicited with little awareness. Taken together, findings support the use of automaticity motives as viable intermediate phenotype for ND and suggest that genetic studies of the complex ND phenotype may benefit from focusing on more discrete smoking behaviors like automatic smoking and motivational profiles. For example, genetic probes of automaticity motives and automatic smoking behavior using neuroimaging or behavioral assays may prove fruitful ground. Further, our results suggest that interventions that target Automaticity by increasing behavioral and cognitive awareness, through either conventional cognitive-behavioral or more novel mindfulness-based approaches, may have particular benefit for this more homogenous group of smokers.
Our results extend prior studies showing associations among variation in the NCAM1-TTC12-ANKK1-DRD2 gene cluster with ND (Bergen et al. 2009; Ducci et al. 2011; Gelernter et al. 2007; Gelernter et al. 2006; Laucht et al. 2008; Morley et al. 2006; Saccone et al. 2007) and extend data from a single prior study demonstrating associations among cholinergic receptor (CHRNA5-A3-B4) gene cluster variants and PDM intermediate phenotypes (Baker et al. 2009) to another principal smoking-related candidate gene region. In a prior study, both haplotype and individual loci analyses suggested that rs4938013 and rs4938015 were associated with ND in EAs (Gelernter et al. 2006). In our study, these correlated ANKK1 SNPs are implicated in an indirect pathway to ND via Automaticity smoking motives. Similarly, with regard to the two SNPs associated with Automaticity outside of the haplotype block, both rs2303380 in TTC12 (Ducci et al. 2011; Gelernter et al. 2006) and rs1079597 in DRD2 (Gelernter et al. 2006) have been previously associated with ND. DRD2 rs1079597 has also been previously shown to predict the severity of withdrawal from smoking (Robinson et al. 2007) further supporting a role for this locus in phenotypes related to the maintenance of smoking behavior. In the current study, we did not find a significant association with rs1800497, the Taq1A variant of significant historical focus, and Automaticity. Thus, across studies data are broadly supportive that a major susceptibility locus for ND or related phenotypes is located in this region, but a specific causal haplotype or variant remains elusive.
In contrast to these prior studies, we did not find an association among loci in this gene cluster and ND, but instead demonstrated specific associations with Automaticity motives as an intermediate phenotype. Unique associations with Automaticity motives along the pathway to ND could be related to the role of region variants on aspects of the DA system seated in the ventral to dorsal striatal pathway, which is the putative substrate for habit learning and the putative transition pathway from motivational to cognitive processes (Everitt and Robbins 2005). For example, in the striatum, where D2 receptors are the most common DA receptor, D2 receptor density is influenced by NCAM1-TTC12-ANKK1-DRD2 cluster variants (Jonsson et al. 1999; Pohjalainen et al. 1998). Thus, NCAM1-TTC12-ANKK1-DRD2 variation may be implicated in ND via an effect on habit learning processes that increase habitual and automatic smoking.
Our findings should be interpreted within the context of several methodological considerations. Although our study used a moderately sized EA sample, as with any candidate genetic approach, associations reported here need to be substantiated through replication. Furthermore, the sample size was not sufficient to include an extensive list of covariates without meaningfully reducing power. In addition, in order maximize power and focus on ND intermediate phenotypes, we analyzed our full sample of EA smokers, which included smokers who ranged from low to severe dependence. The presence of less dependent smokers is a consideration when comparing our results to those of studies using higher dependence thresholds. With regard to the haplotype blocks, we employed a standard LD threshold for haplotype block derivation (Gabriel et al., 2002). However, other block definition algorithms may have resulted in slightly different block formations and there was some ambiguity with rs2303380 coming very close to meet D′ thresholds for Block 1 inclusion. Further, although our genotype and analytic approach served to characterize common variation in the NCAM1-TTC12-ANKK1-DRD2 gene region well, variants measured in the current study explained only ~1% of the variance in targeted phenotypes. Thus, not only is there additional genotypic variance within this region that was not measured in the current study (e.g. both common and rare variants), there are additional mechanisms involved that may act in concert with NCAM1-TTC12-ANKK1-DRD2 common variants in order to influence risk for smoking. We assumed an additive model for the haplotype and individual loci analyses, but this method only accounts for one possible form of genetic effect and alternative models should be explored and tested in future studies. Finally, although our mediational models imply an indirect pathway from genetic variants to ND via Automaticity, this potential causal pathway should be explicitly tested in future work using longitudinal designs.
In sum, our findings using haplotype, individual genotype, and mediation analyses in a single study extend the literature to support a role for NCAM1-TTC12-ANKK1-DRD2 cluster variants in risk for ND and suggest that the effect of variation within the region on ND may exert itself through a highly automatic smoking ritual that can be elicited with little awareness. Automaticity may be a viable intermediate phenotype for understanding genetic contributions to ND that may shed light on the genetic pathways to clinical dependence. Future studies should seek to replicate and extend associations identified here as well as examine the specific functionality of this complex gene cluster.
Acknowledgments
Funding sources
Funding was provided by the following grants: SAPRP 65626 from the Robert Wood Johnson Foundation and K23 AA016936 from NIH to James MacKillop; K23 DA033302 from NIDA to L. Cinnamon Bidwell; a Research Career Development Award from the Medical Research Service of the Department of Veteran Affairs, 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs to John McGeary; and K01 AA021113 from NIAAA to Rohan Palmer; and R01 DA023134 from NIDA to Valerie Knopik. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Dr. MacKillop is the holder of the Boris Chair in Addictions Research, which partially supported his contributions.
Footnotes
conflict of interest declaration:
The authors have no conflicts of interest to report.
Bibliography and References Cited
- (NCI) NCI. National Cancer Institute Tobacco Control Monograph Series No. 20. Bethesda, MD: 2009. Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence; p. 654. [Google Scholar]
- Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, Piper ME, Matsunami N, Smith SS, Coon H, McMahon WM, Scholand MB, Singh N, Hoidal JR, Kim SY, Leppert MF, Cannon DS. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11:785–96. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.ip71. pdb ip71. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Conti DV, Van Den Berg D, Lee W, Liu J, Li D, Guo N, Mi H, Thomas PD, Lessov-Schlaggar CN, Krasnow R, He Y, Nishita D, Jiang R, McClure JB, Tildesley E, Hops H, Tyndale RF, Benowitz NL, Lerman C, Swan GE. Dopamine genes and nicotine dependence in treatment-seeking and community smokers. Neuropsychopharmacology. 2009;34:2252–64. doi: 10.1038/npp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Kaakinen M, Pouta A, Hartikainen AL, Veijola J, Isohanni M, Charoen P, Coin L, Hoggart C, Ekelund J, Peltonen L, Freimer N, Elliott P, Schumann G, Jarvelin MR. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol Psychiatry. 2011;69:650–60. doi: 10.1016/j.biopsych.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ. Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol. 2002;155:478–84. doi: 10.1093/aje/155.5.478. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, Farrer L, Kranzler HR. Genomewide linkage scan for nicotine dependence: identification of a chromosome 5 risk locus. Biol Psychiatry. 2007;61:119–26. doi: 10.1016/j.biopsych.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, Kranzler HR, Farrer L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15:3498–507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- Goldman D, Ducci F. Deconstruction of vulnerability to complex diseases: enhanced effect sizes and power of intermediate phenotypes. Scientific World Journal. 2007;7:124–30. doi: 10.1100/tsw.2007.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- IBM. IBM SPSS Statistics for Windows, Version 19.0. IBM Corp; Armonk, NY: Released 2010. [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–6. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Laucht M, Becker K, Frank J, Schmidt MH, Esser G, Treutlein J, Skowronek MH, Schumann G. Genetic variation in dopamine pathways differentially associated with smoking progression in adolescence. J Am Acad Child Adolesc Psychiatry. 2008;47:673–81. doi: 10.1097/CHI.0b013e31816bff77. [DOI] [PubMed] [Google Scholar]
- Li MD, Ma JZ, Beuten J. Progress in searching for susceptibility loci and genes for smoking-related behaviour. Clin Genet. 2004;66:382–92. doi: 10.1111/j.1399-0004.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- Lu J, Wei Q, Bondy ML, Li D, Brewster A, Shete S, Yu TK, Sahin A, Meric-Bernstam F, Hunt KK, Singletary SE, Ross MI, Wang LE. Polymorphisms and haplotypes of the NBS1 gene are associated with risk of sporadic breast cancer in non-Hispanic white women <or=55 years. Carcinogenesis. 2006;27:2209–16. doi: 10.1093/carcin/bgl077. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Few LR, Murphy JG, Wier LM, Acker J, Murphy C, Stojek M, Carrigan M, Chaloupka F. High-resolution behavioral economic analysis of cigarette demand to inform tax policy. Addiction. 2012;107:2191–200. doi: 10.1111/j.1360-0443.2012.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Munafò M. Genetic Influences on Addiction: An Intermediate Phenotype Approach. MIT Press; Cambridge, MA: 2013. [Google Scholar]
- MacKillop J, Obasi E, Amlung MT, McGeary JE, Knopik VS. The Role of Genetics in Nicotine Dependence: Mapping the Pathways from Genome to Syndrome. Current Cardiovascular Risk Reports. 2010;4:446–453. doi: 10.1007/s12170-010-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol Sci. 2009;18:16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KI, Medland SE, Ferreira MA, Lynskey MT, Montgomery GW, Heath AC, Madden PA, Martin NG. A possible smoking susceptibility locus on chromosome 11p12: evidence from sex-limitation linkage analyses in a sample of Australian twin families. Behavioral Genetics. 2006;36:87–99. doi: 10.1007/s10519-005-9004-0. [DOI] [PubMed] [Google Scholar]
- Mota NR, Araujo-Jnr EV, Paixao-Cortes VR, Bortolini MC, Bau CH. Linking dopamine neurotransmission and neurogenesis: The evolutionary history of the NTAD (NCAM1-TTC12-ANKK1-DRD2) gene cluster. Genet Mol Biol. 2012;35:912–8. doi: 10.1590/s1415-47572012000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine Tob Res. 2004;6:583–97. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Oroszi G, Anton RF, O’Malley S, Swift R, Pettinati H, Couper D, Yuan Q, Goldman D. OPRM1 Asn40Asp predicts response to naltrexone treatment: a haplotype-based approach. Alcohol Clin Exp Res. 2009;33:383–93. doi: 10.1111/j.1530-0277.2008.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajewski NM, Parker SD, Poland GA, Ovsyannikova IG, Song W, Zhang K, McKinney BA, Pankratz VS, Edberg JC, Kimberly RP, Jacobson RM, Tang J, Kaslow RA. The role of HLA-DR-DQ haplotypes in variable antibody responses to anthrax vaccine adsorbed. Genes & Immunity. 2011;12:457–65. doi: 10.1038/gene.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML, Heath AC, Martin NG, Madden PA. Genetic analyses of DSM-IV nicotine withdrawal in adult twins. Psychol Med. 2006;36:963–72. doi: 10.1017/S0033291706007495. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Piper ME, Baker TB. Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives: II. Evidence from a laboratory self-administration assay. J Abnorm Psychol. 2010;119:513–23. doi: 10.1037/a0020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Bolt DM, Kim SY, Japuntich SJ, Smith SS, Niederdeppe J, Cannon DS, Baker TB. Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives. J Abnorm Psychol. 2008;117:747–61. doi: 10.1037/a0013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J Consult Clin Psychol. 2004;72:139–54. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Purcell S. PLINK v1.07 ( http://pngu.mgh.harvard.edu/purcell/plink/)
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Lam CY, Minnix JA, Wetter DW, Tomlinson GE, Minna JD, Chen TT, Cinciripini PM. The DRD2 TaqI-B polymorphism and its relationship to smoking abstinence and withdrawal symptoms. Pharmacogenomics J. 2007;7:266–74. doi: 10.1038/sj.tpj.6500427. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–9. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet. 2005;76:449–62. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate statistics. 4. Allyn & Bacon; 2001. [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]