Abstract
It is generally accepted that complex memories are stored in distributed representations throughout the brain, however the mechanisms underlying these representations are not understood. Here, we review recent findings regarding the subcellular mechanisms implicated in memory formation, which provide evidence for a dendrite-centered theory of memory. Plasticity-related phenomena which affect synaptic properties, such as synaptic tagging and capture, synaptic clustering, branch strength potentiation and spinogenesis provide the foundation for a model of memory storage that relies heavily on processes operating at the dendrite level. The emerging picture suggests that clusters of functionally related synapses may serve as key computational and memory storage units in the brain. We discuss both experimental evidence and theoretical models that support this hypothesis and explore its advantages for neuronal function.
Keywords: Plasticity, active dendrites, memory, synapse clustering
1. Introduction
The ways in which memories are formed and stored in the brain remain one of the most exciting mysteries of neuroscience. While it is generally believed that complex memories are stored in distributed representations throughout the brain (Hübener & Bonhoeffer, 2010; Josselyn, 2010; Lashley, 1950), the mechanisms underlying the formation of these representations are still under intense scrutiny. Powerful new technologies, such as ligand- and light-driven neuronal activation systems, have established that the biophysical correlates of memory (memory engrams) consist of ensembles of neurons which undergo plasticity during learning, and are necessary for the expression of memory (Garner et al., 2012; Han et al., 2007; Josselyn, 2010; J. Kim, Kwon, Kim, Josselyn, & Han, 2014; X. Liu et al., 2012).
The storage of memories in neuronal populations is believed to rely on structural and biophysical changes in the synapses between interconnected neurons. Ramon y Cajal was the first to suggest that synaptic contacts between neurons could play a role in memory storage (Ramón y Cajal & y Cajal, 1893) but it wasn’t until the 1940’s that the synaptic potentiation postulate of Donald Hebb was formulated. According to Hebb’s influential theory, synapses between neurons are potentiated when they are concurrently activated, and this mechanism forms the physiological foundation for learning and memory (Hebb, 1949).
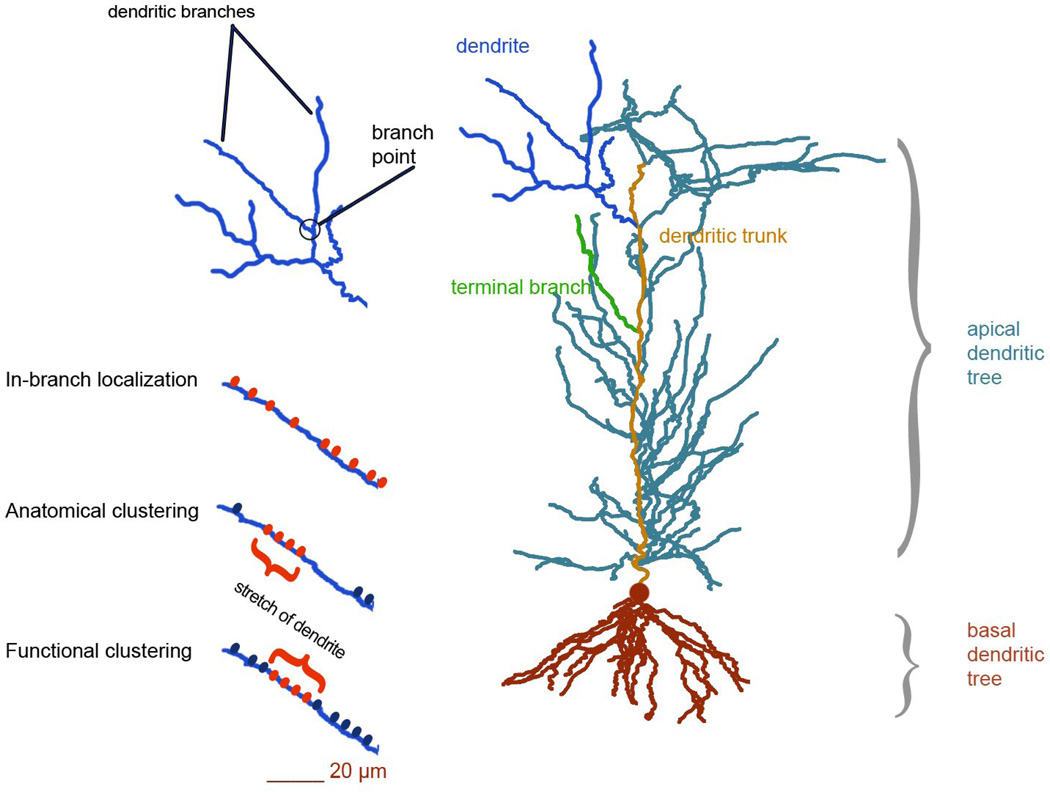
Synaptic modifications however are greatly influenced by the biophysical and anatomical complexity of their hosting structures, namely the dendritic branches (Figure 1). The elaborate morphology together with the rich repertoire of voltage-gated ionic mechanisms found in dendrites (Häusser, Spruston, & Stuart, 2000; Mainen & Sejnowski, 1996; Major, Larkum, & Schiller, 2013; Per Jesper Sjöström, Rancz, Roth, & Häusser, 2008; Spruston, 2008) influences the integration of synaptic signals and their forward propagation to the soma, enabling these structures to exhibit compartmentalized regenerative events (Häusser et al., 2000; Larkum et al., 2009; Nevian, Larkum, Polsky, & Schiller, 2007; Schiller, Schiller, Stuart, & Sakmann, 1997; Wei et al., 2001) and support spatially restricted plasticity (Golding, Staff, & Spruston, 2002; Hardie & Spruston, 2009; Losonczy, Makara, & Magee, 2008; Per Jesper Sjöström et al., 2008). These properties furnish dendrites with the ability to regulate synapse modification in complex, nonlinear ways thus adding another level of complexity in the memory formation process.
Figure 1.
Dendritic structure and plasticity. Each dendritic tree (apical or basal) in pyramidal neurons can be subdivided to a number of dendrites (dendritic subtrees connected to the apical trunk or the soma). Thin terminal branches are the main targets of excitation in the cerebral cortex. There, synaptic inputs can be organized in the following ways: 1) they can be localized in the same dendritic branch without specific spatial arrangement (in-branch localization), 2) they can form anatomical clusters, whereby spines form morphologically distinct groups of several spine heads located in distances less than 5 microns from each other within stretches of a given branch and 3) they can form functional clusters where spine density is uniform but nearby synapses (located within 10 – 20 microns) are activated synchronously.. The implications of these different arrangements of connectivity at the dendritic level are discussed in section 2.
Within dendrites, an array of plasticity processes, which include Hebbian long-term potentiation or depression (LTP/LTD), synaptic tagging and capture (STC), plasticity of intrinsic excitability, local homeostasis etc., govern the structural re-organization of synaptic contacts that takes place during memory formation. Many of these processes act locally, at the level of a dendritic branch or even a stretch of a dendritic branch (Zhang & Linden, 2003). Spatially-restricted changes in synaptic properties have been proposed to underlie the formation of local groups or clusters of synaptic connections in dendritic compartments (Branco & Häusser, 2010). This hypothesis, termed clustered plasticity, has been associated with increased storage capacity (Poirazi & Mel, 2001) and feature binding (Govindarajan, Kelleher, & Tonegawa, 2006; Legenstein & Maass, 2011), both of which are important attributes of memory.
On top of it all, global balancing mechanisms, like homeostatic plasticity together with the plasticity of inhibitory connections (Kullmann, Moreau, Bakiri, & Nicholson, 2012; Turrigiano & Nelson, 2004; Zhang & Linden, 2003), ensure a smooth running operation of the neuronal circuits that capture, encode and store the life events or skills that we call memories.
In light of the evidence that memory trace formation is governed by plasticity processes operating at multiple levels, existing memory theories are being revised and refined (Branco & Häusser, 2010; Chklovskii, Mel, & Svoboda, 2004; Govindarajan et al., 2006; M. P. Jadi, Behabadi, Poleg-Polsky, Schiller, & Mel, 2014; Redondo & Morris, 2011; Rogerson et al., 2014). In the view proposed here, memories are stored in small and distributed overlapping populations of neurons, in which synaptic clusters in dendritic branches encode for 'related' (in time, space or context) memories (Rogerson et al., 2014; Silva, Zhou, Rogerson, Shobe, & Balaji, 2009). Thus, the formation of a new memory trace does not only result in alterations of the connectivity strengths between neurons in a network, but also in the spatial arrangement of synapses and the excitability properties of dendritic branches where they reside (Chklovskii et al., 2004; Silva et al., 2009; Zhang & Linden, 2003) (see Figure 3). Evidence for this hypothesis implies a mechanistic link between the expression of memory representations at the neuronal circuit level and the underlying cellular, dendritic and synaptic components that participate in their formation.
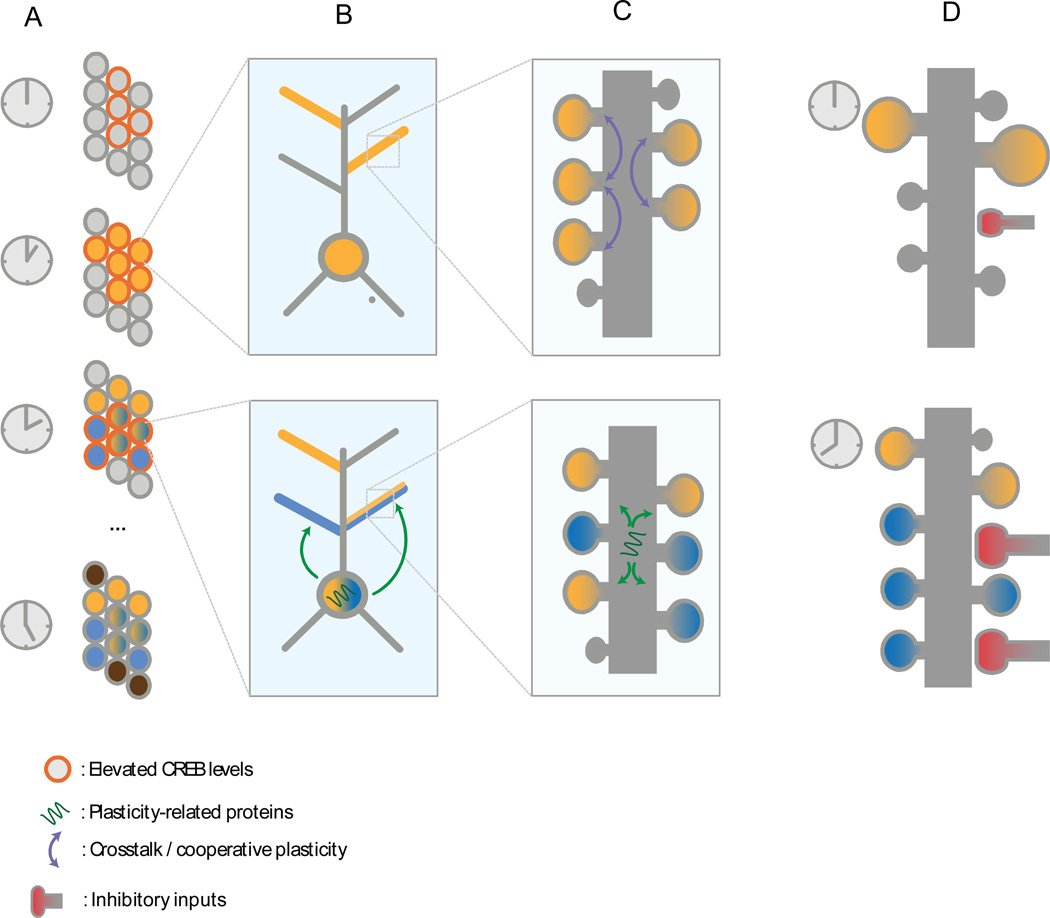
Figure 3. Memory allocation across multiple temporal and spatial scales.
A. Increased CREB levels (red outline) make these cells more likely to be engaged in the formation of upcoming memory representations (yellow cells). Engagement of cells in memory leads to increased CREB levels for a given time period (red outline), increasing the probability that these cells will take part in subsequent learning (yellow/blue cells). This does not happen if learning occurs much later (brown).
B. Intrinsic plasticity mechanisms can regulate excitability locally in branches (e.g. branch strength potentiation) allow neural cells to compartmentalize storage. Additionally, synaptic tagging and capture that depends on nuclear protein production (green arrows) can enhance cooperativity across branches.
C. Synaptic crosstalk and plasticity cooperativity acts locally in branches, enabling the formation of synaptic clusters (top, purple arrows). Additionally, synaptic tagging and capture that occurs within branches can allow the binding of different memories/features in clusters (bottom, green arrows).
D. Homeostatic processes act in longer timescales, fine tuning synaptic integration, normalizing excitation and balancing excitatory and inhibitory inputs in order to prevent runaway excitation or silencing of neurons.
In this review, we discuss experimental and computational findings that examine how dendritic non-linearlities in conjunction with local and global plasticity processes shape memory formation in neuronal circuits.
2. Dendritic branches as key computational elements
In neocortical pyramidal neurons, dendritic branches provide the physical substrate where synapses are formed and modified through plasticity operations. Equipped with an array of biophysical mechanisms, dendrites1 can dynamically modulate local voltage responses. This allows them to integrate the excitatory postsynaptic potentials (EPSPs) of the afferent synapses that impinge upon them in sublinear, linear or supralinear ways (Ariav, Polsky, & Schiller, 2003; Branco & Häusser, 2011; Häusser, Mel, & Hausser, 2003; Longordo, To, Ikeda, & Stuart, 2013; Losonczy & Magee, 2006; Poirazi, Brannon, & Mel, 2003a; Silver, 2010; Yuste, 2011). On one hand, the elaborated biophysical profile of dendrites, which includes multiple types of voltage-gated conductances, has been postulated to mediate the linear integration of distributed synaptic inputs, irrespectively of their location within the neuron (Cash & Yuste, 1999; Yuste, 2011). On the other hand, the ability of dendritic branches in pyramidal and other neuron types to support local electrogenesis, evidenced by the generation of dendritic spikes, has been shown to underlie the non-linear integration of synaptic inputs.
Based on their primary source, dendritic spikes are distinguished in 3 main types: sodium, calcium and NMDA (N-methyl-D-aspartate) spikes, all of which have been extensively documented in pyramidal neurons both in vitro (Ariav et al., 2003; Gasparini, Migliore, & Magee, 2004; Golding et al., 2002; S. Kim, Guzman, Hu, & Jonas, 2012; Losonczy & Magee, 2006; Makara & Magee, 2013; Nevian et al., 2007; Polsky, Mel, & Schiller, 2004; Schiller et al., 1997) and in vivo (Lavzin, Rapoport, Polsky, Garion, & Schiller, 2012; S. L. Smith, Smith, Branco, & Häusser, 2013). They are characterized as nonlinear, all-or-none dendritic responses which can propagate actively for some distance and are often confined within the generating branch (Antic, Zhou, Moore, Short, & Ikonomu, 2010; Larkum & Zhu, 2002; Schiller, Major, Koester, & Schiller, 2000b; Schiller et al., 1997). This allows the branch, the dendrite or the neuron to integrate synaptic signals over much longer timescales than passive integration would allow.
Since the processing capabilities of pyramidal neuron dendrites are discussed in several excellent reviews (Branco & Häusser, 2010; Häusser et al., 2003; Major et al., 2013; I. Segev, 2000; Silver, 2010; Spruston, 2008), we highlight just a few of their key features. Cortical dendrites, perform synaptic integration non-uniformly, with distal inputs within the same branch being amplified over larger time windows compared to proximal ones (Branco & Häusser, 2011). This difference is attributed, by computational models, to the generation of NMDA-dependent dendritic spikes which are facilitated when synapses are located towards the tip of a dendritic branch (Branco & Häusser, 2011; Sidiropoulou & Poirazi, 2012). As a result, distal synapses, which are individually too weak to significantly influence the somatic voltage, can act cooperatively to affect the output of the neuron (Schiller, Major, Koester, & Schiller, 2000a). A similar nonlinearity that serves as a mechanism for coincidence detection also depends on NMDA conductances, this time in the apical tuft dendrites of layer 5 pyramidal neurons (Larkum et al., 2009). The initiation of dendritic spikes and their amplitude is, in turn, determined by the magnitude and location of inhibition that these neurons receive (M. Jadi, Polsky, Schiller, & Mel, 2012).
The above are just a few examples of modeling and experimental studies suggesting that local spikes enable dendritic branches to implement nonlinear integration modes (Mel 1993, Häusser, Spruston et al. 2000, Gasparini, Migliore et al. 2004, Polsky, Mel et al. 2004, Gasparini and Magee 2006, Losonczy and Magee 2006, Makara and Magee 2013), thus conferring enhanced flexibility in neuronal information processing. In order to exploit this additional processing power of nonlinear dendrites, synaptic input should be such that the whole range of possible dendritic responses are explored, including the generation of dendritic spikes. As discussed in sections 2.1 and 2.2, the spatial arrangement of synaptic inputs in dendritic branches can serve as a way to realize this goal.
2.1 Effect of spatial synaptic arrangement on dendritic integration: distributed connectivity and linear integration
Distributed synaptic inputs, irrespectively of their location within the neuron, have been suggested to summate linearly, a result attributed to the elaborated biophysical profile of pyramidal neuron dendrites (Cash & Yuste, 1999; Yuste, 2011). This linear integration mode may be particularly useful when synaptic input is dispersed uniformly throughout the dendritic tree, for example as a result of an essentially random connectivity between neurons that is dictated by anatomical constraints (Braitenberg & Schüz, 1998). According to this model, the connectivity of neuronal circuits is determined by the overlap of dendritic arbors and axonal processes, as dictated by Peters’ rule (Peters, Paley, & Webster, 1976). A series of in vivo imaging studies in sensory areas has provided indirect support for this model (X. Chen, Leischner, Rochefort, Nelken, & Konnerth, 2011; Jia, Rochefort, Chen, & Konnerth, 2010; Varga, Jia, Sakmann, & Konnerth, 2011) by showing that neighboring synapses are tuned to apparently random input features (but see section 2.4.3 for an alternative interpretation).
In a distributed connectivity model like the one discussed above, a large number of synapses must be activated in order to induce a postsynaptic spike. The integration of multiple distributed synaptic inputs within a neuron, however, is negatively affected by membrane dynamics that create an interference problem. Synaptic depolarization causes the opening of membrane conductances, thus lowering the membrane resistance and making the neuron less excitable in response to subsequent synaptic input. Dendritic spines with high electrical neck resistance have been suggested to counteract these effects by isolating synaptic inputs (Idan Segev & Rall, 1998). Alternatively, spatially segregating the synaptic contacts throughout the dendritic tree may serve as a mechanism for implementing linear local summation of incoming signals (Yuste, 2011), however the requirement for a large number of distributed inputs would also lead to shunting effects. The distributed connectivity and linear summation model has inspired a large portion of the early artificial neural network research in the past decades (Hopfield, 1988; Minsky & Papert, 1969). While these artificial neuron models overly simplify the function of neurons, they have been instrumental in studying memory storage in artificial neuronal populations. These models have established synaptic weight changes caused by plasticity as a valid mechanism of learning in artificial neural networks (McClelland & Rumelhart, 1986).
2.2 Effect of spatial synaptic arrangement on dendritic integration: clustering and supralinear integration
The distributed connectivity model presented above has been challenged by findings in several brain regions, including the hippocampus and the cerebral cortex. As detailed in section 2.4., an increasing number of studies have shown that synaptic contacts can group together within a short stretch of the dendritic branch, forming anatomical clusters2 (Makino & Malinow, 2011; Yadav et al., 2012) and/or create functional clusters whereby several neighboring synapses are activated synchronously (Fu, Yu, Lu, & Zuo, 2012; Kleindienst, Winnubst, Roth-Alpermann, Bonhoeffer, & Lohmann, 2011; Takahashi et al., 2012), in the absence of obvious spine density changes This patterned spatial synaptic arrangement along with concrete evidence of dendritic spike generation both in vitro (Ariav et al., 2003; Häusser et al., 2000; S. Kim et al., 2012; Larkum & Nevian, 2008; Losonczy & Magee, 2006; Makara & Magee, 2013; Nevian et al., 2007; Schiller et al., 2000a) and in vivo (Lavzin et al., 2012; Major et al., 2013; S. L. Smith et al., 2013), are not accounted for by the distributed connectivity and linear integration model, challenging the traditional view of synaptic weight-based learning. An alternative theory entails that, changes in the spatial wiring of synaptic connections together with dendritic excitability modulation, can serve as additional memory reservoirs in the brain (Chklovskii et al., 2004).
In particular, the spatial arrangement of incoming inputs within active dendrites of both simplified (B W Mel, 1993; Poirazi & Mel, 2001) and biophysically detailed neurons (Poirazi et al., 2003a, 2003b) was theoretically predicted to influence the dendritic and neuronal output by differentially engaging local conductances. For example, synchronous activation of synapses within the same apical branch (hereby termed in-branch localization, see Figure 1) of a biophysically realistic pyramidal neuron model was predicted to result in supralinear responses while stimulation of the same number of synapses distributed in different branches resulted in linear summation (Poirazi et al., 2003a). This prediction was verified experimentally in L5 neocortical pyramidal neurons (Polsky et al., 2004), highlighting the effect of synapse placement on neuronal output. Supralinearity in this case resulted from the induction of dendritic spikes, a phenomenon that was not seen when synapses were stimulated across different branches.
Similar supralinearities were also found in oblique dendrites of CA1 pyramidal cells, upon stimulation of synapses within individual radial oblique branches (Losonczy & Magee, 2006). In this case, synchronous stimulation of nearby synapses (mimicking functional clustering, see Figure 1) had the same effect as synchronous stimulation of the same number of synapses distributed uniformly within the branch (in-branch localization), suggesting that these structures act as single, nonlinear integrative compartments, as predicted by previous modeling work (Poirazi et al., 2003a, 2003b). These dendrites have also been suggested to act as coincidence detectors (Ariav et al., 2003; Gómez González, Mel, & Poirazi, 2011; Losonczy & Magee, 2006) and serve as detectors of asynchronous bursty inputs (Gómez González et al., 2011), via the induction of fast or slow, respectively, dendritic spikes. Evidence of such independent integrative compartments provides support for a 2-stage model of neuronal processing (Katz et al., 2009; Poirazi et al., 2003b), with multiple implications with respect to information processing (for a recent review on the 2-layer model, see (M. P. Jadi et al., 2014)).
The placement of synapses at different parts (proximal vs. distal) of dendritic branches has also been predicted and experimentally evidenced to influence dendritic and neuronal responses (Behabadi, Polsky, Jadi, Schiller, & Mel, 2012; Branco & Häusser, 2011). For example the amplitude of EPSPs and the supralinearity of electrical integration during the stimulation of multiple synapses varies from the base to the tip of a single dendrite. The tip displays both higher EPSP amplitude, higher gain, and higher EPSP supralinearity compared to the base or the middle section of the dendrite (Branco & Häusser, 2011). Moreover, the amplitude and threshold of basal dendritic spikes is affected by the positioning of excitation along the dendrite (Behabadi et al., 2012). Distal excitation lowers the threshold for dendritic spike generation in more proximal inputs, while proximal excitation lowers the threshold and increases the voltage gain of distal inputs.
In sum, models and experiments have shown that the location of any given synapse influences its effective “weight” (i.e. its impact on dendritic and neuronal depolarization), since co-activation of neighboring synapses will result in a much larger depolarization than if the particular synapse is activated in isolation. These findings suggest that the spatial organization of synaptic contacts is also likely to have a key role in plasticity processes that underlie learning and memory formation, as detailed in the next session.
2.3 LTP cooperativity in nearby inputs
Beyond spatiotemporal integration, dendritic depolarization and dendritic spikes have a strong effect on Long Term Potentiation (LTP), a form of synaptic plasticity which is believed to play a key role in learning and memory formation. In CA1 pyramidal neurons, local synaptic depolarization that results in dendritic spikes can induce LTP even in the absence of somatic spiking (Golding et al., 2002; Hardie & Spruston, 2009). In addition, this form of LTP is stronger when paired synaptic inputs are both located in the apical dendrites than if they are separated in the apical and basal trees. This suggests that the large and long lasting dendritic depolarization generated by the activation of spatially proximal synapses is more effective in inducing strong LTP than the pairing of dendritic input with back-propagating action potentials. Where does this difference stem from? Possibly from the ability of spatially close synapses to undergo plasticity in a cooperative manner.
Cooperativity is the ability of multiple activated synapses to collectively overcome the threshold for plasticity and is a characteristic property of LTP that is presumed to be mediated by NMDA calcium influx (Bliss & Collingridge, 1993; P J Sjöström, Turrigiano, & Nelson, 2001). Synaptic input which leads to LTP in dendrites initiates complex biochemical signaling cascades in the dendritic region, triggered by the influx of calcium and the elevation of its local concentration (Baudry, Bi, Gall, & Lynch, 2011). Some of these pathways facilitate the cooperativity of LTP at nearby synapses, and this can lead to the coordinated potentiation of neighboring synapses, thus promoting synaptic clustering. For example, the MAPK (mitogen-activated protein kinase) and mTOR (mechanistic target of rapamycin) cascades remain active for several minutes after their initial activation (G.-Y. Wu, Deisseroth, & Tsien, 2001). This prolonged activation allows the spread of proteins and kinases to nearby synapses, thus facilitating their plasticity. The Ras GTPase, which is part of the MAPK signaling pathway and is correlated with increased spine volume during LTP induction, has been shown to spread and invade nearby spines (Harvey, Yasuda, Zhong, & Svoboda, 2008). In addition, the RhoAGTPase was found to spread out of stimulated dendritic spines undergoing structural plasticity related to LTP for about 5 µm along the dendrite (Murakoshi, Wang, & Yasuda, 2011). These molecular mechanisms support the cooperative potentiation of synaptic clusters at the spatial scale of < 20 µm and are detailed in several excellent reviews (Hering & Sheng, 2001; Patterson & Yasuda, 2011; Winnubst, Lohmann, Jontes, Wang, & Niell, 2012).
Another mechanism that enables local cooperativity of LTP is the activation of ‘silent’ synapses. Silent synapses contain only NMDA receptors and are blocked by Mg2+ ions when the local membrane is in its resting state. Activation of nearby synapses, however, can remove the Mg2+ block, allowing these synapses to become conductive. Under a Hebbian plasticity protocol, this could eventually lead to the insertion of AMPA receptors in the synapse, thus ‘unsilencing’ the synapse (Liao, Hessler, & Malinow, 1995).
Clusters can also be formed by the addition of new synapses near existing ones (Fu et al., 2012), which effectively changes the wiring diagram, however such changes are typically slower as they require the restructuring of neural tissue (Chklovskii et al., 2004; Trachtenberg et al., 2002). Since both synapse formation and elimination are processes that persist in the adult brain (Trachtenberg et al., 2002), it may be possible that LTP cooperativity interacts with synapse formation or the conversion of filopodia to dendritic spines and biases the formation of anatomical synaptic clusters.
The abovementioned evidence indicates that LTP cooperativity in nearby synapses can lead to the formation and stabilization of functional and anatomical (Figure 1) clusters of synapses within frequently stimulated dendrites. This clustering may serve as a mechanism for effective wiring, whereby connections are established by sharing protein products, thus saving energy and molecular recourses, while at the same time dendritic non-linearities are fully exploited via the selective induction of dendritic spikes (Winnubst et al., 2012). Based on this evidence, the clustered plasticity hypothesis has been put forward, which proposes that inputs with correlated activity patterns (presumably sharing some functional features), are more likely to be organized in functional and/or anatomical clusters within the dendrites of pyramidal neurons (Govindarajan et al., 2006; Harvey & Svoboda, 2007; Poirazi & Mel, 2001). This view has gradually been gaining experimental support, through the advent of modern imaging methods which allow the detailed mapping of synapses in dendritic arbors. In the following section we summarize the most important experimental findings that support this hypothesis.
2.4 Experimental evidence for synaptic clustering
2.4.1 Anatomical clustering
Evidence for anatomical synapse clustering was first shown in the dendrites of the adaptive microcircuit of the barn owl auditory localization circuit (McBride, Rodriguez-Contreras, Trinh, Bailey, & DeBello, 2008). Barn owls reared with prismatic spectacles develop an adaptive zone that does not exist in normally reared animals and is a result of the animal’s abnormal experience. The experimenters found both increased clustering (contacts located within < 20 microns) of axodendritic contacts (potential synapses) in the adaptive zone (presumably a result of the physiological and behavioral adaptation caused by the abnormal experience of the prism) and decreased clustering in the normal zone, indicating that dendritic synapse clustering is correlated with this type of learning (McBride et al., 2008). Importantly, the total number of contacts per dendrite remained constant throughout the experiment, indicating that synaptic contacts were both created and eliminated. Thus, reorganization of the local circuitry during development is accompanied by synaptic clustering.
Assessing the connectivity between neuronal populations with advanced fine-scale circuit mapping methods has also provided evidence that, during development synapses tend to cluster in dendritic domains. Specifically, (Druckmann et al., 2014) found that the connectivity between the CA3-CA1 hippocampal neurons is highly structured and clustered both at the neuronal and at the dendritic branch level. By examining pairs of neurons that shared the same neurogenesis and synaptogenesis time window, the authors found exceptionally high anatomical synaptic clustering (five times larger than “normal” or “random” clustering), indicating selective and highly clustered synapse formation between neurons which share the same developmental history.
Anatomical spine clustering has also been documented during learning. By imaging the formation of spines in motor cortex dendrites, Fu et al. analyzed the spine changes that occur during the learning of a motor task that was repeated over multiple days (Fu et al., 2012). During this learning protocol, the majority of new spines that were formed in adjacent positions in the dendrite were more clustered than control spines (in distances < 5 µm), and the process was dependent on the activation of NMDA receptors. This study showed that newly formed spines, are highly likely to be added to the existing clusters, thus contributing to the refinement or reinforcement of motor learning. In addition, clustered spines were more stable than isolated ones, implying that the arrangement of synapses in clusters may promote the stability of long-term memories. Increased anatomical clustering of potentiated synapses has also been observed in an in vitro study which simulated spaced learning in the hippocampus (Kramár et al., 2012).
Further evidence for anatomical synapse clustering has been provided by the in vivo visualization of plasticity-induced receptor trafficking during learning. Makino &Malinow (Makino & Malinow, 2011) used fluorescently tagged glutamate receptor type 1 (GluR1) subunits to visualize the trafficking of AMPA receptors during normal sensory experience as well as during sensory deprivation (whisker removal). Normal experience (e.g., whisking) triggers coordinated trafficking of GluR1 subunits to nearby synapses in the dendritic tree of somatosensory neurons in mice. The authors estimated the size of synaptic potentiation at approximately 8 µm of dendritic length. The clustering of GluR1 subunits was not evident on sensory-deprived mice, indicating that rich sensory experience results in higher clustering. In addition, mutations in GluR1 subunits that render them insensitive to modulation signals induced by LTP cooperativity prevented the coordinated potentiation of nearby synapses in dendritic branches.
Anatomical clustering has also been documented in the primate cortex (Yadav et al., 2012). Analysis of the locations of spines in the prefrontal cortex of rhesus monkeys confirmed the preference for spatial spine clustering. The clustering of spines was concentrated in the terminal branches, which receive the majority of synaptic inputs and the clusters were found to predominantly contain mushroom and stubby-shaped spines.
Finally, an imaging study which studied the allocation of new spines in dendritic spines related to memory consolidation after sleep may provide evidence to how the allocation of spines can be compartmentalized (Yang et al., 2014). This study showed that different learning tasks cause spine formation on different sets of dendritic branches after sleep consolidation. The formation of these spines was dependent on the reactivation during sleep of the same population of neurons that were active during learning. It appears thus that sleep may promote learning and enable the allocation of synapses encoding for different memories to different dendrites.
2.4.2 Functional clustering
Co-activation of neighboring synapses in the absence of profound differences in spine density, termed functional clustering, has also been documented both in vitro and in vivo, during development and as a result of learning. Using calcium imaging, (Kleindienst et al., 2011) investigated the connectivity and activation patterns in organotypic cultures of the developing hippocampus of rats. The authors found that synapses located within a distance of 16 µm had correlated activity, indicating that synapses tended to activate in clusters. This clustered activation of nearby synapses was crucially depended on neuronal spiking and NMDA receptor activation. In this particular study no evidence was provided regarding the possibility or exclusion of anatomical clustering of co-active synapses.
In vivo imaging also allows the functional mapping of active synapses. By visualizing the synaptic activation of neurons in the barrel cortex of mice, Takahashi et al. found that activated synapses tended to form functional ‘assemblets’ which were synchronized and locally confined (Takahashi et al., 2012). Specifically, nearby spines were found to be significantly co-activated and tended to form functional synaptic which consisted of groups of synapses (2–12 synapses within < 10 µm) in dendritic branches. The spines which participated in these assemblets were larger in size compared to spines that did not form assemblets, indicating that assemblets might have been formed by LTP mechanisms. Indeed, the authors found reduced clustered activation of synapses in tissue that was cultivated in the presence of NMDA receptor antagonist. This experiment also studied the source of the observed clustered co-activation of synapses. The authors found that the clustered activation is attributed to the concurrent activation of the afferent axons which impinge on the synapses. The fact that these afferents synapse in a clustered fashion indicates that synaptic plasticity may have molded a synaptic cluster through either remodeling of the connectivity (i.e. the creation of new synapses) or through the cooperative potentiation of synapses that happened to be proximal to each other.
Overall, the large number of studies providing experimental evidence for both anatomical and functional synaptic clustering (some examples are shown in Figure 2) suggests that clustering may be a common pattern of organization conserved across different brain areas and species (DeBello et al., 2014).
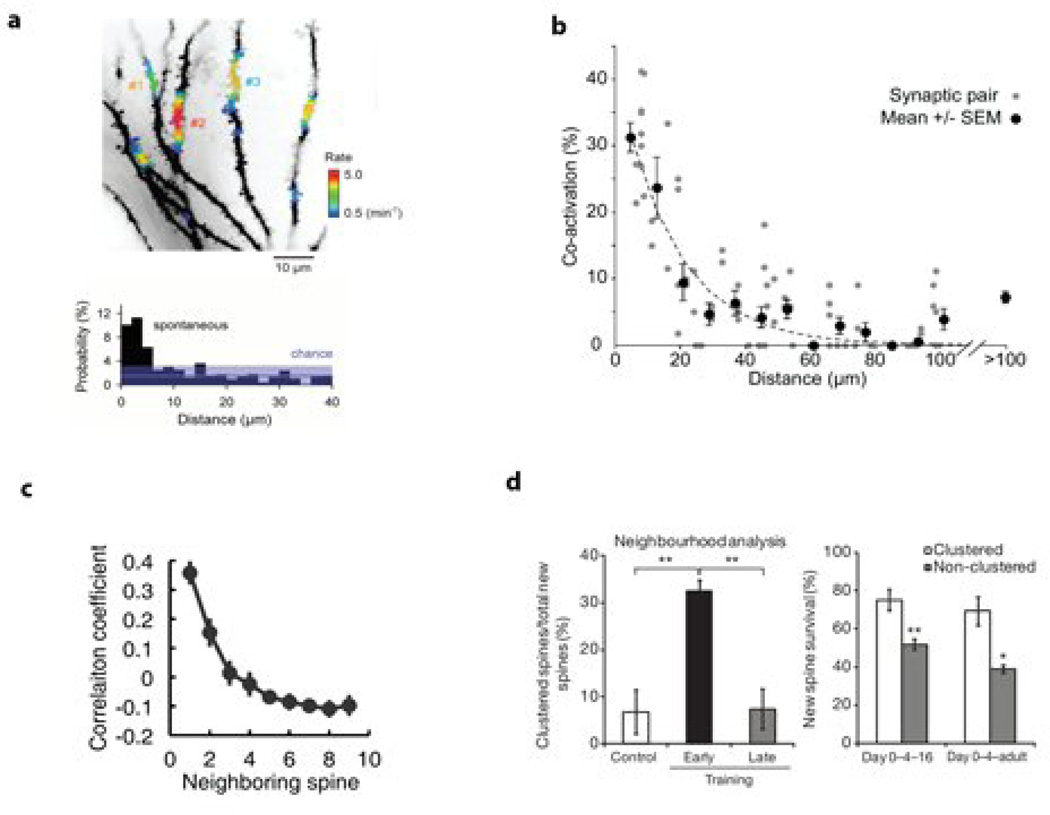
Figure 2. Evidence for synaptic clustering.
a) Top: Observation of functional synaptic clusters ex vivo. Color rate code is the frequency with which these “assemblets” are activated. Bottom: Probability of observing co-activated spines as a function of the inter-spine distance compared to chance level (shaded), as observed in vivo in the mouse barrel cortex. Reproduced with permission (Takahashi, Kitamura et al. 2012).
b) Co-activation of synapse pairs in a developing hippocampal neuron observed ex vivo as a function of the distance between pairs of synapses. Reproduced with permission (Kleindienst, Winnubst et al. 2011).
c) AMPA enrichment is correlated in nearby synapses, as observed using a fluorescently tagged AMPA receptor in vivo. Reproduced with permission (Makino and Malinow 2011)
d) New Spines formed during learning a repetitive motor task are more likely to form clusters. Additionally, clustered spines have a higher survival rate over a 16-day period. Reproduced with permission (Fu, Yu et al. 2012).
2.4.3 Functional properties of neighboring synapses
In addition to identifying the presence of anatomical or functional synaptic clusters, a number of mapping experiments have examined the coding or tuning features of neighboring synapses in sensory cortices. These experiments show that synapses in nearby spines do not necessarily share the same or similar sensory tuning features as one would expect, but instead the tuning of synapses varies widely along the same dendritic branch without an apparent orderly arrangement. More specifically, combining high-speed 2-photon imaging with electrophysiological recordings in the visual (Jia et al., 2010), auditory (X. Chen et al., 2011) and barrel (Varga et al., 2011) cortices, it was shown that synapses on nearby spines in dendrites of pyramidal neurons code for seemingly unrelated orientations, sound frequencies or whiskers and whisker combinations, respectively. These findings appear to contradict the hypothesis of synaptic clustering, which predicts that synapses, which carry correlated information, would form functional synaptic clusters in dendrites. This contradiction can be reconciled with the clustering model, however, if we accept that functional clusters of synapses do not code for a continuum of elementary sensory features (e.g. subsequent letters of the alphabet) but for combinations of such features which form conceptual entities of behaviorally relevant natural stimuli (e.g. words). For example, a functional synapse cluster in the primary auditory cortex could be composed of synapses that are tuned to the frequencies contained in natural speech.
As these frequencies vary over a wide range, the tuning of synapses in a functional synaptic cluster which would respond to natural speech would thus reflect this wide range of frequencies. It would be interesting to investigate experimentally the spatial organization of synapses in response to presentation of combinations of input features that are relevant to the animal (e.g. frequencies contained in behaviorally relevant sounds) to test this hypothesis.
In sum, the experimental evidence provided above suggests that, although synaptic clustering remains an active area of research, there is considerable evidence for spatial synapse clustering, either anatomical, functional or both, as a result of learning. As described in section 2, this clustering provides advantages for memory storage by, for example, ensuring the propagation of ‘strong’ or ‘important’ signals as opposed to noise via the facilitation of nonlinear responses and dendritic spikes. Further experiments are expected to clarify the roles of distributed and clustered connectivity, as well as the range of its functional implications.
2.4.4 A cautionary note
It should be noted that dendritic function and plasticity have been studied at different spatial scales and therefore the role of synaptic clustering in enabling dendritic braches or stretches inside them to act as computational elements remains unclear. On the one hand, whole dendritic branches have been proposed to be elementary units of memory function and storage (Branco & Häusser, 2010; Govindarajan et al., 2006). The level of compartmentalization of function and plasticity in this case is limited by a) the extent in which local signals can be integrated nonlinearly (e.g. in order to generate dendritic spikes) and b) by the spatial spread of biochemical signaling which would allow cooperative plasticity. In this context, dendritic ‘subunits’, which represent electrically independent thin terminal branches (receiving the bulk of incoming synaptic connections (Megıas, Emri, Freund, & Gulyas, 2001)), have been studied theoretically and shown to provide an additional level of computation in the cell (Archie & Mel, 2000; M. P. Jadi et al., 2014; Migliore, Novara, & Tegolo, 2008; Poirazi et al., 2003b; X. E. Wu & Mel, 2009). This model is corroborated by experiments which show nonlinear synaptic integration at distances < 40 µm (Polsky et al., 2004) and studies of synaptic tagging which find LTP cooperativity within dendritic segments < 60 µm (Govindarajan, Israely, Huang, & Tonegawa, 2011). Importantly, both synaptic integration and synaptic plasticity at dendritic subunits have been found to be relatively isolated between branch points (Govindarajan et al., 2011; Polsky et al., 2004). On the other hand, the computational properties of smaller-scale synaptic interactions which occur at < 20 µm have also been theorized to provide computational advantages in learning (Bartlett W. Mel, 1992), pattern discrimination (Bartlett W Mel, 1991) and orientation tuning (Bartlett W. Mel, Ruderman, & Archie, 1998) and such synaptic arrangements could arise from STDP (Iannella & Tanaka, 2006).
It is yet unclear how the properties of synapse clustering at the microscopic level affect the dendritic-subunit compartmentalization. However, the experimental evidence discussed in section 2.4 shows that the spatial arrangement of activated synapses within dendritic branches is, in many cases, neither uniform nor random. More experiments are needed to clarify how this spatial clustering influences the ability of dendritic subunits (whether branches or stretches of a branch) to act as independent computational elements.
2.5 Plasticity of dendritic excitability
In parallel with synaptic clustering, synaptic activity can change the conductance of ionic currents which determine the excitability of neuronal membranes. This dynamic adaptation of intrinsic excitability can influence the way dendrites integrate synaptic inputs and consequently affect the neuronal output. Lasting changes in excitation properties are a form of plasticity called plasticity of intrinsic excitability, which can be induced by electrical stimulation in vitro, or through exposure to an enriched environment. For instance, A-type currents are persistently downregulated after LTP-inducing excitatory stimulation of CA1 pyramidal neurons, leading to increased dendritic excitability (Frick, Magee, & Johnston, 2004). In addition, LTP and LTD protocols in CA1 result in the increase and decrease, respectively, of the linear summation of postsynaptic responses. This bidirectional plasticity of excitability reflects changes in the hyperpolarization-activated Ih currents and NMDA receptors (Wang, Xu, Wu, Duan, & Poo, 2003). In the latter study, while blockade of both Ih and IA channels had similar effects in increasing the linearity of synaptic summation, the increase in summation that follows LTP was mainly attributed to modulation of Ih.
The plasticity of intrinsic excitability can be locally restricted to dendritic branches via alterations in branch coupling strengths: repeatedly triggering dendritic spikes in a dendrite in vitro leads to a slow but long-lasting increase in the coupling strength of the dendrite to the somatic depolarization which is mediated by downregulation of A-type potassium currents (Losonczy et al., 2008). The regulation of dendritic excitability may thus be exploited as a compartmentalized memory storage mechanism during learning. Indeed, it has been shown that exposure of rats to an enriched environment leads to the enhancement of dendritic spike propagation selectively in a subset of dendritic branches of CA1 neurons (Makara, Losonczy, Wen, & Magee, 2009).
In the above-mentioned studies, the localized alteration of dendritic excitability was attributed to the activity-dependent regulation of ionic currents. It is not clear however if the plasticity of dendritic excitability requires synaptic input or synaptic plasticity. To investigate this issue, a recent study tested the plasticity of dendritic excitability using photostimulation of hippocampal dendrites in neurons infected with a channelrhodopsin-2 (ChR2) vector (Labno, Warrier, Wang, & Zhang, 2014). Interestingly, the pairing of dendritic photocurrent with somatic spiking induced localized depression of excitability. This depression was not dependent on synaptic activation or LTP induction, but was sensitive to calcium. Moreover, the depression was conferred by changes in the A-type potassium current, similarly to the case of branch strength potentiation. These two examples suggest a key role of the A-type K+ channel in regulating the local intrinsic excitability of pyramidal neuron dendrites.
Taken together, these results indicate that dendritic excitability is a dynamic property which can undergo long-term potentiation or depression in response to specific stimulation protocols, and it can be dissociated from synaptic plasticity. Therefore, the plasticity of dendritic excitability can serve as a mechanism that modulates localized synaptic activity and contributes to localized memory storage, so that it can be considered part of the memory engram (Legenstein & Maass, 2011; Per Jesper Sjöström et al., 2008; Zhang & Linden, 2003).
Overall, section 2 provided compelling evidence that both synaptic plasticity and the plasticity of intrinsic ionic conductances enhance the flexibility of dendritic responses, endowing dendritic branches, or even membrane stretches inside them, with key computational features that support a pivotal role in memory formation. The molecular processes that may underlie such a model of localized information storage are discussed next.
3. Synaptic Consolidation and Protein Capture
The mechanisms which determine the effect of synaptic plasticity and the resulting changes in connectivity on memory formation are numerous and complex. Indeed, the induction of synaptic plasticity involves networks of signaling cascades and kinase activation which have timescales that vary from seconds to hours (Bhalla, 2011; Citri & Malenka, 2008). Nevertheless, a high-level model of memory consolidation can capture important aspects of memory encoding and its protein dependence. The synaptic tagging and capture model, described in the next section is such a powerful framework for characterizing the role of late-LTP processes in memory encoding and provides the foundation for models of localized, clustered memory storage (Govindarajan et al., 2006; Rogerson et al., 2014).
3.1 The Synaptic Tagging and Capture (STC) model
According to the model of synaptic tagging and capture (Frey & Morris, 1997; Redondo & Morris, 2011), the consolidation of synaptic potentiation which is believed to underlie permanent memory storage occurs in a number of phases. Initially, synaptic plasticity sets a local synaptic tag in the synapse targeted for potentiation or depression. The synthesis of plasticity related proteins (PRPs), which are required for synaptic potentiation, takes place over a period of hours after learning. Finally, the synapses that were tagged capture the synthesized PRPs in order to stabilize their synaptic strengths.
The synaptic tagging and capture (STC) model was initially proposed based on LTP experiments showing that protein-synthesis-dependent LTP could be induced under conditions of protein synthesis inhibition, given that stimulation of a different pathway occurred within a few hours (Frey & Morris, 1997; Reymann & Frey, 2007). The phenomenon was observed by the facilitation of late-LTP in weakly stimulated synapses through the activation of a second strongly stimulated set of synapses (Frey & Morris, 1997). Weak stimulation normally results in early-LTP, a form of LTP that decays after a few hours. As a consequence of the strong stimulus, however, weakly stimulated (but tagged) synapses, -which would normally only express early-LTP- can capture the PRPs generated by strong stimulation of the second set of synapses and thus express late-LTP, as observed in synaptic cross-capture experiments (Redondo & Morris, 2011; Sajikumar & Frey, 2004).
The implications of the STC model for learning and memory concern the interactions that are expected to arise between learning events that occur within a defined time horizon. This interaction was tested in behavioral experiments which involved pairing a weak learning protocol with a strong form of learning or environmental novelty. By pairing a weak learning protocol, which normally induces short-term memory, with environmental novelty, it was found that novelty –considered a strong event- promotes the formation of long-term memory, presumably through the mechanisms of STC (Ballarini, Moncada, Martinez, Alen, & Viola, 2009; de Carvalho Myskiw, Benetti, & Izquierdo, 2013; Moncada & Viola, 2007). Indeed, the memory enhancement was prevented when the protein synthesis inhibitor anisomycin was introduced along with the environmental novelty. These experiments suggested that there are alternative sources of memory-related proteins needed for late-LTP, some of which can be localized within dendritic branches as discussed in the next session.
3.2 STC and local protein synthesis
Synaptic plasticity involves numerous kinases, phosphatases, as well as various molecular signaling pathways (Citri & Malenka, 2008), the activation of which may be spatially constrained. This suggests that molecular signaling cascades may underlie the cooperativity effects observed in plasticity induction within nearby sites in dendrites (Bhalla, 2011; Govindarajan et al., 2011; Harvey et al., 2008; Murakoshi et al., 2011). In addition, the PRPs required for plasticity can be synthesized by the protein synthesis machinery existing in the cell soma, or they may be translated locally by ribosomes which exist in dendritic arbors. Several studies have established the existence of ribosomes, in hippocampal dendrites (Bodian, 1965; Bourne & Harris, 2011; OSWALD Steward & Levy, 1982; Sutton & Schuman, 2006). These ribosome complexes were found to be near synaptic sites, thus positioned appropriately to facilitate plasticity. Moreover, a large number of mRNAs have been found in hippocampal dendrites and many of those mRNAs code for known synaptic proteins (Cajigas et al., 2012; O Steward & Schuman, 2007). This evidence suggests that dendrites may support local forms of plasticity that do not depend on transcription or somatic protein synthesis by sustaining their own protein synthesis which is triggered by local signaling pathways. Dendritic protein synthesis was first identified to be a requirement for rapid synaptic potentiation under exposure to BDNF (Kang & Schuman, 1996) and has since been found to be required for many forms of synaptic plasticity (Sutton et al., 2006).
Based on these observations, it has been proposed that the phenomenon of STC may occur at the dendritic level. In this case, it can lead to LTP interactions and to the generation of activity associations at the dendritic level, via the strengthening and stabilization of neighboring synapses, thus facilitating synaptic clustering (Govindarajan et al., 2006; Kelleher, Govindarajan, & Tonegawa, 2004; Rogerson et al., 2014). A recent in vitro study confirmed that STC can take place at the level of the dendritic branch (Govindarajan et al., 2011). Using glutamate uncaging and two-photon imaging, it was shown that local protein synthesis induced in a synaptic spine could convert the early-LTP of a nearby spine to late-LTP via synaptic capture mechanisms. This conversion of early-LTP to late-LTP was dependent on the time interval between the stimulation and protein synthesis and on the distance between the two spines. The strength of this synaptic cross-capture was inversely proportional to the distance and it did not occur for distances larger than 70µm on the same dendritic branch or larger than 50µm when the synapses were placed in sister branches. In addition, the authors found that during LTP consolidation, tagged synapses compete for the capture of available proteins, indicating that the availability of synaptic proteins is a limiting factor for dendritic STC.
It should be noted, however, that certain forms of LTP require gene transcription along with protein synthesis, such as the long-term LTP induced during theta burst stimulation and serotonin application in hippocampal slices (Y.-Y. Huang & Kandel, 2007). It is thus possible that different forms of LTP are employed by different brain areas, and/or under different stages of memory consolidation (Izquierdo et al., 2006), which could lead to differential spatial distributions of potentiated synapses (i.e. clustered vs. distributed). While this is the dominant view of the role of protein synthesis in learning and memory, there are alternative views where protein synthesis plays a more passive role. Protein synthesis may be more peripherally involved in the formation of memories where it is needed to replace proteins ‘consumed’ by learning or the inhibition of protein synthesis impairs the general well-being of neurons, leading to an inability to deliver resources needed for memory formation (Gold, 2008; Routtenberg & Rekart, 2005). This view suggests that protein synthesis is necessary to just replenish resources that are depleted by memory formation mechanisms.
3.3 STC and memory formation
An intriguing consequence of dendritic STC is that it can become a mechanism for associating temporally close memories, which are expected to form memory representations captured by nearby synapses. This mechanism would result in the generation of functional and/or anatomical clusters of synapses that code for memories that are temporally related over large time frames, defined by the temporal overlap between the life time of the synaptic tag and the upregulation of PRPs. According to such a model, the cross-capture of proteins between synapses that express either LTP or LTD can lead to clustered formation of memory engrams (Govindarajan et al., 2006). As described by previous modeling work, clustered formation of memory engrams whereby synapses with correlated activity are grouped within dendritic branches greatly expands the information storage capacity of neural tissue (Poirazi & Mel, 2001). Moreover, synaptic clustering resulting from STC has been hypothesized to mediate the cellular and behavioral binding of memories that are temporally related (Rogerson et al., 2014; Silva et al., 2009). More studies are needed to investigate the validity and consequences of this hypothesis.
4. Plasticity of Inhibition Influences Dendritic Integration
The vast majority of plasticity studies have focused on the plasticity of excitatory connections. However, a significant body of recent work has shown that inhibitory connections are also plastic (Z. J. Huang et al., 1999; Kullmann et al., 2012), and often follow the patterns of excitatory contacts (J. L. Chen et al., 2012).
Inhibition plays a major role in shaping neuronal output throughout the brain, and displays significant variability in its magnitude and targeting (Klausberger and Somogyi 2008). For example, dendritically-targeted inhibition regulates the input-output-transformations in CA1 pyramidal cells and increases the threshold for dendritic spiking, while perisomatic inhibition controls oscillatory activity and suppresses the amplitude of dendritic spikes (M. Jadi et al., 2012; Lovett-Barron et al., 2012). Thus, neurons can tailor their output by adjusting the location of inhibition that they receive in different dendritic pathways. Importantly, computational modeling suggests that local inhibition can regulate the plasticity of excitatory connections, by controlling calcium influx through the postsynaptic voltage (Bar-Ilan, Gidon, & Segev, 2012). Indeed, somatostatin-expressing inhibitory neurons exert local compartmentalized control over the Ca2+ signals within individual spines, in a way that can directly affect the biochemical signaling of plasticity processes (Chiu et al., 2013). Inhibitory synaptic boutons, on the other hand, have been found to be unstable and are believed to continuously probe the postsynaptic membrane for synapse formation (Schuemann, Klawiter, Bonhoeffer, & Wierenga, 2013).
In relation to the clustering hypothesis, a recent study examined the dynamics of inhibitory synapses along with the dynamics of spines in the mouse visual cortex (J. L. Chen et al., 2012). The authors found that inhibitory synapses made on dendritic spines were more dynamic than inhibitory synapses on dendritic shafts. Importantly, these spines followed closely the arrangements of other dynamic spines within ~10µm which were presumably excitatory, thus indicating that inhibitory synapses exhibit the same clustered plasticity pattern of excitatory synapses. In addition, inhibitory synapses in spines had different remodeling kinetics during altered sensory experience. These findings show that inhibitory synapses closely follow the spatial arrangement of excitatory synapses, and therefore they are likely to form anatomical clusters with them.
The coordinated plasticity of excitatory and inhibitory connections has been suggested to play a major role in the stability of simulated cortical networks, where a “detailed balance” of excitation/inhibition is required (Tim P Vogels & Abbott, 2009).
5. Regulation of synaptic plasticity by local homeostasis
Apart from inhibition, homeostatic plasticity is another major balancing mechanism which acts continuously to regulate synaptic plasticity in the long term. The effect of homeostatic regulation on synaptic clustering and dendritic excitability is thus critical for in a model of memory formation where dendritic branches play a key role. Homeostatic phenomena include changes in the intrinsic membrane excitability, the regulation of presynaptic transmitter release, the balancing between excitation and inhibition as well as alterations in neuronal connectivity and modulation of synaptic strengths (Turrigiano & Nelson, 2004). As the focus of this review is synapse clustering, we briefly discuss evidence regarding local homeostasis taking place within dendrites. For a more in-depth discussion on homeostatic plasticity mechanisms we direct the reader to a number of excellent reviews (Abraham, 2008; Pozo & Goda, 2010; Turrigiano & Nelson, 2004).
Homeostatic plasticity can be local, thus regulating only the synapses located within a specific branch (Rabinowitch & Segev, 2008). Such specificity may be critical for the maintenance of existing memory engrams during the continuous formation of new ones. Recent studies have identified forms of homeostatic plasticity which operate at the level of the synapse and/or the branch. Hou et al. found that the increasing the presynaptic firing that drives a synapse, caused a selective downregulation of GluA1 receptors in the postsynaptic site (Hou, Gilbert, & Man, 2011). This indicates a synapse-specific homeostatic regulation mechanism that compensates for increased synaptic input. Another study used a combination of two-photon glutamate uncaging and imaging to show that individual synapses can compensate for changes in their input via homeostatic regulation that is independent of their neighboring synapses (Béïque, Na, Kuhl, Worley, & Huganir, 2011). In this case, homeostatic plasticity was found to require the immediate early gene Arc, which is known to be implicated in synaptic plasticity. The functional role of localized or synapse-specific homeostatic plasticity is not straightforward, as it seems to be a rule that counters the action of LTP in individual synapses, thus leading to erasure of information. A computational study, on the other hand, has shown that a local form of homeostasis which acts on groups of nearby synapses in dendrites can mediate normalization of responses without disrupting synaptic plasticity (Rabinowitch & Segev, 2008).
Along with the homeostasis of excitatory connectivity, homeostasis of inhibitory connections acts in concert with synaptic potentiation to regulate the strength of inhibition (Echegoyen, Neu, Graber, & Soltesz, 2007). At the dendritic level, the dynamic interplay between excitatory and inhibitory synaptic inputs depends on their spatial proximity (G. Liu, 2004) while at the synaptic level AMPA receptor expression at single synapses is homeostatically regulated under conditions that increase either inhibition or excitation (Hou et al., 2011). The latter indicates the ability of synapses to self-regulate their synaptic potency.
How homeostasis, synaptic plasticity, plasticity of excitability and plasticity of inhibition interact to regulate the action of synapses at the dendritic level is not clear, as these processes have different timescales and roles. Intrinsic excitability appears to positively enhance Hebbian plasticity, while homeostasis provides a form of negative feedback to synaptic action (Per Jesper Sjöström et al., 2008). Interestingly, dendrite-specific LTP coupled with homeostatic depression was computationally predicted to maximize the learning capacity of a medial temporal lobe model implementing online learning (X. E. Wu & Mel, 2009). The dendritic learning rules led to an order-of-magnitude increase in the capacity of the network compared to Hebbian learning.
Homeostatic mechanisms provide the final touch in the delicate interplay of local and global factors that oversee the formation of memory representations, starting at synaptic mechanisms and including dendritic, neuronal and network processes. In the following section we discuss how computational models can be used to dissect and/or integrate the roles of different plasticity mechanisms in neuronal function and memory formation.
6. Computational modeling of memory-related neuronal functions: the role of active dendrites and synapse clustering
Computational models have been instrumental in the quest to understand memory functions and particularly the role of synaptic and dendritic processes in memory formation. In this section we review some of the most important computational models that have been proposed over the last few decades to underlie learning and memory formation in the brain. We focus on models taking into account active dendritic processes and plasticity mechanisms that may influence both the strength and the spatial arrangement of synapses.
6.1 Computational models investigating the role of synapse clustering in neuronal function
The ability of neocortical pyramidal neurons to selectively respond to spatially inhomogeneous patterns of synaptic activation (i.e. randomly distributed vs. spatially clustered activation of synapses) was first predicted by Bartlett Mel, using computational modeling (B W Mel, 1993; Bartlett W. Mel, 1992; Bartlett W Mel, 1991), and was termed “cluster sensitivity”. These early studies identified the boosting provided by the clustered spatial arrangement of synapses and predicted a key role of NMDA conductances in this phenomenon. This effect was found to be robust for a wide range of distributions of active conductances in dendrites. In addition, the author identified the conditions which would cancel the effects of clustering, namely, the high resistance of spine necks, the large synaptic conductances, and the high baseline levels of activity. The advantage of synaptic clustering in the form of in-branch localization (whereby co-active synapses were positioned uniformly within a given branch) for the discrimination and memory capacity of neurons was studied by the same group later on, using theoretical neuron models (Poirazi & Mel, 2001). When dendritic nonlinearities and in-branch localization were taken into account, the pattern discrimination capacity of simplified model neurons and neural networks expanded dramatically, suggesting that synapse clustering could serve as a mechanism for maximizing storage capacity in the brain. Moreover, dendritic nonlinearities and in-branch localization were also predicted by the same group to underlie translation-invariant orientation tuning in visual “complex” cells (Bartlett W. Mel et al., 1998). This latter work was the first to predict a key role of dendritic supralinearities in orientation tuning of single neurons in the visual cortex, a prediction that recently received experimental support from in vivo experiments (Lavzin et al., 2012; S. L. Smith et al., 2013)
A follow-up study using a detailed biophysical model of a CA1 pyramidal cell was able to tease out the mathematical formula underlying “cluster sensitivity”. The authors found that the terminal apical dendrites of these neurons summate synaptic inputs nearly independent from each other, using a sigmoidal (or thresholding) activation function (Poirazi et al., 2003a). This prediction has been verified experimentally for the basal dendrites of cortical pyramidal neurons (Polsky et al., 2004) as well as the radial oblique dendrites of CA1 pyramidal cells (Losonczy & Magee, 2006). Moreover, this finding led to the proposal of a “2-layer” model of neuronal integration, according to which, the firing rate of a CA1 pyramidal neuron in response to a large range of synaptic stimuli can be predicted by a two layer mathematical abstraction, in which terminal dendrites act as independent nonlinear thresholding units whose combined output goes through a second thresholding unit at the cell body (Poirazi et al., 2003b). This 2-stage model was recently demonstrated to match the processing of basal trees in cortical pyramidal neurons (Behabadi & Mel, 2014) and has received experimental support based on anatomical findings in CA1 pyramidal cells (Katz et al., 2009). For an extensive discussion on the 2-layer neuronal model please see an excellent recent review (M. P. Jadi et al., 2014).
An extension of the 2-layer hypothesis put forward by Hausser and colleagues entails that the interaction between proximal and distal integrative regions of a pyramidal cell may allow for an additional layer of integration, which is multiplicative in nature (Häusser et al., 2003). A different augmented version of the 2-layer model for cortical pyramidal neurons, where basal and apical tuft regions are treated as independent compartments and dendritic responses depend on the spatial arrangement of both excitatory and inhibitory inputs is put forward in (M. P. Jadi et al., 2014). Finally, based on the ability of dendrites to release neurotransmitters and neuromodulators, it has been proposed that a neuron may have multiple outputs, with each dendritic subunit performing local integration. In this model, morphology and biophysical properties determine the hierarchical arrangement of dendritic subunits (Branco & Häusser, 2010; Ludwig, 2005).
Overall, the above are a few modeling studies that establish the role of dendritic nonlinearities, which are maximally expressed when synaptic contacts are activated in clusters, in the functioning of neuronal cells and circuits.
6.2 Computational models investigating the role of dendritic nonlinearities and synapse clustering in memory functions
The functional implications of synapse clustering and dendritic nonlinearities which relate explicitly to memory functions have also been modeled in a number of computational studies. Models that included plasticity mechanisms are discussed in the next session. In a single cell model of a layer 5 PFC pyramidal neuron, working memory was simulated via the induction of persistent (beyond the end of the stimulus) activity. It was found that positioning of synapses towards the tips of the basal dendrites increased the probability of persistent firing due to the generation of larger, NMDA-dependent, dendritic spikes (Sidiropoulou & Poirazi, 2012). Similar findings were seen at the microcircuit level, whereby NMDA-dependent dendritic spikes were predicted to support persistent activity induction within a group of just a few L5 PFC cells. In the absence of NMDA dendritic spikes, the size of the network required to support persistent activity, the cellular correlate of working memory, increased dramatically (Papoutsi, Sidiropoulou, Cutsuridis, & Poirazi, 2013; Papoutsi, Sidiropoulou, & Poirazi, 2012). In a computational study using realistic neuronal morphologies and active properties, Migliore et al (Migliore et al., 2008) examined the role of active dendrites of CA1 neurons in the binding of multiple inputs which arrive at their radial oblique dendrites. Their results suggest that CA1 neurons have a preferred number of radial dendritic inputs that maximizes their capacity to recognize multiple features. The authors suggest thus a link between this number and the well known limitation of short term memory to 7 items. Finally, another computational study investigated the role of dendritic function in the spatial working memory circuit by varying the mode of nonlinearity and the configuration of inhibition (Morita, 2008). In this case dendritic compartmentalization enabled the formation of accurate memory that was dependent on the contrast of the external input, but not its intensity. The requirement for this was the existence of either tuned global dendritic inhibition or local dendritic inhibition tuned with global somatic inhibition.
It should be noted that none of the above mentioned studies incorporated synaptic or dendritic plasticity rules. Neuron models with nonlinear dendrites that implement realistic plasticity rules can help investigate memory-related phenomena that go beyond working memory and make predictions about the properties of the resulting memory traces (Clopath, Ziegler, Vasilaki, Büsing, & Gerstner, 2008; Cutsuridis, Cobb, & Graham, 2010; Govindarajan et al., 2006; Legenstein & Maass, 2011; Morita, 2008). While the mechanisms of these plasticity rules are not entirely known, models can incorporate simple phenomenological abstractions to study their effect in memory. Only few studies, however, have explicitly examined how active dendritic properties in combination with local plasticity rules can shape memory engram formation.
Wu & Mel explored the capacity of the monosynaptic pathway from Schaffer collaterals to CA2 pyramidal dendrites for “online” (one-shot) learning (X. E. Wu & Mel, 2009). This study found the dendritic-specificity of LTP, along with homeostatic depression, enables efficient memory utilization. The learning rules which that enabled this CA3-to-CA1 system to perform online learning include a dual-threshold LTP, sparse synaptic plasticity, binary synaptic weight values and the co-occurrence of homeostatic depression and LTP as all-or-none phenomena. Using a model of nonlinear dendrites in combination with the branch strength potentiation (BSP), a computational study showed how nonlinear dendrites could be used to bind combinations of multiple features in dendritic subunits (Legenstein & Maass, 2011). The branch strength potentiation rule induced a competition between dendrites, which allow a neuron to become part of multiple memory traces. In this model, branch strength potentiation enables neurons to specialize in the binding of specific combinations of input features, which could represent the unique characteristics of objects. By inducing competition, a single neuron is thus able to store multiple such combinations in separate dendritic domains. Another recent study examined the implications of the spatial patterning of plasticity proteins in memory using a simplified model of memory consolidation which incorporated simplified dendritic domains (O’Donnell, Sejnowski, & O’Donnell, 2014). The spatial patterning of protein synthesis was found led to the consolidation of memories selectively, even when other events occur simultaneously and are represented by the same neuronal populations. Based on this function, the authors propose a model for selective memory generalization during sleep.
6.3 Creating predictive models of dendritic function
Reliable and predictive modeling of dendritic properties and function requires that models can be constrained well by experimental data. As the study of dendrites is an active area of research, our knowledge of dendritic function and synaptic plasticity is only partially complete. For example, both the size and the spatial extent of functional and/or anatomical dendritic synapse clusters can only be inferred with imaging methods such as calcium imaging, immunolabeling or electron microscopy (Kleindienst et al., 2011; Makino & Malinow, 2011; Takahashi et al., 2012; Yadav et al., 2012). Similarly, the temporal constraints related to synaptic capture, plasticity protein production and homeostatic mechanisms can be found in relevant studies (Govindarajan et al., 2011; Hou et al., 2011; Losonczy et al., 2008). Although recent research has provided a wealth of data at an unprecedented level of detail, much remains to be discovered regarding the particular functions of memory storage. For example, the extent of LTP cooperativity, the protein dependence of LTP consolidation, the ability for dendritic protein synthesis, the molecular basis of synaptic tagging are parameters that crucially affect the properties of memory formation (O’Donnell et al., 2014). However, even with our current knowledge of these mechanisms, we can generate predictive models of memory formation. As knowledge accumulates, these models will be continuously refined, generating more interesting and accurate predictions. Their ability to integrate data and mechanisms that operate at different levels, as well as to separately characterize the contribution of each of these mechanisms, will not only further our understanding of memory functions but also point to new avenues for experimental investigations.
7. Perspective
A large body of experimental studies has recently revealed detailed information about the existence and possible manipulation of memory representations at the cellular level (Silva, Zhou et al. 2009, Josselyn 2010, Rogerson, Cai et al. 2014). In parallel, multiple lines of evidence suggest that the plasticity underlying learning and memory acts at multiple levels: fast spine dynamics are shaped by synaptic activity, dendritic branch excitation properties are regulated by activity and homeostatic mechanisms, neuronal excitability is affected by previous learning, etc. (Figure 3). To complete the picture, the excitability of networks is also controlled by several factors including the plasticity of GABA-mediated inhibition. These findings challenge the old view whereby synapses are considered as independent and elementary units of plasticity. Dendritic branches now emerge as semi-independent units of function and plasticity (Branco and Häusser 2010). The observation of functional and/or anatomical synaptic clusters within dendritic branches implies that synapses may act in groups, formed by cooperative plasticity and local protein synthesis, which exert a nonlinear effect on the output of the cell (Poirazi, Brannon et al. 2003 Poirazi & Mel 2001, Mel 1992, Mel 1993, Govindarajan, Kelleher et al. 2006, Rogerson, Cai et al. 2014). The size of synaptic clusters and their spatial extent can be defined based on observations. The limited experimental evidence that is currently available suggests that even clusters of 2 synapses are functionally relevant (Fu, Yu et al. 2012, Takahashi, Kitamura et al. 2012). Based on a variety of biochemical and electrophysiological data, we can at least estimate the spatial proximity necessary between synapses to receive facilitation of plastic events. Examples include LTP facilitation due to shared protein synthesis within 50–70 microns of a dendritic branch (and its sister) (Govindarajan, Kelleher et al. 2006, Govindarajan, Israely et al. 2011) and diffusion of activated GTPases within 5–10 microns within a dendritic branch (Harvey & Svoboda, 2007; Harvey et al., 2008; Murakoshi et al., 2011; Murakoshi & Yasuda, 2012)
The effect of synaptic clustering on neuronal output is in turn modulated by intrinsic plasticity mechanisms that modify the excitability properties of dendritic branches where groups of synapses reside (Zhang and Linden 2003). The emerging picture suggests that clusters of functionally related synapses, which are formed within dendritic branches under the influence of local activity-dependent and homeostatic mechanisms, are likely to serve as a key computational and memory storage unit in the brain. The time is ripe for undertaking the obvious challenge, namely to design experiments that test the implications of this idea at the behavioral level.
For example, it would be critical to know whether manipulations that do not disrupt canonical synaptic plasticity processes, but interfere with dendritic synaptic clustering alter or disrupt learning and memory at a behavioral level (Lavzin, Rapoport et al. 2012). Furthermore, is there a clinical consequence of abnormal clustering for episodic memory in animals and humans? For example, episodic memory representations require a certain degree of independence from each other to allow for accurate pattern separation and specificity. At the same time, memories must be richly connected for a sequence of experiences to be recalled together. Excessive or hyper-connectedness between each memory may result in the activation of overlapping networks of neurons, dendritic branches or synapses for every memory. This is likely to lead to a lack of pattern separation between memories and poor episodic recall or even catastrophic forgetting (i.e. interference between all memories resulting in no recall). In contrast, if events are too sparsely connected, memory for individual details might be extremely accurate but the general context and relatedness between items will be lost. These extremes can be captured by either over- or under-clustering of synapses, or neurons related to a set of memories (Figure 4).
Figure 4. The Impact of Clustering on Neurological Disease.
Clustering in the normal cortex is balanced between the benefits of associativeness and distinctiveness of episodic memories. Memories are spatially segregated with a moderate degree of overlap. Over-clustering between memories predicts reduced spatial segregation, decreased resolution of individual episodic memories, and the intrusion of remote associations as seen in schizophrenia. Under-clustering is characterized by autonomous spatial arrangements that increase the capacity for distinct episodic memories at the cost of reduced relatedness between memories and/or knowledge domains, as seen in autism. These principles are represented above, in which three distinct memories that share a temporal context are represented in red, blue and green. The Venn diagrams illustrate the general concept of under-clustering in autistics, average clustering in normally-developed brains, and over-clustering in schizophrenics. Synaptic level cross-talk between intracellular signaling cascades during synaptic tagging and capture is illustrated. Dendritic level clustering shows the degree of spatial segregation of synapses within a dendritic branch that results in the formation of multiple processing units. At the cellular level, distinct memories may be allocated to networks of neurons whose overlap may predict the degree of relatedness between them.
Predictions can be made for performance profiles that lie along the clustering continuum. On the extremes, over-clustering should present as an increase in associative thinking but with poor episodic memory due to a lack of pattern separation and increased interference between memories. Schizophrenia is characterized as having a looseness of association of ideas (Bleuler & Zinkin, 1950), and patients perform better than controls on tasks that favor highly associative thinking (Manschreck et al., 1988; Poljakov, 1973). However, on tests of episodic memory, specifically pattern separation, schizophrenics typically perform poorly (Das, Ivleva, Wagner, Stark, & Tamminga, 2014). This is what would be predicted if the associative memory deficit were due to over-clustering. In this case, the encoding of new events activates a normal spread of neurons or dendrites in a network. However, with each subsequent episodic event, a highly redundant, overlapping network is activated, causing interference between events. Consequently, cueing a single event stimulates recall of multiple memories and performance fails.
In contrast, too little clustering would produce normal or even superior episodic memory for individual items, but poor contextual or relational processing. In the case of autism, numerous studies show that performance on recognition memory tests of individual items using a variety of nonsocial stimuli is unimpaired and occasionally superior in autistic patients compared with normal controls (Boucher et al., 2005; D M Bowler, Gardiner, & Grice, 2000; Salmond et al., 2005; Williams, Goldstein, & Minshew, 2006). On the other hand, autistics have difficulty with integrative thinking and contextual processing, such as using semantically-related words to probe contextual processing (D M Bowler, Matthews, & Gardiner, 1997; Dermot M Bowler, Gaigg, & Gardiner, 2010; B. J. Smith, Gardiner, & Bowler, 2007), or categorical information to improve recall of semantically-related words (B. J. Smith et al., 2007). The extreme case of savants is a profound example of a highly localized knowledge that does not generalize nor associate to any other knowledge domain within the individual (Treffert, 2009). Mechanistically, rather than a string of events clustering together to create contextual and temporal meaning, each new experience would stimulate a separate and autononomous pattern of activation, which could strengthen access to each event, and weaken connectivity between events. This could explain how individuals with low cognitive skills and poor performance on some intelligence tests might still display talent in a restricted domain (O’Connor & Hermelin, 1988).
Although these hypothetical, clinical examples of over- and under-clustering have little direct evidence as to their mechanistic explanation, they point to the need for computational models that capture the complexity of nonlinear dendritic properties and make predictions that can be tested in behaving animals and humans. In years to come, experimental and theoretical approaches will have to work side-by-side in unraveling and testing hypotheses concerning the basic dendritic rules that underlie the formation of memory representations in the brain.
In the last twenty years a plethora of approaches, including transgenic mice, have established the importance of stable long-lasting changes in synaptic function in learning and memory (Lee & Silva, 2009). We are now able to explore the behavioral implications of dendritic processing rules. This daunting task will require all of the tools at our disposal, including new computational approaches, optogenetics, in vivo 2-photon imaging, in vivo whole-cell and dendritic recordings, multi-electrode high resolution recordings, etc. We will also need to develop new behavioral tools specifically designed to test the implications of changes in the electrophysiological and structural properties of dendrites, imaging methods paired with neuroinformatics tools that will allow us to track and characterize the dendritic activation patterns of neuronal networks in behaving animals, molecular tools that will inducibly manipulate individual synaptic molecular components in single synapses of spine clusters, fluorescent tags that will make it possible to image the very molecular events that trigger, filter, stabilize, modify and maintain synaptic clusters, etc. Importantly, all of these developments may only be a heart-beat away (if not here already). Experimentalists will need simple and predictive computational models that they can use to design the behavioral-based dendritic clustering experiments that seem impossibly complex today, but that undoubtedly will be routine tomorrow. It is both sobering and enormously exciting that the tools and results described here are bringing us closer than ever to understanding one of the greatest mysteries of all times: how experiences shape our memories and the very core of who we are as individuals.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R37-AG13622 and P50-MH0779720) to AJS and (1F32MH097413) to DJC, the European Research Council Starting Grant dEMORY (GA 311435) to PP and the BIOSYS project, Action KRIPIS, (No MIS-448301, 2013SE01380036) of the General Secretariat for Research and Technology, Ministry of Education, Greece and the European Regional Development Fund (Sectoral Operational Programme: Competitiveness and Entrepreneurship, NSRF 2007–2013)/ European Commission. We would like to thank Vassilis Cutsuridis, Athanasia Papoutsi, Adam Frank and Tristan Shuman for their insightful comments.
List of Abbreviations
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APA
American Psychological Association
- BDNF
Brain-derived neurotrophic factor
- CA1
CornuAmmonis area 1
- CA3
CornuAmmonis area 3
- ChR2
Channel rhodopsin 2
- CREB
(cAMP response element-binding protein)\
- EPSP
Excitatory post-synaptic potential
- fMRI
Functional magnetic resonance imaging
- GluA1
Glutamate receptor 1
- GluR1
Glutamate receptor type 1
- HFA
High-functioning autistics
- IPSP
Inhibitory post-synaptic potential
- LTD
Long-term depression
- LTP
Long-term potentiation
- MAPK
Mitogen-activated protein kinase
- mGRASP
Mammalian GFP reconstitution across synaptic partners
- MLFA
Moderately low functioning autistics
- mTOR
Mechanistic target of rapamycin
- NMDA
N-methyl-D-aspartate receptor
- PFC
Prefrontal cortex
- PRPs
Plasticity related proteins
- STC
Synaptic Tagging and Capture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are several definitions for the word “Dendrite”, depending on the cited papers. According to Wikipedia, Dendrites (from Greek δένδρον déndron, "tree") are the branched projections of a neuron that act to propagate the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. We consider as a single “dendritic branch” the section contained within two consecutive branch points (or one branch point and the tip for terminal dendrites) that is not part of trunk.
As shown in Figure 1, there are two major types of synaptic clustering: (a) anatomical clustering, whereby spines form morphologically distinct groups of several spine heads located in distances less than 5 microns from each other and (b) functional clustering, where spine density is uniform but nearby synapses (located within 10 – 20 microns) are activated synchronously.
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews. Neuroscience. 2008;9(5):387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Antic SD, Zhou W-LW-L, Moore AR, Short SM, Ikonomu KD. The decade of the dendritic NMDA spike. Journal of Neuroscience Research. 2010;88(14):2991–3001. doi: 10.1002/jnr.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archie KA, Mel BW. A model for intradendritic computation of binocular disparity. Nature Neuroscience. 2000;3(1):54–63. doi: 10.1038/71125. [DOI] [PubMed] [Google Scholar]
- Ariav G, Polsky A, Schiller J. Submillisecond precision of the input-output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons. The Journal of Neuroscience. 2003;23(21):7750–7758. doi: 10.1523/JNEUROSCI.23-21-07750.2003. Retrieved from http://www.jneurosci.org/content/23/21/7750.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14599–14604. doi: 10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ilan L, Gidon A, Segev I. The role of dendritic inhibition in shaping the plasticity of excitatory synapses. Frontiers in Neural Circuits. 2012 Apr;6:118. doi: 10.3389/fncir.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Bi X, Gall C, Lynch G. The biochemistry of memory: The 26year journey of a “new and specific hypothesis”. Neurobiology of Learning and Memory. 2011;95(2):125–133. doi: 10.1016/j.nlm.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behabadi BF, Mel BW. Mechanisms underlying subunit independence in pyramidal neuron dendrites. Proceedings of the National Academy of Sciences. 2014;111(1):498–503. doi: 10.1073/pnas.1217645111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behabadi BF, Polsky A, Jadi M, Schiller J, Mel BW. Location-Dependent Excitatory Synaptic Interactions in Pyramidal Neuron Dendrites. PLoS Computational Biology. 2012;87:e1002599. doi: 10.1371/journal.pcbi.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque J-C, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proceedings of the National Academy of Sciences. 2011;108(2):816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla US. Multiscale interactions between chemical and electric signaling in LTP induction, LTP reversal and dendritic excitability. Neural Networks : The Official Journal of the International Neural Network Society. 2011;24(9):943–949. doi: 10.1016/j.neunet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Bleuler E, Zinkin J. Dementia Praecox or the Group of Schizophrenias. New York: International University Press; 1950. [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bodian D. A suggestive relationship of nerve cell RNA with specific synaptic sites. Proceedings of the National Academy of Sciences of the United States of America. 1965;53(2):418. doi: 10.1073/pnas.53.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Cowell P, Howard M, Broks P, Farrant A, Roberts N, Mayes A. A combined clinical, neuropsychological, and neuroanatomical study of adults with high functioning autism. Cogn Neuropsychiatry. 2005;10(3):165–213. doi: 10.1080/13546800444000038. doi:P01U93L300864771 [pii] 10.1080/13546800444000038. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Nanoscale analysis of structural synaptic plasticity. Current Opinion in Neurobiology. 2011;22(3):1–11. doi: 10.1016/j.conb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler DM, Gaigg SB, Gardiner JM. Multiple list learning in adults with autism spectrum disorder: Parallels with frontal lobe damage or further evidence of diminished relational processing? Journal of Autism and Developmental Disorders. 2010;40(2):179–187. doi: 10.1007/s10803-009-0845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler DM, Gardiner JM, Grice SJ. Episodic memory and remembering in adults with Asperger syndrome. J Autism Dev Disord. 2000;30(4):295–304. doi: 10.1023/a:1005575216176. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11039856. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Matthews NJ, Gardiner JM. Asperger’s syndrome and memory: similarity to autism but not amnesia. Neuropsychologia. 1997;35(1):65–70. doi: 10.1016/s0028-3932(96)00054-1. doi:S0028-3932(96)00054-1 [pii] [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Schüz A. Cortex: Statistics and Geometry of Neuronal Connectivity. Springer; 1998. Cortical architectonics; pp. 135–137. [Google Scholar]
- Branco T, Häusser M. The single dendritic branch as a fundamental functional unit in the nervous system. Current Opinion in Neurobiology. 2010;20(4):494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Branco T, Häusser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron. 2011;69(5):885–892. doi: 10.1016/j.neuron.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajigas J, Tushev G, Will TJJ, Dieck S, Fuerst N, Schuman EMM, tom Dieck S. The Local Transcriptome in the Synaptic Neuropil Revealed by Deep Sequencing and High-Resolution Imaging. Neuron. 2012;74(3):453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S, Yuste R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron. 1999;22(2):383–394. doi: 10.1016/s0896-6273(00)81098-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10069343. [DOI] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PTC, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74(2):361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Leischner U, Rochefort NLNL, Nelken I, Konnerth A. Functional mapping of single spines in cortical neurons in vivo. Nature. 2011;475(7357):501–505. doi: 10.1038/nature10193. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GCR, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340(6133):759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DBB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431(7010):782–788. doi: 10.1038/nature03012. Retrieved from http://www.nature.com/nature/journal/v431/n7010/abs/nature03012.html. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Clopath C, Ziegler L, Vasilaki E, Büsing L, Gerstner W. Tag-trigger-consolidation: a model of early and late long-term-potentiation and depression. PLoS Computational Biology. 2008;4(12):e1000248. doi: 10.1371/journal.pcbi.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsuridis V, Cobb S, Graham BP. Encoding and retrieval in a model of the hippocampal CA1 microcircuit. Hippocampus. 2010;20(3):423–446. doi: 10.1002/hipo.20661. [DOI] [PubMed] [Google Scholar]
- Das T, Ivleva EI, Wagner AD, Stark CEL, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophrenia Research. 2014;159(1):193–197. doi: 10.1016/j.schres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho Myskiw J, Benetti F, Izquierdo I. Behavioral tagging of extinction learning. Proceedings of the National Academy of Sciences. 2013;110(3):1071–1076. doi: 10.1073/pnas.1220875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBello WM, McBride TJ, Nichols GS, Pannoni KE, Sanculi D, Totten DJ. Input clustering and the microscale structure of local circuits. Frontiers in Neural Circuits. 2014;8:112. doi: 10.3389/fncir.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S, Feng L, Lee B, Yook C, Zhao T, Magee JC, Kim J. Structured Synaptic Connectivity between Hippocampal Regions. Neuron. 2014 doi: 10.1016/j.neuron.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Echegoyen J, Neu A, Graber KD, Soltesz I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: synaptic scaling, intrinsic plasticity and age-dependence. PloS One. 2007;2(8):e700. doi: 10.1371/journal.pone.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RGMG. Synaptic tagging and long-term potentiation. Nature. 1997;385(6616):533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nature Neuroscience. 2004;7(2):126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;1(7387):92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science (New York NY) 2012;335(6075):1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S, Migliore M, Magee JC. On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24(49):11046–11056. doi: 10.1523/JNEUROSCI.2520-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Protein synthesis inhibition and memory: formation vs amnesia. Neurobiology of Learning and Memory. 2008;89(3):201–211. doi: 10.1016/j.nlm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long- term potentiation. Nature. 2002;418(6895):326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gómez González JF, Mel BW, Poirazi P. Distinguishing Linear vs. Non-Linear Integration in CA1 Radial Oblique Dendrites: It’s about Time. Frontiers in Computational Neuroscience. 2011;5:44. doi: 10.3389/fncom.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Israely I, Huang S-Y, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69(1):132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nature Reviews. Neuroscience. 2006;7(7):575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- Han J-H, Kushner Sa, Yiu AP, Cole CJ, Matynia A, Brown Ra, Josselyn Sa. Neuronal competition and selection during memory formation. Science (New York NY) 2007;316(5823):457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Hardie J, Spruston N. Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2009;29(10):3233–3241. doi: 10.1523/JNEUROSCI.6000-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450(7173):1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science (New York NY) 2008;321(5885):136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Mel B, Hausser M. Dendrites: bug or feature? Current Opinion in Neurobiology. 2003;13(3):372–383. doi: 10.1016/s0959-4388(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Häusser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290(5492):739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. The Organization of Behavior. Wiley; 1949. [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nature Reviews. Neuroscience. 2001;2(12):880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hopfield JJ. Artificial neural networks. Circuits and Devices Magazine, IEEE. 1988;4(5):3–10. [Google Scholar]
- Hou Q, Gilbert J, Man H-Y. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72(5):806–818. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Y, Kandel ER. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. The Journal of Neuroscience. 2007;27(12):3111–3119. doi: 10.1523/JNEUROSCI.3908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hübener M, Bonhoeffer T. Searching for engrams. Neuron. 2010;67(3):363–371. doi: 10.1016/j.neuron.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Iannella N, Tanaka S. Synaptic efficacy cluster formation across the dendrite via STDP. Neuroscience Letters. 2006;403(1):24–29. doi: 10.1016/j.neulet.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LRM, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends in Neurosciences. 2006;29(9):496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Jadi MP, Behabadi BF, Poleg-Polsky A, Schiller J, Mel BW. An Augmented Two-Layer Model Captures Nonlinear Analog Spatial Integration Effects in Pyramidal Neuron Dendrites. 2014 doi: 10.1109/JPROC.2014.2312671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadi M, Polsky A, Schiller J, Mel BW. Location-dependent effects of inhibition on local spiking in pyramidal neuron dendrites. PLoS Comput Biol. 2012;8(6):e1002550. doi: 10.1371/journal.pcbi.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464(7293):1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- Josselyn S. Continuing the search for the engram: examining the mechanism of fear memories. Journal of Psychiatry & Neuroscience. 2010;35(4):221–228. doi: 10.1503/jpn.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273(5280):1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Katz Y, Menon V, Nicholson Da, Geinisman Y, Kath WL, Spruston N. Synapse distribution suggests a two-stage model of dendritic integration in CA1 pyramidal neurons. Neuron. 2009;63(2):171–177. doi: 10.1016/j.neuron.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44(1):59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kim J, Kwon J-T, Kim H-S, Josselyn SA, Han J-H. Memory recall and modifications by activating neurons with elevated CREB. Nature Neuroscience. 2014;17(1):65–72. doi: 10.1038/nn.3592. [DOI] [PubMed] [Google Scholar]
- Kim S, Guzman SJ, Hu H, Jonas P. Active dendrites support efficient initiation of dendritic spikes in hippocampal CA3 pyramidal neurons. Nat Neurosci. 2012;15(4):600–606. doi: 10.1038/nn.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Article Activity-Dependent Clustering of Functional Synaptic Inputs on Developing Hippocampal Dendrites. Neuron. 2011;72(6):1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Kramár Ea, Babayan AH, Gavin CF, Cox CD, Jafari M, Gall CM, Lynch G. Synaptic evidence for the efficacy of spaced learning. Proceedings of the National Academy of Sciences. 2012;109(13):5121–5126. doi: 10.1073/pnas.1120700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012;75(6):951–962. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Labno A, Warrier A, Wang S, Zhang X. Local Plasticity of Dendritic Excitability Can Be Autonomous of Synaptic Plasticity and Regulated by Activity-Based Phosphorylation of Kv4. 2. PloS One. 2014;9(1):e84086. doi: 10.1371/journal.pone.0084086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Nevian T. Synaptic clustering by dendritic signalling mechanisms. Current Opinion in Neurobiology. 2008;18(3):321–331. doi: 10.1016/j.conb.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J, Larkum Nevian T, Sandler M, Polsky A, Schiller J, M E. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science. 2009;325(5941):756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ. Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. The Journal of Neuroscience. 2002;22(16):6991–7005. doi: 10.1523/JNEUROSCI.22-16-06991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS. In search of the engram. Symposia of the Society for Experimental. 1950 Retrieved from http://homepage.mac.com/sanagnos/lashley1950.pdf. [Google Scholar]
- Lavzin M, Rapoport S, Polsky A, Garion L, Schiller J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature. 2012;490(7420):397–401. doi: 10.1038/nature11451. [DOI] [PubMed] [Google Scholar]
- Lee Y-S, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10(2):126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legenstein R, Maass W. Branch-Specific Plasticity Enables Self-Organization of Nonlinear Computation in Single Neurons. Journal of Neuroscience. 2011;31(30):10787–10802. doi: 10.1523/JNEUROSCI.5684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375(6530):400–403. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci. 2004;7(4):373–379. doi: 10.1038/nn1206. doi:10.1038/nn1206 nn1206 [pii] [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longordo F, To M, Ikeda K, Stuart G. Sublinear integration underlies binocular processing in primary visual cortex. Nature Neuroscience. 2013;16(6):714–723. doi: 10.1038/nn.3394. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50(2):291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452(7186):436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun X-H, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nature Neuroscience. 2012;15(3):423–430. S1–S3. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- Ludwig M. Dendritic neurotransmitter release. Springer; 2005. [DOI] [PubMed] [Google Scholar]
- Mainen Z, Sejnowski T. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996 doi: 10.1038/382363a0. Retrieved from http://papers.cnl.salk.edu/PDFs/Influence of Dendritic Structure on Firing Pattern in Model Neocortical Neurons 1996-3205.pdf. [DOI] [PubMed] [Google Scholar]
- Major G, Larkum ME, Schiller J. Active properties of neocortical pyramidal neuron dendrites. Annual Review of Neuroscience. 2013;36:1–24. doi: 10.1146/annurev-neuro-062111-150343. [DOI] [PubMed] [Google Scholar]
- Makara JK, Losonczy A, Wen Q, Magee JC. Experience-dependent compartmentalized dendritic plasticity in rat hippocampal CA1 pyramidal neurons. Nature Neuroscience. 2009;12(12):1485–1487. doi: 10.1038/nn.2428. [DOI] [PubMed] [Google Scholar]
- Makara JK, Magee JC. Variable Dendritic Integration in Hippocampal CA3 Pyramidal Neurons. Neuron. 2013;80(6):1438–1450. doi: 10.1016/j.neuron.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R. Article Compartmentalized versus Global Synaptic Plasticity on Dendrites Controlled by Experience. Neuron. 2011;72(6):1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Milavetz JJ, Ames D, Weisstein CC, Schneyer ML. Semantic priming in thought disordered schizophrenic patients. Schizophrenia Research. 1988;1(1):61–66. doi: 10.1016/0920-9964(88)90041-2. doi:0920-9964(88)90041-2 [pii] [DOI] [PubMed] [Google Scholar]
- McBride TJ, Rodriguez-Contreras A, Trinh A, Bailey R, DeBello WM. Learning drives differential clustering of axodendritic contacts in the barn owl auditory system. The Journal of Neuroscience. 2008;28(27):6960–6973. doi: 10.1523/JNEUROSCI.1352-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Rumelhart DE. A distributed model of human learning and memory. Parallel Distributed Processing: Explorations in the Microstructure of Cognition. 1986;2:170–215. [Google Scholar]
- Megıas M, Emri ZS, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102(3):527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mel BW. The clusteron: toward a simple abstraction for a complex neuron. Advances in neural information processing systems. 1991:35–42. [Google Scholar]
- Mel BW. NMDA-based pattern discrimination in a modeled cortical neuron. Neural Computation. 1992;4(4):502–517. [Google Scholar]
- Mel BW. Synaptic integration in an excitable dendritic tree. Journal of Neurophysiology. 1993;70(3):1086–1101. doi: 10.1152/jn.1993.70.3.1086. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8229160. [DOI] [PubMed] [Google Scholar]
- Mel BW, Ruderman DL, Archie KA. Translation-invariant orientation tuning in visual “complex” cells could derive from intradendritic computations. The Journal of Neuroscience. 1998;18(11):4325–4334. doi: 10.1523/JNEUROSCI.18-11-04325.1998. Retrieved from http://www.jneurosci.org/cgi/content/abstract/18/11/4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Novara G, Tegolo D. Single neuron binding properties and the magical number 7. Hippocampus. 2008;18(11):1122–1130. doi: 10.1002/hipo.20480. [DOI] [PubMed] [Google Scholar]
- Minsky M, Papert S. Perceptron: an introduction to computational geometry. Vol. 19. Cambridge: The MIT Press; 1969. p. 88. Expanded Edition. [Google Scholar]
- Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2007;27(28):7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K. Possible role of dendritic compartmentalization in the spatial working memory circuit. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28(30):7699–7724. doi: 10.1523/JNEUROSCI.0059-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472(7341):100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Yasuda R. Postsynaptic signaling during plasticity of dendritic spines. Trends in Neurosciences. 2012;35(2):135–143. doi: 10.1016/j.tins.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Larkum ME, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nature Neuroscience. 2007;10(2):206–214. doi: 10.1038/nn1826. [DOI] [PubMed] [Google Scholar]
- O’Connor N, Hermelin B. Low intelligence and special abilities. J Child Psychol Psychiatry. 1988;29(4):391–396. doi: 10.1111/j.1469-7610.1988.tb00732.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3063716. [DOI] [PubMed] [Google Scholar]
- O’Donnell C, Sejnowski TJ, O’Donnell C. Selective memory generalization by spatial patterning of protein synthesis. Neuron. 2014;82(2):398–412. doi: 10.1016/j.neuron.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi A, Sidiropoulou K, Cutsuridis V, Poirazi P. Induction and modulation of persistent activity in a layer V PFC microcircuit model. Frontiers in Neural Circuits. 2013 Oct;7:161. doi: 10.3389/fncir.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi A, Sidiropoulou K, Poirazi P. Memory Mechanisms in Health and Disease: Mechanistic Basis of Memory. 1st ed. World Scientific Pub Co Inc.; 2012. Memory Beyond Synaptic Plasticity: The Role of Intrinsic Neuronal Excitability; p. 444. Retrieved from http://www.amazon.com/Memory-Mechanisms-Health-Disease-Mechanistic/dp/9814366692. [Google Scholar]
- Patterson M, Yasuda R. Signalling pathways underlying structural plasticity of dendritic spines. Spine. 2011;1 doi: 10.1111/j.1476-5381.2011.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Paley SL, Webster H, de F. The Fine Structure of the Nervous System. Philadelphia: Saunders; 1976. [Google Scholar]
- Poirazi P, Brannon T, Mel BW. Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell. Neuron. 2003a;37(6):977–987. doi: 10.1016/s0896-6273(03)00148-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12670426. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Brannon T, Mel BW. Pyramidal neuron as two-layer neural network. Neuron. 2003b;37(6):989–999. doi: 10.1016/s0896-6273(03)00149-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12670427. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of Active Dendrites and Structural Plasticity on the Memory Capacity of Neural Tissue. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Poljakov J. Schizophrenie und Erkenntnistatigkeit. Stuttgart, Germany: Hippokrates; 1973. [Google Scholar]
- Polsky A, Mel BW, Schiller J. Computational subunits in thin dendrites of pyramidal cells. Nature Neuroscience. 2004;7(6):621–627. doi: 10.1038/nn1253. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch I, Segev I. Two opposing plasticity mechanisms pulling a single synapse. Trends in Neurosciences. 2008;31(8):377–383. doi: 10.1016/j.tins.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal SRy, y Cajal SR. Neue Darstellung vom histologischen Bau den Centralnervensystems. Arch. Anat. Entwick. 1893:319–428. [Google Scholar]
- Redondo RL, Morris RGM. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12(1):17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Reymann KG, Frey JU. The late maintenance of hippocampal LTP: requirements, phases, “synaptic tagging”, “late-associativity” and implications. Neuropharmacology. 2007;52(1):24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Rogerson T, Cai DJ, Frank A, Sano Y, Shobe J, Lopez-Aranda MF, Silva AJ. Synaptic tagging during memory allocation. Nature Reviews Neuroscience. 2014;15:157–169. doi: 10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends in Neurosciences. 2005;28(1):12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiology of Learning and Memory. 2004;82(1):12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Connelly A, Friston KJ, Gadian DG, Vargha-Khadem F. The role of the medial temporal lobe in autistic spectrum disorders. Eur J Neurosci. 2005;22(3):764–772. doi: 10.1111/j.1460-9568.2005.04217.x. doi:EJN4217 [pii] 10.1111/j.1460-9568.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites. 2000a;1261(1997):285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000b;404(6775):285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Schiller J, Schiller Y, Stuart G, Sakmann B. Calcium action potentials restricted to distal apical dendrites of rat neocortical pyramidal neurons. The Journal of Physiology. 1997;505(3):605–616. doi: 10.1111/j.1469-7793.1997.605ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuemann A, Klawiter A, Bonhoeffer T, Wierenga CJ. Structural plasticity of GABAergic axons is regulated by network activity and GABAA receptor activation. Frontiers in Neural Circuits. 2013 Jun;7:113. doi: 10.3389/fncir.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev I. Untangling Dendrites with Quantitative Models. Science. 2000;290(5492):744–750. doi: 10.1126/science.290.5492.744. [DOI] [PubMed] [Google Scholar]
- Segev I, Rall W. Computational study of an excitable dendritic spine. Journal of Neurophysiology. 1988;60(2):499–523. doi: 10.1152/jn.1988.60.2.499. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2459320. [DOI] [PubMed] [Google Scholar]
- Segev I, Rall W. Excitable dendrites and spines: earlier theoretical insights elucidate recent direct observations. Trends in Neurosciences. 1998;21(11):453–460. doi: 10.1016/s0166-2236(98)01327-7. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Brayton RK, Miller JP, Segev I, Rinzel J, Rall W. Signal enhancement in distal cortical dendrites by means of interactions between active dendritic spines. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(7):2192–2195. doi: 10.1073/pnas.82.7.2192. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=397519&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulou K, Poirazi P. Predictive Features of Persistent Activity Emergence in Regular Spiking and Intrinsic Bursting Model Neurons. PLoS Comput Biol. 2012;8(4):e1002489. doi: 10.1371/journal.pcbi.1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science (New York NY) 2009;326(5951):391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA. Neuronal arithmetic. Nature Reviews. Neuroscience. 2010;11(7):474–489. doi: 10.1038/nrn2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Rancz EA, Roth A, Häusser M. Dendritic excitability and synaptic plasticity. Physiological Reviews. 2008;88(2):769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32(6):1149–1164. doi: 10.1016/s0896-6273(01)00542-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11754844. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Gardiner JM, Bowler DM. Deficits in free recall persist in Asperger’s syndrome despite training in the use of list-appropriate learning strategies. J Autism Dev Disord. 2007;37(3):445–454. doi: 10.1007/s10803-006-0180-4. [DOI] [PubMed] [Google Scholar]
- Smith SL, Smith IT, Branco T, Häusser M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature. 2013;503(7474):115–120. doi: 10.1038/nature12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nature Reviews. Neuroscience. 2008;9(3):206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. The Journal of Neuroscience. 1982;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Handbook of Neurochemistry and Neurobiology. 2007;7 doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125(4):785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127(1):49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y. Locally synchronized synaptic inputs. Science (New York NY) 2012;335(6066):353–356. doi: 10.1126/science.1210362. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Miles R, Whittington MA, Toth K. A branching dendritic model of a rodent CA3 pyramidal neurone. J. Physiol. 1994;481(Pt_1):79–95. doi: 10.1113/jphysiol.1994.sp020420. Retrieved from http://jp.physoc.org/cgi/content/abstract/481/Pt_1/79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffert DA. The savant syndrome: an extraordinary condition. A synopsis: past, present, future. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1522):1351–1357. doi: 10.1098/rstb.2008.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature Reviews. Neuroscience. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Varga Z, Jia H, Sakmann B, Konnerth A. Dendritic coding of multiple sensory inputs in single cortical neurons in vivo. PNAS. 2011;108(37) doi: 10.1073/pnas.1112355108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Abbott LF. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nature Neuroscience. 2009;12(4):483–491. doi: 10.1038/nn.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Sprekeler H, Zenke F, Clopath C, Gerstner W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science (New York NY) 2011;334(6062):1569–1573. doi: 10.1126/science.1211095. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xu N, Wu C, Duan S, Poo M. Bidirectional Changes in Spatial Dendritic Integration Accompanying Long-Term Synaptic Modifications. Neuron. 2003;37(3):463–472. doi: 10.1016/s0896-6273(02)01189-3. [DOI] [PubMed] [Google Scholar]
- Wei DS, Mei YA, Bagal A, Kao JP, Thompson SM, Tang CM. Compartmentalized and binary behavior of terminal dendrites in hippocampal pyramidal neurons. Science (New York NY) 2001;293(5538):2272–2275. doi: 10.1126/science.1061198. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. The profile of memory function in children with autism. Neuropsychology. 2006;20(1):21–29. doi: 10.1037/0894-4105.20.1.21. doi:2006-01440-003 [pii] 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnubst J, Lohmann C, Jontes J, Wang H, Niell C. Synaptic clustering during development and learning: the why when, and how. Frontiers in Molecular Neuroscience. 2012 May;5:70. doi: 10.3389/fnmol.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G-Y, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nature Neuroscience. 2001;4(2):151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Wu XE, Mel BW. Capacity-enhancing synaptic learning rules in a medial temporal lobe online learning model. Neuron. 2009;62(1):31–41. doi: 10.1016/j.neuron.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Gao YZ, Rodriguez A, Dickstein DL, Susan L, Luebke JI, Wearne SL. Morphologic evidence for spatially clustered spines in apical dendrites of monkey neocortical pyramidal cells. The Journal of Comparative Neurology. 2012;520(13):2888–2902. doi: 10.1002/cne.23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan W-B. Sleep promotes branch-specific formation of dendritic spines after learning. Science (New York NY) 2014;344(6188):1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Dendritic spines and distributed circuits. Neuron. 2011;71(5):772–781. doi: 10.1016/j.neuron.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4(11):885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]