Abstract
Objective
The association of high-density lipoprotein particle (HDL-P) with atherosclerosis may be stronger than that of HDL-cholesterol (HDL-C) and independent of conventional cardiovascular risk factors. Whether associations persist in populations at low risk of coronary heart disease (CHD) remains unclear. This study examines the associations of HDL-P and HDL-C with carotid intima-media thickness (cIMT) and plaque counts among Japanese men, who characteristically have higher HDL-C levels and a lower CHD burden than those in men of Western populations.
Methods
We cross-sectionally examined a community-based sample of 870 Japanese men aged 40-79 years, free of known clinical cardiovascular disease (CVD) and not on lipid-lowering medication. Participants were randomly selected among Japanese living in Kusatsu City in Shiga, Japan.
Results
Both HDL-P and HDL-C were inversely and independently associated with cIMT in models adjusted for conventional CHD risk factors, including low-density lipoprotein cholesterol (LDL-C) and diabetes. HDL-P maintained an association with cIMT after further adjustment for HDL-C (P<0.01), whereas the association of HDL-C with cIMT was noticeably absent after inclusion of HDL-P in the model. In plaque counts of the carotid arteries, HDL-P was significantly associated with a reduction in plaque count, whereas HDL-C was not.
Conclusion
HDL-P, in comparison to HDL-C, is more strongly associated with measures of carotid atherosclerosis in a cross-sectional study of Japanese men. Findings demonstrate that, HDL-P is a strong correlate of subclinical atherosclerosis even in a population at low risk for CHD.
Keywords: High-density lipoprotein (HDL) particle, High-density lipoprotein (HDL) cholesterol, subclinical atherosclerosis, carotid intima-media thickness (cIMT), plaque count
Introduction
Many studies have reported an inverse association between high-density lipoprotein cholesterol (HDL-C) and coronary heart disease (CHD).1-3 This has led to the notion that cardiovascular risk may drop significantly once HDL-C levels are increased. 4 However, recent trials involving pharmacological increases in HDL-C levels have reported no significant effects on the reduction of carotid intima-media thickness (cIMT)5, the progression of coronary atherosclerosis,6 or any other cardiovascular measurement.7, 8 Also, a large mendelian randomization study has shown that some polymorphisms associated with genetically higher HDL-C levels do not lower risk of myocardial infarction9. Lack of improved cardiovascular outcomes with increased HDL-C has stressed the view that increasing HDL-C levels may not directly translate to decreases in cardiovascular risk10 and, thus has led to a surge of interest in identifying other features of HDL that can be targeted for assessing cardiovascular risk.
Recently, total HDL particle (HDL-P) concentration has been shown to be a marker of reduced cardiovascular risk11-13 and some evidence suggests that this is independent of HDL-C12. However, studies on HDL-P were largely limited to Western populations, which are known to have a higher risk of CHD and lower levels of HDL-C than less vulnerable regions of Asia, particularly Japan.14-16 Whether associations persist in these regions at lower risk for CHD and with higher HDL-C levels remains unclear. Our objective is to evaluate the association of HDL-C and HDL-P with subclinical atherosclerosis in a population-based sample of Japanese men.
Methods
Study participants
The Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA) aims to examine various factors associated with subclinical atherosclerosis. The design of this study is described elsewhere.17 In brief, from 2006 to 2008, 1,094 Japanese men aged 40 to 79 years were randomly selected from the general population in Kusatsu City, Shiga, Japan. After excluding those on lipid-lowering medications (n=168) and missing information on HDL-P, HDL-C or lipid-lowering medications (n=56), 870 remained for analysis in the current report. All participants provided written informed consent. The study complies with the Declaration of Helsinki and was approved by the Institutional Review Board of Shiga University of Medical Science, Otsu, Japan.
Factors collected through physical examinations include height, weight, blood pressure, and a variety of other measures. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Blood pressure was measured twice in a seated position after a 5 minute rest, using an automated sphygmomanometer (BP-8800; Omron Colin, Tokyo, Japan). The average of two measurements was used.
Hypertension was defined as systolic blood pressure (SBP) ≥140mm Hg, diastolic blood pressure (DBP) ≥90mm Hg, or as the use of antihypertensive medications. Diabetes mellitus (DM) was defined as a hemoglobin A1c (HbA1c) ≥6.1% (Japan Diabetes Society criteria; equivalent to HbA1c≥6.5% in National Glycohemoglobin Standardization Program)18 a fasting glucose ≥6.99 mmol/l (126 mg/dL), or the use of antidiabetic medications.
A self-administered questionnaire was used to collect data on medical history, medication use, smoking, alcohol intake, and other lifestyle behaviours with confirmation by trained technicians.
Laboratory Measurements
Blood samples were drawn from participants after a 12-hour fast and centrifuged soon after coagulation. Standard lipids, including total cholesterol and triglycerides (TG), were measured using enzymatic techniques. HDL-C was measured after heparin-calcium precipitation. Measurements were standardized according to guidelines from the Center for Disease Control and Prevention/Cholesterol Reference Method Laboratory Network (CDC/CRMLN).19 Friedewald's formula was used to estimate low-density lipoprotein cholesterol (LDL-C) levels in men with TG <4.52mmol/l (400 mg/dl). For higher TG levels, LDL-C was treated as missing.
HDL-P concentration was determined by nuclear magnetic resonance (NMR) spectroscopy using serum samples stored at −80°C,20 and shipped on dry ice to LipoScience Inc ( Raleigh, North Carolina, US). Concentrations were obtained from amplitudes of distinct spectroscopic NMR signals of the lipid methyl group, characteristic of each subclass. Reproducibility of NMR-measured HDL-P has been examined and measurements have a coefficient of variation <2%. 21
Intima-media thickness and plaque counts of carotid arteries
Ultrasound measurements of the carotid arteries were performed by sonographers following an established protocol of the Ultrasound Research Laboratory at the University of Pittsburgh.17, 22 A Toshiba XarioSSA-660A scanner (Toshiba Medical Systems, Japan), equipped with a 7.5MHz linear-array imaging probe, was used for high-resolution B-mode ultrasound of the carotid arteries. Sonographers scanned both right and left carotid arteries.
In both arteries, the IMT of the common carotid artery (CCA), carotid bulb, and internal carotid artery were measured. For the CCA segment, both near and far walls were examined 1 cm proximal to the bulb. For the bulb and internal carotid artery segments, only far walls were examined. cIMT was defined as the mean of the eight IMT values measured in both arteries.
Plaque was defined as focal thickening lesion (>10% protrusion compared to adjacent areas) with an IMT of ≥1mm. The total number of plaques in CCA, bulb, and internal carotid artery of both left and right carotid arteries were counted.
Statistical Analyses
Participant demographics were described according to quartile of HDL-P and HDL-C. P-values for trend across the quartiles were determined either using linear regression when a response variable is continuous (such as age), or using logistic regression when it is categorical (such as current smoker or not).
A dose-response relationship between HDL measures and subclinical atherosclerosis was investigated by obtaining adjusted means of cIMT and plaque counts across quartiles of HDL-P and HDL-C using linear regression. We then calculated a difference in cIMT per 1 standard deviation (SD) increase in HDL-P or HDL-C, treating them as continuous variables.
For carotid plaque, we modeled plaque count as an overdispersed integer response following a negative binomial distribution. Regression coefficients have been transformed to percentages, indicating the percent reduction (or excess) in plaque counts per 1 SD increase in HDL-P or HDL-C.
In regression models, we chose the following adjusting covariates as they are established cardiovascular risk factors: age (years), SBP (mmHg), hypertension medication (yes/no), current smoker (yes/no), current alcohol intake (g/day), DM (yes/no), LDL-C (mmol/l) [this set was defined as “base covariates”] and HDL-P or HDL-C (mmol/l).
Analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina) and two-tailed P-values of <0.05 were considered significant.
Results
Study Participants and characteristic trends with HDL-P and HDL-C
Characteristics of study participants according to quartiles of HDL-P and HDL-C are displayed in Table 1A and 1B. Mean (SD) characteristics of all participants included 63.3(10) years for age, 834 (184)μm for cIMT and 2.4(2.4) for plaque count (75.4% of all participants had presence of plaque ≥1). Men with higher HDL-P tended to be younger, leaner, have less prevalence of DM, and consumed more alcohol. The same was also true for HDL-C with the exception of age. Additionally, men with higher HDL-C tended to have less prevalence of hypertension and were less likely to be current smokers. Among lipids, HDL-P and HDL-C were positively related to each other. LDL-C was negatively associated with both HDL-P and HDL-C.
Table 1A. Characteristics of participants (n=870), aged 40-79 years, across quartiles of HDL particle concentration, 2006 - 2008, Kusatsu, Shiga, Japan.
| Characteristic | Quartile of HDL-P | P trend | |||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | ||
|
|
|
|
|
|
|
| Age, years | 68.2 ± 8.3 | 64.4 ± 9.7 | 60.7 ± 10.2 | 60.0 ± 9.6 | <0.001 |
| Body mass index, kg/m2 | 23.9 ± 3.2 | 23.1 ± 2.8 | 23.3 ± 3.0 | 23.1 ± 2.8 | 0.016 |
| SBP, mmHg | 138 ± 16 | 133 ± 19 | 133 ± 19 | 140 ± 22 | 0.089 |
| Hypertension, %† | 57.7 | 47.0 | 46.1 | 53.2 | 0.803 |
| Diabetes, % (Type 2) | 21.8 | 17.7 | 16.6 | 17.0 | 0.033 |
| Current smoker, % | 34.6 | 36.3 | 27.2 | 35.8 | 0.512 |
| Alcohol intake (g/day) | 12.3 ± 18.4 | 17.3 ± 20.8 | 23.4 ± 25.2 | 43.4 ± 34.1 | <0.001 |
| Triglycerides, mmol/l | 1.36 ± 0.70 | 1.31 ± 0.74 | 1.38 ± 1.00 | 1.53 ± 1.11 | 0.010 |
| LDL-C, mmol/l‡ | 3.31 ± 0.87 | 3.33 ± 0.80 | 3.33 ± 0.71 | 3.05 ± 0.83 | <0.001 |
| HDL-C, mmol/l | 1.20 ± 0.31 | 1.44 ± 0.34 | 1.62 ± 0.36 | 1.88 ± 0.47 | <0.001 |
Values are mean ± SD, or % (as indicated).
Hypertension is defined as SBP ≥140mmHg or DBP ≥ 90mmHg or use of anti- hypertensive medication. Diabetes is defined as glycated hemoglobin ≥ 6.5% (NGSP) or fasting glucose ≥ 6.99 mmol/l or use of anti-diabetic medication.
LDL-C was calculated by Friedewald equation. [LDL-C (mg/dl) = total cholesterol (mg/dl) - HDL cholesterol (mg/dl) -triglyceride(mg/dl)/5] SBP, systolic blood pressure; HDL, high-density lipoprotein; HDL-C, HDL cholesterol; HDL-P, HDL particle; LDL, low-density lipoprotein cholesterol.
Quartiles of HDL-P are as follows (1): 13.9 to 29.8μmol/l; n=220, (2): 29.9 to 33.4 μmol/l; n=215, (3): 33.5 to 37.8 μmol/l; n=217, and (4): 37.9 to 68.9 μmol/l; n=218. P-values for trend were obtained using linear regression (for continuous variables) or logisitc regression (for categorical variables) as per 1 unit increase in HDL-P.
Table 1B. Characteristics of participants (n=870), aged 40-79 years, across quartiles of HDL cholesterol concentration, 2006 - 2008, Kusatsu, Shiga, Japan.
| Characteristic | Quartile of HDL-C | P trend | |||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | ||
|
|
|
|
|
|
|
| Age, years | 64.1 ± 9.5 | 63.7 ± 9.5 | 62.2 ± 10.6 | 63.4 ± 10.3 | 0.129 |
| Body mass index, kg/m2 | 24.6 ± 3.0 | 23.9 ± 2.8 | 23.0 ± 2.9 | 21.9 ± 2.6 | <0.001 |
| SBP, mmHg | 137 ± 17 | 136 ± 19 | 136 ± 18 | 135 ± 23 | 0.168 |
| Hypertension, %† | 55.2 | 50.5 | 56.4 | 41.4 | 0.008 |
| Diabetes, % (Type 2) | 20.8 | 22.0 | 20.5 | 9.5 | 0.001 |
| Current smoker, % | 42.5 | 32.7 | 35.0 | 23.3 | <0.001 |
| Alcohol intake (g/day) | 18.4 ± 23.8 | 19.0 ± 24.2 | 27.9 ± 30.0 | 30.9 ± 30.9 | <0.001 |
| Triglycerides, mmol/l | 1.91 ± 1.01 | 1.49 ± 1.11 | 1.23 ± 0.63 | 0.96 ± 0.43 | <0.001 |
| LDL-C, mmol/l‡ | 3.36 ± 0.85 | 3.47 ± 0.82 | 3.18 ± 0.72 | 3.02 ± 0.79 | <0.001 |
| HDL-P, μmol/l | 28.9 ± 4.6 | 32.5 ± 4.2 | 36.4 ± 5.7 | 39.0 ± 7.1 | <0.001 |
Values are mean ± SD, or % (as indicated).
Hypertension is defined as SBP ≥140mmHg or DBP ≥ 90mmHg or use of anti- hypertensive medication. Diabetes is defined as glycated hemoglobin ≥ 6.5% (NGSP) or fasting glucose ≥ 6.99 mmol/l or use of anti-diabetic medication.
LDL-C was calculated by Friedewald equation. [LDL-C (mg/dl) = total cholesterol (mg/dl) - HDL cholesterol (mg/dl) -triglyceride(mg/dl)/5]
Quartiles of HDL-C are as follows (1): 0.67 to 1.19 mmol/l; n=212, (2): 1.22 to 1.45 mmol/l; n=214, (3): 1.46 to 1.78 mmol/l; n=234, and (4): 1.81 to 3.88 mmol/l; n=210. P-values for trend were obtained using linear regression (for continuous variables) or logisitc regression (for categorical variables) as per 1 unit increase in HDL-C.
HDL-P and HDL-C associations with cIMT and carotid plaque
Results of quartile analyses are depicted in Figure 1. With adjustment for base covariates, higher quartiles of HDL-P and HDL-C were both associated with smaller cIMT (panels A & B, dashed blue lines). The overall inverse relationship of HDL-P was maintained with further adjustments for HDL-C (panel A, solid red line). In contrast, the observed inverse association of HDL-C was noticeably absent after adjustments for HDL-P (panel B, solid red line). Higher quartiles of HDL-P were associated with lower mean plaque count in both models (panel C), with and without adjustments for HDL-C. Across quartiles of HDL-C, an association with plaque counts was absent (panel D).
Figure 1.
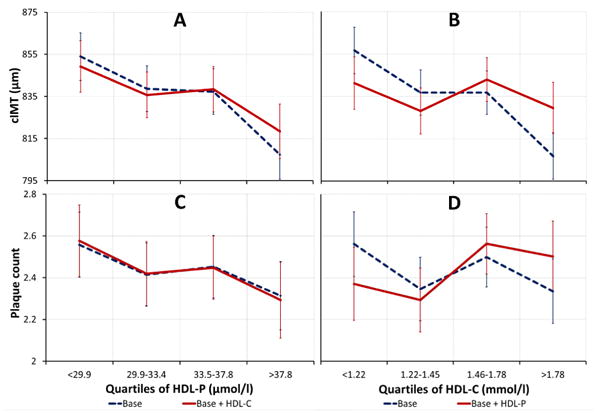
Adjusted mean cIMT (n=870) and plaque count across quartiles of HDL-P and HDL-C. Means were adjusted for base covariates [Base]: age (years), systolic blood pressure (mmHg), hypertension medication (yes/no), smoking status (yes/no), alcohol intake (g/day), diabetes (yes/no) and LDL-C (mg/dl), or adjusted for base covariates and HDL-C (mmol/l) or HDL-P (μmol/L) [Base + HDL-C or HDL-P]. P-values are for linear trend. All linear trends are significant p<0.05, except for HDL-C models: Base + HDL-P (p=0.886) for cIMT and Base (p=0.763) and Base + HDL-P (p=0.187) for plaque counts. Error bars represent standard error of mean.
In Table 2, a 1 SD increase in HDL-P and HDL-C was associated with 47.2μm and 22.1μm lower cIMT, respectively, (unadjusted models). In models adjusted for base covariates, 22.1 μm and 11.1 μm lower cIMT was estimated per 1 SD increase in HDL-P and HDL-C, respectively. After adjustment for HDL-C, the estimated cIMT differences in relation to HDL-P remained significant. In contrast, differences in cIMT with HDL-C were absent when adjusted for HDL-P.
Table 2. Estimated reduction (-) or excess (+) in cIMT per 1 standard deviation increase in HDL-P or HDL-C (n=870), 2006 - 2008.
| Parameter | Model | cIMT (μm) | 95% CI | P value |
|---|---|---|---|---|
| HDL-P | Unadjusted | -47.2 | -59.0 , -35.3 | <0.001 |
| Base covariates | -22.1 | -34.4 , -9.9 | <0.001 | |
| Base covariates + HDL-C | -22.8 | -37.9 , -7.7 | 0.003 | |
|
| ||||
| HDL-C | Unadjusted | -22.1 | -34.3 , -10.0 | <0.001 |
| Base covariates | -11.1 | -22.1 , 0.0 | 0.050 | |
| Base covariates + HDL-P | +1.0 | -12.6 , +14.6 | 0.886 | |
Base covariates include: age, SBP, hypertension medication (yes/no), and smoking status (yes/no), alcohol intake, LDL-C, diabetes (yes/no) and HDL-P or HDL-C. 1 standard deviation of HDL-P = 6.7 μmol/l and of HDL-C = 0.45 mmol/l.
cIMT, carotid intima-media thickness; CI, confidence interval.
Table 3 depicts the estimated reduction or excess in total number of carotid artery plaque counts per 1 SD increase in HDL-P and HDL-C. In unadjusted models, a 1 SD increase in HDL-P and HDL-C was associated with 20.4% and 8.8% reduction in total plaques, respectively. HDL-P was associated with significant reductions in plaque counts even after adjustment for base covariates and HDL-C. Here, a 1 SD increase in HDL-P was associated with 10.4% reduction in number of plaques in the final model adjusted for HDL-C. In contrast, HDL-C had no significant associations with carotid artery plaque in any of the adjusted models.
Table 3. Estimated percent reduction (-) or excess (+) in total number of carotid artery plaque count per 1 standard deviation increase in HDL-P or HDL-C (n=870), 2006-2008.
| Parameter | Model | Estimate (%) | 95% CI | P value |
|---|---|---|---|---|
| HDL-P | Unadjusted | -20.4 | -27.4 , -13.5 | <0.001 |
| Base covariates | -7.8 | -15.1 , -0.5 | 0.037 | |
| Base covariates + HDL-C | -10.4 | -19.7 , -1.1 | 0.029 | |
|
| ||||
| HDL-C | Unadjusted | -8.8 | -15.7 , -1.9 | 0.012 |
| Base covariates | -2.0 | -8.5 , +4.5 | 0.552 | |
| Base covariates + HDL-P | +3.7 | -4.5 , +11.9 | 0.380 | |
Base covariates include: age, SBP, hypertension medication (yes/no), and smoking status (yes/no), alcohol intake, LDL-C, diabetes (yes/no) and HDL-P or HDL-C. 1 standard deviation of HDL-P = 6.7 μmol/l and of HDL-C = 0.45 mmol/l.
Discussion
HDL and carotid atherosclerosis
In this cross-sectional study of Japanese men, free of clinical CVD and not on lipid-lowering medication, the inverse association of HDL-P with cIMT was independent of conventional cardiovascular risk factors, including HDL-C. In contrast, the association of HDL-C with cIMT was attenuated with adjustments for these factors and was absent after adjustment for HDL-P. Furthermore, higher HDL-P, but not higher HDL-C, was inversely and independently associated with lower number of carotid artery plaque after adjustment for cardiovascular risk factors. We demonstrated stronger associations of HDL-P, compared to HDL-C, with two different measures of carotid atherosclerosis (i.e. cIMT and carotid plaque) among a community-based sample of Japanese men. Whether effects of HDL-P are more noticeable in the higher ranges of HDL-C, normally thought to be atheroprotective, warrants consideration.
Our findings are consistent with those of other studies.11-13 The Multi-Ethnic Study of Atherosclerosis (MESA),12 in the United States, for example, reported a significant inverse association of cIMT with HDL-P, but not with HDL-C after adjustments for each other and known risk factors. The Woman's Health Study (WHS), however, did not find a significant inverse association of HDL-P with CHD 23 and instead, only confirmed the inverse association between HDL-C and cardiovascular risk. Possible explanations for the difference in findings may not only be due to the sample population, but also to the randomized clinical trial study design of WHS, involving low-dose aspirin and vitamin E in primary prevention of CVD and cancer.
It is noteworthy that Japanese populations have higher HDL-C levels16 and lower risk of CHD compared to populations of Western countries.24 In addition, we previously reported significantly lower measurements of cIMT and higher levels of HDL-P among Japanese men compared to Caucasian men in the US.15 Despite having a different cardiovascular risk profile, we found that in Japanese men, HDL-P, but not HDL-C, was significantly inversely associated with two measures of carotid atherosclerosis. Hence, our finding, together with results of other studies, suggests that HDL-P may be a novel marker for, and may possibly play a biological role against, the pathogenesis of atherosclerosis.
We have also analyzed HDL size subclass: small (7.3-8.2 nm), medium (8.2-8.8 nm), and large (8.8-13 nm) and their associations with cIMT and carotid plaque counts. However, we found no significant associations of any size with either measure of subclinical atherosclerosis in models adjusted for HDL-C. An atheroprotective effect of subclass size is also controversial 25 and is in need of focused attention.
Potential Mechanisms
The failure of recent randomized controlled trials on HDL-C-increasing drugs for CVD prevention resulted in questioning a causal protective role of HDL-C, which may only be an indicator of cardioprotective mechanisms at work. Nevertheless, the cardioprotective association of HDL is far from being ruled out. It has been suggested that increased particle concentrations of HDL may be indicative of higher reverse cholesterol transport activity.12 The reverse cholesterol transport pathway mediates the efflux of cholesterol from peripheral cells to the liver 26 which is believed to be a key process in preventing plaque formation and progression27 and, thus, many CVDs. Indeed, macrophage-specific cholesterol efflux was found to have a strong inverse association with cIMT and CHD. 28 Furthermore, recent studies have found that HDL-P, and not HDL-C, concentrations are positively associated with cholesterol efflux in patients with type 2 diabetes29 and patients undergoing coronary angiography. 30 These findings parallel our results, with total HDL-P having inverse associations with cIMT. It may be that serum HDL-P concentration is more closely related to the performance rate of cholesterol efflux in the reverse cholesterol transport pathway than HDL-C, with more particles being analogous to increased pathway activity. How HDL affects the cholesterol transport and protects against CVD may depend on its structure and composition, leading to a variety of biological activities, such as anti-inflammation, antioxidation, and vasodilation,25 all of which cannot be assessed by HDL-C alone.26, 31
Limitations and strengths
As our study is cross-sectional and observational, causality cannot be proven in the associations of HDL-P with cIMT and carotid plaque counts. Other limits of our study include the study population being restricted to men of a single ethnic group. However, this is not without its advantage, as homogeneity in a population minimizes confounding from genetic variation. The size of carotid plaques was also not taken into account. Total plaque count may not define the grade or vulnerability of plaques, nevertheless it has been reported that the presence of plaque, alone, in the carotid arteries is positively associated with increased risk of cardiovascular events.34 Thus, carotid plaque count can be used as an alternate indicator of subclinical atherosclerosis.35 The main protein component of HDL particle, apolipoprotein A-1, is a strong predictor of CHD and a key player in reverse cholesterol transport.32, 33 Unfortunately, apolipoprotein A-1 levels were not measured in our serum samples and thus we were unable to look at possible confirmatory associations of HDL-P with cIMT and plaque.
This is the first study, of which we are aware of, to report a significant and inverse association of HDL-P, and not HDL-C, with plaque count in the carotid arteries, even after adjustments for conventional risk factors. This finding, as well as the confirmatory finding of cIMT associations, is in agreement with most literature published on HDL-P and both clinical CVD and subclinical atherosclerosis.11, 12, 36 The fact that we could identify a relationship of HDL-P with cIMT and plaque in a population at low risk of CHD, indicates that HDL-P may be an important predictor of subclinical atherosclerosis and perhaps even more so in populations at high risk. Presently, there are few population-based studies on HDL-P, let alone any on a Japanese population, that characteristically has higher serum HDL-C levels compared to those of Western populations. Thus, our findings provide additional information to the current modest body of knowledge in this area.
Conclusion
In a community-based sample of Japanese men, free of clinical CVD, HDL-P was associated with measures of carotid atherosclerosis (cIMT and plaque count) independent of lipids or lipoproteins and other traditional CVD risk factors. In contrast, associations with HDL-C were absent after accounting for HDL-P. There is need for more scrutiny towards the properties of HDL in general, in order to better understand its involvement in CVD risk processes.
Highlights.
We examined a cross-sectional sample of Japanese men aged 40-79 years.
HDL-P was associated with subclinical atherosclerosis (cIMT and carotid plaque).
The association persisted after adjustment for other factors including HDL-C.
In contrast, HDL-C was unrelated to either measure after HDL-P adjustment.
HDL-P is a stronger correlate of subclinical atherosclerosis than HDL-C.
Acknowledgments
Funding: This work was supported by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology Japan [(A) 13307016, (A) 17209023, (A) 21249043, (A) 23249036, and (A) 25253046]; Glaxo-Smith Klein; and by National Institutes of Health (NIH), USA [R01HL068200].
Research was supported in part by Ichiro Kanehara Foundation Scholarship for Foreign Nationals in Japan [12RY006] to MZ, for the 2013 fiscal year.
Footnotes
Institution where work was performed: Shiga University of Medical Science, Seta Tsukinowa-cho, Otsu, Shiga Japan, 520-2192
The SESSA Research Group: Chairperson: Hirotsugu Ueshima (Center for Epidemiologic Research in Asia, Department of Public Health, Shiga University of Medical Science, Otsu, Shiga).
Co-chairperson: Katsuyuki Miura (Department of Public Health, Shiga University of Medical Science, Otsu, Shiga).
Research members: Minoru Horie, Yasutaka Nakano, Takashi Yamamoto (Department of Cardiovascular and Respiratory Medicine, Shiga University of Medical Science, Otsu, Shiga), Emiko Ogawa (Health Administration Center, Shiga University of Medical Science, Otsu, Shiga), Hiroshi Maegawa, Itsuko Miyazawa (Division of Endocrinology and Metabolism, Department of Medicine, Shiga University of Medical Science, Otsu, Shiga), Kiyoshi Murata (Department of Radiology, Shiga University of Medical Science, Otsu, Shiga), Kenichi Mitsunami (Shiga University of Medical Science, Otsu, Shiga), Kazuhiko Nozaki (Department of Neurosurgery, Shiga University of Medical Science, Otsu, Shiga), Akihiko Shiino (Biomedical MR Science Center, Shiga University of Medical Science, Otsu, Shiga), Isao Araki (Kusatsu Public Health Center, Kusatsu, Shiga) Teruhiko Tsuru (Department of Urology, Shiga University of Medical Science, Otsu, Shiga), Ikuo Toyama (Unit for Neuropathology and Diagnostics, Molecular Neuroscience Research Center, Shiga University of Medical Science, Otsu, Shiga), Hisakazu Ogita, Souichi Kurita (Division of Medical Biochemistry, Department of Biochemistry and Molecular Biology, Shiga University of Medical Science, Otsu, Shiga), Toshinaga Maeda (Central Research Laboratory, Shiga University of Medical Science, Otsu, Shiga), Naomi Miyamatsu (Department of Clinical Nursing Science Lecture, Shiga University of Medical Science, Otsu, Shiga), Toru Kita (Kobe City Medical Center General Hospital, Kobe, Hyogo), Takeshi Kimura (Department of Cardiovascular Medicine, Kyoto University, Kyoto), Yoshihiko Nishio (Department of Diabetes, Metabolism, and Endocrinology, Kagoshima University, Kagoshima), Yasuyuki Nakamura (Department of Living and Welfare, and Cardiovascular Epidemiology, Kyoto Women's University, Kyoto), Tomonori Okamura (Department of Preventive Medicine and Public Health, School of Medicine, Keio University, Tokyo), Akira Sekikawa, Emma JM Barinas-Mitchell (Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA), Daniel Edmundowicz (Department of Medicine, Section of Cardiology, School of Medicine, Temple University, Philadelphia, PA, USA), Takayoshi Ohkubo (Department of Hygiene and Public Health, Teikyo University School of Medicine, Tokyo), Atsushi Hozawa (Preventive Medicine, Epidemiology Section, Tohoku University, Tohoku Medical Megabank Organization, Sendai, Miyagi), Nagako Okuda (Department of Health and Nutrition, University of Human Arts and Sciences, Saitama, Japan), Aya Kadota (Department of Public Health, Shiga University of Medical Science, Otsu, Shiga), Aya Higashiyama (Research and Development Initiative Center, National Cerebral and Cardiovascular Center, Suita, Japan), Shinya Nagasawa (Department of Epidemiology and Public Health, Kanazawa Medical University, Kanazawa, Ishikawa), Yoshikuni Kita_(Tsuruga Nursing University), Akira Fujiyoshi, Naoyuki Takashima, Takashi Kadowaki, Sayaka Kadowaki (Department of Public Health, Shiga University of Medical Science, Otsu, Shiga), Yoshitaka Murakami (Department of Medical Statistics, Faculty of Medicine, Toho University, Tokyo, Japan), Robert D. Abbott, Seiko Ohno (Center for Epidemiologic Research in Asia, Shiga University of Medical Science, Otsu, Shiga), Takashi Hisamatsu (Center for Epidemiologic Research in Asia, Department of Public Health, Shiga University of Medical Science, Otsu, Shiga), Naoko Miyagawa, Sayuki Torii, and Yoshino Saito (Department of Public Health, Shiga University of Medical Science, Otsu, Shiga).
Conflict of Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emerging Risk Factors C. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W Atherosclerosis Risk in Communities Study G. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108–13. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–8. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, Shear CL, Duggan WT, Vicari RM, Grobbee DE, Kastelein JJ. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370(9582):153–60. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- 6.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356(13):1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 9.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D'Agostino RB, Sr, Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7(5):484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 11.El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150(2):84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 12.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-Density Lipoprotein Cholesterol and Particle Concentrations, Carotid Atherosclerosis, and Coronary Events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60(6):508–16. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128(11):1189–97. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekikawa A, Miura K, Lee S, Fujiyoshi A, Edmundowicz D, Kadowaki T, Evans RW, Kadowaki S, Sutton-Tyrrell K, Okamura T, Bertolet M, Masaki KH, Nakamura Y, Barinas-Mitchell EJ, Willcox BJ, Kadota A, Seto TB, Maegawa H, Kuller LH, Ueshima H Group EJS. Long chain n-3 polyunsaturated fatty acids and incidence rate of coronary artery calcification in Japanese men in Japan and white men in the USA: population based prospective cohort study. Heart. 2014;100(7):569–73. doi: 10.1136/heartjnl-2013-304421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekikawa A, Ueshima H, Sutton-Tyrrell K, Kadowaki T, El-Saed A, Okamura T, Takamiya T, Ueno Y, Evans RW, Nakamura Y, Edmundowicz D, Kashiwagi A, Maegawa H, Kuller LH. Intima-media thickness of the carotid artery and the distribution of lipoprotein subclasses in men aged 40 to 49 years between whites in the United States and the Japanese in Japan for the ERA JUMP study. Metabolism. 2008;57(2):177–82. doi: 10.1016/j.metabol.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueshima H, Iida M, Shimamoto T, Konishi M, Tanigaki M, Nakanishi N, Takayama Y, Ozawa H, Kojima S, Komachi Y. High-density lipoprotein-cholesterol levels in Japan. JAMA. 1982;247(14):1985–7. [PubMed] [Google Scholar]
- 17.Kadota A, Miura K, Okamura T, Fujiyoshi A, Ohkubo T, Kadowaki T, Takashima N, Hisamatsu T, Nakamura Y, Kasagi F, Maegawa H, Kashiwagi A, Ueshima H. Carotid Intima-Media Thickness and Plaque in Apparently Healthy Japanese Individuals with an Estimated 10-Year Absolute Risk of CAD Death According to the Japan Atherosclerosis Society (JAS) Guidelines 2012: The Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA) J Atheroscler Thromb. 2013;20(10):755–66. doi: 10.5551/jat.17244. [DOI] [PubMed] [Google Scholar]
- 18.Kashiwagi A, K M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H. Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society: International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura M, Sato S, Shimamoto T. Improvement in Japanese clinical laboratory measurements of total cholesterol and HDL-cholesterol by the US Cholesterol Reference Method Laboratory Network. J Atheroscler Thromb. 2003;10(3):145–53. doi: 10.5551/jat.10.145. [DOI] [PubMed] [Google Scholar]
- 20.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48(3-4):171–80. [PubMed] [Google Scholar]
- 21.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–70. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Sutton-Tyrrell K, Wolfson SK, Jr, Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23(2):215–20. doi: 10.1161/01.str.23.2.215. [DOI] [PubMed] [Google Scholar]
- 23.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931–9. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y, Okamura T. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118(25):2702–9. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17(10):594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Vickers KC, Remaley AT. HDL and cholesterol: life after the divorce? J Lipid Res. 2014 Jan;55(1):4–12. doi: 10.1194/jlr.R035964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21(3):229–38. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan HC, Tai ES, Sviridov D, Nestel PJ, Ng C, Chan E, Teo Y, Wai DC. Relationships between cholesterol efflux and high-density lipoprotein particles in patients with type 2 diabetes mellitus. J Clin Lipidol. 2011;5(6):467–73. doi: 10.1016/j.jacl.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Linsel-Nitschke P, Jansen H, Aherrarhou Z, Belz S, Mayer B, Lieb W, Huber F, Kremer W, Kalbitzer HR, Erdmann J, Schunkert H. Macrophage cholesterol efflux correlates with lipoprotein subclass distribution and risk of obstructive coronary artery disease in patients undergoing coronary angiography. Lipids Health Dis. 2009;8:14. doi: 10.1186/1476-511X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90(2):89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 32.Andrikoula M, McDowell IF. The contribution of ApoB and ApoA1 measurements to cardiovascular risk assessment. Diabetes Obes Metab. 2008;10(4):271–8. doi: 10.1111/j.1463-1326.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien T, Nguyen TT, Hallaway BJ, Hodge D, Bailey K, Holmes D, Kottke BA. The role of lipoprotein A-I and lipoprotein A-I/A-II in predicting coronary artery disease. Arterioscler Thromb Vasc Biol. 1995;15(2):228–31. doi: 10.1161/01.atv.15.2.228. [DOI] [PubMed] [Google Scholar]
- 34.Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, O'Leary DH. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2(2):e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290–6. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125(20):2469–78. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]


