Abstract
Background
Adolescence is a period of developmental flux when brain systems are vulnerable to influences of early substance use, which in turn relays increased risk for substance use disorders. Our study intent was to assess adolescent regional cerebral blood flow (rCBF) as it relates to current and future alcohol use. The aim was to identify brain-based predictors for initiation of alcohol use and onset of future substance use disorders.
Methods
Quantitative rCBF was assessed in 100 adolescents (age 12-15). Prospective behavioral assessments were conducted annually over a three-year follow-up period to characterize onset of alcohol initiation, future drinking patterns and use disorders. Comparisons amongst use groups (i.e., Current-, Future-, and Non-alcohol Using adolescents) identified rCBF associated with initiation of alcohol use. Regression by future drinking patterns identified rCBF predictive of heavier drinking. Survival analysis determined whether or not baseline rCBF predicted later development of use disorders.
Results
Baseline rCBF was decreased to the parietal cortex and increased to mesolimbic regions in adolescents currently using alcohol as well as those who would use alcohol in the future. Higher baseline rCBF to the left fusiform gyrus and lower rCBF to the right inferior parietal cortex and left cerebellum was associated with future drinking patterns as well as predicted the onset of alcohol and substance use disorders in this cohort.
Conclusions
Variations in resting rCBF to regions within reward and default mode or control networks appear to represent trait markers of alcohol use initiation and is predictive of future development of use disorders.
Keywords: adolescence, alcohol use, substance use, regional cerebral blood flow, ASL MRI
1. INTRODUCTION
Adolescence is a period of developmental change marked by significant brain maturation and vulnerability of the brain to influences of environmental factors and exposure to substances (Grant et al., 2005). The latter is particularly relevant for adolescents with 9-15% meeting criteria for substance abuse and 5% for substance dependence (period prevalence, National Survey on Drug Use and Health for 2002-2008). These data underscore this adolescent public health problem given the potentially toxic effects of substances on brain development (Guerri and Pascual, 2010).
Most neuroimaging studies have investigated brain function in chronic use among adults (Bjork et al., 2008). The few studies focusing on younger populations have involved adolescents already using substances (Jacobus et al., 2012; Squeglia et al., 2014b). Imaging studies of familial risk for alcoholism in adolescents have focused on brain structure (e.g., Squeglia et al., 2015) and function (Cservenka et al., 2013; Wetherill et al., 2012) in fronto-parietal networks and reward circuitry. These studies indicate susceptibility to ethanol-related brain damage, largely localized in the gray matter of the hippocampus and prefrontal cortex (Squeglia et al., 2014b), as well as reduction in white matter integrity (c.f., De Bellis et al., 2008; Squeglia et al., 2014a). However, because these differences co-occurred with use, they cannot clarify whether effects are present prior to use disorder onset, or are the result of use. Recent prospective studies have identified smaller gray matter volume in left paralimbic cingulate, inferior frontal and right cerebellum (Cheetham et al., 2014; Squeglia et al., 2014c) and differences in task-related fronto-parietal activity (Squeglia et al., 2012), proposing these as brain-based risk markers for future substance use.
Neuroimaging metrics that track resting-state, regional cerebral blood flow (rCBF) can provide unique information about the developing brain. rCBF measured with Arterial Spin Labeled (ASL) MRI is useful in characterizing tonic neural activity, allowing for inference to maturation given the need for blood flow to support processes like neuronal proliferation and pruning (Uhlhaas et al., 2010). Until the fairly recent advent of ASL MRI methods, rCBF has not been readily assessed in adolescents because previous methods required radioactive exposure; therefore there is a dearth of information about rCBF in adolescence.
There are few ASL MRI prospective studies of brain development (e.g., De Vis et al., 2013 in neonates), but rCBF can be inferred via studies using functional MRI (fMRI), which assesses blood flow fluctuations and their coherence amongst functionally related regions (i.e., functional connectivity). The correlation between blood flow measured with ASL MRI or as functional connectivity is spatially heterogeneous (Khalili-Mahani et al., 2014), but the following fMRI studies are presented as a proxy to understanding rCBF in adolescence. For example, functional connectivity is altered (Gordon et al., 2010; Lorenz et al., 2014; Sherman et al., 2014) and more diffuse (Jolles et al., 2011; Sherman et al., 2014) in adolescents than in young adults. Development of functional connectivity tends to follow a short-to-long distance trajectory (Fair et al., 2008) and the relationships between different networks (e.g., default mode and cognitive control) become distinct (Bray et al., 2014; Sherman et al., 2014). Maturation of connectivity patterns occurs throughout adolescence (Dosenbach et al., 2008) and into young adulthood, changing again in late adulthood (Lorenz et al., 2014), highlighting the sensitivity of blood flow and functional connectivity to age-related changes in brain structure and function. Importantly, functional connectivity patterns in adolescence can predict impulsivity scores (Shannon et al., 2011), working memory performance (Lorenz et al., 2014), and intelligence (Sherman et al., 2014) suggesting that maturation, supported by alterations in blood flow, can mark personality, mood, and other individualistic pieces of an adolescent’s adult brain.
Quantitative measures of rCBF show alcohol-related shifts providing a basis for understanding the physiology of regions affected by alcohol exposure. For example, increased global perfusion is seen with acute alcohol administration, is greater in females than males (Marxen et al., 2014), and is particularly elevated in the cingulate, frontal and parietal cortex and the hippocampi (Khalili-Mahani et al., 2011; Strang et al., 2014; Tolentino et al., 2011). Among chronic alcohol abusers, there is persistent reduced blood flow and atrophy in these same regions (Suzuki et al., 2010). These data demonstrate sensitivity of certain regions to alcohol, but it is unclear how they are affected in early experimentation or continued use in adolescence.
Early initiation of alcohol use is associated with higher risk to develop alcohol use disorders (Behrendt et al., 2009; Grant et al., 2005), suggesting that traits may exist that predispose one to substance experimentation or sensitivity to exposure. This population is of particular interest because, although alcohol abuse and dependence is rare in adolescence, the population prevalence of these disorders jumps to 6% by late adolescence (Cohen et al., 1993; Rohde et al., 1996), particularly when they are comorbid with mood, stress-related or behavioral disorders (Aseltine et al., 1998; Clark et al., 1998), which also increase in prevalence during adolescence (Birmaher et al., 1996). Alcohol tends to be the substance most available and used by adolescents (Kirby and Barry, 2012), and its initiation is often followed by experimentation with other substances.
The hypotheses underlying this study are driven by fMRI findings of altered frontal and parietal function in substance-naïve offspring of alcoholics (Hanson et al., 2011; Wetherill et al., 2012) and altered reward network activity with chronic alcohol use and addiction (Haber and Knutson, 2010; Suzuki et al., 2010). Our primary hypothesis is that the developing brain will show altered rCBF to frontal, parietal and mesolimbic regions associated with initiation of alcohol use in adolescents who are currently using, or will initiate use, relative to those who remain alcohol naïve. A related hypothesis is that these findings will relate to future drinking patterns as neural predictors of heavier drinking. A secondary hypothesis is that baseline rCBF to some, if not all of these regions, will have prognostic significance to predict alcohol initiation and future alcohol use disorders in a cohort of adolescents who are just beginning to initiate alcohol use.
2. METHOD
2.1. Subjects
Three hundred thirty-two adolescents were recruited into the Teen Alcohol Outcomes Study (TAOS; Williamson: R01AA016274) via commercial phone lists containing families living within a 30-mile radius of the University of Texas Health Science Center at San Antonio (UTHSCA). Healthy adolescents, without dental appliances, and their parent/guardian completed self-report assessments and diagnostic interviews. All subjects assented to participate and their parent/guardian provided informed consent per UTHSCSA Institutional Review Board protocol.
2.2. Screening and Exclusion Criteria
The “kiddie” Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present, Lifetime version (K-SADS-PL, Kaufman et al., 2000) was administered at baseline and at annual follow-up sessions to assess lifetime Axis I psychiatric diagnoses. Adolescents were excluded from study if they met DSM-IV criteria for mood disorder other than anxiety, substance abuse/dependence, had binge drank (4+ alcoholic drinks on one occasion for females, 5+ for males), had a learning disability or had a lifetime externalizing disorder. Additionally, the K-SADS-PL was used to assess onset of substance abuse or dependence over the three-year study period. Follow-up interviews focused on the time since the last assessment (~12-14 months).
Subjects must have participated in each of the 3-year follow-up assessments to ensure that they were correctly grouped by use – i.e., Non-Users had not initiated use of any substance other than caffeine for the study period.
2.3. Dimensional Measures of Mood and Stress Assessment
Dimensional self- and parent-report measures of behavior, mood, anxiety, and past and current stress or trauma were administered annually. These measures assessed depressive symptoms for the prior two weeks, past month anxiety, behavioral or emotional problems, childhood trauma, and life stressors within the past year. Details about these scales and the scores used in the present analyses are in Table 1 and Supplementary Materials1.
Table 1.
Demographic information and scores on self- and parent-report dimensional measures are provided for each of the Use Groups.
| Non-Users | Future Users |
Current Users |
||
|---|---|---|---|---|
| Statistic | N or Mean | N or Mean | N or Mean | |
| n | 44 | 35 | 21 | |
| Age | F(2,97) = 2.228, p = 0.113 | 13.67 ± 0.89 |
13.76 ± 0.99 |
14.17 ± 0.84 |
| Gender (M:F)a | χ2 df=2 = 5.2, p = 0.075 | 25:19 | 15:20 | 6:15 |
| Ethnicity | χ2 df=2 = 4.780, p = 0.092 | |||
| Hispanic | 15 (33%) | 13 (37%) | 7 (33%) | |
| White | 26 (59%) | 21 (60%) | 13 (62%) | |
| Other | 3 (7%) | 1 (3%) | 1 (5%) | |
| Mood and Feelings Questionnaire – Child Report | F(2,97) = 3.12, p = 0.048 | 8.8 ± 6.8a | 9.0 ± 9.8b | 14.3 ± 10.7c |
| Mood and Feelings Questionnaire – Parent Report | F(2,97) = 1.02, p = 0.362 | 3.3 ± 5.9 | 2.4 ± 3.3 | 3.7 ± 3.5 |
| Childhood Trauma Questionnaire | F(2,97) = 1.01, p = 0.367 | 25.2 ± 4.2 | 26.5 ± 9.7 | 28.3 ± 7.4 |
| Stressful Life Events Schedule | F(2,97) = 2.26 p = 0.109 | 3.17 ± 1.2 | 3.33 ± 1.2 | 3.97 1.1 |
| Screen for Childhood Anxiety RElated Disorders – Child Report |
F(2,97) = 1.20, p = .306 | 16.8 ± 8.5 | 13.3 ± 9.0 | 15.2 ± 9.3 |
| Screen for Childhood Anxiety RElated Disorders – Parent Report |
F(2,97) = 1.95, p = 0.147 | 7.1 ± 6.4 | 5.0 ± 4.1 | 13.4 ± 22.3 |
| Youth Self Report – Internalizing Symptoms | F(2,97) = 1.83, p = 0.166 | 9.4 ± 5.8 | 8.0 ± 5.9 | 10.3 ± 5.2 |
| Child Behavioral Checklist – Internalizing Symptoms (Parent Report) |
F(2,97) = 1.24, p = .293 | 3.6 ± 3.7 | 2.4 ± 2.7 | 3.6 ± 3.2 |
| Youth Self Report – Externalizing Symptoms | F(2,97) = 7.69, p = 0.001 | 7.57 ± 3.9a | 9.6 ± 5.8b | 14.19 ± 7.8c |
| Child Behavioral Checklist – Externalizing Symptoms (Parent Report) |
F(2,97) = 2.65, 0.075 | 2.6 ± 2.9 | 2.4 ± 2.7 | 5.8 ± 6.0 |
| Use of Alcohol + Other Substancesd | t(54) = −.61, p = 0.543 | . | 17 (48%) | 12 (57%) |
| Maximum Use Metric | t(54) = −1.46, p = .15 | . | 8 ± 10 | 13 ± 11 |
| Future Alcohol Use Disordere | 0 | 5 | 3 | |
| Future Substance Use Disorderf | 0 | 11 | 8 |
Though there was a trend for more males in the Non-User group and more females in the Current User group, this difference in distribution was not significant. Similarly, there were more females than males who were alcohol-only users (7 males, 20 females), the gender distribution amongst poly-drug users was almost equal (14 males, 15 females); therefore, a gender by poly-drug use approached significance (χ2df=2=2.98, p=0.073) such that males who initiated alcohol use were also more likely than females to initiate use of other substances.
Non-User and Future User group contrast not significant.
Current significantly different that the others. p < 0.05.
Twenty-nine of the Future Initiators and Current Users reported also initiating use of other substances: nicotine (76%), marijuana (93%), inhalants (two subjects), hallucinogens (one subject), or cocaine (one subject). Only one of these subjects had initiated use of both alcohol and marijuana at baseline.
Five of the 8 subjects who developed a future alcohol use disorder also met criteria for substance use disorder.
Of the 19 subjects who developed a future substance use disorders, 5 met DSM-IV criteria for both alcohol and substance use disorders, 3 for alcohol use disorders, and 11 for substance use disorders.
2.4 Substance Use Measurement
The Substance Use Questionnaire (Molina et al., 2007) was administered at all interviews. This measure quantifies duration and frequency of use within the past month and year. Dimensional scaling of frequency of use (past month or past year) and amount of use were multiplied to calculate a Use Metric. A score of >50 is equivalent to regular use or abuse, e.g., 11-15 drinks 2-3 times per month or four drinks twice a day. The Maximum Use Metric indexed the highest rate of drinking for each subject over the three-year follow-up period.
Subjects were classified into three alcohol use-groups: 1) Non-Users: naïve to alcohol or any other substance use at baseline and no use of any substance over the three-year follow-up period (n = 44); 2) Future Users: substance naïve at baseline but initiated alcohol use during the three-year follow-up period (n = 35); or 3) Current Users: initiated some alcohol and limited other substance use at baseline (n = 21) (Table 1).
2.5. Imaging Methods
Arterial spin labeled magnetic resonance imaging (ASL-MRI, Wang et al., 2003) is a metric of tonic neurophysiologic function sensitive to regional abnormalities in psychiatric disorders (e.g., Monkul et al., 2011). Analysis of ASL images entails spatial co-registration to a high-resolution structural MRI image used for anatomical localization. The following parameters were used for high-resolution structural and ASL-MRI scans:
2.5.1. T-1 weighted structural MRI
Subjects were scanned on a 3T Siemens Trio MRI scanner (Siemens, Erlangen, Germany) with an eight-channel phase array coil. T1-weighted image parameters were: TR = 2200ms, TE = 2.8ms, tip angle = 13°, slice thickness =0.8mm, in-plane resolution = 0.8 × 0.8mm2.
2.5.2. ASL-MRI image acquisition
A pulsed arterial spin labeling sequence with interleaved images with and without labeling was used to acquire 13 slices (7.5mm thickness with 1.5mm gap) for 100 repetitions with the following parameters: FOV = 24cm, in-plane matrix size = 64 × 64, TR = 2440ms, TE = 19ms, delay time (TI1) = 700ms, label time (TI2) = 1000ms, flip angle = 90°. The equilibrium brain tissue magnetization (M0) was measured using the following parameters: TR/TI1/TI2 = 8000/5000/6000ms and repetition = 4.
2.5.3. ASL-MRI Image Processing
Quantitative CBF was calculated using MATLAB 7 scripts based on Wang et al. (2003). Raw quantitative ASL images (ΔM) were obtained by subtracting labeled and unlabeled images. The voxel-by-voxel CBF mapping was then computed by the following equation:
where λ = 0.9 is the blood/tissue water partition coefficient, T1a = 1.6s is the longitudinal relaxation time of blood at 3T, α = 0.95 is the inversion efficiency.
2.5.4. Spatial Normalization and Statistical Analysis of ASL Images
Cerebral blood flow maps were registered to the Montreal Neurological Institute (MNI) coordinate system (Jenkinson et al., 2002). Images were normalized to a global mean of 1000 to remove inter-individual differences in whole-brain blood flow. These images were smoothed with a 8mm Gaussian filter. Voxel-by-voxel, whole-brain, full factorial design (Statistical Parametric Mapping 8 - http://www.fil.ion.ucl.ac.uk/spm) explored the main effects of Use Group. Post-hoc between-group t-tests identified the group and direction of the effects contributing to the group effect. Second, a multiple regression identified the association of the Maximum Use Metric to rCBF, covaried for age and gender. These exact analyses were replicated with the inclusion of all dimensional measures of mood, stress and behavior as covariates to identify effects unique to alcohol use. For all contrasts, significant clusters of supra-threshold voxels were defined as those in gray matter with a peak-level at p < 0.001 in greater than six contiguous voxels (48mm3), uncorrected for multiple comparisons. Anatomical localizations for the regional effects were labeled using the Anatomy toolbox in SPM8 (Eickhoff et al., 2005).
Average blood flow to each of the clusters associated with the main effects of Use Group or associated with the Maximum Use Metric was sampled and entered, along with all of the log-transformed dimensional measures of mood, stress, and behavior, into SPSS 21 to compute a right-censored (three-year follow-up period) Cox proportional hazards survival analysis. The analysis was time-dependent on the age of onset for those who developed a use disorder (n = 19) to determine whether or not baseline rCBF is associated with alcohol and/or substance use disorder onset.
3. RESULTS
3.1. Demographic and Behavioral Correlates of Alcohol Use
One hundred TAOS participants met criteria for study and had ASL MRI images at baseline. This nested cohort was slightly older (TAOS: 14.3 years of age, nested cohort: 13.7 years of age; t[329] = −3.52, p < .0001), but demonstrated the same gender and ethnicity distributions (gender: Χ2df = 1 = .47, p = .49; ethnicity: Χ2df = 3 = 1.82, p = .61) as the larger group.
The Use Groups did not differ for demographic or behavioral measures. Current Users demonstrated more self-reported depressive symptoms and externalizing problems than the other two groups (Table 1).
Alcohol use at baseline was low, only in the Current User group, with the average Use Metric across the group being 2 (frequency = 1 or 1-3 times in the past 12 months; amount = 2, one standard drink). Only three of the Current Users had experimented with other substances at baseline (light use of nicotine, marijuana, and one had tried inhalants and LSD once each). The Maximum Use Metric ranged from 0 in Non-Users, to 8 in Future Users and 13 in Current Users (roughly 0 to 1 drink every 2-3 months). Over the following 3-year period, Use Metrics for Future and Current Users increased, ranging from 2 to 49 (roughly six drinks 2-3 times per week); though, the average metric for the entire cohort remained low (mean = 10, mode = 2, median = 6). By the third year, 29 Future Users and Current Users also reported use of other substances – primarily, nicotine and marijuana (Table 1).
Finally, 19 adolescents developed use disorders (five with alcohol + substance use disorders, three with alcohol use disorders, and 11 with other substance use disorders). These 19 differed from the rest of the adolescents at baseline, reporting higher self- (F[1,97] = 10.30, p = 0.002) and parent-reports (F[1,97] = 7.303, p = 0.008) of externalizing problems and heavier drinking patterns over time (F[1,97] = 67.77, p < .0001).
Use Group and Maximum Use Metric analyses were covaried for age, gender, and all dimensional measures. This was done because rCBF was associated with each of the dimensional measures of mood, stress, and behavior assessed (Supplemental Table 12), and because many of the constructs measured are suggested to increase risk for use disorders (c.f., De Bellis et al., 2008; Hussong et al., 2011). By controlling for these variables, the analyses elucidate effects unique to the initiation of alcohol use.
3.2. Is baseline rCBF altered in adolescents who are current or future users of alcohol relative to those who remain alcohol-naïve?
3.2.1. Current Users of Alcohol
Current Users had increased rCBF to frontal, temporal, posterior cingulate, insula and cerebellar regions, and decreased rCBF to the bilateral temporal, parietal and occipital cortex relative to Non-Users (Table 2, Figure 1). Current Users only differed from Future Users with higher rCBF in left cerebellum (x = −42, y = −68, z = −28, t = 3.52, extent = 72mm3) and left middle temporal gyrus (x = −62, y = −54, z = 10, t = 3.57, extent = 48mm3).
Table 2.
Use Group contrastsa.
| Current Users vs. Non-Users | Future Users vs. Non-Users | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Region | Extent mm3 |
MNIb
Coordinates |
T | Extent mm3 |
MNIb
Coordinates |
T | Group Contrast | ||||
|
| |||||||||||
| x | y | z | x | y | z | ||||||
|
| |||||||||||
| Frontal | |||||||||||
| Left Superior Frontal Gyrus | 88 | −26 | 12 | 66 | 3.44 | Current > Non | |||||
| Right Superior Frontal Gyrus | 7304 | 18 | 64 | 0 | 5.12 | Current > Non | |||||
| −12 | 62 | 0 | 4.66 | ||||||||
| 32 | 60 | −2 | 4.08 | ||||||||
| Left Mid Orbital Gyrus | 2304 | −8 | 62 | −2 | 4.19 | Future > Non | |||||
| 2 | 58 | 2 | 3.79 | ||||||||
| Left Inferior Frontal Gyrus | 168 | −52 | 20 | 0 | 3.44 | Non > Future | |||||
| −52 | 14 | −4 | 3.33 | ||||||||
|
| |||||||||||
| Temporal | |||||||||||
|
| |||||||||||
| Left Superior Temporal Gyrus | 72 | −56 | −2 | 14 | 3.52 | Non > Future | |||||
| Right Superior Temporal Gyrus | 1680 | 64 | 2 | −2 | 4.77 | Non > Current | |||||
| 56 | 14 | −12 | 3.46 | ||||||||
| Left Middle Temporal Gyrus | 48 | −62 | −54 | 10 | 3.57 | 5544 | −54 | −58 | 12 | 4.57 | Current & Non > Future |
| −54 | −44 | 28 | 4.17 | ||||||||
| −40 | −54 | 14 | 4.13 | ||||||||
| −38 | −56 | 18 | 3.81 | ||||||||
| −36 | −58 | 32 | 3.29 | ||||||||
| 320 | −50 | −42 | 4 | 3.49 | Non > Future | ||||||
| Right Temporal Pole | 56 | 34 | 12 | −24 | 3.42 | 272 | 56 | 10 | −14 | 3.71 | Current > Non > Future |
|
| |||||||||||
| Parietal | |||||||||||
|
| |||||||||||
| Right Temporo-Parietal Cortex | 15272 | 38 | −56 | 48 | 4.52 | Non > Future | |||||
| 46 | −44 | 50 | 4.36 | ||||||||
| 46 | −48 | 20 | 4.01 | ||||||||
| 52 | −44 | 32 | 3.88 | ||||||||
| 50 | −44 | 24 | 3.85 | ||||||||
| 26 | −64 | 50 | 3.8 | ||||||||
| 42 | −58 | 8 | 3.7 | ||||||||
| 40 | −50 | 8 | 3.56 | ||||||||
| 44 | −62 | 32 | 3.41 | ||||||||
| Left Superior Parietal Lobule | 64 | −30 | −66 | 48 | 3.26 | Non > Future | |||||
| Left Parieto-Occipital Cortex | 10584 | −24 | −66 | 44 | 4.24 | 624 | −36 | −50 | 48 | 3.57 | Non > Current & Future |
| −32 | −70 | 24 | 4.14 | ||||||||
| −20 | −76 | 44 | 3.83 | ||||||||
| −14 | −72 | 42 | 3.82 | ||||||||
| −16 | −82 | 22 | 3.52 | ||||||||
| −14 | −88 | 30 | 3.42 | ||||||||
| Right Postcentral Gyrus | 3224 | 58 | −20 | 30 | 5.16 | Non > Future | |||||
| Right Precuneus | 4504 | 0 | −48 | 70 | 4.76 | 144 | 24 | −58 | 26 | 3.48 | Non > Current & Future |
| −14 | −56 | 70 | 4.19 | ||||||||
| −26 | −44 | 70 | 3.94 | ||||||||
| 24 | −52 | 70 | 3.76 | ||||||||
|
| |||||||||||
| Occipital | |||||||||||
| Right Occipito-Parieto-Temporal Cortex |
21216 | 42 | −64 | 28 | 4.93 | Non > Current | |||||
| 42 | −50 | 40 | 4.49 | ||||||||
| 48 | −54 | 20 | 4.44 | ||||||||
| 52 | −42 | 36 | 4.39 | ||||||||
| 46 | −50 | −6 | 4.07 | ||||||||
| 44 | −54 | −14 | 3.92 | ||||||||
| 20 | −60 | 48 | 3.86 | ||||||||
| 26 | −56 | 42 | 3.58 | ||||||||
| 24 | −60 | 28 | 3.43 | ||||||||
| Right Cuneus | 352 | 20 | −74 | 32 | 3.51 | Non > Future | |||||
| Left Lingual Gyrus | 144 | −6 | −86 | −12 | 3.91 | Future > Non | |||||
|
| |||||||||||
| Limbic | Current > Non | ||||||||||
| Left Posterior Cingulate Cortex | 56 | −2 | −46 | 14 | 3.53 | ||||||
|
| |||||||||||
| Sub-Lobar | |||||||||||
|
| |||||||||||
| Left Insula | 712 | −24 | 14 | −20 | 4.6 | Current > Non | |||||
|
| |||||||||||
| Cerebellum | |||||||||||
|
| |||||||||||
| Left Lobule VIIa Crus I | 104 | −20 | −82 | −28 | 3.59 | Non > Future | |||||
Contrasts were whole-brain, voxel-wise T-tests, p<.001 in greater than 6 contiguous voxels, uncorrected for multiple comparisons.
Montreal Neurological Institute coordinates.
Figure 1.
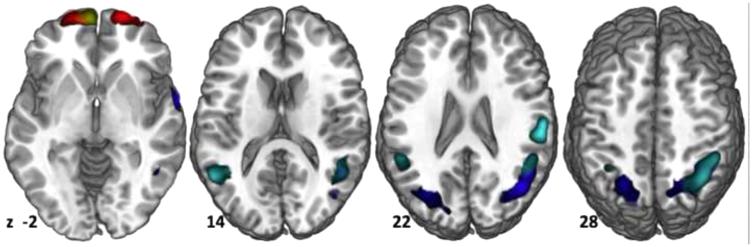
Current and Future Users had reduced rCBF (Current: blue; Future: cyan) to temporo-parietal cortex relative to Non-Users, which was distributed bilaterally to a greater extent into the temporal lobes in the Future than Current Users. Increased rCBF (Current = red; Future = yellow) was seen only in frontal cortex (A) in the Future Users relative to Non-Users, but to a greater extent in the Current Users than the Non-Users. The only difference between the groups was increase rCBF to the left middle temporal gyrus (not pictured) in the Current > Future Users. Images are displayed by z-value, starting on the left at z = −2 and ending on the right at z = 28.
3.2.2. Future Users of Alcohol
Prior to initiating alcohol use, Future Users had increased frontal and lingual rCBF and decreased bilateral temporal and parietal rCBF relative to Non-Users (Figure 1, Table 2).
3.3. Does the Maximum Use Metric relate to baseline rCBF?
Baseline rCBF was increased to medial frontal, mid-cingulate, and left fusiform regions and decreased to bilateral middle frontal, inferior parietal, hippocampal, insular, and cerebellar regions in adolescents with heavier future drinking patterns (Table 3, Figure 2).
Table 3.
Relation of baseline rCBF to future drinking patterns.a
| Location | Extent mm3 |
MNIb
Coordinates |
T-Value | Direction of Effect with Higher Maximum Use Metric |
|||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
|
| |||||||
| Frontal | |||||||
| Superior Frontal Gyrus | 2216 | 0 | 72 | 28 | 6c | ↑ | |
| Left Middle Frontal Gyrus | 4968 | −44 | 52 | 6 | 5.02 | ↓ | |
| −40 | 58 | −10 | 3.99 | ||||
| −48 | 54 | −12 | 3.80 | ||||
| Right Middle Frontal Gyrus | 656 | 50 | 46 | 4 | 3.88 | ↓ | |
| Left Mid Orbital Gyrus | 352 | −8 | 56 | −4 | 3.75 | ↑ | |
| Left Middle Orbital Gyrus | 328 | −34 | 48 | −4 | 3.75 | ↑ | |
|
| |||||||
| Parietal | |||||||
|
| |||||||
| Right Inferior Parietal Lobule | 48 | 4 | −44 | 40 | 3.35 | ↓ | |
|
| |||||||
| Limbic | |||||||
|
| |||||||
| Left Middle Cingulate Cortex | 416 | 2 | −6 | 38 | 4.05 | ↑ | |
| Right Hippocampus (Entorhinal Cortex) | 208 | 16 | −14 | −32 | 3.35 | ↓ | |
|
| |||||||
| Occipital | |||||||
|
| |||||||
| Left Fusiform Gyrus | 296 | −36 | −66 | −14 | 3.63 | ↑ | |
|
| |||||||
| Sub-Lobar | |||||||
|
| |||||||
| Right Insula | 232 | 44 | −4 | −2 | 3.84 | ↓ | |
|
| |||||||
| Cerebellum | |||||||
| Cerebellum (VIIa Crus I) | 504 | −22 | −84 | −26 | 4.57 | ↓ | |
General linear model computed relationship between rCBF and Maximum Use Metric, p<.001 in 6 contiguous voxels, uncorrected for multiple comparisons.
Montreal Neurological Institute coordinates.
Significant at p < .05corrected.
Figure 2.
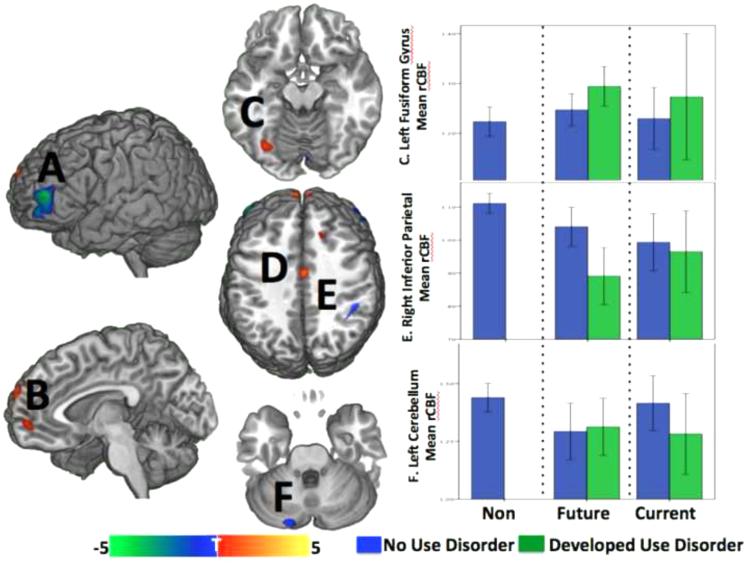
The maximum Use Metric, as an index of future drinking patterns, was associated with decreased (blue) rCBF to bilateral middle frontal gyri (A), right inferior parietal lobule (E), right insula (not pictured) and the left cerebellum (F). Increased rCBF (red) with higher future drinking was observed to superior frontal and mid-orbital gyri (B), mid-cingulate cortex (D), and the left fusiform gyrus (C). Future drinking was correlated with future substance use disorders, as expected, and baseline rCBF to three of these regions in turn predicted future substance use disorders in this sample – 1) left fusiform gyrus rCBF was higher in the Future Users than the other groups, particularly in the Future Users that went on to develop a use disorder (top graph); 2) right inferior parietal cortex rCBF was lower in the Future and Current Users than the Non-Users, and to a greater extent in those Future Users who developed a use disorder in the future (middle graph), and 3) left cerebellar rCBF was lower in the Future Users than the Non Users and in those who went on to develop use disorders from both User groups (bottom graph). Bars represent the 95% confidence interval.
3.4. Does baseline rCBF predict risk for future diagnosis of alcohol and/or substance abuse or dependence?
The survival analysis computed relative risk, right censored for the 3-year period of study. Higher parent-report of externalizing problems (relative risk (RR) = 2.1, 95% CI 1.23 to 3.51), higher rCBF to the left fusiform gyrus (RR = 563.97, 95% CI 4.055 to 78437), and decreased rCBF to the right inferior parietal lobule (RR = .001, 95% CI 0 to .04) and left cerebellum (RR = .137, 95% CI .019 to .983) increased the relative risk for onset of alcohol or substance abuse/dependence.
4. DISCUSSION
We sought to determine: 1) whether adolescents who are, or will be, drinking alcohol demonstrate altered rCBF in fronto-parietal or reward centers of the brain, 2) whether baseline rCBF marks heavier rates of future drinking, and 3) whether these rCBF markers associated with early drinking behavior predict future alcohol or substance use disorders. The analyses to address these goals were controlled for the effects of mood, stress and behavior, factors also associated with onset of substance use initiation. As such, this is among the first studies to account for comorbidities while identifying brain-based markers related to initiation of alcohol use in a large sample of adolescents.
The most striking finding was increased orbito-frontal and decreased bilateral parietal (right > left) rCBF, even in Future Users prior to initiation, nominating this rCBF marker as a brain-based signature of susceptibility to use. Furthermore, only parietal rCBF at baseline was predictive of onset of a use disorder in Future and Current Users, suggesting that decreased rCBF to this area is a risk factor for use disorders. The right hemisphere cortical surface has been implicated in the pathogenesis of familial risk for depression and anxiety disorders (Peterson et al., 2009; Uhlhaas et al., 2010), and the present data suggest a limited area within this cortical surface (i.e., the right inferior parietal cortex) may also mark the pathogenesis of substance use disorders.
This area of the parietal cortex has demonstrated decreased CBF in young, adult female drinkers (Clark et al., 2007; Gordon et al., 2010), decreased activity during a spatial working memory task in young binge drinkers (specifically on the right, (Squeglia et al., 2011), and atrophy in middle-aged, long term abstinent alcoholic men (Fein et al., 2006; Jolles et al., 2011). This parietal area is a hub of the default mode network, which also includes the frontal regions positively related to future drinking patterns, and has been found to associate with substance abuse/dependence (Dalwani et al., 2014). This parietal region, along with dorso-lateral frontal regions, is also a part of the dorsal attention or “control” network implicated in alertness, externally driven cognition, and working memory (Corbetta these networks has been observed in adolescent males with conduct and substance use disorders (Dalwani et al., 2014), drug addiction (Ma et al., 2011), first-episode psychosis (Alonso-Solís et al., 2012; Shannon et al., 2011) and attention deficit disorder (Dickstein et al., 2010), suggesting that its integrity subsumes a developmentally important but subtle behavioral trait that may also relate to predisposition for substance use initiation.
Mesolimbic reward centers of the brain also related to initiation of alcohol use and future drinking patterns. Adolescents who had already initiated alcohol use had elevated rCBF to the left hippocampus and amygdala relative to Non-Users, but this effect was only seen when the effects of mood, stress and behavior were not in the analysis (Supplemental Table 23), suggesting that these group differences may largely be driven by mood or behavioral traits. Nonetheless, baseline rCBF in other mesolimbic regions related to future drinking patterns and predicted later onset of use disorders (i.e., left fusiform gyrus and cerebellum).
Regions of the brain reward network have frequently been associated with alcohol use, even in adolescents (Norman et al., 2011; Suzuki et al., 2010). Findings in regions of this network may relate to their role in dopaminergic and GABAergic function during adolescent brain development. For example, while functional connectivity develops, there are also increases in dopamine synthesis and processing in prefrontal cortex and its projections to mesolimbic regions (Padmanabhan and Luna, 2014; Tomasi and Volkow, 2014). At that developmental stage, the balance between prefrontal and subcortical pruning shifts from subcortical to prefrontal regulation (Woo et al., 1997). Concurrently, GABAergic inhibitory circuits are maturing (Hyde et al., 2011) and their immaturity in those “at risk” may allow uncontrolled dopaminergic activity. As such, it may be that group differences in reward network rCBF and its relationship with future drinking patterns and development of use disorders is a reflection of increased mesolimbic activity in a maturing reward network.
The fact that fronto-parietal and mesolimbic rCBF did not differ between the Current and Future Users is further support of these as markers of proclivity toward using alcohol. A finding more specific to initiation, as opposed to risk for drinking, is found in the left middle temporal gyrus (Brodmann Area 38) where rCBF was higher in Current than Future Users. It is unclear why rCBF to this region of the brain is elevated in adolescents currently using alcohol, though it has been found to be hyper-active in teens with alcohol use disorders (Tapert et al., 2003). This area of the temporal lobe is most often associated with domain-general semantic integration that is specific to social or autobiographical information (Wong and Gallate, 2012). Gray matter density and glucose metabolism to this temporal area is known to be altered in psychopathic and mood disorders (Elizabeth Sublette et al., 2009; Molina et al., 2007). The importance of this region in early drinking onset may relate to these comorbid risk factors or to the particular demands for social or identity development at this point in adolescence (Wong and Gallate, 2012), but longitudinal analysis of the TAOS imaging data will help to clarify its role.
4.1 Limitations of the Present Study
Our use measure was by self-report questionnaire and could have included false positive or negative responses, as with any subjective measure. This measure may have obscured our findings where more objective measures could have yielded lesser or greater effects. In addition, measurement was limited to the three-year study period and initiation of substances after this period in our Non-User group remains possible. Further, our current analyses did not account for familial history of alcohol or substance abuse/dependence, but future analyses could further determine how the brain-based markers reported herein may be attributable to these potential risk factors.
Furthermore, although we controlled for presence of mood, stress, and behavior, and excluded for externalizing disorders, there are behavioral substrates of externalizing disorders present that predict future use disorders in this sample. Those substrates could account for some variability in our measure of rCBF.
We also recognize that rCBF is only one metric of brain function with comparatively poor spatial and temporal resolution. The interpolation required for calculation of quantitative rCBF and the larger voxel sizes introduce noise. Through normalization methods, the data were transformed to 2x2x2mm voxels and aligned to a standard brain (MNI) to improve spatial localization of the effects, but we recognize that the signal to noise ratio of ASL MRI is poorer than in fMRI methods, making the p-values less for a given effect size. Nonetheless, we opted to use a whole brain approach rather than a priori region-of-interest approach in this investigation given the lack of existing literature using the same metrics in a similar population.
Last, while our sample is reasonably large, our groups remain fairly small, particularly for onset of use disorders. It would be beneficial to have a larger cohort of adolescents assessed at baseline in order to observe a greater number who develop alcohol use disorders and to comprehensively examine brain-based predictors of future onset of disorder.
4.2 Conclusions
In summary, adolescents who initiate alcohol use by age 12-15, and those who will initiate use before young adulthood, have increased medial frontal and decreased temporo-parietal rCBF relative to Non-Users. A similar pattern, increased frontal and decreased parietal rCBF, was also predictive of future drinking patterns, with the addition of increased rCBF to mesolimbic brain reward centers. Along with the presence of externalizing problems at age 12-15, rCBF in parietal, fusiform, and cerebellar regions predicted future onset of alcohol and/or substance use disorders. All of these findings were present in the Future User group, i.e., prior to the initiation of use, and therefore may be considered markers of susceptibility to use and risk for abuse or dependence.
Supplementary Material
Highlights.
Adolescents who are using or will use alcohol have decreased blood flow to mesolimbic and parietal cortex, even 3 years prior to beginning to use.
Regional cerebral blood flow (rCBF) to mesolimbic and parietal cortex predicted these adolescents’ future drinking patterns and future onset of alcohol and substance use disorders.
Variation in resting rCBF to reward and control networks represent trait markers for future risk to use alcohol and to develop use disorders.
Acknowledgments
Role of Funding Source:This work funded in part by grants from the National Institute on Alcohol Abuse and Alcoholism to Drs. Williamson (R01AA016274) and Ramage (R03AA020823-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Conflict of Interest: Drs. Ramage, Lin, Olvera and Williamson have no financial disclosures. Dr. Fox has received funding from the following sources over the past two years: equity interest in Cerebral Magnetics, LLC and msMRI, LLC. In addition, he receives royalties from Wiley-Blackwell for serving as the Editor-in-Chief of Human Brain Mapping.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
References
- Alonso-Solís A, Corripio I, de Castro-Manglano P, Duran-Sindreu S, Garcia-Garcia M, Proal E, Nuñez-Marín F, Soutullo C, Alvarez E, Gómez-Ansón B, Kelly C, Castellanos FX. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophren. Res. 2012;139:13–18. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseltine RH, Gore S, Colten ME. The co-occurrence of depression and substance abuse in late adolescence. Dev. Psychopathol. 1998;10:549–570. doi: 10.1017/s0954579498001746. [DOI] [PubMed] [Google Scholar]
- Behrendt S, Wittchen H-U, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol Depend. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B. Childhood and adolescent depression: a review of the past 10 years. Part I. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Smith AR, Hommer DW. Reduced posterior mesofrontal cortex activation by risky rewards in substance-dependent patients. Drug Alcohol Depend. 2008;95:115–128. doi: 10.1016/j.drugalcdep.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Arnold AEGF, Levy RM, Iaria G. Spatial and temporal functional connectivity changes between resting and attentive states. Hum. Brain Mapp. 2014 doi: 10.1002/hbm.22646. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons J, Yücel M, Lubman DI. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology (Berl.) 2014;231:1731–1742. doi: 10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Eyler LT, Drummond SPA, Braun DR, Tapert SF. Decreased perfusion in young alcohol-dependent women as compared with age-matched controls. Am. J. Drug Alcohol Abuse. 2007;33:13–19. doi: 10.1080/00952990601082605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, Rojas M, Brook J, Streuning EL. An epidemiological study of disorders in late childhood and adolescence--I. age- and gender-specific prevalence. J. Child Psychol. Psychiatry. 1993;34:851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2013;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwani MS, Tregellas JR, Andrews-Hanna JR, Mikulich-Gilbertson SK, Raymond KM, Banich MT, Crowley TJ, Sakai JT. Default mode network activity in male adolescents with conduct and substance use disorder. Drug Alcohol Depend. 2014;134:242–250. doi: 10.1016/j.drugalcdep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol. Clin. Exp. Res. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vis JB, Petersen ET, de Vries LS, Groenendaal F, Kersbergen KJ, Alderliesten T, Hendrikse J, Benders MJNL. Regional changes in brain perfusion during brain maturation measured non-invasively with Arterial Spin Labeling MRI in neonates. Eur. J. Radiol. 2013;82:538–543. doi: 10.1016/j.ejrad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, Kelly C, Gee DG, Zuo X-N, Castellanos FX, Milham MP. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol. Psychiatry. 2010;68:839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Elizabeth Sublette M, Milak MS, Hibbeln JR, Freed PJ, Oquendo MA, Malone KM, Parsey RV, John Mann J. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukot. Essent. Fatty Acids. 2009;80:57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Lee PS, Maisog JM, Foss-Feig J, Billington ME, Vanmeter J, Vaidya CJ. Strength of default mode resting-state connectivity relates to white matter integrity in children. Dev. Sci. 2010;14:738–751. doi: 10.1111/j.1467-7687.2010.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, True WR, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol. Med. 2005;36:109. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol. Addict. Behav. 2011;25:127–142. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychol. Addict. Behav. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology (Berl.) 2012;222:675–684. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SARB. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb. Cortex. 2011;21:385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, van Osch MJ, de Rooij M, Beckmann CF, van Buchem MA, Dahan A, van Gerven JM, Rombouts SARB. Spatial heterogeneity of the relation between resting-state connectivity and blood flow: an important consideration for pharmacological studies. Hum. Brain Mapp. 2014;35:929–942. doi: 10.1002/hbm.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili-Mahani N, van Osch MJP, Baerends E, Soeter RP, de Kam M, Zoethout RWM, Dahan A, van Buchem MA, van Gerven JMA, Rombouts SARB. Pseudocontinuous arterial spin labeling reveals dissociable effects of morphine and alcohol on regional cerebral blood flow. J. Cereb. Blood Flow Metab. 2011;31:1321–1333. doi: 10.1038/jcbfm.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T, Barry AE. Alcohol as a gateway drug: a study of US 12th graders. J. School Health. 2012;82:371–379. doi: 10.1111/j.1746-1561.2012.00712.x. [DOI] [PubMed] [Google Scholar]
- Lorenz RC, Gleich T, Beck A, Pöhland L, Raufelder D, Sommer W, Rapp MA, Kühn S, Gallinat J. Reward anticipation in the adolescent and aging brain. Hum. Brain Mapp. 2014;35:5153–5165. doi: 10.1002/hbm.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu X-M, Li N, Wang C-X, Zhang H, Qian R-B, Xu H-S, Hu X, Zhang D-R. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxen M, Gan G, Schwarz D, Mennigen E, Pilhatsch M, Zimmermann US, Guenther M, Smolka MN. Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J. Cereb. Blood Flow Metab. 2014;34:472–479. doi: 10.1038/jcbfm.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, Hechtman L, Jensen PS, Vitiello B, Hoza B, Pelham WE, Elliott GR, Wells KC, Abikoff HB, Gibbons RD, Marcus S, Conners CK, Epstein JN, Greenhill LL, March JS, Newcorn JH, Severe JB, Wigal T. Delinquent behavior and emerging substance use in the mta at 36 months: prevalence, course, and treatment effects. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:1028–1040. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Silva LAP, Narayana S, Peluso MAM, Zamarripa F, Nery FG, Najt P, Li J, Lancaster JL, Fox PT, Lafer B, Soares JC. Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: a 15O-H2O PET study. Hum. Brain Mapp. 2011;33:272–279. doi: 10.1002/hbm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Luna B. Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain Cogn. 2014;89:27–38. doi: 10.1016/j.bandc.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proc. Natl. Acad. Sci. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Psychiatric comorbidity with problematic alcohol use in high school students. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:101–109. doi: 10.1097/00004583-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, Bache K, Calhoun VD, Nigg JT, Nagel BJ, Stevens AA, Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc. Natl. Acad. Sci. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev. Cogn. Neurosci. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Brumback T, Meloy MJ, Tapert SF. White matter integrity in alcohol-naive youth with a family history of alcohol use disorders. Psychol. Med. 2014a;44:2775–2786. doi: 10.1017/S0033291714000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handb. Clin. Neurol. 2014b;125:501–510. doi: 10.1016/B978-0-444-62619-6.00028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain responses to working memory over three years of adolescence: influence of initating heavy drinking. J. Stud. Alcohol Drugs. 2012:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Dev. Cogn. Neurosci. 2014c;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol. Clin. Exp. Res. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Jacobus J, Brumback T, Taylor CT, Tapert SF. Structural connectivity of neural reward networks in youth at risk for substance use disorders. Psychopharmacology (Berl.) 2015 doi: 10.1007/s00213-014-3857-y. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Claus ED, Ramchandani VA, Graff-Guerrero A, Boileau I, Hendershot CS. Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology (Berl.) 2014 doi: 10.1007/s00213-014-3706-z. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Oishi M, Ogawa K, Mizutani T. Atrophy of the parahippocampal gyrus and regional cerebral blood flow in the limbic system in chronic alcoholic patients. Alcohol. 2010;44:439–445. doi: 10.1016/j.alcohol.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch. Gen. Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, Smith TL, Schuckit MA. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol. Clin. Exp. Res. 2011;35:1034–1040. doi: 10.1111/j.1530-0277.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb. Cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Alsop DC, Song HK, Maldjian JA, Tang K, Salvucci AE, Detre JA. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST) Magn. Reson. Med. 2003;50:599–607. doi: 10.1002/mrm.10559. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naïve youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. 2012;1449:94–116. doi: 10.1016/j.brainres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.