Abstract
Objective
To determine the association of circulating P-selectin with prevalent and incident peripheral artery disease (PAD), the ankle brachial index (ABI), and change in the ABI.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective population-based cohort study including 6814 European descent, African American, Hispanic and Chinese men and women aged 45–84 at baseline. Four clinical exams took place after the baseline exam. After excluding those with ABI>1.4, prevalent and incident PAD were defined as an ABI≤0.90. ABI progression was defined as progression from a normal ABI (0.91–1.4) to abnormal (≤0.90 or >1.4) at a later exam.
Results
In adjusted models, each SD (13 ng/mL) higher P-selectin was significantly associated with 0.007 lower ABI (95% CI ((−0.011, −0.004)), p<0.001), and an average change in the ABI of − 0.006 ((−0.010, −0.003, p<0.001). P-selectin was significantly associated with a 1.17-fold greater odds of prevalent PAD ((1.02, 1.33), p=0.03), and a 30% greater risk of incident PAD ((1.11, 1.53), p=0.001), as well as progression from a normal ABI to an ABI≤ 0.90 (p=0.003), but not to an ABI>1.4 (p=0.96). Addition of P-selectin to models containing traditional PAD risk factors and markers of inflammation/coagulation significantly improved the net reclassification for ABI progression (p=0.03), but was only marginally significant for incident PAD (p=0.06).
Conclusions
P-selectin is significantly associated with the development of PAD. However, further research is needed in population-based studies to confirm prospective associations of P-selectin with incident PAD and change in the ABI, as well as its potential predictive ability.
Keywords: P-selectin, prediction, net reclassification improvement, incidence, ankle brachial index, peripheral artery disease
Introduction
Between 2000 and 2010, the global burden of peripheral artery disease (PAD) increased by almost 29% in low and middle income countries and 13% in high income countries[1]. PAD is associated with increased morbidity and mortality[2–5], as well as decreased functional status and quality of life[6–9]. Given the burden and comorbid conditions associated with PAD, there is a continuing need for a thorough study of biomarkers related to obstructive lower extremity atherosclerosis that could possibly lead to therapeutic targets to prevent or treat PAD.
The role of P-selectin in the atherosclerotic process involves the activation, rolling and attachment of leukocytes, as well as bonding of endothelial cells via ligand interaction[10–12]. P-selectin levels correlate with the severity of PAD[13], and there is some evidence for the specificity of P-selectin for PAD [14–16]. For example, among those with PAD, treatment with anti-platelet agents such as clopidogrel, aspirin, and cilostazol[17], as well as atorvastatin,[18] appears to reduce levels of P-selectin effectively.
Only two population-based cohorts have examined the association of P-selectin with lower extremity PAD[19, 20]. In these studies, P-selectin was not significantly associated with prevalent PAD, intermittent claudication, or ABI categories (<0.9, 0.9–1.0, >1.0–1.4)[19, 20]. However, associations of soluble P-selectin with the ABI and PAD, especially in a larger multi-ethnic cohort, are not well characterized. Furthermore, to our knowledge no diverse population-based cohort has examined the prospective association of P-selectin with incident PAD or change in the ABI. Thus, using data from the Multi-Ethnic Study of Atherosclerosis (MESA), we examined the association of P-selectin with prevalent and incident PAD, levels of and change in the ABI, as well as progression from a normal to an abnormal ABI. We examined the interactions of both race/ethnicity, sex, and diabetes with P-selectin for each of these outcomes. Additionally, we sought to determine whether P-selectin contributed to the prediction of PAD above and beyond traditional risk factors, as well as beyond additional markers of inflammation and coagulation.
Methods
Study Participants
MESA participants were recruited from six field sites in the United States – Forsyth County, NC (Wake Forest), Northern Manhattan/Bronx, NY (Columbia), Baltimore/Baltimore County, MD (Johns Hopkins), St. Paul, MN (University of Minnesota), Chicago, IL (Northwestern), and Los Angeles County, CA (UCLA). Details of recruitment have been previously published[21]. MESA complies with the Declaration of Helsinki, and Institutional Review Boards at each field site, as well as the Coordinating Center (University of Washington, Seattle), approved the study. Briefly, MESA recruited 6,814 men and women ages 45 to 84 years free of cardiovascular disease, and the cohort is 53% women with a racial/ethnic composition of approximately 38% non-Hispanic white, 28% African American, 23% Hispanic and 11% Asian, primarily of Chinese descent. The baseline exam (Exam 1) occurred from 2000–02, with Exam 2 from 2002–04, Exam 3 from 2004–05, Exam 4 from 2005–07, and Exam 5 from 2010–2012. The current study includes MESA participants with both P-selectin and ABI measurements, and includes data from Exams 2, 3, and 5.
P-selectin Measurement
Soluble P-selectin was measured at Exam 2 in plasma with the human soluble P-selectin/CD62P Immunoassay kit (R&D Systems, Minneapolis, MN). The minimum detection limit was 0.5ng/mL, and the inter-assay coefficient of variation was 6.7% at a mean concentration of 182 ng/mL.
Ankle Brachial Index and Peripheral Artery Disease
For the ABI, which includes data from Exams 3 and 5 for the current study, systolic blood pressure was measured in both the left and right brachial, dorsalis pedis, and posterior tibial arteries using a hand-held Doppler instrument with a 5-mHz probe. The ABI was calculated for both the left and right sides as maximum systolic blood pressure in the posterior tibial artery and dorsalis pedis, divided by the average of the left and right brachial pressures. As previous studies have shown a strong association between PAD and subclavian stenosis[22], in the event that left and right brachial pressures differed by 10 mmHg or more, the higher of the brachial pressures was used. If a pulse was detected when the cuff was inflated to 300mmHg, the ABI was classified as “incompressible”. For these analyses, minimum of the left and right leg ABI was used, and then those with ABI>1.4 were excluded. However, we also examined associations with the ABI defined by first excluding those with ABI>1.4 on either side, then using the minimum of the left and right side ABI.
Prevalent PAD at Exam 3 was defined as ABI≤0.90, removing those with ABI>1.4. Seven participants had a lower extremity revascularization or angioplasty prior to Exam 3 and also attended Exam 3; these participants were considered to have prevalent PAD regardless of ABI values at Exam 3 and were excluded from ABI analysis. Incident PAD was defined as ABI≤0.90 at Exam 5, removing those with ABI>1.4, as well as prevalent PAD. Participants with no evidence of PAD at Exam 3 were eligible for examination of PAD incidence at Exam 5; ABI≤0.90 at Exam 5 defined incident PAD. ABI progression was defined only for participants with a normal ABI value at Exam 3 (0.91–1.4) as progression to significant lower extremity disease, i.e. an ABI value of ≤0.90 indicating PAD or >1.4 indicating arterial stiffening, at Exam 5.
Covariate Measurement
Information on age, sex, race/ethnicity, and smoking history was obtained via interview and questionnaires. Height was measured by a stadiometer to the nearest 0.1 of a centimeter. Weight was measured to the nearest pound using a platform balance scale. Body mass index (BMI) was calculated as weight in kilograms per height in meters squared. Medication use was obtained via medication inventory. Systolic and diastolic blood pressures were determined by averaging the last two of three measurements taken with the Dinamap automated blood pressure device (GE Healthcare).
Plasma HDL-cholesterol, fasting triglycerides, fasting plasma glucose, C-reactive protein, IL-6, D-dimer and fibrinogen were measured at a central laboratory after a requested 12 hour fast. LDL-cholesterol was calculated using the Friedewald formula in those with triglycerides<400 mg/dL. HDL-cholesterol was measured using the cholesterol oxidase cholesterol method (Roche Diagnostics) after precipitation of non-HDL-cholesterol with magnesium/dextran, and has a laboratory CV of 2.9%. Triglycerides were measured using Triglyceride GB reagent (Roche Diagnostics, Indianapolis, IN 46250) on the Roche COBAS FARA centrifugal analyzer, and have a laboratory CV of 4.0%. Serum fasting glucose was measured by rate reflectance spectrophotometry using thin film adaptation of the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY 14650) with a laboratory CV of 1.1%. Hemoglobin A1c (HbA1c) was analyzed on the Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer (Tosoh Medics, Inc., San Francisco, CA 94080) using an automated high performance liquid chromatography method, with a laboratory CV range of 1.4 – 1.9%. Serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY), with a laboratory CV of 2.2%. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equations[23]. CRP was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL) with an intra-assay CVs range of 2.3 – 4.4% and inter-assay CVs range of 2.1 – 5.7%. IL-6 was measured by ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN) with a laboratory CV of 6.3%. Fibrin fragment D-dimer was measured using an immuno-turbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ) on the Sta-R analyzer (Diagnostica Stago, Parsippany, NJ). Fibrinogen antigen was measured using the BNII nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc., Deerfield, IL) with intra-assay and inter-assay CVs of 2.7% and 2.6%, respectively.
Hypertension was defined as self-report of physician diagnosis AND anti-hypertensive medication use, or systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥ 90 mmHg. Diabetes was defined as use of oral diabetes medication or insulin, or fasting glucose ≥126 mg/dL, or HbA1c > 6.5%.
All covariates used in this analysis were from Exam 3, concurrent with the ABI, except for CRP, IL-6, D-dimer and fibrinogen, which were measured at Exam 1.
Statistical Analysis
Univariate associations of participant characteristics with ABI category at Exam 3 were examined using ANOVA, chi-square, or Kruskal-Wallis tests as appropriate. In order to assess potential cut points for P-selectin in associations with the ABI and PAD, generalized additive models with a smoother that fits cubic B-splines to the data were examined. No significant departures from linearity were detected, so P-selectin was modeled as per standard deviation in all analyses.
To examine the associations of P-selectin with ABI at Exam 3, linear regression was used. To assess change in ABI from Exam 3 to Exam 5, growth curve models were used. Using change in the ABI between Exam 3 and Exam 5 (i.e. Exam 5 ABI – Exam 3 ABI), or for example, (Exam 5 ABI – Exam 3 ABI)/Exam 3 ABI, compounds the measurement error from two time points rather than cancelling it out. Alternatively, growth curve models minimize the error normally encountered by using raw change scores as the outcome, and ensure the greatest precision.
Prevalent PAD, incident PAD and ABI progression were defined as described in the “Ankle Brachial Index and Peripheral Artery Disease” section above. Logistic regression was used for prevalent and Cox regression for incident PAD, with the midpoint between Exam 3 and Exam 5 used as the time to event for incident PAD cases. The proportional hazards assumption was tested and fulfilled for all Cox models using a chi-square test based on summed Schoenfeld residuals. Multinomial regression was used for ABI progression in order to compare participants who progressed to significant disease at Exam 5 (ABI≤0.90 or ABI>1.4) to those who remained in the normal ABI category (0.91–1.4) at Exam 5 (the reference group); this model produces relative risk ratios. For all analyses except ABI progression, participants with ABI>1.4 were excluded. Staged models were examined to adjust for potential confounding and to assess independence. Models were initially adjusted for age, sex, and race/ethnicity, then in a second model adding BMI, ever smoking, diabetes, hypertension, and eGFR. A third model added blood pressure, fasting glucose, HDL cholesterol, LDL cholesterol, triglycerides, while a fourth model added CRP, IL-6, D-dimer and fibrinogen.
Additionally, race/ethnicity, sex, and diabetes-specific associations of P-selectin were examined by stratifying on race/ethnic group, sex group, or diabetes status within fully adjusted global models, and then testing for interaction. Formal tests of interaction were conducted on an additive scale for linear regression, and on a multiplicative scale for logistic and Cox regression.
In order to examine whether P-selectin contributed to prediction above and beyond traditional PAD risk factors, as well as other biomarkers such as D-dimer, IL-6 and CRP, c-statistics and net reclassification improvement (NRI) were calculated for incident PAD and ABI progression.
Results
Overall mean±SD P-selectin was 36.4±13.9 ng/mL and the median (Q1, Q3) was 34.5 (26.6, 44.3). P-selectin levels in this sample ranged from 3.9 to 216.7 ng/mL. Overall mean ±SD ABI at Exam 3 was 1.121±0.127, and at Exam 5 was 1.124±0.152. The mean ± SD time in years between Exams 2 and 3 was 1.6±0.2, between Exams 2 and 5 was 7.8±0.4, and between Exams 3 and 5 was 6.3±0.4. The median (Quartile 1, Quartile 3) time in years between Exams 2 and 3 was 1.5 (1.4, 1.6), between Exams 2 and 5 was 7.8 (7.6, 8.0), and between Exams 3 and 5 was 6.2 (6.0, 6.4).
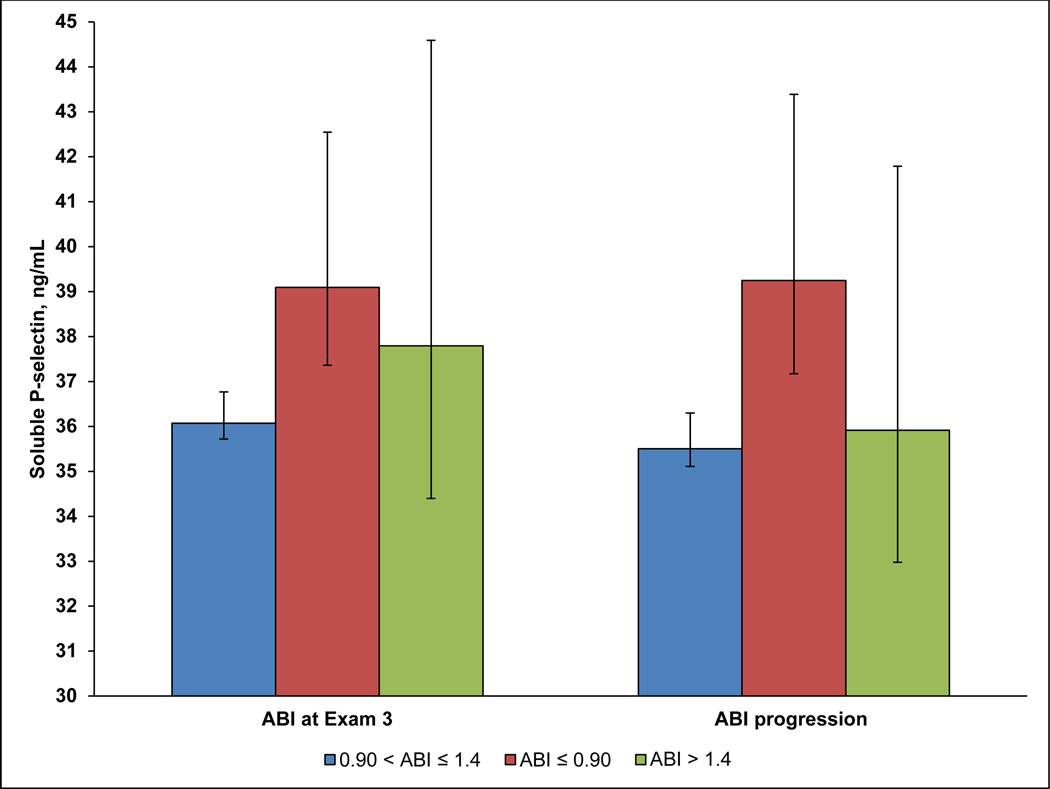
Table 1 displays participant characteristics by ABI category at Exam 3. Only BMI, LDL cholesterol, triglycerides, and anti-platelet agent use were not significantly associated with ABI category (p=0.13, 0.24, 0.84, and 0.07, respectively). Figure 1 displays least squares means of P-selectin levels (95% CI) by ABI category at Exam 3, and by ABI progression categories, adjusted for age, sex, and race/ethnicity. For ABI categories at Exam 3, levels of P-selectin differed significantly between the ABI≤0.90 and 0.91≤ABI≤1.4 groups (p<0.001), but no other pairwise comparisons were significant. Similarly, mean levels of P-selectin differed significantly between participants who progressed to ABI≤0.90 at Exam 5, and participants who remained in the normal ABI category (0.91–1.4) from Exam 3 to Exam 5, p<0.001. Additionally, levels of P-selectin were marginally significantly different between participants who progressed to ABI>1.4 at Exam 5, and participants who progressed to an ABI of ≤ 0.90, p=0.07.
Table 1.
Participant Characteristics by ABI category at Exam 3
| ABI ≤ 0.90 n=228 |
0.91 ≤ ABI ≤ 1.4 n=5415 |
ABI > 1.4 n=57 |
p-value | |
|---|---|---|---|---|
| Age, years | 73 ± 9 | 65 ± 10 | 66 ± 11 | <0.001 |
| Female Sex, n(%) | 118 (52%) | 2847 (53%) | 18 (32%) | 0.007 |
| Race, n(%) | ||||
| White | 68 (30%) | 2202 (41%) | 33 (58%) | <0.001 |
| African-American | 110 (48%) | 1404 (26%) | 10 (18%) | |
| Chinese | 10 (4%) | 661 (12%) | 3 (5%) | |
| Hispanic | 40 (18%) | 1148 (21%) | 11 (19%) | |
| Body Mass Index, kg/m2 | 28 ± 6 | 28 ± 5 | 30 ± 6 | 0.13 |
| Ever Smoker, n(%) | 150 (66%) | 2680 (50%) | 23 (40%) | <0.001 |
| Pack Years Smoking | 23.2 ± 31.9 | 10.6 ± 20.1 | 6.6 ± 11.1 | <0.001 |
| Lipid lowering medications, n(%) | 92 (40%) | 1814 (34%) | 15 (26%) | 0.002 |
| Anti-platelet medications, n (%) | 86 (38%) | 1445 (27%) | 57 (30%) | 0.07 |
| Diabetes, n(%) | 61 (27%) | 768 (14%) | 12 (21%) | <0.001 |
| Fasting glucose, mg/dL | 106 ± 32 | 98 ± 26 | 98 ± 22 | <0.001 |
| Estimated GFR, min/mL/1.73m2 | 67±20 | 79±16 | 75±14 | <0.001 |
| Hemoglobin A1c, % | 6.1±1.5 | 5.7±0.9 | 5.8±1.0 | <0.001 |
| Hypertension, n(%) | 184 (81%) | 2535 (47%) | 21 (37%) | <0.001 |
| Systolic Blood Pressure, mmHg | 135 ± 26 | 123 ± 20 | 118 ± 16 | <0.001 |
| Diastolic Blood Pressure, mmHg | 69 ± 11 | 70 ± 10 | 68 ± 8 | 0.02 |
| LDL cholesterol, mg/dL | 108 ± 34 | 112 ± 32 | 106 ± 29 | 0.24 |
| HDL cholesterol, mg/dL | 51 ± 17 | 52 ± 15 | 47 ± 14 | 0.05 |
| Triglycerides, mg/dL * | 99 (76, 150) | 107 (74, 156) | 117 (78, 141) | 0.84 |
| C-reactive protein (CRP), mg/L*† | 3.10 (1.17, 6.00) | 1.80 (0.80,4.08) | 1.85 (0.87, 4.22) | <0.001 |
| Interleukin (IL)-6, pg/mL*† | 1.70 (1.09, 2.49) | 1.14 (0.75, 1.78) | 1.06 (0.77, 1.87) | <0.001 |
| Fibrinogen, mg/dL*† | 366 (324, 426) | 335 (293, 385) | 338 (274, 366) | <0.001 |
| D-dimer, ug/mL*† | 0.35 (0.20, 0.55) | 0.20 (0.13, 0.35) | 0.23 (0.13, 0.32) | <0.001 |
Median (Q1, Q3), Kruskal-Wallis test used
Measured at Exam 1
Figure 1. Mean Soluble P-selectin levels by ABI Category at Exam 3 and ABI progression from Exam 3 to Exam 5.
Figure 1 shows least squares means of soluble P-selectin, adjusted for age, race/ethnicity and sex, by ABI category at Exam 3, and progression of ABI from normal at Exam 3 (0.91–1.4) to abnormal (≤0.90 or >1.4) at Exam 5. P-selectin in ng/mL is shown on the Y-axis, with ABI categories on the X-axis. ABI≤0.90 is represented by red bars, ABI>1.4 by green bars, and 0.90<ABI≤1.4 by blue bars. Error bars are the 95% CI around the least squares means.
Each standard deviation (SD) higher P-selectin was significantly associated with a 0.007 lower mean ABI at Exam 3 (p<0.001) among 5700 participants with both Exam 3 ABI and Exam 2 P-selectin data, even after adjustment for demographics, lifestyle, comorbidities, lipids, glucose, blood pressure and other markers of coagulation and inflammation (Table 2). After removing 57 participants with ABI>1.4, prevalent PAD at Exam 3 and P-selectin were available on 5643 participants, of which 231 were prevalent PAD cases. Soluble P-selectin levels were also significantly associated with prevalent PAD (Table 2); each SD greater P-selectin was associated with a 1.17-fold higher odds of prevalent PAD. There were no significant interactions of P-selectin with sex or race/ethnicity for ABI at Exam 3 (p=0.25, p=0.76, respectively) or prevalent PAD (p=0.66, p=0.23, respectively) in fully adjusted models. Additionally, there were no significant interactions of diabetes with P-selectin for ABI at Exam 3 (p=0.12) or prevalent PAD (p=0.83).
Table 2.
Association of Soluble P-selectin with the ABI and Prevalent PAD*
| ABI at Exam 3 Beta (95%CI) |
p-value | Prevalent PAD Exam 3 OR (95%CI) |
p-value | |
|---|---|---|---|---|
| Demographics† | −0.009 (−0.013, −0.006) | <0.001 | 1.21 (1.06, 1.37) | 0.004 |
| +Lifestyle and Comorbidities‡ | −0.009 (−0.012, −0.006) | <0.001 | 1.18 (1.04,1.34) | 0.01 |
| +Lipids, glucose, blood pressure§ | −0.008 (−0.011, −0.005) | <0.001 | 1.18 (1.04, 1.35) | 0.01 |
| +CRP, IL-6, D-Dimer, Fibrinogen | −0.007 (−0.011, −0.004) | <0.001 | 1.17 (1.02, 1.33) | 0.03 |
P-selectin per standard deviation (SD), which is 13.9 ng/mL; ABI at Exam 3 excluding ABI>1.4; Prevalent PAD defined as ABI≤0.90, excluding ABI>1.4
Includes age, race, sex
Adds ever smoker, BMI, hypertension, diabetes, estimated glomerular filtration rate
Adds HDL cholesterol, LDL cholesterol, triglycerides, glucose, systolic blood pressure, diastolic blood pressure
Change in the ABI from Exam 3 to Exam 5, and P-selectin at Exam 2 data, were available on 4276 participants, as this definition included participants with ABI>1.4 at either Exam 3 or 5. After removing prevalent PAD cases as well as participants with ABI>1.4 at either Exam 3 or 5, incident PAD and P-selectin data were available on 4096 participants, of which 147 were incident cases. P-selectin was significantly associated with change in ABI, with each SD greater of P-selectin associated with an average change in ABI of −0.006 (p<0.001) in fully adjusted models, as well as 30% greater risk of incident PAD in fully adjusted models (Table 3). There were no significant interactions of P-selectin with sex or race/ethnicity in for change in ABI (p=0.37, p=0.44) or incident PAD (p=0.70, p=0.33, respectively). Additionally, there were no significant interactions of diabetes with P-selectin for change in the ABI (p=0.64) or incident PAD (p=0.52).
Table 3.
Association of soluble P-selectin Change in the ABI and Incident PAD*
| Change in ABI Beta (95%CI) |
p-value | Incident PAD HR (95%CI) |
p-value | |
|---|---|---|---|---|
| Demographics† | −0.008 (−0.011,−0.005) | <0.001 | 1.33 (1.14, 1.54) | <0.001 |
| +Lifestyle and Comorbidities‡ | −0.008 (−0.011, −0.004) | <0.001 | 1.31 (1.12, 1.53) | 0.001 |
| +Lipids, glucose, blood pressure§ | −0.007 (−0.010, −0.003) | <0.001 | 1.28 (1.09, 1.50) | 0.003 |
| +CRP, IL-6, D-Dimer, Fibrinogen | −0.006 (−0.010, −0.003) | <0.001 | 1.30 (1.11, 1.53) | 0.001 |
P-selectin per standard deviation (SD), which is 13.9 ng/mL; change in ABI from Exam 3 to Exam 5 modeled with growth curve analysis; incident PAD is defined as ABI≤0.90, excluding ABI>1.4 at Exam 3 or Exam 5, and prevalent PAD cases
Includes age, race, sex
Adds ever smoker, BMI, hypertension, diabetes, estimated glomerular filtration rate
Adds HDL cholesterol, LDL cholesterol, triglycerides, glucose, systolic blood pressure, diastolic blood pressure
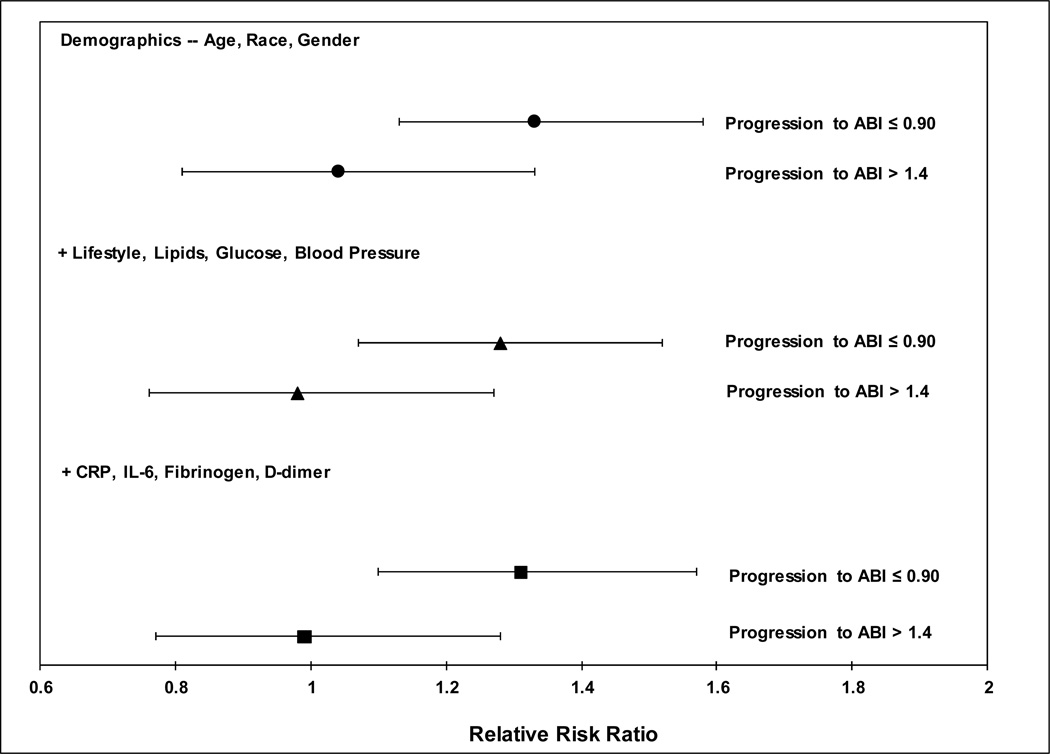
For ABI progression, data on 4124 participants with normal ABI (0.91–1.4) at Exam 3 as well as Exam 5 ABI values were available, with 147 cases progressing to ABI≤0.90, and 72 progressing to ABI>1.4 at Exam 5. P-selectin was significantly associated with progression from a normal ABI (0.91–1.4) to an ABI≤0.90, but not with progression to an ABI>1.4 (Figure 2). Each SD higher P-selectin was associated with a 31% (95% CI (1.10, 1.57, p=0.003) greater risk of progression to an ABI≤0.90 in fully adjusted models. There were no significant interactions of P-selectin with sex or race/ethnicity for ABI progression (p=0.85, p=0.89, respectively). There was also no significant interaction of P-selectin with diabetes (p=0.79).
Figure 2. Association of soluble P-selectin with progression of the ABI from Exam 3 to Exam 5.
Figure 2 is a forest plot that displays the association of P-selectin per standard deviation for ABI progression from Exam 3 to Exam 5. Values shown are odds ratios with 95% CIs for varying levels of model adjustment as described in the text. Only participants with a normal ABI (0.91–1.4) at Exam 3 were included, and were evaluated for progression to an abnormal ABI at Exam 5 (ABI≤0.90 or ABI>1.4), with the reference group as participants who remained in the normal ABI category from Exam 3 to Exam 5 (ABI of 0.91–1.4). Model adjustments include demographics (age, race/ethnicity, and gender), with additional adjustment for body mass index, ever smoking, hypertension, diabetes, estimated glomerular filtration rate, fasting glucose, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure; a final model additionally adjusts for CRP, IL-6, fibrinogen, and D-dimer.
Additional adjustment for lipid-lowering medications, anti-platelet agents, or pack years of smoking did not materially alter results of any model. Using a slightly different definition of the ABI, which first excludes participants with ABI>1.4 in either leg and then using the minimum of the left and right ABI, also did not change the results.
P-selectin did not appear to add significantly to the area under the curve (AUC) for incident PAD in either a model containing traditional PAD risk factors (p=0.28) or traditional risk factors plus markers of inflammation and coagulation (p=0.26) (Table 4). Similarly, P-selectin did not add significantly to the AUC for ABI progression, with p=0.36 for a traditional risk factor model, and p=0.35 for a model containing traditional risk factors plus markers of inflammation and coagulation. Using risk category cut points of 5, 10, and 15%, P-selectin appeared to marginally improve the net reclassification improvement (NRI) for incident PAD for the model adding P-selectin to a model containing traditional and inflammation/coagulation risk factors (p=0.06). For ABI progression, the NRI was significant for the addition of P-selectin to both traditional risk factor (p=0.04), and traditional risk factor plus markers of coagulation and inflammation models (p=0.03).
Table 4.
Predictive Ability of P-selectin for Incident PAD and ABI progression
| Model Comparison | AUCs | P-value for AUC Comparison |
NRI (SE); p‡ |
|---|---|---|---|
| Incident PAD | |||
| Traditional RF vs. Traditional RF+P-selectin* | 0.8069 vs. 0.8122 | 0.28 | 0.06 (0.04); 0.17 |
| Biomarker vs. Biomarker + P-selectin† | 0.8093 vs. 0.8146 | 0.26 | 0.08 (0.04); 0.06 |
| ABI Progression | |||
| Traditional RF vs. Traditional RF+P-selectin* | 0.8074 vs. 0.8155 | 0.36 | 0.08 (0.04); 0.04 |
| Biomarker vs. Biomarker + P-selectin† | 0.8103 vs. 0.8144 | 0.35 | 0.09 (0.04); 0.03 |
Traditional risk factor (RF) models include age, sex, race/ethnicity, ever smoker, BMI, hypertension, diabetes, estimated glomerular filtration rate, blood pressure, fasting glucose, LDL cholesterol, HDL cholesterol, triglycerides
Biomarker model includes traditional risk factors plus CRP, IL-6, D-dimer, fibrinogen
Using risk category cut points of 5, 10, and 15%
Discussion
In MESA, we found that P-selectin measured was significantly associated with the ABI and prevalent PAD. P-selectin also predicted change in the ABI and incident PAD, as well as progression from a normal ABI at Exam 3 to ABI≤0.90 at Exam 5. P-selectin was not associated with progression from a normal ABI at Exam 3 to ABI>1.4 at Exam 5, indicating that P-selectin is not associated with arterial stiffening. There was no evidence of modification by race/ethnicity, sex, or diabetes of associations of P-selectin with the ABI or PAD. Despite the significant association with incident PAD, P-selectin did not appear to add significant incremental predictive ability above and beyond traditional risk factors, or traditional risk factors plus markers of inflammation and coagulation. However, the NRI did indicate significant improvement in reclassification for ABI progression, with marginal significance for incident PAD.
To our knowledge this is the first study in a population-based cohort that examines the prospective association of P-selectin with incident PAD, change in ABI, and progression from normal to abnormal ABI. Only a few studies, with smaller sample sizes than MESA, have examined the associations of P-selectin with ABI, prevalent PAD, or intermittent claudication. In the Framingham Heart Study (FHS) Offspring cohort (n=2800), P-selectin was not associated with prevalent intermittent claudication/revascularization or ABI categories in participants of European descent [19]. In 1791 African-American and European descent participants from the Atherosclerosis Risk in Communities (ARIC) Study, P-selectin was also not significantly associated with prevalent PAD after risk factor adjustment [20]. These results were inconsistent with our study, but there could be several factors involved in the discrepancy. In FHS, Murabito et al[19] examined ABI categories (<0.90, 0.90–1.0, >1.0–1.4), and had 111 cases in the ABI<0.90 group, and only 90 cases of intermittent claudication. Thus, power could have been an issue. In ARIC, the measurement of ABI differed substantially from that of MESA, using oscillometric methods, and measuring blood pressure in one leg chosen randomly[24]. As bilateral lower extremity PAD is not always the case, some misclassification of PAD cases likely occurred.
The selectins are a class of adhesion molecules primarily involved in leukocyte adhesion to the endothelium and migration of leukocytes from the endothelium to the intima, which occurs at the early stages of the atherosclerotic process[12]. In particular, the primary roles of P-selectin in atherosclerotic process involve the activation, rolling and attachment of leukocytes, and bonding of endothelial cells via ligand interaction[10–12]. Specifically in those with already existing lower extremity PAD, P-selectin has been shown to increase leukocyte adhesion to fibrinogen and platelet monolayers[25]. This increased leukocyte adhesion was particularly apparent at low shear sites, indicating that P-selectin could be involved in recruiting leukocytes to sites of vascular injury and contributing to progression of PAD via the generation of new plaques[25].
Several studies support the specificity of p-selection for lower extremity PAD. Nambi et al[15] found significantly higher shear-induced P-selectin in those with PAD compared to those with CAD, possibly indicating a role of P-selectin specific to PAD. Blann et al[26] also found evidence for the specificity of P-selectin to PAD, with elevated P-selectin levels in those with femoral-iliac disease, but not in those with carotid disease, when compared to a control group. Additionally, higher P-selectin expression[13] and soluble P-selectin[16] have been associated with increasing severity of PAD.
The early activation in the atherosclerotic process, as well as the evidence for specificity for PAD, render P-selectin a potentially attractive therapeutic target. Among those with PAD, antiplatelet agents such as clopidigrel, aspirin, cilostazol[17, 27], as well as atorvastatin[18], have been effective in reducing levels of P-selectin. Given the side effects and safety issues with antiplatelet therapy, other treatments have been recently investigated. An anti-P-selectin agent, inclacumab, a monoclonal antibody that works by inhibiting platelet-leukocyte interactions, appears effective in both preclinical and clinical studies including PAD[28]. However, there remains limited data on these agents for secondary prevention in those with PAD[29], and these agents have not yet been tested in the primary prevention of PAD. In the current study, P-selectin does not seem to contribute substantially to the ability to predict incident PAD in terms of the area under the curve, which could call into question the use of drug therapy to reduce P-selectin levels for primary prevention. However, there does appear to be some benefit for reclassifying PAD cases, which does provide evidence for future testing of drug therapies for primary prevention of PAD.
Our study has several strengths, but also some limitations to note. MESA is a large, prospective ethnically diverse cohort of men and women, with a standardized, comprehensive ABI measurement protocol at each clinic examination. Power to detect interactions of P-selectin with race/ethnicity, sex, and diabetes may not have been adequate for prevalent and incident PAD, but we did see similar non-significant interaction associations with the ABI. While a change of 0.007 in the ABI over time may not be a clinically significant finding, this is the average change in the ABI per standard deviation increase (13.9ng/mL) in P-selectin. Given the range of P-selectin in the current study is 3.9 to 216.7 ng/mL, participants with higher levels of P-selectin would indeed potentially have clinically significant changes in the ABI.
In the current study, we found that soluble P-selectin is significantly associated with prevalent PAD, incident PAD, the ABI, and change in the ABI. Further research is needed in population-based studies to confirm prospective associations of P-selectin with incident PAD as well as its potential to increase predictive ability and reclassification. Additionally, the clinical and scientific community could benefit from clinical trials to determine efficacy of drug therapies such as inclacumab as both a primary prevention tool for those at high risk of developing PAD and a secondary prevention tool among those with PAD.
Highlights.
The global burden of PAD warrants continued research on potential therapeutic targets.
Associations of p-selectin with the ABI and PAD are not well characterized.
P-selectin was significantly associated with a decreased ABI and incident PAD.
P-selectin improved reclassification for ABI progression and incident PAD.
Further research is needed to confirm findings and assess P-selectin as a drug target.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources
This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health via MESA contracts N01-HC-95159 through N01-HC-95169, and R01 HL098077 to SJB, and by grants UL1-TR-000040 and UL1-RR-025005 from National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have none to disclose.
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013 doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Guralnik JM, Ferrucci L, Criqui MH, Greenland P, Tian L, et al. Functional decline in lower-extremity peripheral arterial disease: associations with comorbidity, gender, and race. J Vasc Surg. 2005;42:1131–1137. doi: 10.1016/j.jvs.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484–2491. doi: 10.1161/CIRCULATIONAHA.107.736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, et al. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study) J Am Coll Cardiol. 2009;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman SD, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, et al. Baseline lower extremity strength and subsequent decline in functional performance at 6-year follow-up in persons with lower extremity peripheral arterial disease. J Am Geriatr Soc. 2009;57:2246–2252. doi: 10.1111/j.1532-5415.2009.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afshar-Kharghan V, Thiagarajan P. Leukocyte adhesion and thrombosis. Curr Opin Hematol. 2006;13:34–39. doi: 10.1097/01.moh.0000190107.54790.de. [DOI] [PubMed] [Google Scholar]
- 11.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 12.Hope SA, Meredith IT. Cellular adhesion molecules and cardiovascular disease. Part I. Their expression and role in atherogenesis. Intern Med J. 2003;33:380–386. doi: 10.1046/j.1444-0903.2003.00378.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan S, Mckay I, Ford I, Bachoo P, Greaves M, Brittenden J. Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management. J Vasc Surg. 2007;46:485–490. doi: 10.1016/j.jvs.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Blann AD, Seigneur M, Boisseau MR, Taberner DA, McCollum CN. Soluble P selectin in peripheral vascular disease: relationship to the location and extent of atherosclerotic disease and its risk factors. Blood Coagul Fibrinolysis. 1996;7:789–793. [PubMed] [Google Scholar]
- 15.Nambi V, Kimball KT, Bray PF, Bergeron AL, Johnson SL, Morrisett JD, et al. Differences in responses of platelets to fluid shear stress in patients with peripheral artery disease (PAD) and coronary artery disease (CAD) Platelets. 2009;20:199–205. doi: 10.1080/09537100902780643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan KT, Tayebjee MH, Lynd C, Blann AD, Lip GY. Platelet microparticles and soluble P selectin in peripheral artery disease: relationship to extent of disease and platelet activation markers. Ann Med. 2005;37:61–66. doi: 10.1080/07853890410018943. [DOI] [PubMed] [Google Scholar]
- 17.Rao AK, Vaidyula VR, Bagga S, Jalagadugula G, Gaughan J, Wilhite DB, et al. Effect of antiplatelet agents clopidogrel, aspirin, and cilostazol on circulating tissue factor procoagulant activity in patients with peripheral arterial disease. Thromb Haemost. 2006;96:738–743. [PubMed] [Google Scholar]
- 18.Mobarrez F, He S, Broijersen A, Wiklund B, Antovic A, Antovic J, et al. Atorvastatin reduces thrombin generation and expression of tissue factor, P-selectin and GPIIIa on platelet-derived microparticles in patients with peripheral arterial occlusive disease. Thromb Haemost. 2011;106:344–352. doi: 10.1160/TH10-12-0810. [DOI] [PubMed] [Google Scholar]
- 19.Murabito JM, Keyes MJ, Guo CY, Keaney JF, Jr, Vasan RS, D'Agostino RB S, et al. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The Framingham Offspring Study. Atherosclerosis. 2009;203:509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matijevic N, Wu KK, Nidkarni N, Heiss G, Folsom AR. The ARIC carotid MRI study of blood cellular markers: an inverse association of monocyte myeloperoxidase content with peripheral arterial disease. Angiology. 2011;62:237–244. doi: 10.1177/0003319710385336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro 3rd AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125. doi: 10.1016/s0021-9150(97)06089-9. [DOI] [PubMed] [Google Scholar]
- 25.Woollard KJ, Kling D, Kulkarni S, Dart AM, Jackson S, Chin-Dusting J. Raised plasma soluble P-selectin in peripheral arterial occlusive disease enhances leukocyte adhesion. Circ Res. 2006;98:149–156. doi: 10.1161/01.RES.0000199295.14073.69. [DOI] [PubMed] [Google Scholar]
- 26.Blann AD, McCollum CN. Increased soluble P-selectin in peripheral artery disease: a new marker for the progression of atherosclerosis. Thromb Haemost. 1998;80:1031–1032. [PubMed] [Google Scholar]
- 27.Klinkhardt U, Bauersachs R, Adams J, Graff J, Lindhoff-Last E, Harder S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease. Clin Pharmacol Ther. 2003;73:232–241. doi: 10.1067/mcp.2003.13. [DOI] [PubMed] [Google Scholar]
- 28.Kling D, Stucki C, Kronenberg S, Tuerck D, Rheaume E, Tardif JC, et al. Pharmacological control of platelet-leukocyte interactions by the human anti-P-selectin antibody inclacumab--preclinical and clinical studies. Thromb Res. 2013;131:401–410. doi: 10.1016/j.thromres.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 29.McDermott MM, Criqui MH. Aspirin and secondary prevention in peripheral artery disease: a perspective for the early 21st century. JAMA. 2009;301:1927–1928. doi: 10.1001/jama.2009.668. [DOI] [PubMed] [Google Scholar]