Abstract
Objective
To correlate gyrA mutations found on the Genotype MTBDRsl assay in Mycobacterium tuberculosis (MTB) isolates with Minimum Inhibitory Concentrations (MICs) to the fluoroquinolones compounds ofloxacin (OFX) and moxifloxacin (MXF).
Methods
MICs for OFX and MXF were ascertained for 93 archived clinical MTB isolates that showed gyrA mutations at Ala90Val, Ser91Pro, Asp94Ala, Asn/Tyr, Gly and His. Thirty fluoroquinolones susceptible isolates as determined by presence of all wild-type gyrA bands on the Genotype MTBDRsl assay were also included.
Results
gyrA mutations at Ala90Val (n=25), Ser91Pro (n=6), Asp94Ala (n=4), Asp94Asn/Tyr (n=13), Asp94Gly (n=42) and Asp94His (n=3) were observed. Isolates with mutations at Ala90Val or Ser91Pro had MIC90 of 4.0µg/ml and 1.0µg/ml for OFX and MXF, respectively, and isolates with mutations at Asp 94Ala, Asn/Tyr, Gly and His had MIC90 of 8.0µg/ml, and 2.5µg/ml for OFX and MXF, respectively.
Conclusions
MTB MICs were found to be consistently lower for MXF than for OFX among isolates with the same gyrA mutation (e.g. Ala90Val). The majority of MTB isolates containing mutations at Asp94Ala, Asn/Tyr, Gly and His in gyrA were associated with a moderate level of resistance to MXF (MIC=2.5µg/ml), although 3 isolates with the mutations Asp94Asn/Tyr/Gly were associated with a high level of resistance to both fluoroquinolones (MXF MICs = 5.0–8.0µg/ml, OFX MICs = ≥10.0µg/ml).
Keywords: Drug Susceptibility Testing, fluoroquinolones, level of resistance, mutations
1. Introduction
Drug resistance in Mycobacterium tuberculosis (MTB) is a major public health issue. The global burden of tuberculosis (TB) is high [1]. There is growing concern regarding the rise and spread of multi- and extensively drug-resistant TB (M/XDR-TB) [2]. The recent appearance of untreatable, or totally drug-resistant, TB (TDR-TB) has further underscored the urgent need for efficient and effective TB control efforts [3]. Currently, clinical diagnosis of drug-resistant TB relies upon slow growth culture and drug susceptibility testing (DST) of MTB isolates via liquid culture Mycobacteria Growth Indicator Tube (MGIT) platforms, yielding phenotypic resistance profiles of these isolates [4, 5]. Molecular diagnosis of M/XDR-TB, in contrast, is based on the rapid detection of genetic mutations known to confer resistance to the given drugs of interest [7]. Unfortunately, both phenotypic DST at critical concentrations as well as molecular diagnostic methods are qualitative, giving rise to clinical uncertainties regarding the optimal drug dosing required to treat patients who may have different levels of resistance to the different TB treatment drugs [8, 9].
Fluoroquinolones represent an effective class of bactericidal second-line drugs for the treatment of MDR-TB [1, 6]. However, phenotypic resistance to these compounds have been shown to be heterogeneous in MTB isolates, varying from low level resistance (suggesting that infections might still be treated effectively with increased concentrations of, or alternative, fluoroquinolones), to high-level resistance (where MTB cannot be cleared with any fluoroquinolones) [10]. Although phenotypic DST with fluoroquinolones specific Minimum Inhibitory Concentrations (MICs) is a valid way to estimate fluoroquinolones resistance, it is a somewhat inefficient means of characterizing drug resistance due to the time required for such methodology [8] and the laboratory limitations of most low-resource clinical settings. Genetic diagnostic technologies have the ability to overcome these barriers. Correlating specific mutations conferring drug resistance with specific MICs for given drug classes are essential before successful implementation of these technologies become a reality.
There are additional clinical benefits in clearly defining the relationship between resistance-associated mutations found in the gyrA gene and fluoroquinolones phenotypic resistance profiles. When patients with MDR-TB are compared to patients with MDR-TB that have additional fluoroquinolones resistance, those with fluoroquinolones resistance appear to have a more serious form of disease, in that treatment success becomes less common, and the risk of developing XDR-TB increases [1]. Cross-resistance to the fluoroquinolones is frequent and fluoroquinolones resistance in MTB is usually associated with mutations in the conserved quinolone resistance-determining region (QRDR) of gyrA, particularly at codons 90 and 94 [12–15]. Previous studies have confirmed that the presence of these different mutations in MTB genes correlate with different levels of resistance [11], and we sought to further explore this relationship, considering a MICs range for susceptible MTB of 0.0625–0.25µg/ml for moxifloxacin (MXF) and 0.5–2.0µg/ml for ofloxacin (OFX) [16–18].
In this study, we estimated the fluoroquinolones MICs of 123 clinical MTB isolates and correlated those MICs with specific gyrA mutations on the Genotype MTBDRsl assay found within the isolates to determine whether these commonly occurring mutations were associated with different levels of fluoroquinolones resistance.
2. Materials and methods
2.1 Study population
The study was performed at the Mycobacteriology Laboratory of the P. D. Hinduja National Hospital (PDHNH) and Medical Research Centre (MRC), a tertiary care hospital in Mumbai, India with a referral bias towards non-responders [19]. The study was approved by the Institutional Review Board (IRB) of Hinduja Hospital. Written consent was waived for all participants as the study was carried out on 123 archived isolates for which both Genotype MTBDRsl assay and DST at the WHO approved critical concentrations had been performed previously.
2.2 Phenotypic DST
Quantitative drug susceptibility testing was conducted using MGIT960: Preparation of Drugs: A stock solution of OFX (Sigma- Aldrich 08757) was prepared by dissolving the drug in 0.1N NaOH. MXF (Sigma- Aldrich 32477) was prepared by dissolving the compound in distilled water. Both drugs were filtered, further diluted in distilled water, and stored at −80°C for up to 6 months. Preparation of the inoculum, as well as inoculation and incubation were performed as per manufacturer instructions (Becton Dickinson Diagnostic System, Sparks, MD) [20].
2.3 Selection of drug concentrations for MICs
Six MICs were selected in order to fully define the phenotypic resistance profiles of the gyrA MTB isolates. A critical concentration of 2.0µg/ml was used for OFX and 0.25µg/ml was used for MXF, in accordance with WHO recommendations [21]. For estimation of OFX MICs, two concentrations below the critical concentration (0.5 and 1.0µg/ml), and three concentrations above the critical concentration (4.0, 8.0 and 10.0µg/ml), were utilized. For estimation of MXF MICs, two concentrations below the critical concentration (0.0625and 0.125µg/ml), and three concentrations above the critical concentration (0.5, 1.0 and 2.5µg/ml) were used. For MXF, if any isolate demonstrated resistance at 2.5µg/ml, then we also tested those isolates (n=3) at three additional higher concentrations 5, 8 and 10µg/ml.
2.4 MIC50 and MIC90 Calculation
In this study, MIC50 was defined as the fluoroquinolones drug concentration that inhibited 50% of the isolates from each mutation group and MIC90 was defined as the fluoroquinolones drug concentration that inhibited 90% of the Isolates from each mutation group. MIC50 and MIC90 were calculated as follows:
MIC50 = no. of isolates (n) × 0.5 and MIC90 = no. of isolates (n) × 0.9.
2.5 Genotype MTBDRsl assay
A 123 consecutive archived isolates for which both Genotype MTBDRsl assay and DST at the WHO approved critical concentrations had been performed previously were selected.
Genotype MTBDRsl assay was processed as follows:
DNA extraction was performed on all decontaminated patient samples using a GenoLyse kit (Hain Lifescience, Nehren, Germany). Multiplex polymerase chain reaction (PCR) amplification was then conducted utilizing biotinylated primers. PCR was performed with the following cycling conditions: Initial Denaturation 95°C/15 min, [Denaturation 95°C/30 sec, Annealing 58°C/2 min, (10 cycles)], [Denaturation 95°C/25 sec, Annealing 53°C/40 sec, Extension 70°C/40 sec (30 cycles)], and Final Extension 70°C/8 min. Reverse Hybridization/Genotype MTBDRsl assay was performed as per manufacturer instructions (Hain Lifescience, Nehren, Germany) [22].
2.6 Pyrosequencing (PSQ)
Thirty representative isolates of the 123 MTB isolates were sequence confirmed by PSQ. These isolates were selected so as to encompass four isolates of each gyrA mutations, on the Genotype MTBDRsl assay i.e. MUT1, MUT2 and 16 isolates from MUT3 i.e. MUT3A, MUT3B, MUT3C and two isolates of MUT 3D. Four isolates with wild-type characterization were also pyrosequenced.
PSQ consisted of a modified PCR amplification followed by PSQ reaction using one set of MTB-specific gyrAprimers. Reagents from the Hot Start Taq kit and deoxynucleoside triphosphate (dNTP) mixtures (Qiagen, Valencia, CA) were used in the PCR master mix. Each PCR reaction contained 2.5 µL of extracted isolate DNA and 22.5 µL of PCR master mix, comprised of: 1× PCR buffer, 2.5 mM MgCl2, 0.96 mMdNTP mixture, 1× Q-solution, 0.5µM primer, and 1 U of HotStartTaq. The PCR reaction included: initial activation of the Hot Start Taq at 95°C for 15 min, 50 cycles of amplification at 94°C for 15 s, 60°C for 30 s, and 72°C for 20 s, and final extension at 72°C for 5 min. PyroMark Q96 reagents were then employed for PSQ, utilizing the sequence analysis mode of the Pyro Mark Q96 ID system (Qiagen, Valencia, CA) [23].
2.7 Statistical analysis
A Kruskal-Wallis test (GraphPad Prism 6 One-way ANOVA) was performed to evaluate whether different level of resistance is associated with the different gyrA mutations.
3. Results
Of the 123 MTB isolates evaluated in this study, 93 were OFX-resistant isolates, of which 75 were MDR with additional resistance to the fluoroquinolones; two were monoresistant to the fluoroquinolones and 16 were XDR and 30 isolates were fluoroquinolones susceptible. Eighty-eight were resistant to both MXF and OFX at the critical concentrations of 0.25 and 2.0µg/ml, respectively.
Agreement between genotypic Genotype MTBDRsl assay and phenotypic susceptibility assay (using critical concentrations) was 95% for MXF and 100% for OFX. PSQ confirmed that, of the 30 representative isolates, four isolates with an absence of the wild-type2 band and presence of the MUT1 band via Genotype MTBDRsl assay corresponded to a 90GCG-GTG mutation; four isolates with an absence of the wild-type band and presence of a MUT2 band corresponded to a 91TGC-CCG mutation; 16 isolates with an absence of the WT3 band and presence of a MUT3A, MUT3B or MUT3C band corresponded to 94GAC-GCC, 94GAC-AAC/TAC or 94GAC-GGC mutations, respectively; two isolates with the absence of a WT3 band and presence of a MUT3D band corresponded to a 94GAC-CAC mutation; and four isolates with Wild-type bands on Genotype MTBDRsl assay were confirmed to be wild type via PSQ.
Results of MIC50 and MIC90 for gyrA mutations are summarized in Table 1. Isolates harboring the mutations Ala90Val and Ser91Pro were found to have a MIC90 of 4.0µg/ml for OFX and 1.0µg/ml for MXF, whereas Asp94Ala, Asp94Asn/Tyr, Asp94Gly and Asp94His were found to have a MIC90 of 8.0µg/ml for OFX and 2.5µg/ml for MXF (Table 1). Three isolates had MICs >2.5µg/ml for MXF. We performed additional MXF MICs testing on these three isolates utilizing the higher drug concentrations 5, 8 and 10µg/ml. We observed that two of isolates harboring Asp94Asn/Tyr mutations had MICs of 5.0µg/ml while one isolate with an Asp94Gly mutation had an MIC of 8.0µg/ml for MXF.
Table 1.
gyr A Mutations and MICs of MXF and OFX for wild-type isolates 388 and isolates harboring mutations:
| Nucleotide changes |
Genotype MTBDRsl assay |
Mutation | Total no. (%) |
No. of isolates |
MIC | MIC Range | MIC50 | MIC90 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OFX | MXF | OFX | MXF | OFX | MXF | OFX | MXF | |||||
| (µg/ml) | (µg/ml) | (µg/ml) | (µg/ml) | |||||||||
| GCG-GTG | MUT1 | Ala90Val | 25 (26.9) |
13 | 4.0 | 1.0 | 4.0– 8.0 |
0.25– 1.0 |
4.0 | 1.0 | 4.0 | 1.0 |
| 4 | 4.0 | 0.5 | ||||||||||
| 3 | 8.0 | 1.0 | ||||||||||
| 5 | 4.0 | 0.25 | ||||||||||
| TGC-CCG | MUT2 | Ser91Pro | 6 (6.5) |
5 | 4.0 | 1.0 | 4.0– 8.0 |
1.0 | 4.0 | 1.0 | 4.0 | 1.0 |
| 1 | 8.0 | 1.0 | ||||||||||
| GAC-GCC | MUT3A | Asp94Ala | 4 (4.3) |
3 | 8.0 | 2.5 | 4.0– 8.0 |
1.0– 2.5 |
8.0 | 2.5 | 8.0 | 2.5 |
| 1 | 4.0 | 1.0 | ||||||||||
| GAC-AAC/TAC | MUT3B | Asp94Asn/Tyr | 13 (13.9) |
10 | 8.0 | 2.5 | 8.0– >10.0 |
2.5– 5.0 |
8.0 | 2.5 | 8.0 | 2.5 |
| 1 | 10.0 | 2.5 | ||||||||||
| 1 | >10.0 | 5.0 | ||||||||||
| 1 | 10.0 | 5.0 | ||||||||||
| GAC-GGC | MUT3C | Asp94Gly | 42 (45.2) |
26 | 8.0 | 2.5 | 8.0– >10.0 |
1.0– 8.0 |
8.0 | 2.5 | 8.0 | 2.5 |
| 9 | 8.0 | 1.0 | ||||||||||
| 6 | 10.0 | 2.5 | ||||||||||
| 1 | >10.0 | 8.0 | ||||||||||
| GAC-CAC | MUT3D | Asp94His | 3 (3.2) |
1 | 8.0 | 1.0 | 8.0 | 1.0– 2.5 |
8.0 | 2.5 | 8.0 | 2.5 |
| 2 | 8.0 | 2.5 | ||||||||||
| WT | WT | WT | 30 | 26 | 0.5 | 0.0625 | 0.5– 2.0 |
0.0625– 0.25 |
||||
| 2 | 1.0 | 0.125 | ||||||||||
| 2 | 2.0 | 0.25 | ||||||||||
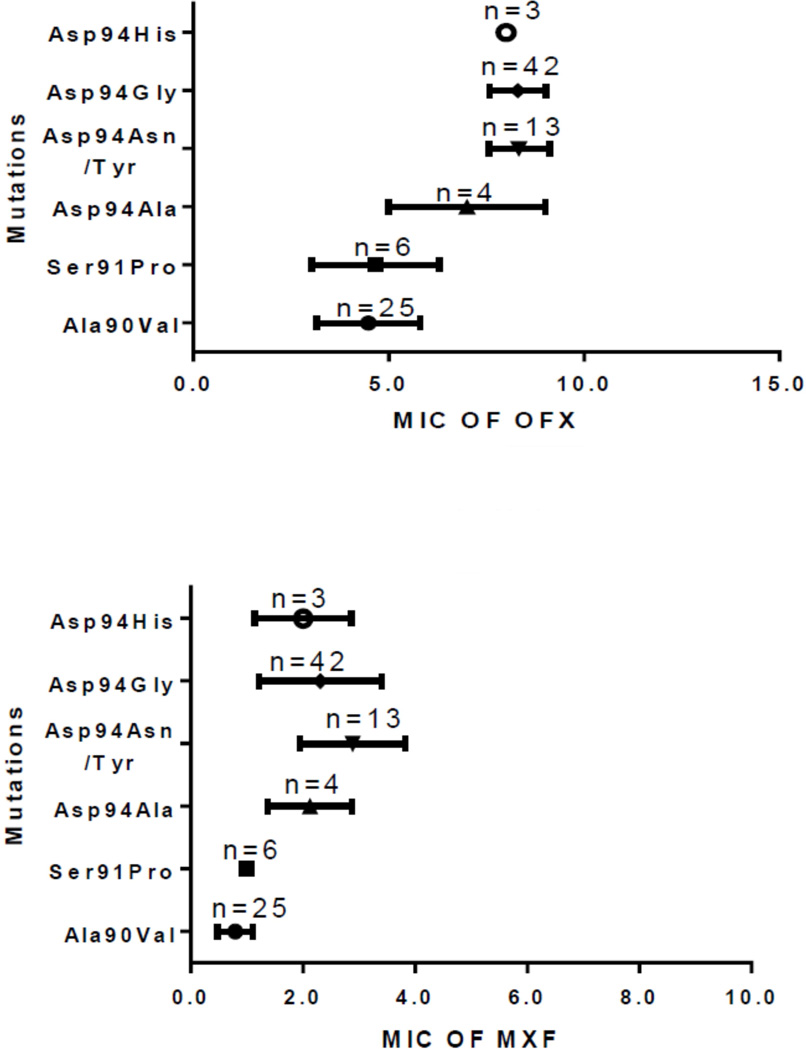
To determine the correlation between fluoroquinolones MICs and gyrA mutations a Kruskal-Wallis test was conducted. The results revealed that the level of resistance to OFX and MXF was significantly associated with specific gyrA mutations. For MXF, low level of resistance was seen with Ala90Val (n=25) and Ser91Pro (n=6), Moderate level of resistance was seen with Asp94Ala (n=3), Asp94Asn/Tyr (n=11), Asp94Gly (n=32) and Asp94His (n=2) and high level were seen with Asp94Asn/Tyr (n=2) and Asp94Gly (n=1). For OFX, Moderate level of resistance was seen with Ala90Val (n=22) and Ser91Pro (n=5) and high level of resistance was seen with Asp94Ala (n=3), Asp94Asn/Tyr (n=13), Asp94Gly (n=42) and Asp94His (n=3). OFX did not show low level of resistance (fig.1). For OFX, the Kruskal-Wallis statistic was 64.66, p value <0.0001 and for MXF it was 60.10, p value <0.0001.
Fig. 1.
Different gyrA mutations with level of resistance for OFX and MXF
4. Discussion
There was a clear correlation between the various gyrA mutations observed with Genotype MTBDRsl assay and the levels of phenotypic resistance seen to OFX and MXF by MGIT. Our results indicated that the MICs for wild type MTB isolates were clearly different from MICs for isolates harboring different mutations. gyrA mutations associated with fluoroquinolones resistance were found in 93 of the 123 MTB isolates that were evaluated. In these 93 fluoroquinolones resistant isolates, we observed seven unique mutations at Ala90Val, Ser91Pro, Asp94Ala, Asp94Asn/Tyr, Asp94Gly and Asp94His.There was a significant correlation between the fluoroquinolones MICs and the gyrA mutation position.
In present study, 88% (22/25) and 100% (25/25) of isolates with the mutation Ala90Val had MICs at or above the critical concentrations of 4.0µg/ml and 0.25–1.0µg/ml for OFX and MXF, respectively. Similarly, 83% (5/6) and 100% (6/6) of isolates with a Ser91Pro mutation showed an MIC of 4.0µg/ml and 1.0µg/ml for OFX and MXF, respectively. A previous study reported, similar findings with a MIC of 4.0µg/ml for OFX and 1.0µg/ml for MXF for the mutations Ala90Val and Ser91Pro [18].
MICs ranging from 8.0 to ≥10.0µg/ml for OFX were seen in 100% (42/42) of isolates with Asp94Gly mutations, in accordance with other studies reporting 94% (16/17) of the isolates with the mutation having MICs ranging from 8.0 to 10.0µg/ml [18]. MICs of 2.5µg/ml for MXF were seen in 76% (32/42) of isolates with an Asp94Gly mutation. A previous study reported that, 82% (14/17) of the isolates had a MICs of 2.0µg/ml for MXF [18]. For other Asp94Asn/Tyr mutations, we found a MIC50 of 2.5µg/ml for MXF and 8.0µg/ml for OFX. This has been noted for gyrA mutation with similar MIC50 of 2.0µg/ml for MXF and 8.0µg/ml for OFX [24].
While most gyrA mutations appear to confer cross-resistance to OFX and MXF, we found clear evidence that the MIC50 associated with specific gyrA mutations were sufficiently different enough, between OFX and MXF to be clinically relevant. For example, the MIC50 for the mutations Ala90Val and Ser91Pro were 1.0µg/ml for MXF, but 4.0µg/ml for OFX; while isolates with the mutation Asp94Gly had an MIC50 of 2.5µg/ml for MXF whereas for OFX it was 8.0µg/ml. These findings confirm results reported in a similar study [24].
The maximum serum concentrations (Cmax) achievable in humans for MXF is 4.3µg/ml with daily oral dosing of 400mg, with a half-life range of 9–12 hours; whereas the Cmax for OFX is 4.0µg/ml with daily oral dosing of 400 mg, with a half-life of 4–5 hours [16, 18]. Cmax of MXF/OFX are similar but the MICs for OFX are consistently almost double those for MXF for any of primary gyrA mutations that are found in fluoroquinolones- resistant isolates, MXF apparently has superior pharmacokinetics and pharmacodynamics (PK/PD) compared to OFX (MXF: Cmax/ MIC=9, AUC24/MIC =96 after a daily dose of 400mg. OFX: Cmax/MIC=2, AUC24/MIC=24 after a daily dosing of 400mg) [16]. It is notable that, despite the presence of resistance-associated mutations in the gyrA gene, most of the MXF MIC levels were below the peak serum concentration while the OFX MICs levels were closer to the peak serum concentration. For example, 20% (5/25) isolates with an Ala90Val mutation had MXF MICs at 0.25µg/ml. For these same isolates, the MICs for OFX was 4.0µg/ml, or two fold higher than the critical concentration. Structural differences between the two fluoroquinolones can explain the phenotypic differences between the two drugs. The MICs of MXF could be the result of the drug’s structure, as it contains a methoxy group at the 8- position and a diazabicyclonyl ring moiety with an S, S configuration at the 7- position. This large hydrophobic moiety at C7 does not allow for efflux the drug [18].
Additionally, as many of the gyrA mutations evaluated in our study had MICs below the Cmax for MXF, it is possible that certain gyrA mutations, detectable by rapid molecular diagnostics, may be used to predict MICs that are above the critical concentration for resistance, but still potentially clinically treatable through increased MXF dosing.
We propose that low level of resistance for MXF (with MIC of ≤1µg/ml) may be treated with normal or higher doses of MXF. Whether moderate level of resistance (with MIC of 2.5µg/ml) can be treated with higher doses of MXF will require a clinical outcome trial. High level of resistance (with MICs of 5.0–8.0µg/ml) perhaps cannot be treated with any of the fluoroquinolones (fig.1).
We believe that this study has important clinical implications. First, it emphasizes the need for performing phenotypic DST for MXF at 2 critical concentrations especially in regions where, MXF is used in the management of MDR TB. Secondly given the superior PK/PD characteristics of MXF, as majority of mutations (including those at gyrA 94) showed an MIC of 2.5µg/ml with moderate level of resistance, clinical outcome studies assessing the efficacy of MXF in these groups are urgently warranted in high MDR TB endemic regions.
5. Conclusions
While it is well known that certain gyrA mutations in MTB confer phenotypic resistance to both MXF and OFX, it appears that the MICs conferred by many gyrA mutations (eg. Ala90Val) are consistently lower for MXF than for OFX. This observation has significant clinical relevance and suggests that MXF will have better PK/PD characteristics than OFX for most gyrA mutations. In our study, the majority of MTB isolates with the mutations Asp94Ala, Asp94Asn/Tyr, Asp94Gly and Asp94His were associated with a moderate level of resistance to MXF (MIC=2.5µg/ml), while three isolates with the mutations Asp94Asn/Tyr or Gly were associated with a high level of resistance to both MXF and OFX (MXF MICs = 5.0–8.0µg/ml, OFX MICs = ≥10.0µg/ml). Our results suggest that clinical MTB isolates shown to have the gyrA mutations Ala90Val and Ser91Pro have the potential to be rapidly diagnosed and treated with standard or increased MXF dosing. However, mutations at 94 gyrA with moderate level of resistance will require clinical outcomes in MDR and XDR patients with MTB isolates harboring theses mutations.
Acknowledgements
We are thankful to National Health and Education Society, Hinduja Hospital and Medical Research Centre for funding this study. Antonino Catanzaro and Timothy C Rodwell were funded by National Institute of Allergy and Infectious Diseases (NIAID) grant U01-AI082229 and grant R01AI111435-04
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization (WHO) Global tuberculosis control: WHO report 2012. World Health Organization. 2012 (WHO/HTM/TB/2012.6)
- 2.Ajbani K, Shetty A, Mehta A, Rodrigues C. Rapid Diagnosis of Extensively Drug-Resistannt Tuberculosis by Use of a Reverse Line Blot Hybridization Assay. J Clin Microbiol. 2011;49:2546–2551. doi: 10.1128/JCM.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udwadia Z, Amale R, Ajbani K, Rodrigues C. Totally drug resistance tuberculosis in India. Clin infect Dis. 2012;54:579–581. doi: 10.1093/cid/cir889. [DOI] [PubMed] [Google Scholar]
- 4.Krüüner A, Yates M, Drobniewski F. Evaluation of MGIT 960-based antimicrobial testing and determination of critical concentrations of first- and second-line antimicrobial drugs with drug-resistant clinical isolates of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:811–818. doi: 10.1128/JCM.44.3.811-818.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S. Drug –Susceptibility testing in tuberculosis: methods and reliability of results. EurRespir J. 2005;25:564–569. doi: 10.1183/09031936.05.00111304. [DOI] [PubMed] [Google Scholar]
- 6.Nosova E, Bukatina A, Isaeva Y. Analysis of mutations in the gyrA and gyrB genes and their association with the resistance of Mycobacterium tuberculosis to levofloxacin, moxifloxacin and gatifloxacin. Journal of medical microbiology. 2013;62:108–113. doi: 10.1099/jmm.0.046821-0. [DOI] [PubMed] [Google Scholar]
- 7.Barnard M, Parsons L, Miotto P, Cirillo D, Feldmann K, Gutierrez C, Somoskovi A. Molecular Detection of Drug- Resistant Tuberculosis by Line probe assay. FIND. 2012:1–229. [Google Scholar]
- 8.Leonid H, Cangelosi G. Drug resistance assays for mycobacterium tuberculosis. Chapter 81 In Antimicrobial drug resistance Edited by Mayers, Douglas. 2009:1161–1170. [Google Scholar]
- 9.Genotype MTB DR plus VER 2.0. Molecular Genetic assay for identification of the M. tuberculosis complex and is resistance to Rifampicin and isoniazid from clinical specimens and cultivated samples. Hain lifescience. IFU-304A-02 [Google Scholar]
- 10.Springer B, Lucke K, Maibach R, Ritter C, Bottger E. Quantitative Drug Susceptibility testing of mycobacterium tuberculosis by use of MGIT 960 and Epicenter Instrumentation. J ClinMicrobiol. 2009;47:1773–1780. doi: 10.1128/JCM.02501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottger E. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin Microbiol infect. 2011;17:1128–1134. doi: 10.1111/j.1469-0691.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 12.Kolyva A, Karakousis P. Old and New TB Drugs: Mechanisms of Action and resistance. Chapter 9 In Understanding Tuberculosis – New Approaches to Fighting against Drug resistance. Edited by pere-joan cardona. 2012:209–232. [Google Scholar]
- 13.Groll A, Martin A, Jureen P, Hoffner S, vandamme P, Portaels F, Palomino J, silva P. Fluoroquinolone resistance in mycobacterium tuberculosis and mutations in gyrA and gyrB. Antimicrob Agents Chemother. 2009;53:4498–4500. doi: 10.1128/AAC.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poissy J, Aubry A, Fernandez C. Should moxifloxacin be used for the treatment of extensively drug-resistant tuberculosis? An answer from a murine model. Antimicrob Agents Chemother. 2010;54:4765–4771. doi: 10.1128/AAC.00968-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruri F, Sterling T, Kaiga A, Blackman A, Heijden Y, Mayer C, Cambau E, Aubry A. A systematic review of gyrasemuationns associated with fluoroquinolones – resistant Mycobacterium tuberculosis and a proposed gyrase numbering system. J Antimicrob Chemother. 2012;67:819–831. doi: 10.1093/jac/dkr566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsburg A, Grosset J, Bishai W. Fluoroquinolones, tuberculosis and resistance. Lancet Infect Dis. 2003;3:432–442. doi: 10.1016/s1473-3099(03)00671-6. [DOI] [PubMed] [Google Scholar]
- 17.Angeby K, Jureen P, Giske C, Chryssanthou E, Sturegard E, Nordvall M, Johansson A, Werngren J, Kahlmeter G, Hoffner S, Schon T. Wild-type MIC disributions of four fluoroquinolones active against mycobacterium tuberculosis in relation to current critical concentrations and available pharmacokinetic and pharmacodynamics data. J AntimicrobChemother. 2012;65:946–952. doi: 10.1093/jac/dkq091. [DOI] [PubMed] [Google Scholar]
- 18.Sirgel F, Warren R, Steicher E, Victor T, Helden P, Bottger E. gyrA mutations and phenotypic susceptibility levels to ofloxacin and moxifloxacin in clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2012;67(5):1088–1093. doi: 10.1093/jac/dks033. [DOI] [PubMed] [Google Scholar]
- 19.Almeida D, Rodrigues C, Udwadia Z, Lalvani A, Gothi G, Mehta P, Mehta A. Incidence of Multidrug-Resistant Tuberculosis in Urban and Rural India and Implications for Prevention. Clinical infectious diseases. 2003;36:152–154. doi: 10.1086/374931. [DOI] [PubMed] [Google Scholar]
- 20.GraceLin S, Desmond E, Bonato D, Gross W, Siddiqi S. Multicenter Evaluation of Bactec MGIT 960 system for Second-line Drug Susceptibility testing of Mycobacterium tuberculosis Complex. J Clin Microbiol. 2009;47:3630–3634. doi: 10.1128/JCM.00803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) Policy Guidance on Drug Susceptibility Testing (DST) of second line Antituberculosis Drugs. 2008 (WHO/HTN/TB/2008.392) [PubMed]
- 22.Ajbani K, Nikam C, Kazi M, Gray C, Boehme C, Balan K, Shetty A, Rodrigues C. Evaluation of Genotype MTBDRsl Assay to Detect Drug Resistance Associated with Fluoroquinolones,Aminoglycosides and Ethambutol on Clinical Sediments. PLOS ONE. 2012 doi: 10.1371/journal.pone.0049433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S, Rodwell T, Rider T, Rider L, Pham L, Catanzaro A, Desmond E. Pyrosequencing for rapid detection of extensively resistant Mycobacterium tuberculosis in clinical isolates. J ClinMicrobiol. 2014;52(2):475–482. doi: 10.1128/JCM.01821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing L, Gao X, Tao L, Jie W, Gang S, Qingyun L, Yuan J, Yangyi Z, Jian M, Qian G. Association of gyrA/B mutations and resistance levels to fluoroquinolones in clinical isolates of Mycobacterium tuberculosis. Emerging Microbes & Infections. 2014 doi: 10.1038/emi.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]