Abstract
Transthyretin-cardiac amyloidoses (ATTR-CA) are an underdiagnosed but increasingly recognized cause of heart failure. Extracellular deposition of fibrillary proteins into tissues due to a variety of inherited transthyretin mutations in ATTRm or due to advanced age in ATTRwt eventually leads to organ failure. In the heart, amyloid deposition causes diastolic dysfunction, restrictive cardio-myopathy with progressive loss of systolic function, arrhythmias, and heart failure. While traditional treatments have consisted of conventional heart failure management and supportive care for systemic symptoms, numerous disease-modifying therapies have emerged over the past decade. From organ transplantation to transthyretin stabilizers (diflunisal, tafamidis, AG-1), TTR silencers (ALN-ATTR02, ISIS-TTR(Rx)), and degraders of amyloid fibrils (doxycycline/TUDCA), the potential for effective transthyretin amyloid therapy is greater now than ever before. In light of these multiple agents under investigation in human clinical trials, clinicians should be familiar with the systemic cardiac amyloidoses, their differing pathophysiology, natural histories, and unique treatment strategies.
Keywords: Cardiac amyloidosis, Transthyretin, Cardiomyopathy, Senile systemic amyloidosis, Familial amyloid polyneuropathy, Diflunisal, ALN-TTR02, ALN-TTRSc, Tafamidis, Doxycycline, TUDCA, siRNA, Oligonucleotides
Introduction
The systemic cardiac amyloidoses (CA) are an uncommon but underdiagnosed cause of heart failure [1]. While the general mechanism of extracellular deposition of fibrillary proteins leading to tissue and organ dysfunction characterizes all amyloid entities, there are at least three different pathophysiologic substrates for cardiac amyloidosis, each with differing natural histories and therapies [2]. In light-chain (AL) cardiac amyloidosis, fibrils are comprised of immunoglobulin light chains produced by a clonal population of plasma cells. Treatment involves chemotherapeutic agents aimed at eradicating the hyperproliferative plasma cell and at times bone marrow transplantation to repopulate a healthy cellular lineage. In contrast, in the transthyretin-related cardiac amyloidoses (ATTR-CA), misfolded monomers and dimers of normally tetrameric transthyretin protein (TTR) from either mutant TTR (ATTRm, also known as familial amyloid cardiomyopathy) or wild-type TTR (ATTRwt, also known as senile systemic amyloidosis) deposit in the myocardium and lead to diastolic and systolic dysfunction, arrhythmias, restrictive cardiomyopathy, and clinical heart failure. Systolic dysfunction can also occur in later stages of the disease, particularly in ATTRwt (see manuscript on Pathogenesis of TTR Amyloid by Liao et al., for further details).
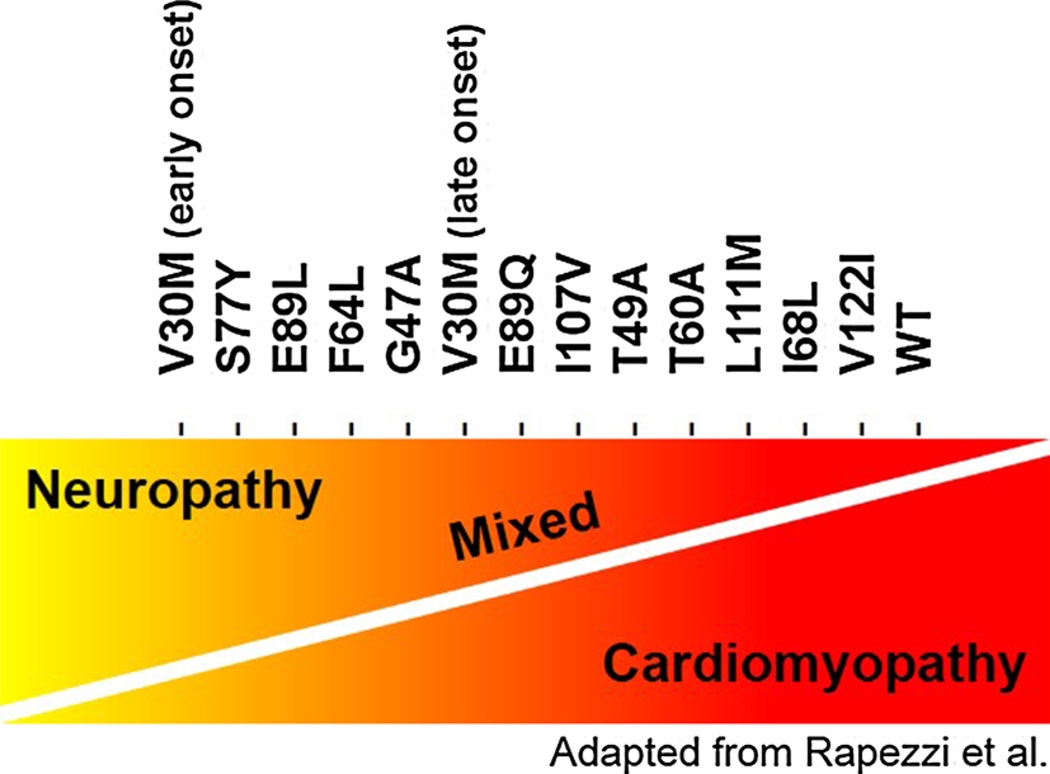
Clinical features of ATTRwt and ATTRm are unique and have been differentiated over the past three decades both from AL CA and from each other. ATTRm is caused by >100 delineated mutations in the TTR gene. These are inherited in an autosomal dominant fashion and manifest with varying geographic distribution and phenotypic expression. The true prevalence of ATTRm is not known, but in the USA, the most common TTR mutation is the substitution of valine for isoleucine at position 122 (V122I). ATTRm V122I is present almost exclusively in African–Americans with an allele frequency ranging 3–3.9 % [3, 4], whereas in parts of Europe and Japan, the V30M allele is endemic [5]. The clinical presentation in ATTRm differs according to the underlying mutation (Fig. 1) with a predominately cardiac phenotype for V122I and L111M, predominate neuropathic phenotype for V30M, and a mixed phenotype for E89Q and T60A. However, genotype–phenotype correlations are also influenced by several other factors including age of onset (e.g., early versus late V30M, the latter of which is associated with a more severe cardiac phenotype), endemic region of origin (which can be associated with earlier onset of disease and a more predominant neurologic phenotype), as well as gender, maternal or paternal inheritance [6], and other not yet delineated factors. Interestingly, in ATTRm owing to the V30M mutation, female gender may be protective against myocardial involvement described in an Italian study of 98 patients although the mechanism is not clear [7]. The reasons for these genotype–phenotype correlations have yet to be fully elucidated.
Fig. 1.
Distribution of phenotypic variation between neuropathic and cardiomyopathic symptoms according to ATTRm mutation
In addition to mutation-specific cardiologic or neurologic predominant phenotypes, a relationship exists between age of disease onset and development of cardio-myopathy. For example, in patients with the V30M mutation, early-onset disease manifests with peripheral neuropathy, also known as familial amyloid polyneuropathy (FAP), while late-onset disease usually presents with a concomitant cardiomyopathic phenotype. Symptomatic conduction disturbances may require permanent pacemaker (PPM) implantation [8]. In advanced systemic disease, ATTRm may lead to gastrointestinal, autonomic, renal, and pulmonary involvement. The most common causes of mortality are cardiomyopathy, malnutrition, and infections occurring approximately 10–15 years after disease onset [9]. Quality of life in symptomatic patients with ATTRm is also severely impaired relative to age-matched non-amyloid controls [10].
While patients with a cardiac phenotype typically have higher left ventricular (LV) septal wall thickness compared with neurologic and mixed phenotypes [11], those with ATTRm V122I have more advanced cardiac dysfunction at diagnosis despite the availability of genetic testing, which should theoretically facilitate early diagnosis [12]. Another theory has suggested that specific amyloid fibril composition may correlate with symptoms [13]. Among patients with ATTRm V30M, those with fragmented ATTR (type A) tend to have late-onset cardiomyopathy, while those with intact ATTR (type B) have early-onset and less myocardial involvement. Indeed, the current dichotomiza-tion of patients into a predominately cardiac, neurologic, or mixed phenotype may be an oversimplification as emerging data suggests that neurologic involvement is found in what was previously thought of as a pure or exclusive cardiac phenotype [14].
While ATTRm can affect individuals of all ages, ATTRwt is a disorder predominately affecting elderly men. On average, ATTRwt patients are older than ATTRm patients at the time of diagnosis [12]. Compared to AL amyloidosis in which development of heart failure can be rapid, cardiomyopathy in ATTRwt progresses slower even though LV wall thickness is greater. Thus, despite an older age and more thickened myocardium at presentation, indicative of more amyloid deposition, heart failure in ATTRwt is typically less severe and patients survive longer than those with AL, generating the hypothesis of additional toxicity attributed specifically to circulating light chains (LC) in the latter disorder [15–17]. Indeed, infusion of LC obtained from AL patients with severe cardiac involvement into Langendorff-perfused isovolumically contracting mouse hearts results in marked impairment of ventricular relaxation with preservation of contractile function compared to saline controls [18]. Direct injection of LC into zebrafish results in impaired cardiac function, pericardial edema, and increased cell death with eventual 100 % mortality relative to LC control samples isolated from multiple myeloma patients [19]. Replication of these studies in ATTR-CA would determine whether TTR amyloid fibrils contribute directly to disease pathogenesis independent of extracellular fibril deposition.
Other clinical features distinguish ATTR from AL CA. Compared with patients with AL, those with ATTR have a higher incidence of carpal tunnel syndrome, lower likelihood of renal disease, and are typically less symptomatic despite greater wall thickness and lower ejection fractions. In African–American patients older than 60 years of age, CA is more than two times more likely to be caused by ATTR than AL [20]. However, many clinical features of ATTR and AL overlap and therefore should not be used alone to distinguish between amyloid subtypes, particularly given differences in treatment strategies, outcomes, and potential harm that can be caused from receiving the wrong treatment [21].
Outcomes in ATTR-cardiac amyloidosis
Morbidity and mortality
The largest prospective, longitudinal investigation to date, the Transthyretin Amyloid Cardiac Study (TRACS) assessed the progression of ATTR-cardiac amyloidosis. Patients were monitored every 6 months for up to 2 years and demonstrated high morbidity and mortality in 29 ATTRwt and ATTR V122I patients [20]. At baseline, no differences in clinical characteristics, biomarkers, or echo parameters were noted between ATTRwt and ATTRm groups. However, after only 15 months, there were 11 deaths and 1 cardiac transplant, with higher mortality and cardiovascular hospitalizations among patients with ATT-Rm. A summary of survival among various other studies of ATTR-CA according to subtype is shown in Table 1.
Table 1.
Summary of survival in ATTR-cardiac amyloidosis according to subtype
| Year | Publication | Subtype (N) | Median survival from diagnosis (months) |
|---|---|---|---|
| 2014 | Quarta et al. [17] | ATTRm (36) | 29 |
| Circulation | ATTRwt (56) | 24 | |
| 2013 | Givens et al. [12] | ATTRmV122I (28) | 36 |
| Aging Health | ATTRwt (37) | 66 | |
| 2013 | Pinney et al. [16] | ATTRwt (102) | 32 |
| J Am Heart Assoc | |||
| 2012 | Ruberg et al. [20] | ATTRmV122I (11) | 25 |
| Am Heart J | ATTRwt (18) | 43 | |
| 2009 | Connors et al. [3] | ATTRmV122I (30) | 27 |
| Am Heart J |
Among the largest and most recent studies, median survival from time of diagnosis in ATTR-cardiac amyloidosis lies between 25 and 41 months [3, 12, 16, 17]. When broken down by subtype in the TRACS study, median survival from time of diagnosis was approximately 25 months in ATTRm and 43 months in ATTRwt. Common causes of death in the TRACS study included heart failure, sudden death, and sepsis. Hospitalizations were about twice as frequent in ATTRm as ATTRwt patients, with the most common reason being congestive heart failure followed by arrhythmias and need for pacemaker implantation. Univariate predictors of mortality included disease duration, heart rate ≥70 beats/min, baseline stroke volume, LVEF <50 %, and ATTRm status. Measurements at 6-month increments demonstrated marked disease progression: mean 6-min hall walk (6MHW) declined by 25.8 m, NT pro-BNP increased 1,816 pg/mL, and LVEF fell by 3.2 %. Overall, disease progressed over a short period of time in TRACS with substantial morbidity and mortality in ATTR-CA, particularly due to V122I, which is relevant given its high prevalence in African–Americans.
Atrial fibrillation, thromboembolic complications, and consideration for anticoagulation
The overall prevalence of atrial fibrillation in ATTR-CA estimated from the TRACS was 64 % and was at least three times more prevalent in ATTRwt compared with ATTRm [20]. While the presence of atrial fibrillation on baseline ECG was not associated with survival in this population, intracardiac thrombosis leading to thrombo-embolism contributes significantly to mortality [22]. In addition to blood stasis in atrial fibrillation, endomyocardial damage and endothelial dysfunction from amyloid deposition are thought to lead subsequently to hypercoagulability and contribute to intracardiac thrombosis, although data to support this are limited [23, 24]. In a study of 116 autopsy specimens and explanted hearts with cardiac amyloidosis, ATTR cases demonstrated a high frequency of intracardiac thrombosis (16 %) and fatal embolic events (8 %) when compared with non-amyloid controls who displayed none, although these complications were notably higher in AL cases (51 and 26 %, respectively). Most intracardiac thrombi were localized to either the left or right atrium.
A follow-up study employed transthoracic (TTE) and transesophageal echocardiography (TEE) to investigate the prevalence of intracardiac thrombosis and response to anticoagulation in cardiac amyloidosis [25]. While only 64 % of the patients studied were in atrial fibrillation, 18 % of patients with ATTR had intracardiac thrombi compared to 35 % in AL. Multivariate analysis showed that LV diastolic dysfunction and low left atrial appendage emptying velocity were, in addition to atrial fibrillation, independently associated with intracardiac thrombosis. These observations fit the hypothesis that a combination of systolic and diastolic ventricular dysfunction and chronic amyloid infiltrate in the atria lead to atrial mechanical dysfunction [26], atrial enlargement, and blood stasis [27]. Such atrial mechanical dissociation at least partially explains why some cardiac amyloid patients develop atrial thrombosis even while in sinus rhythm [26].
Risk for intracardiac thrombosis can be lowered with anticoagulation [25], but in general, the risk assessment for thrombosis and subsequent decision for anticoagulation in CA should not be based upon the CHADS2-VASC score alone [28]. Rather, the decision for anticoagulation ought to reflect a careful comprehensive clinical assessment of high-risk features specific to the CA patient. Early screening for intracardiac thrombosis and preemptive initiation of anticoagulation is recommended in CA for patients with AL subtype or for ATTR with concomitant thromboembolic risk factors including atrial fibrillation, advanced LV diastolic dysfunction, lower left atrial appendage emptying velocity, elevated heart rate, and increased right ventricular (RV) wall thickness reflecting more advanced amyloid deposition with poor RV diastolic function and consequent stasis [29]. Anecdotally, many amyloid centers have also recommended considering anti-coagulation in patients in normal sinus rhythm based on poor atrial function reflected by low atrial velocities on Doppler mitral inflow. Novel oral anticoagulants (NOACS) have been employed for thromboembolic risk reduction, but no studies to date have compared them head to head with warfarin in this population.
Prognostic stratification
Echocardiographic variables for cardiac amyloidosis
Emerging echocardiographic indices of LV structure and function among the cardiac amyloidoses are noteworthy. Despite initial preservation of ejection fraction, standard measures of LV function and strain rate assessed by speckle-tracking imaging worsen as wall thickness increases regardless of subtype [17]. Longitudinal strain (LS) is impaired in ATTRwt and AL amyloidoses compared with ATTRm, but notably, LS is preserved at the apex independent of amyloid subtype or degree of wall thickening. The utility of strain rate imaging is also seen in its incremental ability over traditional tissue Doppler echocardiography to identify a group of patients with probable subclinical cardiac amyloidosis [30]. When assessed by two-dimensional speckle-tracking imaging (STI), longitudinal strain was a negative predictor of survival in CA patients [17]. Another novel measure of myocardial function, myocardial contraction fraction (MCF) [31], shows promise in prognosticating survival in cardiac amyloidosis. Defined as the ratio of stroke volume to myocardial volume, higher MCF has been associated with improved survival in patients with cardiac amyloidosis [32].
Biomarkers
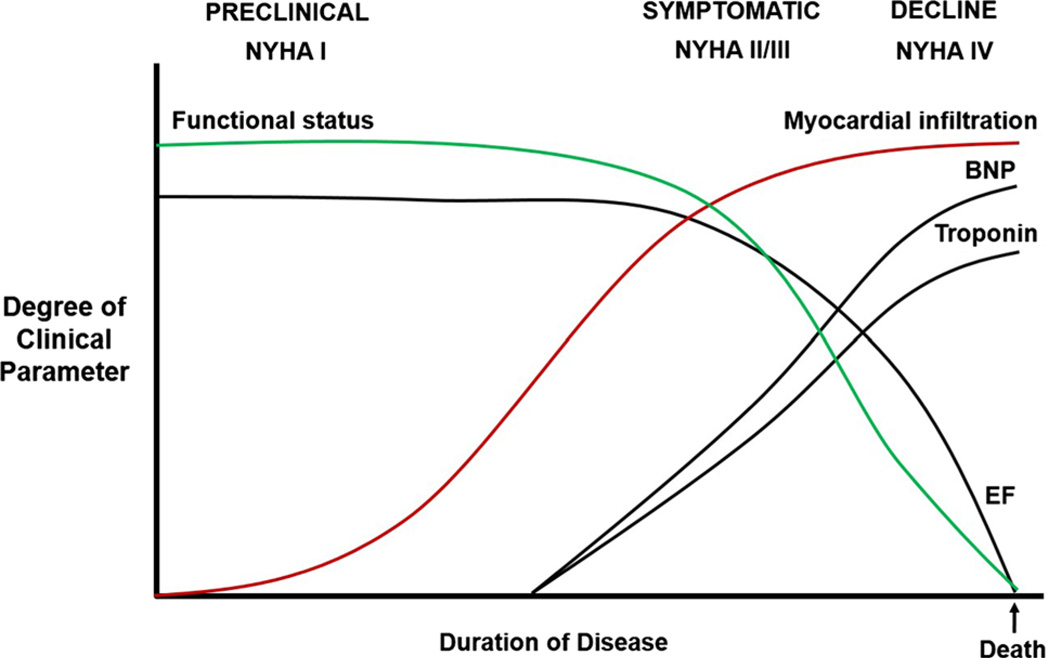
Given the progressive nature of CA and its high lethality in advanced stages, biomarkers are needed for differentiation between amyloid subtypes, monitoring disease progression, response to therapy, and prognosticating outcomes. Cardiac biomarker trends according to the natural history of CA are proposed in Fig. 2. As a diagnostic tool, cardiac biomarkers along with clinical features can be useful in differentiating between amyloid subtypes. An N-terminal pro-brain natriuretic peptide (NT pro-BNP) level ≤1,430 pmol/L and older age at diagnosis ≥70 years have been associated with ATTRwt and can help distinguish it from AL cardiac amyloidosis [16].
Fig. 2.
Proposed progression of ATTR-cardiac amyloidosis by clinical and biomarker parameters. In the preclinical phase, myocardial infiltration is minimal, while functional status and ejection fraction (EF) are normal. Symptomatic patients begin to experience a decline in functional status as amyloid fibrils infiltrate the myocardium and other organs. Ejection fraction initially remains normal, but diastolic function ensues and cardiac biomarkers begin to rise. As myocardial infiltration plateaus, patients continue to decline, functional status advances to a debilitated state, EF eventually drops steeply and then progresses slowly in a terminal phase leading to death
Cardiac biomarkers, similar to genotype, are also useful in that they are associated with specific amyloid phenotypes. Among symptomatic patients in the Transthyretin Amyloid Outcomes Survey (THAOS), the first global multicenter longitudinal study of ATTR pooling data from nearly 1,000 patients with ATTRm and ATTRwt CA from 19 countries [10], brain natriuretic peptide (BNP) and troponin I were significantly elevated in cardiac and mixed phenotype groups compared with the neurologic phenotype [11]. In another THAOS registry study that categorized ATTR-CA patients by interventricular septal wall thickness (IVST—normal, mild, moderate, and severe), patients with moderately and severely increased thickness tended to be older men with cardiac symptoms, a history of heart failure with higher BNP values, conduction abnormalities, low voltage, and pathologic Q waves on electrocardiogram (ECG) [33]. However, other biomarkers including body mass index (BMI), modified body mass index (mBMI, product of BMI and serum albumin to correct for water weight) [34], heart rate, troponin I or T, or the presence of a pacemaker did not seem to differ between IVST categories.
Several retrospective studies have also elucidated the prognostic role of biomarkers in ATTR-CA. In patients with ATTRwt, baseline factors associated with worse outcome have included a positive troponin T, the presence of a pacemaker, and New York Heart Association (NYHA) class IV symptoms [16]. Significant predictors of mortality and progression to orthotopic heart transplantation (OHT) were elevated troponin T, BNP, and depressed systolic function with ejection fraction (EF) ≤35 %, with the strongest independent predictor being troponin T [35]. In multivariable analysis, depressed systolic function (e.g., reduced EF) was an independent predictor of mortality and OHT. Death among patients who did not receive OHT was predicted by lower estimated glomerular filtration rate (eGFR), higher serum potassium level, higher BNP, elevated troponin I, and higher New York Heart Association (NYHA) class III or IV [12]. The cardiac biomarkers tro-ponin T and BNP had less utility as predictors of hospitalization [35].
Supportive non-disease-modifying therapies for ATTR-cardiac amyloidosis
General principles for non-disease-modifying therapy of amyloid cardiomyopathy include symptom management, maintenance of euvolemia, avoidance of polypharmacy particularly in elderly patients, avoidance of medications that may cause symptomatic hypotension, and consideration of pacemaker therapy for symptomatic conduction disturbances (Table 2).
Table 2.
Mainstay of non-disease-modifying therapy for ATTR-cardiac amyloidosis
| Therapy | Considerations in ATTR-CA patients |
|---|---|
| Salt restriction | Recommended restriction to <2 g sodium daily |
| Daily weights | Recommended to gauge adequacy of diuresis |
| Loop diuretics | Recommended, especially bioavailable loop diuretics (e.g., torsemide, bumetanide) to avoid diuretic resistance in advanced cardiomyopathy |
| K+ should be checked within 1–2 weeks of starting |
|
| Aldosterone antagonists | Consider addition of low-dose spironolactone 12.5 mg every other day given the long half- life of bioavailable loop diuretics and risk of hypokalemia |
| Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers |
Usually poorly tolerated due to risk of symptomatic hypotension as disease progresses |
| Beta blockers | Given fixed stroke volume and reliance of higher heart rate to maintain cardiac output, risk of symptomatic hypotension |
| Calcium channel blockers | Contraindicated |
| Hyperphysiologic effects may lead to high-degree heart block and profound negative inotropic effect with resulting cardiogenic shock |
|
| Digoxin | Relatively contraindicated |
| Hypersensitivity may lead to abrupt cardiac rhythm disturbances and sudden death |
|
| Midodrine | May be useful to augment blood pressure in patients with diuretic resistance and symptomatic hypotension |
| Venous compression stockings | Useful in avoiding symptomatic orthostatic hypotension by increasing preload and maintaining blood pressure |
| Helpful in patients with peripheral edema |
Diuretics
Diuretics including aldosterone antagonists and bioavailable loop diuretics along with salt restriction are the mainstay of traditional heart failure therapy in CA. However, patients with advanced cardiomyopathy can often develop diuretic resistance mediated by high central venous pressure and low stroke volume. As the disease progresses and blood pressure falls, there is an increased risk of syncope from a low blood pressure, and in this scenario, use of midodrine may be helpful.
Heart failure drug intolerances
Patients with advanced cardiac amyloidosis often have low baseline blood pressures as a result of decreased stroke volume and may become intolerant to several traditional heart failure therapies including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), and beta blockers (BBs) owing to symptomatic hypotension. These drug classes should generally be avoided in CA but if needed, they should be titrated with extreme caution.
Avoidance of calcium channel blockers
Calcium channel blockers (CCBs) are relatively contrain-dicated due to the high sensitivity observed in patients with CA. The affinity of TTR amyloid fibrils for various bone-seeking technetium-based radionucleotides is thought to be calcium-related, which may suggest a potential mechanism for CCB sensitivity [36], as increased binding of calcium channel blockers to amyloid fibrils promotes hyperphysiologic effects including high-degree heart block and profound negative inotropic effects with resulting shock. Non-dihydropyridine calcium channel blockers (e.g., verapamil and diltiazem in particular) should be avoided in CA patients with hypertension and for rate control in those with atrial fibrillation.
Caution with digoxin
Patients with CA can also be highly sensitive to digoxin, which may cause abrupt cardiac rhythm disturbances or sudden death [37, 38]. An in vitro study demonstrated that isolated amyloid fibrils bind digoxin with a striking degree of affinity with the majority of fibrils binding after only 15 min [39]. A homogenate of cardiac muscle that contained amyloid deposits bound digoxin significantly more than a normal cardiac muscle homogenate confirmed by positive indirect immunofluorescence with antibody to digoxin. This interaction may play some role in the sensitivity to digoxin observed in some patients with CA. Selective binding of digoxin to amyloid fibrils may enhance the severity of disturbances in the myocardium previously attributed to amyloid fibrillary deposits. In general, caution with digoxin administration in patients with CA is recommended, but the adverse effects of digoxin are not as severe as with non-dihydropyridine calcium channel blockers.
Considerations for pacemaker and cardioverter–defibrillator implantation
Permanent pacemakers
Cardiac autonomic dysfunction and progressive conduction disease are common in ATTR-CA, including those with FAP due to ATTR V30M [40]. Among patients with advanced FAP in Sweden, up to 50 % had reduced heart rate variability and 20 % had undetected atrial arrhythmias. In a larger study in over 100 FAP patients, more than 50 % had abnormal 24-h ECG recordings [5]. Conduction disturbances in this population do not seem to be related to IVS thickness indicating that the distribution and extent of infiltration of the heart by amyloid is heterogeneous and related to other factors such as gender, age of onset, and damage to the autonomic nervous control of cardiac function. Overall, due to the high incidence of conduction disturbances, Holter monitoring may be considered even in the absence of a thickened myocardium by TTE.
The need for implantation of a permanent pacemaker (PPM) has been reported in 25 % of patients with ATTRm due to V30M within an 11-year period after disease onset [41] and in as high as 36 % of ATTRm V122I and 43 % of ATTRwt [12]. The majority of arrhythmias necessitating PPM are high-degree conduction block, symptomatic bra-dycardia, and atrial fibrillation with slow ventricular responses. In a large series of patients with FAP V30M with conduction disturbances who underwent electrophysiology studies, prophylactic PPMs were implanted for HIS-ventricular (HV) interval ≥70 ms, >55 ms associated with fascicular block, first-degree atrioventricular block (AVB), or Wenckebach anterograde point ≤100 bpm [42]. Patients who did not receive a PPM were typically younger and had less severe cardiac involvement than those who received a PPM for a class I/IIa indications or prophylactically. Over a 45-month follow-up, permanent AVB was observed in as high as 25 % of patients suggesting that prophylactic PPM prevented a substantial number of potentially symptomatic events.
However, pacemaker implantation in cardiac amyloidosis caries some risk. Placement of a pacemaker lead through the tricuspid valve apparatus can cause tricuspid regurgitation, increase CVP, resulting in reduced right ventricular output and concomitant declines in stroke volume in ATTR-CA patients who already have advanced diastolic dysfunction and are preload dependent. Additionally, in retrospective analyses, PPM implantation has not been associated with a significant change in the likelihood of dying or in time to death [12]. In fact, the presence of PPM at baseline has been associated with worse survival in ATTRwt compared with AL [16], though this does not necessarily prove causality given that patients with conduction abnormalities necessitating a PPM are at higher risk to begin with for sudden cardiac death. Therefore, the presence of a PPM is more likely a marker of advanced disease and subsequent shorter survival after diagnosis. A multicenter study is needed to determine the long-term survival benefit of PPM implantation in ATTR-CA.
In the meantime, we recommend adherence with class I indication practice guidelines for PPM implantation such as sinus node dysfunction (SND) with documented symptomatic bradycardia or chronotropic incompetence, third-degree and advanced second-degree AVB associated with symptomatic bradycardia, and/or chronic bifascicular block [43]. But for class IIa, indications such as asymptomatic second-degree AVB or asymptomatic type II second-degree AVB with a narrow QRS where practice guidelines in the general population would deem PPM implantation to be reasonable, a candid conversation with ATTR-CA patients about the lack of supporting evidence is recommended.
There may be a role for biventricular pacing to mitigate reductions in stroke volume observed in CA. Decreased heart rate variability in FAP appears to be associated with shortened LV filling time and prolonged total isovolumic time reflecting ventricular dyssynchrony despite normal QRS complex [44]. Additionally, ATTR-CA patients often become pacemaker dependent. Chronic RV pacing in addition to well-established autonomic disturbances and baseline ventricular dyssynchrony can lead to further reductions in stroke volume and resulting symptoms. Correction of such disturbed ventricular function by cardiac resynchronization therapy with a biventricular device may reduce symptoms, though additional research in this area is needed.
Implantable cardioverter–defibrillators
Implantable cardioverter–defibrillator (ICD) therapy is not generally advised in cardiac amyloidosis but may play a role in specific patients. Traditionally, AL cardiac amyloidosis has carried a dismal prognosis with median survival less than 1 year once patients present with heart failure [45]. Therefore, due to the direct conflict with practice guidelines recommending ICD placement for the primary prevention of sudden cardiac death (SCD) in patients with a life expectancy greater than 1 year, ICDs typically are not implanted in advanced AL CA [43]. In addition, sudden cardiac death in patients with CA has often been attributed to electromechanical dissociation rather than to a primary arrhythmic cause [45–47], which further argues against the role for ICD in this population.
While an association between CA and ventricular arrhythmias is known and several small case series have reported successful defibrillation in individual patients with ICDs [47–50], overall survival benefit from ICD therapy in this population has not been demonstrated. A study in which malignant ventricular arrhythmias were present in the majority of 34 sampled patients with ATTR (88 % ATTRwt, 12 % ATTRm V122I) demonstrated that the presence of NSVT on 24-h Holter monitor recordings does not confer worse survival [51]. Given the absence of a convincing survival benefit, there is no clear indication for primary prevention ICD implantation in ATTR-cardiac amyloidosis at this time. Careful risk/benefit analysis is recommended in CA patients who might specifically benefit from an ICD, which include those with outpatient telemetry monitoring demonstrating a high burden of NSVT or sustained VT, and patients listed for OHT [52]. The decision to implant a secondary prevention ICD should similarly be individualized.
Organ transplantation for ATTR-cardiac amyloidosis
Liver transplantation
Because 95 % of TTR protein is produced by the liver, orthotopic liver transplantation (OLT) has been employed in ATTRm to replace amyloidogenic mutant with wild-type TTR and theoretically arrest amyloid formation [34]. The first liver transplant for ATTRm was performed in Sweden in 1990 on a patient with the V30M mutation with an encouraging result [53, 54]. In the modern era, the majority of OLTs are performed in patients with a primary neuropathic phenotype, most commonly due to the V30M mutation [34, 55]. In these patients, OLT is typically considered a preventative measure before the development of painful lower extremity sensorimotor neuropathy or multiple organ involvement including the gastrointestinal tract, kidneys, heart, and autonomic nerves that would impair functional status and outcomes [56–58].
Survival after transplantation in V30M patients is approximately 75 % at 5 years [34]. Risk factors that should be taken into account during candidate selection reflect evidence of systemic disease and include modified BMI <600 (direct contraindication), the presence of malnutrition in combination with bile acid malabsorption and severe autonomic denervation, and predisposition to thrombosis given the known complication of hepatic artery thrombosis after OLT [59]. Further complicating OLT outcomes are an increasing number of reports of progressive amyloidosis after transplant. Normal wild-type TTR from the transplanted liver can buildup on already deposited mutant TTR present at the time of transplantation in the heart and nerves, leading to amyloid cardiomyopathy or progression of polyneuropathy [60–62]. The precise mechanism for the development of cardiomyopathy after OLT is unknown, but one group proposed that ATTR fibril type may play a role. Patients with fragmented type A ATTR fibrils developed heart failure and reduced EF after OLT, while those who had intact type B ATTR did not deteriorate to the same degree [63]. Additionally, OLT does not prevent the development of heart arrhythmias necessitating PPM implantation, suggesting that amyloid already deposited in the conduction system at the time of OLT independently contributes to arrhythmia after transplantation regardless of suppression of mutant TTR synthesis [41].
Patients who undergo OLT should therefore have routine cardiac evaluations with yearly electrocardiograms and biomarkers as well as evaluation for a progressive peripheral neuropathy with a neurologist. Given the drawbacks of OLT (e.g., risk for hepatic artery thrombosis, recurrent amyloid deposition, and long-term immunosup-pression-related diabetes, dyslipidemia, infection, renal failure, and malignancies), it is recommended that it be reserved for patients with amyloidosis who are young, with early manifestations and a neuropathic mutation, as it typically takes decades for disease to occur in the heart, which is typically longer than the mean survival after OLT.
Domino liver transplantation
Patients born with ATTRm generally do not develop clinical symptoms until at least their third decade of life and given variations in disease penetrance; some allele carriers may never become symptomatic. Long disease latency subsequently led to the idea that rather than discarding an otherwise well-functioning ATTRm liver, it could be used in the organ sharing pool for sequential transplantation into a different patient. The first domino liver transplant was performed in Quimbra, Portugal and has since gained popularity [64–66]. No amyloid disease or deposits on biopsy specimens were reported in the first recipients of domino amyloid livers within 3.5 years after transplantation, but several groups have since reported de novo amyloidosis within 10 years post-transplantation [67, 68]. Emerging data suggest that most domino liver recipients eventually develop amyloidosis, the timing of which is dependent of several factors including type of mutation, age of recipient, and other yet undefined factors. Therefore, as the experience of domino liver transplantation continues, we recommend that all amyloid liver recipients undergo routine evaluations for clinical amyloidosis on a yearly basis.
Orthotopic heart and combined heart/liver transplantation
In younger individuals with ATTRm and severe cardiomyopathy, orthotopic heart transplantation (OHT) may be an option if no other systemic disease manifestations are present. In patients with mutations that lead to FAP who develop advanced cardiomyopathy but have not yet developed neuropathy or other systemic symptoms, combined OHT and OLT may be considered.
Heart transplantation in patients with cardiac amyloidosis was first reported in 1988 [69] but remains controversial due to lower survival compared with patients who undergo OHT for other forms of cardiomyopathy. At many centers, cardiac amyloidosis is considered a contraindication to OHT because it poses additional complications [70]. In addition to the conventional drawbacks of OHT including the requirement of lifelong immunosuppression, surgical risk in already hemodynamically compromised patients, and high expense, the amyloid population is further limited by advanced age at the time of diagnosis, comorbid conditions such as dangerous arrhythmias, renal failure, neuropathy, and enteropathy, and by the potential for amyloid progression after OHT [71]. Additionally, for the vast majority of older adults with cardiac amyloidosis, transplantation of any organ is not feasible or ethical given the shortage of donor organs and concomitant comorbidities of advanced age. For these reasons, the experience of OHT in patients with advanced heart failure secondary to cardiac amyloidosis has been limited to a few case series at single centers. Nevertheless, among available therapies for ATTR-cardiac amyloidosis, OHT possibly with concurrent OLT currently offers the greatest chance of long-term survival and is reserved for patients with intractable and advanced heart failure at high risk for short-term mortality.
Disease-modifying therapeutic opportunities: stabilizers, silencers, and degraders
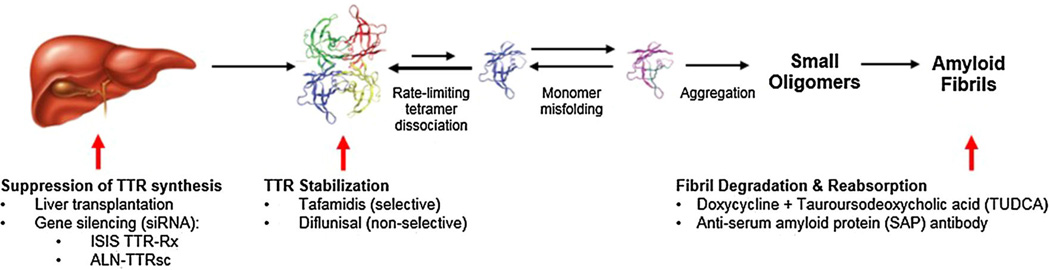
As no Food and Drug Administration-approved drugs are currently available for treatment of the ATTR-cardiac amyloidoses, the development of medications that prevent TTR-mediated organ toxicity has been an important therapeutic goal. Opportunities under investigation for treating TTR amyloidosis are described in Fig. 3.
Fig. 3.
Emerging disease-modifying therapeutic opportunities within the TTR amyloidogenesis cascade target suppression/silencing of TTR synthesis, stabilization, and degradation of TTR
Transthyretin stabilizers: diflunisal, tafamidis, AG-10
Diflunisal
Several transthyretin stabilizing agents have been developed and are in various stages of clinical trials. Diflunisal, a nonsteroidal antiinflammatory drug (NSAID), binds, and stabilizes common familial TTR variants against acidmediated fibril formation in vitro and has been tested in animal safety studies and human clinical trials [72–76]. Use of diflunisal in ATTR-CA is controversial given known consequences of chronic inhibition of cyclooxygenase (COX) enzymes including gastrointestinal bleeding (COX-1), renal dysfunction (COX-2), fluid retention, and hypertension that may precipitate heart failure (COX-2) in vulnerable individuals [77–82].
In patients with ATTR-CA, a single-arm, open-label cohort study suggested that at low dose and with careful monitoring, diflunisal along with a prophylactic proton pump inhibitor (PPI) could be safely administered to compensated patients [83], although an association was observed between chronic diflunisal use and adverse changes in renal function, suggesting that severe renal dysfunction may be prohibitive to diflunisal administration. A larger study in FAP patients with peripheral or autonomic neuropathy randomly assigned patients to a diflunisal or placebo arm [84]. Despite a high attrition rate approaching 50 %, diflunisal was reported to reduce the rate of progression of neurologic impairment and preserve quality of life over a 2-year follow-up. Echocardiography demonstrated a prevalence of presumptive cardiac amyloidosis in over 50 % of patients, but further prospective study of cardiac outcome measures are needed [85].
Tafamidis
Currently under clinical trial investigation, Tafamidis (Pfizer Inc, New York, NY, USA) is a novel compound that binds to the thyroxine-binding sites of the TTR tetramer, inhibiting its dissociation into monomers [86] and blocking the rate-limiting step in the TTR amyloidogenesis cascade (Fig. 3) [87]. In an 18-month double-blind, placebo-controlled trial in patients with early-stage ATTRm due to V30M, tafamidis stabilized V30M TTR tetramers and slowed progression of neurologic symptoms [88]. A phase 2, open-label, single-treatment arm safety and efficacy study in 21 patients with ATTRm due to eight different mutations showed some worsening of neurologic function, but stable health-related quality of life, cardiac biomarker NT-proBNP, echocardiographic parameters, and mBMI over a 12-month period [89]. Therefore, tafamidis appears to effectively stabilize TTR in several non-V30M variants.
When focusing on the cardiac phenotype, a phase 2 open-label trial assessing progression of cardiomyopathy showed that tafamidis treatment effectively achieved and maintained TTR stabilization was well tolerated and stabilized cardiac disease as measured by biochemical and echocar-diographic parameters [90]. A similar study investigated both cardiac and neurologic disease progression among individuals with ATTRm without V30M or V122I mutations treated with tafamidis, and it also showed TTR stabilization with good tolerability [89]. Five-year safety data have been reassuring in that tafamidis was generally well tolerated. Efficacy data at 3.5 years showed that clinically stabilized patients (defined as those who remained alive without changes form baseline in regard to cardiovascular hospitalization, worsening NYHA class, increase NT-proBNP ≥1,000 pg/mL, increase troponin I ≥0.1 ng/mL, and decrease 6-min hall walk test ≥50 m) had shorter disease duration, lower cardiac biomarkers, less myocardial thickening, and higher EF than those who progressed [90]. Overall, long-term tolerability and efficacy data are needed to determine the full range of the therapeutic effects among ATTRm variants. A phase 3 trial currently underway with a recruitment goal of 400 patients will investigate the efficacy, safety, and tolerability of tafamidis oral dose of 20 or 80 mg compared with placebo [91]. Primary outcome measures will include all-cause mortality and frequency of cardiovascular-related hospitalizations (Table 3).
Table 3.
Ongoing clinical trials for TTR cardiomyopathy therapeutics
| Clinical trial | Design | Estimated enrollment (N) |
Intervention (s) | Primary outcome |
|---|---|---|---|---|
| Tafamidis | ||||
| A multicenter, international, phase 3, double-blind, placebo-controlled, randomized study to evaluate the efficacy, safety, and tolerability of daily oral dosing of tafamidis meglumine (PF-06291826) 20 or 80 mg in comparison with placebo in subjects diagnosed with transthyretin cardiomyopathy |
Phase 3 | 400 | Tafamidis 20 or 80 mg PO QD × 30 months |
All-cause mortality and frequency of cardiovascular-related hospitalization |
| Multicenter | ||||
| Multinational | Secondary outcome measure change in 6MWT and KCCQ, cardiovascular- related mortality, TTR stabilization at 1 month |
|||
| Randomized | Placebo PO QD × 30 months |
|||
| Double blind | ||||
| Placebo- controlled | ||||
| (NCT01994889) | ||||
| ALN-TTR02 | ||||
| A phase 3 multicenter, multinational, randomized, double-blind, placebo- controlled study to evaluate the efficacy of ALN-TTR02 in transthyretin (TTR)- mediated polyneuropathy |
Phase 3 | 200 | ALN-TTR02 IV infusion |
Difference between ALN-TTR02 and placebo in the change from baseline of mNIS+7 |
| Multicenter | ||||
| Multinational | Sterile normal saline (0.9 % NaCl) IV infusion |
|||
| Randomized | ||||
| (NCT01960348) | Double blind | |||
| Placebo- controlled |
||||
| A phase 2, multicenter, open-label, extension study to evaluate the long-term safety, clinical and pharmacokinetics of ALN- TTR02 in patients with familial amyloidotic polyneuropathy who have previously received ALN-TTR02 |
Phase 2 | 28 | ALN-TTR02 IV infusion |
Safety and tolerability of long-term dosing with ALN-TTR02 in ATTR patients |
| Multicenter | ||||
| Open label | ||||
| (NCT01961921) | ||||
| ALN-TTRSc | ||||
| A phase 2, open-label trial to evaluate the safety, pharmacokinetics, pharmacodynamics and exploratory clinical activity of ALN-TTRSC in patients with transthyretin (TTR) cardiac amyloidosis |
Phase 2 | 25 | ALN-TTRSC SQ QD × 5 days, then qwk × 5 weeks |
Pharmacodynamic activity through 90 days |
| Multicenter | ||||
| Open label | Clinical activity through TTE, 6MWT, NYHA class, CMR, NT-proBNP, KCCQ, EQ-5D |
|||
| ISIS-TTR(Rx) | ||||
| A phase 2/3 randomized, double-blind, placebo-controlled study to assess the efficacy and safety of ISIS-TTR(Rx) in familial amyloid polyneuropathy |
Phase 3 | 195 | ISIS-TTR(Rx) 300 mg SQ TID × 1 week, then qwk × 64 weeks |
Efficacy measured by change from baseline in mNIS+7 and Norfolk Quality of Life Diabetic Neuropathy Questionnaire |
| Randomized | ||||
| Double blind | ||||
| (NCT01737398) | Placebo- controlled |
Placebo SQ TID × 1 week, then qwk for 64 weeks |
Exploratory analysis of TTE measures, NT-proBNP, and polyneuropathy disability score |
|
| Doxycycline + TUDCA | ||||
| A single center, 12-month, open-label, prospective study followed by a 6-month withdrawal period to evaluate the efficacy, tolerability, safety, and pharmacokinetics of doxycycline in combination with TUDCA in transthyretin amyloidosis |
Phase 2 | 40 | Doxycycline 100 mg BID + TUDCA 250 mg TID for 12 months |
mBMI reduction <10 % and change in NIS-LL <2 in subjects with peripheral neuropathy |
| Single center | ||||
| Non- randomized |
mBMI reduction <10 % and increase in NT-proBNP concentration <30 % or <300 pg/mL in subjects with isolated cardiomyopathy |
|||
| Open label | ||||
| (NCT01171859) | ||||
| An 18-month, open-label study of the tolerability and efficacy of a combination of doxycycline and TUDCA in patients with transthyretin amyloid cardiomyopathy |
Phase 1, 2 | 40 | Doxycycline 100 mg BID + TUDCA 250 mg TID for 18 months |
Rate of progression TTR-cardiac amyloidosis measured by changes in longitudinal strain echocardiography assessed every 6 months compared with historical controls |
| Single center | ||||
| Open label | ||||
| (NCT01855360) |
mBMI modified body mass index, 6MWT 6-min walk test, NYHA New York Heart Association, CMR cardiac magnetic resonance, KCCQ Kansas City Cardiomyopathy Questionnaire, EQ-5D patient-reported quality of life, NIS-LL Neurologic Impairment Score-Lower Limbs, mNIS+7 modified Neuropathy Impairment Score
AG10
In a high-throughput screen for TTR ligands, the compound AG10 was identified as a selective stabilizer of WT and TTR-V122I in vitro [92, 93]. AG10 prevents the dissociation of TTR-V122I in serum obtained from patients with ATTRm cardiomyopathy, and it protects human cardiomyocytes from TTR amyloid toxicity [93]. In human serum, AG10 increases stability of TTR-WT against acid-mediated dissociation that would otherwise accelerate amyloidogenesis.
Though AG10 is only in early phases of investigation, potential advantages relative to other TTR stabilizers include its potency of stabilization for TTR-V122I and TTR-WT. The association may exceed the ability of tafamidis and diflunisal in whole serum, though in vivo assays have not yet been reported. The oral bioavailability of AG10 and its encouraging pharmacokinetic properties with respect to selectivity and affinity also makes it a promising candidate for stabilizing ATTR cardiomyopathy.
Importantly, AG10, like tafamidis, has no significant interactions with a number of cellular receptors and enzymes including COX-1 or COX-2 (an inevitable target of the NSAID, diflunisal), thyroid hormone nuclear receptor, the potassium ion channel hERG (human ether-ago-go-related gene), and a number of cytochrome P450 isozymes. Crystallographic studies of AG10 bound to TTR-V122I give valuable insights into how AG10 achieves such effective kinetic stabilization, which also offers promise for designing effective TTR stabilizers [93].
Silencers—siRNA and oligonucleotides
siRNA: ALN-TTR01, ALN-TTR02, and ALN-TTRSc
RNA interference (RNAi) has emerged as an endogenous cellular mechanism for controlling gene expression in which small interfering RNAs (siRNAs) bound to the RNA-induced silencing complex (RISC) mediates the cleavage of target messenger RNA (mRNA) [94, 95]. Formulations of lipid nanoparticles and newer triantennary N-acetylgalactosamine (GalNAc) conjugates have emerged as agents to deliver siRNAs to hepatocytes [96, 97]. Lipid nanoparticles require IV infusion while GalNAc-siRNA conjugates are administered via subcutaneous injection. The GalNAc carbohydrate cluster has affinity for asialoglycoprotein receptor (ASGPR), which clears serum glycoproteins via clathrin-mediated endocytosis on the surface of hepatocytes. These agents have resulted in a robust and durable reduction in genetic expression (knockdown) of a variety of hepatocyte targets across multiple species. They appear to be well suited for receptor-mediated targeted delivery to suppress TTR gene expression.
ALN-TTR01 and ALN-TTR02 are first- and second-generation formulations of lipid nanoparticles as agents to deliver siRNAs. Each formulation encapsulates an identical siRNA that targets a conserved sequence in the 3′ untranslated region of non-mutant and mutant mRNA in TTR, thereby affecting both forms of TTR. They have similar physiochemical properties, but different ionizable lipid components resulting in ALN-TTR02 being exceptionally more potent than ALN-TTR01. In two sequential multicenter, randomized, placebo-controlled, dose-ranging phase 1 trials, patients with biopsy proven ATTR or healthy adult volunteers were given ALN-TTR01 or ALN-TTR02, respectively, to evaluate safety and efficacy (Table 3) [98].
After a single dose of ALN-TTR01, significant lowering from baseline TTR levels was observed as early as day 1, with a mean reduction of 38 % in TTR levels by day 7. But significant variability in TTR knockdown was measured among participants. Four participants with the V30M mutation and one with the S77Y mutation experienced peak knockdown ranging from 16 to 41 % and recovered close to predose baseline levels by day 28. However, one patient with the S77F mutation had a robust response, with more than 50 % TTR knockdown at day 2, a nadir level of 81 % by day 10, persistence greater than 50 % knockdown by day 28, and recovery to baseline level at day 70. In contrast, after a single dose of ALN-TTR02, TTR knockdown was rapid, potent, and durable across all three dose levels tested with highly significant changes compared with placebo lasting through day 28. Little variability among participants was observed in the kinetics of response with more than 50 % TTR knockdown by day 3, nadir level by day 10, continued suppression more than 50 % by day 28, and full recovery by day 70.
These siRNA lipid nanoparticles appear to be safe in that they have not resulted in any significant changes in hematologic, liver, renal, or thyroid function measures, and there were no drug-related serious adverse events or study drug discontinuations owing to adverse events. Since the main role of TTR is to bind retinol-binding protein (RBP), assays of serum RBP and vitamin A (retinol) provide additional measures of pharmacodynamic activity [99]. While RBP and retinol levels decrease in a dose-responsive fashion, no clinically meaningful correlate of visual symptoms has been observed. Nevertheless, it may be advisable for patients to take low doses of supplemental vitamin A until further long-term data on RBP suppression are available. Of note, mild-to-moderate infusion-related reactions have occurred in approximately 1/5th of participants receiving ALN-TTR01 and resolved spontaneously. A single participant who received ALN-TTR02 experienced a moderate infusion reaction, which readily responded to temporary interruption of the infusion without need for additional glucocorticoids and subsequent resumption of drug administration at a slower rate.
Another study of TTR siRNA conjugated to GalNAc, ALN-TTRSc, is now recruiting participants for a phase 2, open-label trial to evaluate safety, pharmacokinetics, pharmacodynamics, and exploratory clinical activity in patients with ATTR-CA [100]. While ALN-TTRSc is under investigation, harnessing RNAi technology with ALN-TTR02 suppresses hepatic TTR production, reduces circulating levels of both mutant and non-mutant TTR, and appears to hold great promise for treating patients with ATTR amyloidosis. The ability of ALN-TTR02 to lower both mutant and non-mutant proteins may also provide a major advantage over liver transplantation, which affects the production of only mutant protein.
Antisense oligonucleotides
Antisense oligonucleotides (ASOs) are also under clinical investigation for their role in inhibiting hepatic expression of amyloidogenic TTR. In a human transgenic mouse model (hTTR I84S) expressing high levels of the mutated human TTR I84S protein, TTR ASOs suppressed hepatic TTR mRNA levels and serum TTR levels by as much as 80 % [101, 102]. Second-generation antisense technology has led to the development of ISIS-TTR(Rx) for treating TTR amyloidosis [103]. When tested in the hTTR I84S transgenic mouse, ISIS-TTR(Rx) showed a dose-dependent 80 % reduction of human TTR at both the mRNA and protein levels. In cynomolgus monkeys, ISIS-TTR(Rx) similarly reduces liver TTR mRNA and plasma TTR protein levels in a dose-dependent manner by approximately 80 %. Treatment with ISIS-TTR(Rx) also produced a significant decrease in plasma RBP4 levels that correlated with reductions in TTR levels. Rodents and monkeys seem to tolerate ISIS-TTR(Rx) well.
Currently, ISIS-TTR(Rx) is under investigation in a phase 2/3 multicenter, randomized, double-blind, placebocontrolled clinical trial in patients with FAP [104]. The primary objective is to evaluate its efficacy as measured by change from baseline in modified neuropathy impairment score +7 (mNIS+7) relative to placebo. Secondary objectives will evaluate efficacy in regard to change from baseline in QOL, mBMI, modified neuropathy impairment score (NIS), pharmacodynamic effect on RBP4, in addition to safety and tolerability. Exploratory objectives in a subset of patients with LV wall thickness ≥13 mm without a history of persistent HTN will also include change from baseline compared to placebo in regard to echocardiographic parameters, NT-proBNP, and polyneuropathy disability score. An echocardiographic substudy collected data on cardiac structure and function in this trial and will facilitate analysis of the effect of TTR suppression on the cardiac phenotype.
Degraders—doxycycline/TUDCA and anti-SAP antibodies
Doxycycline/TUDCA
Treatment of systemic amyloidoses will always require reducing amyloid precursor protein production, but the ability to remove already deposited amyloid would be an important therapeutic advance. The utility of combined doxycycline and tauroursodeoxycholic aid (TUDCA) has been studied for TTR degradation (Fig. 3) [105]. In older TTR V30M transgenic mouse models, doxycycline administered in the drinking water for 3 months removed stomach TTR amyloid deposits. Doxycycline also resulted in a decrease in the levels of extracellular matrix remodeling protein that accompany fibrillary amyloid deposition. TUDCA, a biliary acid administered to the same mouse model, was also effective in lowering deposited non-fibrillary TTR and unlike doxycycline and also lowered levels of markers associated with pre-fibrillary TTR in young mice.
Combined cycled doxycycline and TUDCA administered to mice with amyloid deposition is more effective than either individual compound alone in significantly lowering TTR deposition and associated tissue markers [106]. A minimum period of 15 days of treatment resulted in fibril removal and a maximum suspension period of 15 days maintained tissues amyloid free. This observed synergistic effect of doxycycline/TUDCA in transgenic TTR mice given at human tolerable dose ranges has prompted further investigation in human clinical trials to test the safety and efficacy of the drug, particularly in early stages of disease.
Doxycycline and TUDCA appear to have an acceptable safety profile. In a population of 20 patients (17 ATTRm, 2 ATTRwt), a phase 2 open-label study of doxycycline/TUDCA showed no serious adverse events and no clinical progression of cardiac or neuropathic involvement over 1 year [107]. An active phase 2, single center, open-label, 12-month study will assess primary outcome measures including mBMI, neurologic impairment score, and NT-proBNP [108]. Secondary outcome measures will include number of patients experiencing treatment-related adverse events, change in QOL, doxycycline pharmacokinetics, response in autonomic dysfunction, sensory–motor peripheral neuropathy, visceral organ involvement, neurologic response, and incidence of patients discontinuing the study due to clinical or laboratory adverse events. Another study actively recruiting will examine the tolerability and efficacy of doxycycline/TUDCA over 18 months in patients with ATTR-cardiac amyloidosis [109].
Monoclonal anti-SAP antibodies
To further the unmet need for therapy that safely promotes the clearance of established amyloid deposits, the role of a normal, non-fibrillar glycoprotein present in all human amyloid deposits, serum amyloid P component (SAP) [110], is currently under intense investigation. Administration of an antihuman SAP antibody to mice with amyloid deposits containing human SAP triggered an unprecedented and potent giant cell reaction that efficiently removed massive visceral amyloid deposits without adverse effects [111]. In humans, anti-SAP antibody treatments could be theoretically feasible because circulating human SAP can be depleted by the bis-D proline compound CPHPC [112], thereby clearing the way for injected anti-SAP antibodies to reach residual SAP in amyloid deposits. If developed into an amyloid therapy, a major advantage of anti-SAP antibodies would be their potential to target all forms of amyloid deposits in all tissues.
Conclusion
Transthyretin-cardiac amyloidoses occurring both due to inherited mutations in ATTRm and older age in ATTRwt are an increasingly recognized cause of heart failure. Clinicians should be aware of their unique pathophysiology, clinical manifestations, and natural history as the treatments and outcomes are distinctly subtype dependent. The diverse array of emerging disease-modifying agents under investigation in human clinical trials has the potential to herald a new era for the treatment of cardiac amyloidosis.
Acknowledgments
We acknowledge the patients with cardiac amyloidosis who continue to participate in clinical trials and wait patiently for the development of effective treatments.
Dr. Drachman has received funding as a scientific advisor to ISIS Pharmaceuticals. Dr. Judge has received funding as a scientific advisor to both Pfizer and Alnylam Pharmaceuticals. Dr. Maurer serves on the advisory board of the Transthyretin Amyloid Outcomes Survey (THAOS), which is funded by Pfizer, Inc., and has received unrestricted educational grant support from Alnylam Pharmaceuticals.
Footnotes
Conflict of interest Dr. Castaño has no conflicts of interest or financial ties to disclose.
Contributor Information
Adam Castaño, Email: ac3220@cumc.columbia.edu, adam.castano@gmail.com, Center for Advanced Cardiac Care, Columbia College of Physicians and Surgeons, New York City, NY, USA; Division of Cardiology, Columbia University Medical Center, 622 W 168th St. PH 10-203, New York, NY 10032, USA.
Brian M. Drachman, Division of Cardiology, University of Pennsylvania, Philadelphia, PA, USA
Daniel Judge, Division of Cardiology, Johns Hopkins University, Baltimore, MD, USA.
Mathew S. Maurer, Center for Advanced Cardiac Care, Columbia College of Physicians and Surgeons, New York City, NY, USA
References
- 1.Dharmarajan K, Maurer MS. Transthyretin cardiac amyloidoses in older North Americans. J Am Geriatr Soc. 2012;60(4):765–774. doi: 10.1111/j.1532-5415.2011.03868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapezzi C, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 3.Connors LH, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607–614. doi: 10.1016/j.ahj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson DR, et al. Revised transthyretin Ile 122 allele frequency in African-Americans. Hum Genet. 1996;98(2):236–238. doi: 10.1007/s004390050199. [DOI] [PubMed] [Google Scholar]
- 5.Hornsten R, et al. Heart complications in familial transthyretin amyloidosis: impact of age and gender. Amyloid. 2010;17(2):63–68. doi: 10.3109/13506129.2010.483114. [DOI] [PubMed] [Google Scholar]
- 6.Bonaiti B, et al. TTR familial amyloid polyneuropathy: does a mitochondrial polymorphism entirely explain the parent-of-origin difference in penetrance? Eur J Hum Genet. 2010;18(8):948–952. doi: 10.1038/ejhg.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapezzi C, et al. Gender-related risk of myocardial involvement in systemic amyloidosis. Amyloid. 2008;15(1):40–48. doi: 10.1080/13506120701815373. [DOI] [PubMed] [Google Scholar]
- 8.Suhr OB, et al. Myocardial hypertrophy and function are related to age at onset in familial amyloidotic polyneuropathy. Amyloid. 2006;13(3):154–159. doi: 10.1080/13506120600876849. [DOI] [PubMed] [Google Scholar]
- 9.Suhr O, et al. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med. 1994;235(5):479–485. doi: 10.1111/j.1365-2796.1994.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 10.Coelho T, Maurer MS, Suhr OB. THAOS—the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63–76. doi: 10.1185/03007995.2012.754348. [DOI] [PubMed] [Google Scholar]
- 11.Maurer MS, et al. Cardiac biomarkers in patients with transthyretin amyloidosis as documented in THAOS: the transthyretin amyloidosis survey. J Am Coll Cardiol. 2013;61(10):E1244–E1244. [Google Scholar]
- 12.Givens RC, et al. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging Health. 2013;9(2):229–235. doi: 10.2217/ahe.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihse E, et al. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol. 2008;216(2):253–261. doi: 10.1002/path.2411. [DOI] [PubMed] [Google Scholar]
- 14.Khella S, Drachman B, Divito P, Polydefkis M, Brannagan T, Judge D, Maurer MS. IXth international symposium on familial amyloid polyneuropathy; VIIIth international symposium on liver transplantation in familial amyloid polyneuropathy. Rio de Janeiro, Brazil: 2013. Neurologic involvement in Val122Ile familial amyloidosis. [Google Scholar]
- 15.Ng B, et al. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165(12):1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 16.Pinney JH, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. doi: 10.1161/JAHA.113.000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quarta CC, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840–1849. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
- 18.Liao R, et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001;104(14):1594–1597. [PubMed] [Google Scholar]
- 19.Mishra S, et al. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am J Physiol Heart Circ Physiol. 2013;305(1):H95–H103. doi: 10.1152/ajpheart.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruberg FL, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the transthyretin amyloidosis cardiac study (TRACS) Am Heart J. 2012;164(2):222–228. e1. doi: 10.1016/j.ahj.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 22.Feng D, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116(21):2420–2426. doi: 10.1161/CIRCULATIONAHA.107.697763. [DOI] [PubMed] [Google Scholar]
- 23.Berghoff M, et al. Endothelial dysfunction precedes C-fiber abnormalities in primary (AL) amyloidosis. Ann Neurol. 2003;53(6):725–730. doi: 10.1002/ana.10552. [DOI] [PubMed] [Google Scholar]
- 24.Yood RA, et al. Bleeding manifestations in 100 patients with amyloidosis. JAMA. 1983;249(10):1322–1324. [PubMed] [Google Scholar]
- 25.Feng D, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119(18):2490–2497. doi: 10.1161/CIRCULATIONAHA.108.785014. [DOI] [PubMed] [Google Scholar]
- 26.Dubrey S, et al. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br Heart J. 1995;74(5):541–544. doi: 10.1136/hrt.74.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy L, Falk RH. Left atrial kinetic energy in AL amyloidosis: can it detect early dysfunction? Am J Cardiol. 2000;86(2):244–246. doi: 10.1016/s0002-9149(00)00870-5. [DOI] [PubMed] [Google Scholar]
- 28.Lip GY, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 29.Klein AL, et al. Comprehensive doppler assessment of right ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;15(1):99–108. doi: 10.1016/0735-1097(90)90183-p. [DOI] [PubMed] [Google Scholar]
- 30.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107(19):2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 31.King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40(2):325–329. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 32.Tendler AHA, Maurer MS. IX international symposium on familial amyloidotic polyneuropathy (ISFAP) and the VIII international symposium on liver transplantation in familial amyloidotic polyneuropathy. Rio de Janeiro, Brazil: 2013. Myocardial contraction fraction is superior to the myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with cardiac amyloidosis. [Google Scholar]
- 33.Damy T, Plante-Bordeneuve V, Karayal O, Mundayat R, Kristen AV. European Society of Cardiology. Amsterdam, Netherlands: 2013. Clinical and echocardiographic signs associated with increased interventricular thickness (IVST) due to TTR related amyloidosis. [Google Scholar]
- 34.Suhr OB, et al. Liver transplantation for hereditary transthyretin amyloidosis. Liver Transpl. 2000;6(3):263–276. doi: 10.1053/lv.2000.6145. [DOI] [PubMed] [Google Scholar]
- 35.Tsay DM, Maurer MS. Biomarkers in ATTR cardiac amyloidosis (in submission) 2014 [Google Scholar]
- 36.Bokhari S, et al. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6(2):195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassidy JT. Cardiac amyloidosis. Two cases with digitalis sensitivity. Ann Intern Med. 1961;55:989–994. doi: 10.7326/0003-4819-55-6-989. [DOI] [PubMed] [Google Scholar]
- 38.Pomerance A. Senile cardiac amyloidosis. Br Heart J. 1965;27(5):711–718. doi: 10.1136/hrt.27.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63(6):1285–1288. doi: 10.1161/01.cir.63.6.1285. [DOI] [PubMed] [Google Scholar]
- 40.Wiklund U, et al. Abnormal heart rate variability and subtle atrial arrhythmia in patients with familial amyloidotic polyneuropathy. Ann Noninvasive Electrocardiol. 2008;13(3):249–256. doi: 10.1111/j.1542-474X.2008.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto S, et al. Continuous development of arrhythmia is observed in Swedish transplant patients with familial amyloidotic polyneuropathy (amyloidogenic transthyretin Val30Met variant) Liver Transpl. 2011;17(2):122–128. doi: 10.1002/lt.22184. [DOI] [PubMed] [Google Scholar]
- 42.Algalarrondo V, et al. Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm. 2012;9(7):1069–1075. doi: 10.1016/j.hrthm.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Epstein AE, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm. 2008;5(6):934–955. doi: 10.1016/j.hrthm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, et al. Left ventricular dyssynchrony is associated with reduced heart rate variability in familial amyloidotic polyneuropathy. Int J Cardiol. 2012;155(2):273–278. doi: 10.1016/j.ijcard.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Dubrey SW, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141–157. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 46.Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984;3(1):107–113. doi: 10.1016/s0735-1097(84)80436-2. [DOI] [PubMed] [Google Scholar]
- 47.Kristen AV, et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5(2):235–240. doi: 10.1016/j.hrthm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Dhoble A, et al. Cardiac amyloidosis treated with an implantable cardioverter defibrillator and subcutaneous array lead system: report of a case and literature review. Clin Cardiol. 2009;32(8):E63–E65. doi: 10.1002/clc.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin G, Dispenzieri A, Brady PA. Successful termination of a ventricular arrhythmia by implantable cardioverter defibrillator therapy in a patient with cardiac amyloidosis: insight into mechanisms of sudden death. Eur Heart J. 2010;31(12):1538. doi: 10.1093/eurheartj/ehp592. [DOI] [PubMed] [Google Scholar]
- 50.Lin G, et al. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2013;24(7):793–798. doi: 10.1111/jce.12123. [DOI] [PubMed] [Google Scholar]
- 51.Garan AR, Kolluri S, Lombardo I, Maurer MS. XIII international symposium on amyloidosis. Groningen, Netherlands: 2012. The prevalence of Holter abnormalities in ATTR cardiac amyloidosis. [Google Scholar]
- 52.Varr BC, et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;11(1):158–162. doi: 10.1016/j.hrthm.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Holmgren G, et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30) Clin Genet. 1991;40(3):242–246. doi: 10.1111/j.1399-0004.1991.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 54.Holmgren G, et al. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet. 1993;341(8853):1113–1116. doi: 10.1016/0140-6736(93)93127-m. [DOI] [PubMed] [Google Scholar]
- 55.Jonsen E, et al. Early liver transplantation is essential for familial amyloidotic polyneuropathy patients’ quality of life. Amyloid. 2001;8(1):52–57. doi: 10.3109/13506120108993814. [DOI] [PubMed] [Google Scholar]
- 56.Sandgren O, et al. Vitreous involvement in familial amyloidotic neuropathy: a genealogical and genetic study. Clin Genet. 1991;40(6):452–460. doi: 10.1111/j.1399-0004.1991.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 57.Olofsson BO. Cardiac involvement in familial amyloidosis with polyneuropathy. Int J Cardiol. 1983;4(3):379–382. doi: 10.1016/0167-5273(83)90101-8. [DOI] [PubMed] [Google Scholar]
- 58.Steen LE, Ek BO. Familial amyloidosis with polyneuropathy. Aspects of the relationship between gastrointestinal symptoms, EMG findings, and malabsorption studies. Scand J Gastroenterol. 1984;19(4):480–486. [PubMed] [Google Scholar]
- 59.Bispo M, et al. High incidence of thrombotic complications early after liver transplantation for familial amyloidotic polyneuropathy. Transpl Int. 2009;22(2):165–171. doi: 10.1111/j.1432-2277.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P, et al. Outcome of liver transplantation for familial amyloidotic polyneuropathy. Liver Transpl. 2003;9(12):1273–1280. doi: 10.1016/j.lts.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 61.Liepnieks JJ, Benson MD. Progression of cardiac amyloid deposition in hereditary transthyretin amyloidosis patients after liver transplantation. Amyloid. 2007;14(4):277–282. doi: 10.1080/13506120701614032. [DOI] [PubMed] [Google Scholar]
- 62.Liepnieks JJ, Zhang LQ, Benson MD. Progression of transthyretin amyloid neuropathy after liver transplantation. Neurology. 2010;75(4):324–327. doi: 10.1212/WNL.0b013e3181ea15d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gustafsson S, et al. Amyloid fibril composition as a predictor of development of cardiomyopathy after liver transplantation for hereditary transthyretin amyloidosis. Transplantation. 2012;93(10):1017–1023. doi: 10.1097/TP.0b013e31824b3749. [DOI] [PubMed] [Google Scholar]
- 64.Furtado A, et al. Sequential liver transplantation. Transplant Proc. 1997;29(1–2):467–468. doi: 10.1016/s0041-1345(96)00206-0. [DOI] [PubMed] [Google Scholar]
- 65.Furtado L, et al. Maximum sharing of cadaver liver grafts composite split and domino liver transplants. Liver Transpl Surg. 1999;5(2):157–158. doi: 10.1002/lt.500050204. [DOI] [PubMed] [Google Scholar]
- 66.Tome L, et al. Sequential liver transplantation: 27 cases in 25 patients. Transplant Proc. 2001;33(1–2):1430–1432. doi: 10.1016/s0041-1345(00)02540-9. [DOI] [PubMed] [Google Scholar]
- 67.Bolte FJ, et al. Evaluation of domino liver transplantations in Germany. Transpl Int. 2013;26(7):715–723. doi: 10.1111/tri.12110. [DOI] [PubMed] [Google Scholar]
- 68.Abdelfatah MM, Hayman SR, Gertz MA. Domino liver transplantation as a cause of acquired familial amyloid polyneuropathy. Amyloid. 2014;21(2):136–137. doi: 10.3109/13506129.2014.885894. [DOI] [PubMed] [Google Scholar]
- 69.Conner R, et al. Heart transplantation for cardiac amyloidosis: successful one-year outcome despite recurrence of the disease. J Heart Transplant. 1988;7(2):165–167. [PubMed] [Google Scholar]
- 70.Kpodonu J, et al. Outcome of heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant. 2005;24(11):1763–1765. doi: 10.1016/j.healun.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 71.Rapezzi C, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7(7):398–408. doi: 10.1038/nrcardio.2010.67. [DOI] [PubMed] [Google Scholar]
- 72.Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13(4):236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 73.Tojo K, et al. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci Res. 2006;56(4):441–449. doi: 10.1016/j.neures.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 74.Johnson SM, et al. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol. 2012;421(2–3):185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller SR, Sekijima Y, Kelly JW. Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab Invest. 2004;84(5):545–552. doi: 10.1038/labinvest.3700059. [DOI] [PubMed] [Google Scholar]
- 76.Adamski-Werner SL, et al. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem. 2004;47(2):355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- 77.Kearney PM, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332(7553):1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Epstein M. Non-steroidal anti-inflammatory drugs and the continuum of renal dysfunction. J Hypertens Suppl. 2002;20(6):S17–S23. [PubMed] [Google Scholar]
- 79.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol Sci. 1999;20(11):465–469. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 80.Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691–703. doi: 10.1053/bega.2001.0229. [DOI] [PubMed] [Google Scholar]
- 81.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 82.Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an under-recognized public health problem. Arch Intern Med. 2000;160(6):777–784. doi: 10.1001/archinte.160.6.777. [DOI] [PubMed] [Google Scholar]
- 83.Castano A, et al. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail. 2012;18(6):315–319. doi: 10.1111/j.1751-7133.2012.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berk JL, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quarta CCF, Solomon RH, Suhr SD, Obici OB, Perlini L, Lindqvist S, Koyama P, Sekijima J, Zeldenrust Y, Yamashita SR, Horibata T, Miller Y, Gorevic F, Merlini P, Ando G, Ikeda Y, Ruberg S, Berk F. International symposium on amyloidosis. Indianapolis, USA: 2014. The prevalence of cardiac amyloidosis in familial amyloidotic polyneuropathy with predominant neuropathy: the diflunisal trial; pp. 88–89. [Google Scholar]
- 86.Bulawa CE, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammarstrom P, et al. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coelho T, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–792. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merlini G, et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30-Met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013;6(6):1011–1020. doi: 10.1007/s12265-013-9512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maurer MSJDP, Rosas GR, Mandel FS, Aarts J. International symposium on amyloidosis. Indianapolis, USA: 2014. Interim analysis of long-term, open-label tafamidis treatment in transthyretin amyloid cardiomyopathy after up to 5 years of treatment. [Google Scholar]
- 91.Pfizer. Safety and efficacy of tafamidis in patients with transthyretin cardiomyopathy (ATTR-ACT) 2014 Available from: http://www.clinicaltrials.gov/show/NCT01994889.
- 92.Alhamadsheh MM, et al. Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity. Sci Transl Med. 2011;3(97) doi: 10.1126/scitranslmed.3002473. 97ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Penchala SC, et al. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci USA. 2013;110(24):9992–9997. doi: 10.1073/pnas.1300761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans . Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 95.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 96.Delivery, A.R.R.C. Available from: http://www.alnylam.com/capella/roundtables/rnai-roundtable-conjugate-delivery-dec-14/
- 97.Kanasty R, et al. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 98.Coelho T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 99.van Bennekum AM, et al. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem. 2001;276(2):1107–1113. doi: 10.1074/jbc.M008091200. [DOI] [PubMed] [Google Scholar]
- 100.Pharmaceuticals A. Phase 2 study to evaluate ALN-TTRSC in patients with transthyretin (TTR) cardiac amyloidosis. 2014 [Google Scholar]
- 101.Benson MD, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33(5):609–618. doi: 10.1002/mus.20503. [DOI] [PubMed] [Google Scholar]
- 102.Benson MD, et al. Antisense oligonucleotide therapy for TTR amyloidosis. Amyloid. 2011;18(Suppl 1):60. doi: 10.3109/13506129.2011.574354021. [DOI] [PubMed] [Google Scholar]
- 103.Ackermann EJ, et al. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19(Suppl 1):43–44. doi: 10.3109/13506129.2012.673140. [DOI] [PubMed] [Google Scholar]
- 104.Pharmaceuticals I. Efficacy and safety of ISIS-TTRRx in familial amyloid polyneuropathy. 2013 Available from: http://www.clinicaltrials.gov/ct2/show/NCT01737398?term=ISIS+amyloid&rank=1.
- 105.Cardoso I, Saraiva MJ. Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. FASEB J. 2006;20(2):234–239. doi: 10.1096/fj.05-4509com. [DOI] [PubMed] [Google Scholar]
- 106.Cardoso I, et al. Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med. 2010;8:74. doi: 10.1186/1479-5876-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Obici L, et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid. 2012;19(Suppl 1):34–36. doi: 10.3109/13506129.2012.678508. [DOI] [PubMed] [Google Scholar]
- 108.Matteo IPS. Safety, efficacy and pharmacokinetics of doxycycline plus tauroursodeoxycholic acid in transthyretin amyloidosis. 2011 Available from: http://www.clinicaltrials.gov/ct2/show/NCT01171859?term=%EF%82%A7%09NCT01171859&rank=1.
- 109.Hospital, B.a.W.s. Tolerability and efficacy of a combination of doxycycline and TUDCA in patients with transthyretin amyloid cardiomyopathy. 2013 Available from: http://www.clinicaltrials.gov/ct2/show/NCT01855360?term=NCT01855360&rank=1.
- 110.Pepys MB, Dash AC. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet. 1977;1(8020):1029–1031. doi: 10.1016/s0140-6736(77)91260-0. [DOI] [PubMed] [Google Scholar]
- 111.Bodin K, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468(7320):93–97. doi: 10.1038/nature09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pepys MB, et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417(6886):254–259. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]