Abstract
Introduction
The structural integrity of platelet receptors is essential for platelets to function normally in hemostasis and thrombosis in response to physiological and pathological stimuli. The aim of this study was to examine the shedding of two key platelet receptors, glycoprotein (GP) Ibα and GPVI, after exposed to the non-physiological high shear stress environment which commonly exists in blood contacting medical devices and stenotic blood vessels.
Materials and Methods
In this in vitro experiment, we exposed healthy donor blood in our specially designed blood shearing device to three high shear stress levels (150, 225, 300Pa) in combination with two short exposure time conditions (0.05 and 0.5 sec.). The expression and shedding of platelet GPIbα and GPVI receptors in the sheared blood samples were characterized using flow cytometry. The ability of platelet aggregation induced by ristocetin and collagen related to GPIbα and GPVI in the sheared blood samples, respectively, was evaluated by aggregometry.
Results and Conclusions
Compared to the normal blood, the surface expression of platelet GPIbα and GPVI in the sheared blood significantly decreased with increasing shear stress and exposure time. Moreover, the platelet aggregation induced by ristocetin and collagen reduced remarkably in a similar fashion. In summary non-physiological high shear stresses with short exposure time can induce shedding of platelet GPIbα and GPVI receptors, which may lead platelet dysfunction and influence the coagulation system. This study may provide a mechanistic insight into the platelet dysfunction and associated bleeding complication in patients supported by certain blood contacting medical devices.
Keywords: Shear stress, platelet receptor shedding, platelet dysfunction, platelet aggregation, blood contacting medical devices
Introduction
Cardiovascular disease is the largest cause of global death. Heart failure (HF) affects more than 5 million Americans and contributed to 274,601 deaths in 2009. In spite of medical advances near 50% diagnosed HF patients will succumb by 5 years. The annual expenditure on HF in the US is daunting $34.4 billion [1]. In order to treat the cardiovascular disease, significant progress in the development and use of prosthetic cardiovascular devices has been made in the past decades. Ventricular assist devices (VADs) are one of the cardiovascular devices and have emerged as a standard therapy for patients with advanced HF waiting for heart transplant or a bridge to myocardial recovery. Recent advance has enabled VADs to be used as a permanent therapy for life extension in HF patients who are not heart transplant candidates [2–4]. Unfortunately, device-related thrombotic and bleeding complications remain the major obstacles for this technology to be accepted widely for improvement of quality of life in millions of HF patient [5, 6]. Many investigations have been carried to understand device-induced platelet activation and coagulation system alteration associated with the use of VAD in patients, which are closely related to thrombotic events [7–9]. Because of thrombotic risk, patients implanted with VADs take the anti-coagulated drugs, which may tilt hemostatic balance towards bleeding in these patients [10, 11]. Two major types of VADs, pulsatile-flow and continuous-flow (CF), have been used in clinics [12]. It has been reported that patients with CF-VADs experienced higher rate of bleeding than those with pulsatile-flow devices although anticoagulation was not different among these two groups of patients. We believe that the increased bleeding risk in patients supported with CF-VADs is associated with the unique high shear stress environment in contemporarily available CF-VADs which has been reported in the literature when compared to pulsatile VADs [10] [13–20].
Platelets are anucleate blood cells that play a critical role in hemostasis. At the site of vascular injury, the subendothelial layer of matrix proteins, specifically Von Willebrand factor (vWF) and collagen, are exposed to blood. The exposed vWF and collagen activate the platelet adhesion receptors glycoprotein Ibα (GPIbα), which belongs to the GPIb-IX-V complex with VWF binding, and glycoprotein VI (GPVI), which is a crucial platelet receptor for collagen binding [21, 22]. Both the activation pathways trigger intracellular signaling cascades leading to final platelet aggregation to form a primary hemostatic plug to stop bleeding. The shedding of these two key receptors would lead the platelet dysfunction and affect hemostasis [23–26]. Receptor shedding is a mechanism for irreversible removal of transmembrane cell surface receptors by proteolysis of the receptor at a position near the extracellular surface of the plasma membrane. This process generates a soluble ectodomain fragment and a membrane-associated remnant fragment, and is distinct from loss of receptor surface expression by internalization or microparticle release or secretion of alternatively spliced soluble forms of receptors lacking a transmembrane domain. Platelet receptor shedding provides a mechanism for down regulating surface expression resulting in loss of ligand binding, decreasing the surface density affecting receptor cross linking and signaling, and generation of proteolytic fragments that may be functional and/or provide platelet-specific biomarkers.
Previous investigations had shown that platelet GPIbα and GPVI could be shed by the shear stress with very long exposure times [27, 28]. The cone-plate viscometers were used to in these studies to expose platelets to shear stress for extended duration. The levels of shear stresses were relatively low and exposure time was long. The relevance of the results from these studies to CF-VADs is limited. In some regions of a typical CF-VAD, blood would experience shear stresses higher than 100 Pa (1000 dyne/cm2) with very short exposure time less than 1 sec[16, 29]. No studies have been conducted to investigate the impact of the higher shear stress and shorter exposure time conditions in these ranges on the shedding of the two functional platelet receptors GPIbα and GPVI, and their aggregation potential upon stimulation by ristocetin and collagen.
We believe that shear induced hemostatic dysfunction imparted by the non-physiological high mechanical shear of high speed rotary CF-VADs, especially the shedding of platelet receptors (GPIbα and GPVI) may be responsible for the morbid bleeding, which is the most common postoperative complication after VADs implantation and a leading manifestation of the disordered coagulation [30–32]. In order to test our hypothesis, an experiment was designed to investigate the shedding of GPIbα and GPVI under high shear stress and short exposure time conditions. During the present study, the blood draw from the donor would be shed by a novel blood-shearing device which could create flow conditions with high shear stress and short exposure time.
Materials and methods
Blood-shearing device
The Blood-shearing device used in this experiment was an axial flow-through Couette device adapted from the adult Jarvik 2000 blood pump (Jarvik Heart, Inc., New York, NY, USA). In this device, the shape of the inner rotor is a spindle which is supported by a pair of tiny pin-bearings and could rotate between 8000 and 12 000 rpm and there is a narrow gap with a uniform width of 100 μm between the inner rotor and the outer housing (shown in figure 1). The detail design of this device could be found in the reference [33]. For a fluid with typical viscosity of 0.0036 Pa·s, a uniform shear region with the corresponding shear stress between 117 and 338 Pa could be created using this device. As shown in figure 1, during the experiment, a syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA) was connected with the blood-shearing device and the whole blood was pressure-driven to pass through the narrow gap in the axial direction, which could be used to control the shearing exposure time of blood. Therefore, the high shear stress and short exposure time could be created by adjusting the spindle rotor speed and the axial flow rate. Also, it should be mentioned that, the shear stress in the other region of this device might had some influence on blood, but it could be negligible. Our early computational fluid dynamics analysis showed that the shear stress in the other region was much smaller than that in the narrow gap region [33].
Figure 1.
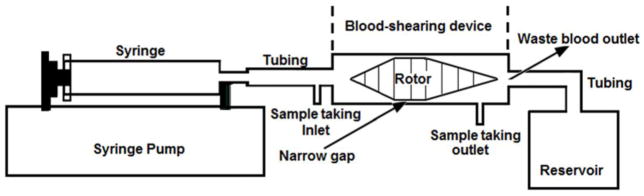
The sketch of Blood shearing system; it is consist of syring pump used to control the flow rate, syring, tubing, blood shearing device including 100μm narrow gap between the inner rotor and the outer housing, blood sample taking inlet and outlet, waste blood outlet and reservoir.
Experimental procedure
Nine healthy human blood donors were recruited to participate in the present study. All the donors gave informed consent and did not take aspirin or other platelet active medication 10 days prior to the blood donation. The blood collection was carried out in accordance to the protocol approved by the Institutional Review Board of the University of Maryland, Baltimore. Fresh blood drawn from these donors mixed with anticoagulant acid citrate dextrose (ACD-A) with the volume ratio of 9 to 1.
Before each experiment, the viscosity of the blood preparation was measured by semi-micro viscometers (Cannon Instrument Company, State College, PA). The rotational speed of spindle rotor and the axial flow rate were decided by the expected shear stresses and exposure times respectively [33]. During the experiment, a baseline blood sample was collected at the inlet of the shearing device and used as the control sample for comparison with the sheared blood samples. Three levels of high shear stresses (150, 225, 300 Pa) and two different short exposure times (0.05 and 0.5 sec.) were chosen in the present study[19, 34]. For each condition with a combination of shear stress and exposure time, the sheared blood sample was collected from the sample outlet of the device. For each condition, the blood shearing experiments would be repeated for nine times. After all the samples were collected, the shedding of platelet receptors GPIbα and GPVI of the baseline and sheared blood samples was measured with flow cytometry. In parallel, the ristocetin and collagen induced platelet aggregation of these blood samples were quantified with whole blood aggregometry.
Flow cytometric assays of shedding of platelet GPIbα and GPVI receptors
The shedding of platelet receptors GPIbα and GPVI in the blood samples after exposed to the non-physiological shear stress conditions was analyzed by flow cytometry. Phycoerythrin (PE) conjugated anti-CD41 antibody (BioLegend, San Diego, CA), Fluorescein isothiocyanate (FITC) conjugated anti-CD42b antibody (BioLegend, San Diego, CA) and Fluorescein isothiocyanate (FITC) conjugated IgGIK antibody (BioLegend, San Diego, CA) were used to identify the platelet population, to determine the level of expression of platelet receptor GPIbα and to serve the negative control for expression of platelet receptor GPIbα, respectively. 5μl whole blood samples were incubated with 25 μl of 10 mM HEPES buffer mixed with 10μl anti-CD41 antibody and 10μl anti-CD42b antibody for 30 min at room temperature in dark. In parallel, a 5 μl whole blood sample was incubated with 25 μl HEPES buffer mixed with 10μl anti-CD41 antibody and 10μl IgGIK antibody and used as the negative control.
For the GPVI shedding, PE conjugated anti-CD 41 antibody (BioLegend, San Diego, CA), eFluor 660 conjugated anti-Human Glycoprotein VI (eBioscience, San Diego, CA) and eFluor 660 conjugated Mouse IgG1K Isotype Control (eBioscience, San Diego, CA) were used to identify the platelets, to determine the level of expression of platelet receptor GPVI and to serve the negative control, respectively. 5μl whole blood samples were incubated with 25 μl of 10 mM HEPES buffer mixed with 5μl anti-CD41 antibody and 5μl anti-Glycoprotein VI antibody for 30min at room temperature in dark. A 5μl whole blood sample was incubated with 25 μl HEPES buffer mixed with 5μl anti-CD41 antibody and 5μl Mouse IgG1K Isotype antibody and used to be the negative control.
After the above labeling steps, 1 ml of 1% paraformaldehyde (PFA) in PBS was used to fix the samples for 30 min at 4 degree in dark. The flow cytometric data collection of the blood samples was performed with a four color flow cytometer (FACS Calibur, BD Bioscience, San Jose, CA). The data were analyzed offline using the software FCS Express 4.0 (De Novo Software, Los Angeles, CA).
Platelet Aggregation test
If the platelet receptors (GPIbα and GPVI) were shed after being exposed to the non-physiological high shear stresses generated by the blood shearing device, the platelet aggregation induced by the agonists stimulating platelet GPIbα and GPVI would decrease. In order to test this hypothesis, the platelet aggregation test of the baseline and sheared blood samples was performed using an aggregometer (Chrono-Log, Havertown, PA) by using ristocetin and collagen as the agonists. The ristocetin induces the platelet aggregation by causing VWF to bind with the platelet receptor GPIbα. The collagen induces the platelet aggregation by binding with the platelet receptor GPVI to induce platelet activation.
According to the manufacturer’s instruction, 0.5ml whole blood sample was added into the preheated polystyrene cuvette containing 0.5ml 0.9% saline and a stir bar. The 1 ml mixed blood sample was heated in the aggregometer and stirred at a stirring rate of 1200 rpm. Then 8μl restocetin with the final concentration of 1mg/ml or 5μl collagen with the final concentration of 4μg/ml was added into the mixed blood sample. After adding the agonists, the platelets were stimulated to adhere on two electrodes which caused the impedance between the electrodes to increase. Once the agonist reagent was added, the time course of the impedance was recorded for 6 minutes. The integral of the impedance curve (area under the impedance curve, AUC) which was the overall metric for platelet aggregation was used to represent the agonist induced platelet aggregation in this study.
Statistical analysis
The data are presented as mean ± SE (standard Error) and statistically analyzed using SPSS statistical software (Statistical Package for Social Sciences for windows, release 18.0; SPSS Inc., Chicago, IL, USA). Statistical differences were determined by using Student’s t-test. Statistical significance was assigned at p < 0.05.
Results
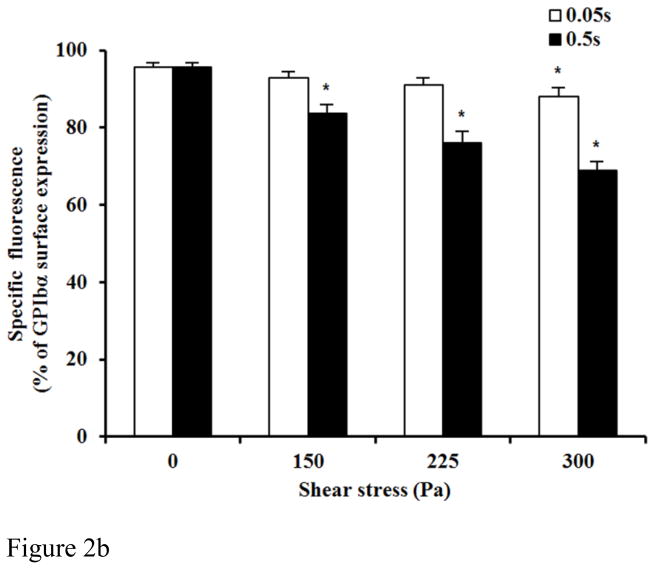
Figure 2A and 2B show typical flow cytometry dot plots of platelet GPIbα and CD-41 expression in the baseline blood and two sheared blood samples and the quantification of reduced platelet GPIbα surface expression by the non-physiological shear stresses. The platelets in the blood samples were identified by the PE conjugated anti-CD41 antibody and the level of the surface expression of GPIbα on each platelet was indicated by fluorescent intensity of FITC (conjugated anti-CD42b). Figure 2A clearly shows the increase in the percentage of platelets (CD-41 positive) with reduced CD-42b expression in the two sheared blood samples compared with that in the baseline blood sample, indicating the shear-induced shedding of the platelet receptor GPIbα after subjected to the non-physiological shear stress. Figure 2B exhibits the levels of the shear-induced shedding of the platelet receptor GPIbα in the normal blood and the six blood samples sheared by the three different levels of high shear stress (150, 225, 300 Pa) for the two exposure times (0.05 and 0.5 sec.). As indicated in these figures, the shedding of the platelet receptor GPIbα in the blood sample sheared by the shear stress levels of 150Pa and 225Pa for the exposure time of 0.05 sec increased slightly, but not significantly compared with the baseline blood. However when the shear stress level increased to 300 Pa, the shedding of the platelet receptor GPIbα for the exposure time of 0.05 sec became significant (P<0.05). With the increase in the exposure time to 0.5 sec, the shedding of the platelet receptor GPIbα in the sheared blood samples was significant for the three levels of shear stresses when compared with the baseline blood. Overall, the shedding of the platelet receptor GPIbα in the sheared blood samples increased with increasing shear stress and exposure time.
Figure 2.
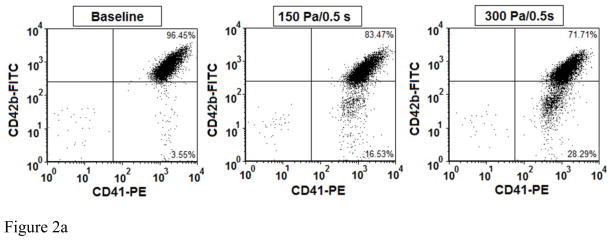
Figure 2A: The flow cytometry dot plots of comparing of platelet receptor GPIbα surface expression among base sample and two sheared samples (150Pa/0.5s and 300Pa/0.5s). The percent of platelet receptor GPIbα surface expression is indicated by both positive antibodies of FITC conjugated anti-CD42b (CD42b-FITC) and PE conjugated anti-CD41 (CD41-PE).
Figure 2B: The average comparing of the percentage of GPIbα surface expression in platelet between base unsheared blood samples (0Pa) and sheared blood samples under three levels of high shear stress (150, 225, 300Pa) for two durations (0.05 and 0.5 sec.)(n=9).
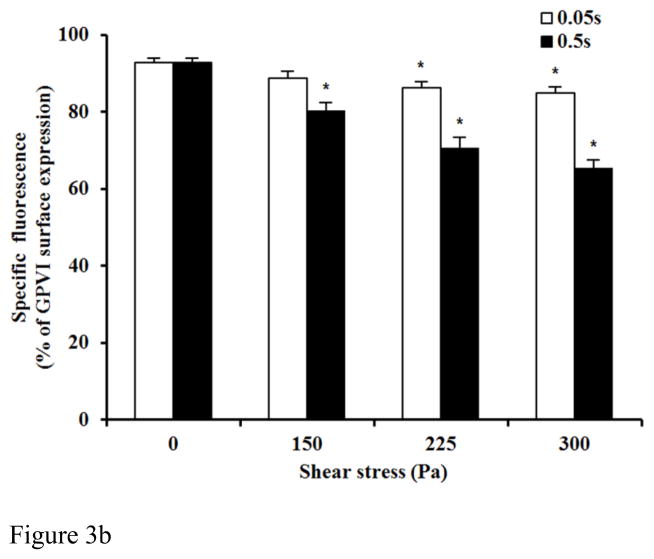
Figure 3A and 3B show typical flow cytometry dot plots of platelet GPVI and CD-41 expression in the baseline and two sheared blood samples and the quantification of the reduced platelet GPVI surface expression by the non-physiological shear stresses. As shown in the flow cytometry dot plots (Figure 3A), the percentage of platelets with reduced GPVI surface expression, as indicated by fluorescent intensity of eFluor 660 (conjugated anti-GPVI), increased with increasing the level of shear stress. Figure 3B exhibits the levels of the shear-induced shedding of the platelet receptor GPVI in the normal blood and the six blood samples sheared by the three levels of shear stress (150, 225, 300 Pa) for the two exposure times (0.05 and 0.5 sec.). In general, the shedding of the platelet receptor GPVI in the sheared blood samples increased with increasing shear stress and exposure time. The percentage of platelets with reduced GPVI surface expression increased significantly (P<0.05) in all the sheared blood samples except one sheared blood sample by 150Pa for the exposure time of 0.05 sec compared with that of the normal blood.
Figure 3.
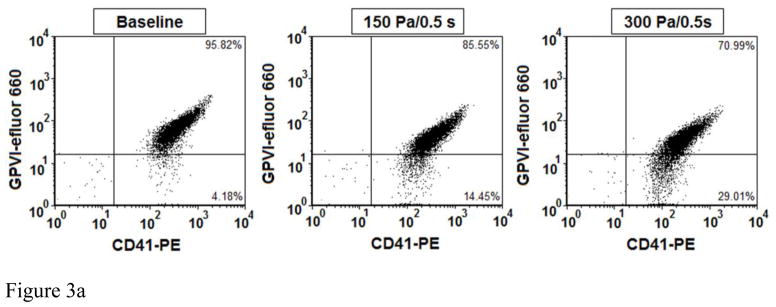
Figure 3A: The flow cytometry dot plots of comparing of platelet receptor GPVI surface expression among base sample and two sheared samples (150Pa/0.5sec. and 300Pa/0.5sec.). The percent of platelet receptor GPVI surface expression is indicated by both positive antibodies of efluor 660 conjugated anti-Human Glycoprotein VI (GPVI-efluor 660) and PE conjugated anti-CD41 (CD41-PE).
Figure 3B: The average comparing of the percentage of GPVI surface expression in platelet between base unsheared blood samples (0Pa) and sheared blood samples under three levels of high shear stress (150, 225, 300Pa) for two durations (0.05 and 0.5 sec.)(n=9).
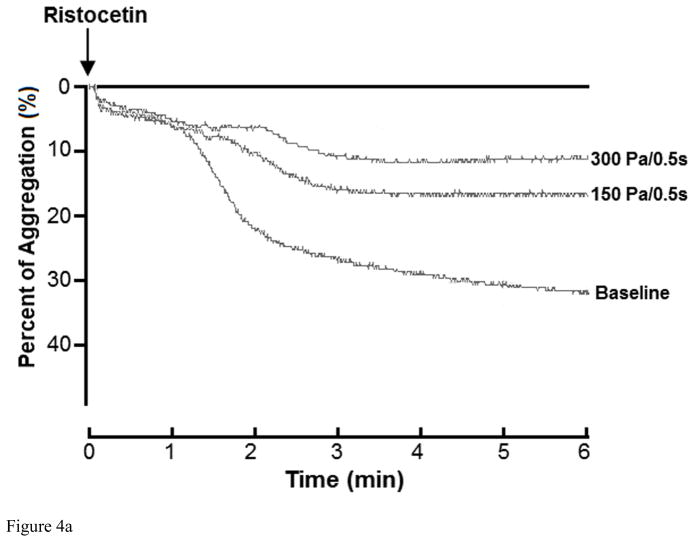
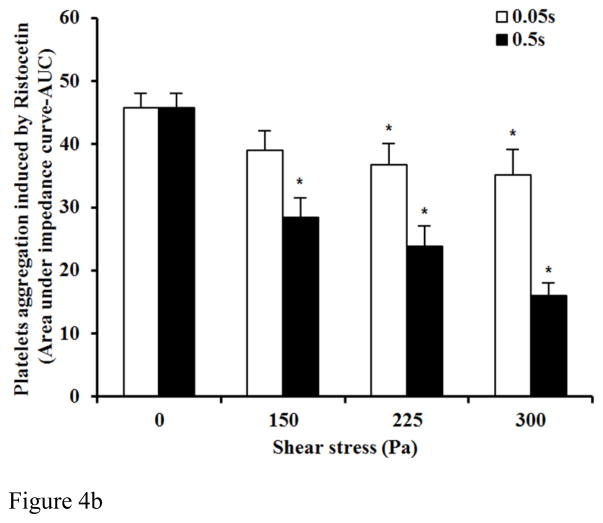
To further confirm the shear-induced shedding of the platelet receptor GPIbα and GPVI, ristocetin and collagen were added to the sheared blood samples to evaluate their platelet receptor GPIbα and GPVI associated aggregation ability, respectively. Figure 4A and 4B show typical traces of ristocetin-induced aggregation of the baseline and two sheared blood samples and the quantitative comparison of the ristocetin-induced aggregation among the baseline blood and the six blood samples sheared by the three levels of shear stress for the exposure times. Figure 4A clearly shows that the non-physiological shear stress caused reduction in the ristocetin-induced aggregation. The degree of the reduction increased with exposure time. Since the platelet aggregation induced by ristocetin is via stimulating the binding of platelet receptor GPIbα with vWF, the shear-induced shedding of the platelet receptor GPIbα should be the reason for the reduction of the ristocetin-induced platelet aggregation under high shear stress and short exposure time conditions. Figure 4B show the levels of the shear-induced reduction in the vWF mediated platelet GPIbα associated aggregation of the baseline and the six blood samples sheared by the three levels of shear stress for the two exposure times. For the exposure time of 0.05 sec, the reduction of the platelet GPIbα associated aggregation of the sheared blood samples by the shear stress level of 150 Pa was not significant compared to the normal blood. However, the reduction in the platelet GPIbα associated aggregation became significant with increasing the shear stress level to 225 and 300 Pa (P<0.05). When the exposure time was increased to 0.5 sec, the platelet GPIbα associated aggregation of the blood samples sheared by the three levels of shear stress reduced significantly (P<0.05). We noticed that there was a slight decrease in the platelet counts with increasing the level of shear stress and exposure time, but not statistically significant. The slight reduction of platelet counts in samples should be expected because we found the aggregation between platelets and monocytes increased with increasing shear stress and exposure time in our previous study [35].
Figure 4.
Figure 4A: The changing of impedance curve (aggregation curve) of the platelets aggregation induced by ristocetin among base sample and two sheared samples (150Pa/0.5s, 300Pa/0.5s). The platelet aggregation is indicated by area under the impedance curve. The time course of the platelets aggregation curve changing was recorded for 6 minutes
Figure 4B: The average comparing of platelet aggregation induced by ristocetin between base unsheared blood samples (0Pa) and sheared blood samples under three levels of high shear stress (150, 225, 300Pa) for two durations (0.05 and 0.5 sec.)(n=9). The platelet aggregation induced by ristocetin in y-axis is indicated by area under the impedance curve.
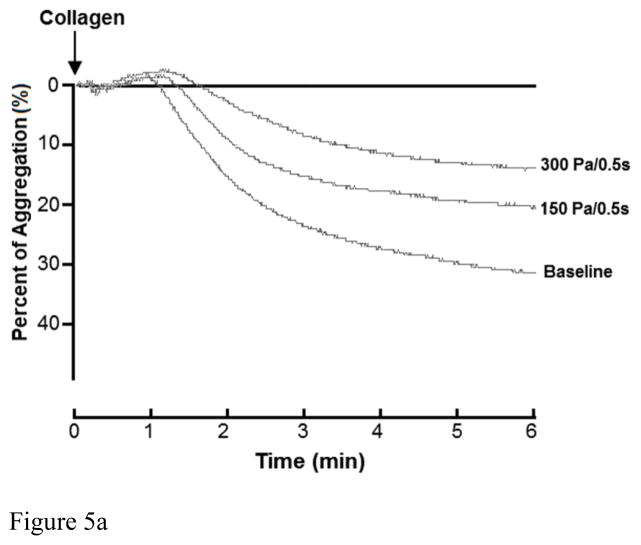
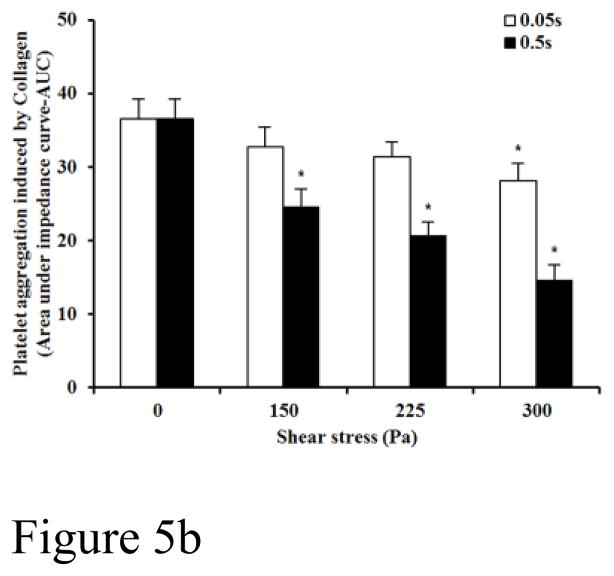
Figure 5A and 5B show typical traces of the collagen-induced aggregation of the baseline blood and two sheared blood samples and the quantitative comparison of the collagen-induced aggregation among the baseline blood and the six blood samples sheared by the three levels of shear stress for the exposure times. Figure 5A shows that the non-physiological shear stress causes reduction in the collagen-induced aggregation. The degree of the reduction increased with exposure time. Since the platelet aggregation induced by collagen is via stimulating the binding of platelet receptor GPVI and platelet activation, the shear-induced shedding of the platelet receptor GPVI should be the reason for the reduction of the collagen-induced platelet aggregation under high shear stress and short exposure time conditions. Figure 5B shows the levels of the shear-induced reduction in the collagen mediated platelet GPVI associated aggregation of the baseline and the six blood samples sheared by the three levels of shear stress for the two exposure times. For the exposure time of 0.05 sec, the collagen-induced platelet aggregation decreased with increasing the shear stress from 150 and 300Pa, but did became significant until the shear stress level reached to 300 Pa (P<0.05) compared to that of the normal blood. For the exposure time of 0.5 sec, the three levels of shear stress caused significant reduction in the collagen-induced platelet aggregation (P<0.05).
Figure 5.
Figure 5A: The changing of impedance curve (aggregation curve) of the platelets aggregation induced by collagen among base sample and two sheared samples (150Pa/0.5s, 300Pa/0.5s). The platelet aggregation is indicated by area under the impedance curve. The time course of the platelets aggregation curve changing was recorded for 6 minutes
Figure 5B: The average comparing of platelet aggregation induced by collagen between base unsheared blood samples (0Pa) and sheared blood samples under three levels of high shear stress (150, 225, 300Pa) for two durations (0.05 and 0.5 sec.)(n=9). The platelet aggregation induced by collagen in y-axis is indicated by area under the impedance curve.
Discussion
The non-physiologic high shear stress often exists in blood contacting medical devices. In particular, the shear stress level above 100 Pa has been found in some region within blood pumping devices, such as the blade region [16, 29]. However, the duration for the blood exposed to high shear stress was very short and less than one sec. Shear-induced platelet activation and aggregation have been the major concerns for use of these devices in clinical setting. Thus a complex anticoagulant drug regimen is often prescribed to mitigate thrombotic risk. The current anticoagulation treatment may incur vulnerability to bleeding and hemorrhage which are the most common postoperative complication associated with circulatory assist devices, ECMO and heart valves and cardiopulmonary bypass. Until recently, bleeding associated with blood contacting medical devices has been often attributed to the anticoagulation treatment. However, several recent reports suggested that bleeding associated one type of device was higher than others[10], for example, patients with CF-VADs experienced higher rate of bleeding than those with pulsatile-flow devices although anticoagulation was not different among these two groups of patients. Thus we believe that the underlying fluid mechanic shear stress might contribute to the increased bleeding.
In present study, human blood was subjected to the three levels of high shear stress for short exposure time. The shedding of platelet receptor GPIbα and GPVI and their associated aggregation were examined. The results clearly showed that the shedding of the platelet receptor GPIbα and GPVI occurred in the sheared blood samples with high shear stress for a short exposure time. It was found that both the platelet aggregations induced by ristocetin and collagen decreased in the sheared blood samples. Both the degree in the shear-induced shedding of the platelet receptor GPIbα and GPVI increased with increasing the shear stress level and exposure time. In contrast, the platelet aggregation associated with these two platelet receptors decreased with increasing the shear stress level and exposure time.
Since the increased bleeding was reported to be associated with CF-VADs [10], many suggestions had been offered to explain this clinical observation, such as overuse of anticoagulation therapy, loss of large vWF multimers and GI tract angiodysplasia formation [5, 36, 37]. However, the hemorrhagic events was a complex processes including many pathological mechanisms, in which the causation is still unclear. Based on the results of the present study, we believe that the non-physiological shear stress damage the integrity of platelets, especially the shedding of platelet receptors, leading to platelet dysfunction and inability of adhesion and aggregation to vWF and collagen. The platelet receptor GPIbα and GPVI are two key receptors for hemostasis. When a blood vessel is injured, vWF and collagen of injured endothelium tissue are exposed to the blood whereas the binding between GPIbα with VWF and adhesion connection between GPVI with collagen initiate the platelet adhesion and aggregation process. In our study, two approaches had been used to investigate the shedding of the platelet receptor GPIbα and GPVI. One was the flow cytometry test to study the percentage of receptor expression in platelets surface. The other was the platelet aggregation test. The data obtained using these two approaches exhibited that the shedding of GPIbα and GPVI occurred under high shear stress and short exposure time. Interestingly, it was found that, even in such short exposure time (0.05s and 0.5s), if the shear stress was higher enough, the platelet receptor GPIbα and GPVI could be shed. In patients implanted with CF-VADs, because of the continued circulation, the platelets would pass through the non-physiological shear region frequently in the high speed shear-inducing rotating pumps. The shedding of platelet receptors GPIbα and GPVI should be enhanced. Therefore, the platelet receptor shedding which would lead platelet dysfunction should be carefully examined in the bleeding events in patients implanted with CF-VADs.
Bleeding is a very dangerous clinical pathological complication, which could be induced by many factors. Only the in vitro experiment is not strong enough to make the conclusion that bleeding is related with receptor shedding induced by non-physiological high shear stress. In our future studies, some in vivo animal experiments would be performed to investigate the influence of the combination with non-physiological high shear stress and anticoagulant drugs on the platelet adhesion, aggregation and hemostasis.
Conclusion
It was proven that the platelet receptors GPIbα and GPVI could be shed by non-physiological high shear stress for short exposure conditions. These sheared platelets exhibited an impaired aggregation capacity of platelets in response to ristocetin and collagen stimulation. The shear-induced shedding of the two key platelet receptors may offer a new perspective to explain the reason of increased bleeding events in patient implanted with those high shear CF-VADs.
Highlights.
High shear stress with short exposure time induces platelet receptors shedding.
Non-physiological high shear stress induces reduction of platelet aggregation.
High shear stress in VADs is related with bleeding complication in patients.
Acknowledgments
Source of Funding: The described research was partially sponsored by the National Institutes of Health (Grant R01 HL 088100).
Footnotes
Conflict of Interest Statement
The authors declared that there are no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. New England Journal of Medicine. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Martin J, Friesewinkel O, Benk C, Sorg S, Schultz S, Beyersdorf F. Improved durability of the HeartMate XVE left ventricular assist device provides safe mechanical support up to 1 year but is associated with high risk of device failure in the second year. The Journal of heart and lung transplantation. 2006;25:384–90. doi: 10.1016/j.healun.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 4.John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. The Annals of thoracic surgery. 2008;86:1227–35. doi: 10.1016/j.athoracsur.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, Rogers JG. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circulation: Heart Failure. 2011;4:779–84. doi: 10.1161/CIRCHEARTFAILURE.111.962613. [DOI] [PubMed] [Google Scholar]
- 6.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–47. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 7.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. The Journal of heart and lung transplantation. 2009;28:881–7. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Bruckner BA, DiBardino DJ, Ning Q, Adeboygeun A, Mahmoud K, Valdes J, et al. High incidence of thromboembolic events in left ventricular assist device patients treated with recombinant activated factor VII. The Journal of heart and lung transplantation. 2009;28:785–90. doi: 10.1016/j.healun.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 9.John R, Kamdar F, Liao K, Colvin-Adams M, Miller L, Joyce L, et al. Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. The Journal of thoracic and cardiovascular surgery. 2008;136:1318–23. doi: 10.1016/j.jtcvs.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 10.Crow S, John R, Boyle A, Shumway S, Liao K, Colvin-Adams M, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. The Journal of thoracic and cardiovascular surgery. 2009;137:208–15. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Kasirajan V, McCarthy PM, Hoercher KJ, Starling RC, Young JB, Banbury MK, et al. Clinical experience with long-term use of implantable left ventricular assist devices: indications, implantation, and outcomes. Seminars in thoracic and cardiovascular surgery. 2000;12(3):229–37. doi: 10.1053/stcs.2000.9667. [DOI] [PubMed] [Google Scholar]
- 12.Loor G, Gonzalez-Stawinski G. Pulsatile vs. continuous flow in ventricular assist device therapy. Best Practice & Research Clinical Anaesthesiology. 2012;26(2):105–15. doi: 10.1016/j.bpa.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Karmonik C, Partovi S, Schmack B, Weymann A, Loebe M, Noon GP, et al. Comparison of hemodynamics in the ascending aorta between pulsatile and continuous flow left ventricular assist devices using computational fluid dynamics based on computed tomography images. Artificial organs. 2014;38:142–8. doi: 10.1111/aor.12132. [DOI] [PubMed] [Google Scholar]
- 14.Avrahami I, Rosenfeld M, Einav S. The hemodynamics of the Berlin pulsatile VAD and the role of its MHV configuration. Annals of biomedical engineering. 2006;34:1373–88. doi: 10.1007/s10439-006-9149-x. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch S, Tarbell JM, Manning KB, Rosenberg G, Fontaine AA. Experimental fluid mechanics of pulsatile artificial blood pumps. Annu Rev Fluid Mech. 2006;38:65–86. [Google Scholar]
- 16.Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. Journal of biomechanical engineering. 2012;134:081002. doi: 10.1115/1.4007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern DR, Kazam J, Edwards P, Maybaum S, Bello RA, D’Alessandro DA, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. Journal of cardiac surgery. 2010;25:352–6. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JB, Wood HG, Allaire PE, McDaniel JC, Olsen DB, Bearnson G. Numerical studies of blood shear and washing in a continuous flow ventricular assist device. ASAIO Journal. 2000;46:486–94. doi: 10.1097/00002480-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Song X, Throckmorton AL, Wood HG, Antaki JF, Olsen DB. Quantitative evaluation of blood damage in a centrifugal VAD by computational fluid dynamics. Journal of fluids engineering. 2004;126:410–8. [Google Scholar]
- 20.Song X, Untaroiu A, Wood HG, Allaire PE, Throckmorton AL, Day SW, et al. Design and transient computational fluid dynamics study of a continuous axial flow ventricular assist device. ASAIO Journal. 2004;50:215–24. doi: 10.1097/01.mat.0000124954.69612.83. [DOI] [PubMed] [Google Scholar]
- 21.Furie B, Furie BC. Mechanisms of thrombus formation. New England Journal of Medicine. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 22.Moroi M, Jung SM, Nomura S, Sekiguchi S, Ordinas A, Diaz-Ricart M. Analysis of the involvement of the von Willebrand factor glycoprotein Ib interaction in platelet adhesion to a collagen-coated surface under flow conditions. Blood. 1997;90:4413–24. [PubMed] [Google Scholar]
- 23.Andrews RK, Gardiner EE, Shen Y, Whisstock JC, Berndt MC. Glycoprotein Ib-IX-V. The international journal of biochemistry & cell biology. 2003;35:1170–4. doi: 10.1016/s1357-2725(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 24.Nesbitt WS, Giuliano S, Kulkarni S, Dopheide SM, Harper IS, Jackson SP. Intercellular calcium communication regulates platelet aggregation and thrombus growth. The Journal of cell biology. 2003;160:1151–61. doi: 10.1083/jcb.200207119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesbitt WS, Kulkarni S, Giuliano S, Goncalves I, Dopheide SM, Yap CL, et al. Distinct glycoprotein Ib/V/IX and integrin alpha IIb beta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. Journal of Biological Chemistry. 2002;277:2965–72. doi: 10.1074/jbc.M110070200. [DOI] [PubMed] [Google Scholar]
- 26.Andrews RK, Karunakaran D, Gardiner EE, Berndt MC. Platelet receptor proteolysis A mechanism for downregulating platelet reactivity. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1511–20. doi: 10.1161/ATVBAHA.107.141390. [DOI] [PubMed] [Google Scholar]
- 27.Al-Tamimi M, Tan CW, Qiao J, Pennings GJ, Javadzadegan A, Yong ASC, et al. Pathologic shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood. 2012;119:4311–20. doi: 10.1182/blood-2011-10-386607. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Yan R, Li S, Yuan Y, Liu J, Ruan C, et al. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibα shedding. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297:H2128–H35. doi: 10.1152/ajpheart.00107.2009. [DOI] [PubMed] [Google Scholar]
- 29.Taskin ME, Fraser KH, Zhang T, Wu C, Griffith BP, Wu ZJ. Evaluation of Eulerian and Lagrangian models for hemolysis estimation. ASAIO Journal. 2012;58:363–72. doi: 10.1097/MAT.0b013e318254833b. [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Mondal NK, Sorensen EN, Cai L, Fang H-B, Griffith BP, et al. Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. The Journal of heart and lung transplantation. 2014;33:71–9. doi: 10.1016/j.healun.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein VI-related clinical defects. British journal of haematology. 2007;139:363–72. doi: 10.1111/j.1365-2141.2007.06799.x. [DOI] [PubMed] [Google Scholar]
- 32.Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad Re, Rackebrandt K, Cazenave J-P, et al. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. The Journal of experimental medicine. 2001;193:459–70. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Taskin ME, Fang HB, Pampori A, Jarvik R, Griffith BP, et al. Study of Flow-Induced Hemolysis Using Novel Couette-Type Blood-Shearing Devices. Artificial organs. 2011;35:1180–6. doi: 10.1111/j.1525-1594.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 34.Leverett LB, Hellums JD, Alfrey CP, Lynch EC. Red blood cell damage by shear stress. Biophysical journal. 1972;12:257–73. doi: 10.1016/S0006-3495(72)86085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding J, Chen ZS, Niu SQ, Zhang JF, Mondal NK, Griffith BP, et al. Quantification of Shear-Induced Platelet Activation: High Shear Stresses for Short Exposure Time. Artificial Organs. 2015 doi: 10.1111/aor.12438. In Press. [DOI] [PubMed] [Google Scholar]
- 36.Geisen U, Heilmann C, Beyersdorf F, Benk C, Berchtold-Herz M, Schlensak C, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. European Journal of Cardio-Thoracic Surgery. 2008;33:679–84. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 37.Meyer AL, Malehsa D, Bara C, Budde U, Slaughter MS, Haverich A, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circulation: Heart Failure. 2010;3:675–81. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]