Abstract
Introduction
The aim of this study was to examine whether the substitution of the Lys linker with the aminooctanoic acid (Aoc) and polyethylene glycol (PEG) linker could substantially decrease the non-specific renal uptake of 99mTc-labeled Arg-Gly-Asp-conjugated α-melanocyte stimulating hormone (α-MSH) hybrid peptides.
Methods
The RGD motif {Arg-Gly-Asp-DTyr-Asp} was coupled to [Cys3,4,10, D-Phe7, Arg11]α-MSH3–13 via the Aoc or PEG2 linker to generate RGD-Aoc-(Arg11)CCMSH and RGD-PEG-(Arg11)CCMSH. The biodistribution results of 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH were examined in M21 human melanoma-xenografted nude mice.
Results
The substitution of Lys linker with Aoc and PEG2 linker significantly reduced the renal uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH by 58% and 63% at 2 h post-injection. The renal uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH was 27.93 ± 3.98 and 22.01 ± 9.89% ID/g at 2 h post-injection. 99mTc-RGD-Aoc-(Arg11)CCMSH displayed higher tumor uptake than 99mTc-RGD-PEG2-(Arg11)CCMSH (2.35 ± 0.12 vs. 1.71 ± 0.25% ID/g at 2 h post-injection). The M21 human melanoma lesions could be clearly visualized by SPECT/CT using 99mTc-RGD-Aoc-(Arg11)CCMSH as an imaging probe.
Conclusions
The favorable effect of Aoc and PEG2 linker in reducing the renal uptake provided a new insight into the design of novel dual receptor-targeting radiolabeled peptides.
Keywords: Arg-Gly-Asp-conjugated, alpha-melanocyte stimulating hormone hybrid peptide, dual receptor-targeting human melanoma imaging
INTRODUCTION
Over the past decade, both radiolabeled alpha-melanocyte stimulating hormone (α-MSH) peptides and Arg-Gly-Asp (RGD) peptides have been reported for receptor-targeting melanoma imaging [1–16]. Specifically, the radiolabeled α-MSH peptides have been utilized to target melanocortin-1 (MC1) receptors [1–11], whereas the radiolabeled RGD peptides have been employed to target αvβ3 integrin receptors [12–16]. In consideration of the expressions of MC1 and αvβ3 integrin receptors on human melanoma cells, we developed a RGD-conjugated α-MSH hybrid peptide which could target both MC1 and αvβ3 integrin receptors for melanoma detection [17]. We demonstrated that the unique dual receptor-targeting 99mTc-RGD-Lys-(Arg11)CCMSH {cyclic(Arg-Gly-Asp-DTyr-Asp)-Lys-(99mTc-[Cys3,4,10, D-Phe7, Arg11]α-MSH3–13)} hybrid peptide displayed significantly higher tumor uptake than 99mTc-labeled α-MSH or RGD peptide targeting only the MC1 or αvβ3 integrin receptor [17]. Flank M21 human melanoma-xenografted tumors were clearly visualized by SPECT/CT using 99mTc-RGD-Lys-(Arg11)CCMSH as an imaging probe [17].
Despite the success of 99mTc-RGD-Lys-(Arg11)CCMSH for human melanoma imaging, 99mTc-RGD-Lys-(Arg11)CCMSH displayed extremely high non-specific renal uptake of 90.80 ± 19.35% ID/g at 4 h post-injection in M21 human melanoma-xenografted nude mice [17]. It is desirable to decrease the renal uptake to facilitate the potential therapeutic application when labeling the peptide with therapeutic 188Re/186Re (β-emitters). Interestingly, the co-injection of L-Lysine reduced the renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH by 52% at 2 h post-injection without affecting the tumor uptake [18], suggesting that the overall positive charge of 99mTc-RGD-Lys-(Arg11)CCMSH substantially contributed to its non-specific renal uptake. Thus, we hypothesized that the reduction of the overall positive charge of 99mTc-RGD-Lys-(Arg11)CCMSH would decrease the non-specific renal uptake. Clearly, three Arg residues and one Lys linker represent the positive charges of 99mTc-RGD-Lys-(Arg11)CCMSH. However, three Arg residues are critical to the MC1 or αvβ3 integrin receptor binding. Thus, we substituted the positively-charged Lys linker with neutral aminooctanoic acid (Aoc) and polyethylene glycol (PEG) linker to decrease the overall positive charge of the peptide to determine whether such change could substantially reduce the non-specific renal uptake.
We synthesized two new RGD-conjugated α-MSH hybrid peptides with the Aoc and PEG2 linkers. Specifically, the RGD motif {Arg-Gly-Asp-DTyr-Asp} was coupled to [Cys3,4,10, D-Phe7, Arg11]α-MSH3–13 peptide via the Aoc or PEG2 linker to generate RGD-Aoc-(Arg11)CCMSH and RGD-PEG-(Arg11)CCMSH. We determined their receptor binding affinities in M21 human melanoma cells. Then, we radiolabeled RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH with 99mTc and examine their tumor targeting and biodistribution properties in M21 human melanoma-xenografted nude mice.
EXPERIMENTAL PROCEDURES
Chemicals and reagents
Amino acid and resin were purchased from Advanced ChemTech (Louisville, KY, USA) and Novabiochem (San Diego, CA, USA). 99mTcO4− was purchased from Cardinal Health (Albuquerque, NM). 125I-Tyr2-(Nle4, dPhe7)-α-MSH [125I-(Tyr2)-NDP-MSH] and 125I-Echistatin were obtained from PerkinElmer (Shelton, CT, USA) for competitive receptor binding assay. All other chemicals used in this study were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and used without further purification. The M21 human melanoma cells were supplied by Dr. David A. Cheresh from the Department of Pathology, Moores University of California-San Diego Cancer Center.
Peptide Synthesis
RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH were synthesized using fluorenylmethyloxy carbonyl (Fmoc) chemistry, purified by reverse phase-high performance liquid chromatography (RP-HPLC) and characterized by liquid chromatography-mass spectrometry (LC-MS). Basically, 70 µmol of resin and 210 µmol of each Fmoc-protected amino acid were used for the synthesis. Briefly, the intermediate scaffolds of H2N-Arg(Pbf)-Ala-Asp(OtBu)-dTyr(tBu)-Asp(O-2-phenylisopropyl)-Aoc/PEG2-Cys(Trt)-Cys(Trt)-Glu(OtBu)-His(Trt)-dPhe-Arg(Pbf)-Trp(Boc)-Cys(Trt)-Arg(Pbf)-Pro-Val were synthesized on H2N-Sieber amide resin by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY). The protecting groups of 2-phenylisopropyl were removed and the peptides were cleaved from the resin treating with a mixture of 2.5% of trifluoroacetic acid (TFA) and 5% of triisopropylsilane. After the precipitation with ice-cold ether and characterization by LC-MS, the protected peptides were dissolved in H2O/CH3CN (50:50) and lyophilized to remove the reagents such as TFA and triisopropylsilane. The protected peptides were further cyclized by coupling the carboxylic group from the Asp with the alpha amino group from the Arg at the N-terminus. The cyclization reaction was achieved by overnight reaction in dimethylformamide (DMF) using benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium-hexafluorophosphate (PyBOP) as a coupling agent in the presence of N,N-diisopropylethylamine (DIEA). After characterization by LC-MS, the cyclized protected peptides were dissolved in H2O/CH3CN (50:50) and lyophilized to remove the reagents. The protecting groups were totally removed by treating with a mixture of trifluoroacetic acid (TFA), thioanisole, phenol, water, ethanedithiol and triisopropylsilane (87.5:2.5:2.5:2.5:2.5:2.5) for 2 h at room temperature (25 °C). The peptides were precipitated and washed with ice-cold ether for four times, purified by RP-HPLC and characterized by LC-MS.
In vitro competitive binding assay
The IC50 values of RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH peptides were determined in M21 human melanoma cells. The receptor binding assay was replicated in triplicate for each peptide. 125I-(Tyr2)-NDP-MSH and 125I-Echistatin were used as radioligands for MC1 and αvβ3 integrin receptors, respectively. For MC1 receptor binding assay, the M21 cells (1.5×105 cells/well, n=3) were incubated at 37 °C for 2 h with approximately 30,000 counts per minute (cpm) of 125I-(Tyr2)-NDP-MSH in the presence of 10−12 to 10−5 M of each peptide in 0.3 mL of binding medium {Modified Eagle’s medium with 25 mM N-(2-hydroxyethyl)-piperazine-N’-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline}. The binding medium was aspirated after the incubation. The cells were rinsed twice with 0.5 ml of ice-cold pH 7.4, 0.2% BSA/0.01 M phosphate buffered saline (PBS) and lysed in 0.5 mL of 1 N NaOH for 5 min. The cells were harvested and measured in a Wallac 1480 automated gamma counter (PerkinElmer, NJ). The IC50 values of the peptides for the MC1 receptor were calculated using the Prism software (GraphPad Software, La Jolla, CA, USA).
For αvβ3 integrin receptor binding assay, the M21 cells (1×105 cells/well, n=3) were seeded in Millipore 96-well filter multiscreen DV plates (0.65 µm pore size) and incubated at 25 °C for 2 h with approximately 30,000 counts per minute (cpm) of 125I-Echistatin in the presence of 10−11 to 10−4 M of each peptide in 0.2 mL of binding medium. After the incubation, the plates were filtered through a multiscreen vacuum manifold and rinsed twice with 0.5 ml of ice-cold pH 7.4, 0.2% BSA/0.01 M PBS. The hydrophilic polyvinylidenedifluoride filters were collected and counted in a Wallac 1480 automated gamma counter. The IC50 values of the peptides for the αvβ3 integrin receptor were calculated using the Prism software.
Peptide radiolabeling
RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH peptides were labeled with 99mTc via a direct reduction reaction using SnCl2. Briefly, 10 µL of 1 mg/mL SnCl2 in 0.1 M HCl, 40 µL of 0.5 M NH4OAc (pH 5.2), 100 µL of 0.2 M Na2tartate (pH 9.2), 100 µL of fresh 99mTcO4− solution (37–74 MBq), and 10 µL of 1 mg/mL of each peptide in aqueous solution were added into a reaction vial and incubated at 25 °C for 20 min to form 99mTc-labeled peptide. Each 99mTc-peptide was purified to a single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytic column (Deerfield, IL) using a 20-min gradient of 16–26% acetonitrile in 20 mM HCl aqueous solution at a flow rate of 1 mL/min. Each purified peptide was purged with N2 gas for 20 min to remove the acetonitrile. The pH of final peptide solution was adjusted to 7.4 with 0.1 N NaOH and sterile normal saline for animal studies.
Biodistribution studies
All the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The biodistribution of 99mTc-RGD-Aoc-(Arg11)CCMSH was determined in M21 melanoma-xenografted nude mice (Harlan, Indianapolis, IN). Each nude mouse was subcutaneously inoculated with 5×106 M21 human melanoma cells on the right flank. The weight of tumors reached approximately 0.1–0.2 g 21 days post cell inoculation. Each melanoma-xenografted nude mouse was injected with 0.037 MBq of 99mTc-RGD-Aoc-(Arg11)CCMSH via the tail vein. Groups of 5 mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. The receptor specificity of the tumor uptake was determined by co-injecting 99mTc-RGD-Aoc-(Arg11)CCMSH with unlabeled RGD-Aoc-(Arg11)CCMSH. Each melanoma-xenografted nude mouse was injected with 0.037 MBq of 99mTc-RGD-Aoc-(Arg11)CCMSH with 6.1 nmol of unlabeled RGD-Aoc-(Arg11)CCMSH via the tail vein. A group of 5 mice were sacrificed at 2 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight. The biodistribution of 99mTc-RGD-PEG2-(Arg11)CCMSH was only determined in M21 melanoma-xenografted nude mice at 2 h post-injection for comparison.
Imaging human melanoma with 99mTc-RGD-Aoc-(Arg11)CCMSH
We determined the melanoma imaging property of 99mTc-RGD-Aoc-(Arg11)CCMSH. Approximately 9.2 MBq of 99mTc-RGD-Aoc-(Arg11)CCMSH was injected into a M21 human melanoma-xenografted nude mouse via the tail vein. The mouse was euthanized for small animal SPECT/CT (Nano-SPECT/CT®, Bioscan, Washington DC) imaging at 2 h post-injection. The 9-min CT imaging was immediately followed by the SPECT imaging of whole-body. The SPECT scans of 24 projections were acquired. Reconstructed data from SPECT and CT were visualized and co-registered using InVivoScope (Bioscan, Washington DC).
Statistical methods
Statistical analysis was performed using the Student’s t-test for unpaired data to determine the significant differences between the groups in the biodistribution and peptide blocking studies described above. Differences at the 95% confidence level (p<0.05) were considered significant.
RESULTS
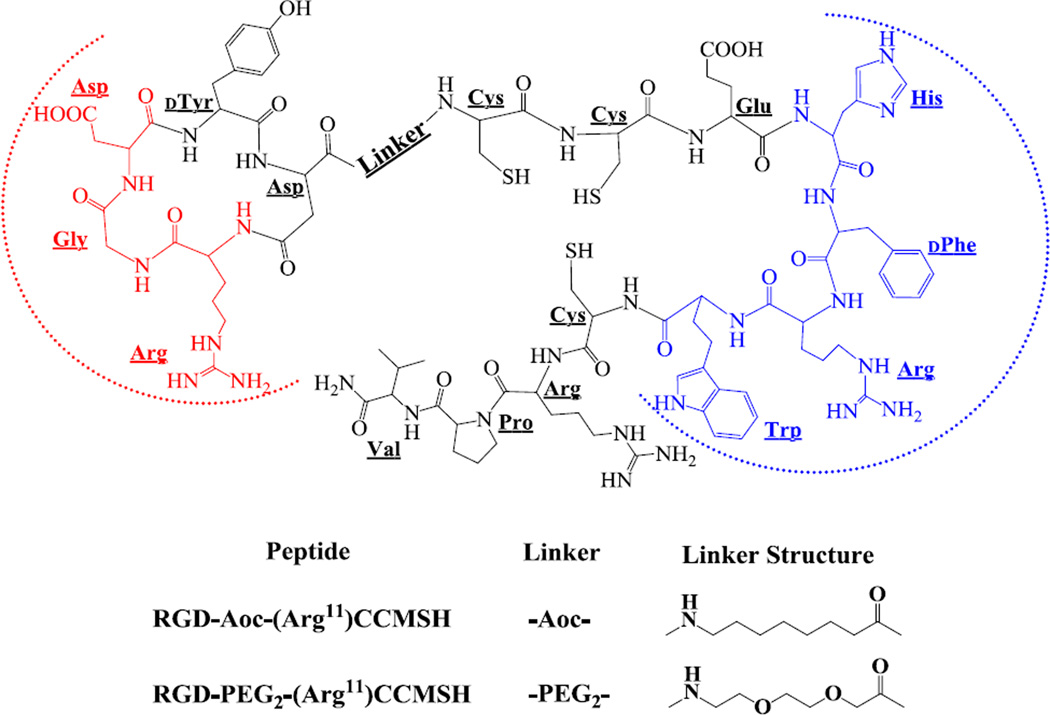
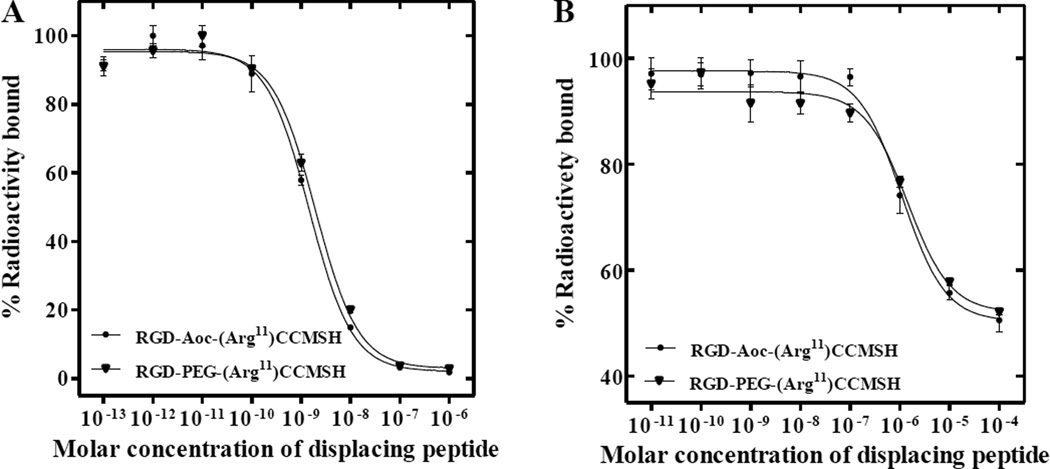
The schematic structures of RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH are shown in Figure 1. RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH were synthesized and purified by RP-HPLC. The overall synthetic yields were approximately 28–30% for RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH. Both peptides displayed greater than 95% purity after HPLC purification. The identities of RGD-Aoc-(Arg11)CCMSH and RGDPEG2-(Arg11)CCMSH were confirmed by mass spectrometry. The found molecular weights matched the calculated molecular weights. The competitive binding curves of the peptides for MC1 and αvβ3 integrin receptors are presented in Figure 2. The IC50 values of RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH were 1.5 ± 0.2 and 1.9 ± 0.2 nM for the MC1 receptor. The IC50 values of RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH were 1125 ± 26 and 1479 ± 74 nM for the αvβ3 integrin receptor. RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH were easily labeled with 99mTc with greater than 95% radiolabeling yields. 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH showed greater than 98% radiochemical purities after the HPLC purification. 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH were completely separated from their excess non-labeled peptides by RP-HPLC. The specific activities of 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH were 1.9259 × 1013 and 1.9259 × 1013 MBq/mol, respectively. The retention times of 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH were 20.5 and 14.1 min, respectively.
Figure 1.
Schematic structures of RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH hybrid peptides. The binding sequences for MC1 and αvβ3 integrin receptors were highlighted with blue and red dashed half circles, respectively.
Figure 2.
The MC1 (A) and αvβ3 integrin (B) receptor competitive binding curves of RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH hybrid peptides in M21 human melanoma cells. The IC50 values of RGD-Aoc-(Arg11)CCMSH and RGD-PEG2-(Arg11)CCMSH were 1.5 ± 0.2, 1.9 ± 0.2 nM for the MC1 receptor and 1125 ± 26, 1479 ± 74 nM for αvβ3 integrin receptor, respectively.
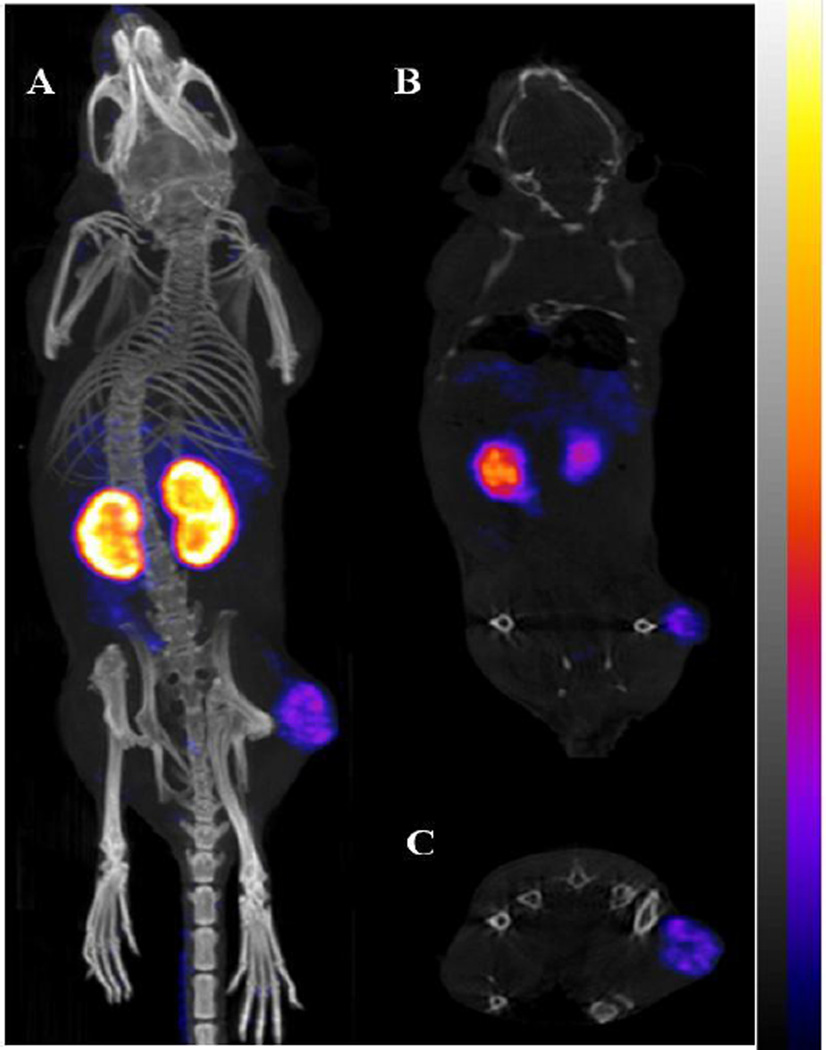
RGD-Aoc-(Arg11)CCMSH displayed stronger receptor binding affinity than RGD-PEG2-(Arg11)CCMSH in M21 cells. Thus, the melanoma targeting and biodistribution properties of 99mTc-RGD-Aoc-(Arg11)CCMSH were determined in M21 melanoma-xenografted nude mice at 0.5, 2, 4 and 24 h post-injection. The melanoma targeting property of 99mTc-RGD-PEG2-(Arg11)CCMSH was determined in M21 melanoma-xenografted nude mice at 2 h post-injection only for comparison. The biodistribution results are shown in Table 1. 99mTc-RGD-Aoc-(Arg11)CCMSH exhibited rapid tumor uptake in M21 melanoma-xenografted nude mice. The tumor uptake was 2.42 ± 0.23% ID/g at 0.5 h post-injection. There were 2.35 ± 0.12 and 1.56 ± 0.26% ID/g of the 99mTc-RGD-Aoc-(Arg11)CCMSH activity remaining in the tumors 2 and 4 h post-injection. At 24 h post-injection, the tumor uptake was 0.64 ± 0.31% ID/g. Co-injection of unlabeled RGD-Aoc-(Arg11)CCMSH blocked the tumor uptake by 50% (p<0.05), demonstrating that the tumor uptake was receptor-mediated. Whole-body clearance of 99mTc-RGD-Aoc-(Arg11)CCMSH was rapid, with approximately 60% of the injected radioactivity cleared through the urinary system by 2 h post-injection (Table 1). The accumulation of 99mTc-RGD-Aoc-(Arg11)CCMSH in normal organs was lower than 5% ID/g except for the kidneys after 2 h post-injection. The renal uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH reached its highest value of 37.05 ± 1.76% ID/g at 4 h post-injection and decreased to 14.32 ± 2.37% ID/g at 24 h post-injection. Co-injection of unlabeled RGD-Aoc-(Arg11)CCMSH did not affect the renal uptake, indicating that the renal uptake was non-specific. 99mTc-RGD-PEG2-(Arg11)CCMSH exhibited significantly (p<0.05) lower tumor uptake than that of 99mTc-RGD-Aoc-(Arg11)CCMSH. However, the renal uptake was similar between 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH at 2 h post-injection. The melanoma imaging property of 99mTc-RGD-Aoc-(Arg11)CCMSH was examined in M21 human melanoma-xenografted nude mice (Figure 3). Flank M21 human melanoma tumors were visualized clearly by 99mTc-RGD-Aoc-(Arg11)CCMSH at 2 h post-injection.
Table 1.
Biodistribution of 99mTc-RGD-Aoc-(Arg11)CCMSH (A) and 99mTc-RGD-PEG2-(Arg11)CCMSH (B) in M21 human melanoma-xenografted nude mice. The data was presented as percent injected dose/gram or as percent injected dose (mean ± SD, n = 5)
| A | B | |||||
|---|---|---|---|---|---|---|
| Tissue | 0.5 h | 2 h | 2 h blockade | 4 h | 24 h | 2 h |
| Percent injected dose/gram (%ID/g) | ||||||
| Tumor | 2.42 ± 0.23 | 2.35 ± 0.12 | 1.17 ± 0.15b | 1.56 ± 0.26 | 0.64 ± 0.31 | 1.71 ± 0.25c |
| Brain | 0.24 ± 0.04 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.07 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Blood | 5.01 ± 0.47 | 1.90 ± 0.21 | 1.39 ± 0.28 | 1.54 ± 0.14 | 0.17 ± 0.03 | 1.52 ± 0.01 |
| Heart | 2.87 ± 0.17 | 1.28 ± 0.14 | 0.99 ± 0.13 | 1.04 ± 0.25 | 0.42 ± 0.12 | 0.20 ± 0.01 |
| Lung | 7.61 ± 1.05 | 3.85 ± 0.41 | 3.62 ± 0.49 | 2.73 ± 0.33 | 1.80 ± 0.10 | 0.79 ± 0.53 |
| Liver | 7.05 ± 0.91 | 4.69 ± 0.36 | 4.34 ± 0.61 | 4.82 ± 0.81 | 2.27 ± 0.61 | 1.12 ± 0.05 |
| Skin | 5.08 ± 0.46 | 2.20 ± 0.14 | 2.16 ± 0.31 | 1.90 ± 0.24 | 1.13 ± 0.28 | 1.16 ± 0.38 |
| Spleen | 2.83 ± 0.47 | 1.65 ± 0.25 | 1.25 ± 0.51 | 1.54 ± 0.23 | 1.37 ± 0.30 | 0.44± 0.27 |
| Stomach | 4.49 ± 0.38 | 4.98 ± 0.45 | 3.16 ± 0.16 | 4.78 ± 0.50 | 5.10 ± 0.87 | 0.66 ± 0.19 |
| Kidneys | 25.85 ± 9.14 | 27.93 ± 3.98 | 25.56 ± 3.30 | 37.05 ± 1.76 | 14.32 ± 2.37 | 22.01 ± 9.89 |
| Muscle | 1.01 ± 0.32 | 0.31 ± 0.13 | 0.32 ± 0.18 | 0.25 ± 0.02 | 0.14 ± 0.04 | 0.14 ± 0.16 |
| Pancreas | 0.90 ± 0.39 | 0.53 ± 0.13 | 0.28 ± 0.25 | 0.41 ± 0.14 | 0.21 ± 0.11 | 0.09 ± 0.04 |
| Bone | 1.55 ± 0.14 | 0.84 ± 0.16 | 0.66 ± 0.23 | 0.60 ± 0.10 | 0.30 ± 0.08 | 0.27 ± 0.09 |
| Percent injected dose (%ID) | ||||||
| Intestines | 4.22 ± 0.51 | 4.44 ± 0.55 | 3.49 ± 0.39 | 5.67 ± 0.16 | 4.68 ± 0.41 | 1.66 ± 0.37 |
| Bladder | 41.20 ± 8.69 | 59.87 ± 4.41 | 64.58 ± 5.43 | 63.71 ± 3.84 | 80.55 ± 6.26 | 81.36 ± 1.72 |
| Uptake ratio of tumor/normal tissue | ||||||
| Tumor/Blood | 0.48 ± 0.08 | 1.24 ± 0.09 | 0.84 ± 0.12 | 1.01 ± 0.18 | 3.76 ± 1.24 | 1.13 ± 0.08 |
| Tumor/Kidneys | 0.09 ± 0.04 | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.08 ± 0.01 |
| Tumor/Lung | 0.32 ± 0.07 | 0.62 ± 0.07 | 0.32 ± 0.08 | 0.57 ± 0.09 | 0.36 ± 0.17 | 2.16 ± 1.43 |
| Tumor/Liver | 0.34 ± 0.05 | 0.50 ± 0.03 | 0.27 ± 0.04 | 0.32 ± 0.07 | 0.28 ± 0.17 | 1.53 ± 0.14 |
| Tumor/Muscle | 2.40 ± 0.91 | 7.58 ± 3.79 | 3.70 ± 1.31 | 6.12 ± 0.70 | 4.75 ± 2.20 | 12.21 ± 7.85 |
p<0.05, significance comparisons on tumor and renal uptake between 99mTc-RGD-Aoc-(Arg11)CCMSH (A) with/without peptide blockade at 2 h post-injection.
p<0.05, significance comparisons on tumor and renal uptake between 99mTc-RGD-Aoc-(Arg11)CCMSH (A) and 99mTc-RGD-PEG2-(Arg11)CCMSH (B) at 2 h post-injection.
Figure 3.
Representative three-dimensional (A), coronal (B) and transversal (C) SPECT/CT images of M21 human melanoma xenografts at 2 h post-injection of 9.2 MBq of 99mTc-RGD-Aoc-(Arg11)CCMSH.
DISCUSSION
We are interested in developing novel hybrid peptides to target both MC1 and αvβ3 integrin receptors for melanoma imaging because both receptors are over-expressed on melanoma cells. For instance, the receptor densities of MC1 and αvβ3 integrin receptors were 1,281 and 96,555 receptors/cell on M21 human melanoma cells [17]. In the mean time, several potential advantages are associated with dual receptor-targeting hybrid peptide as compared to single receptor-targeting peptide. Firstly, the overall receptor density increases for the dual receptor-targeting hybrid peptide because it can target both MC1 and αvβ3 integrin receptors. Thus, targeting dual receptors will capture a larger audience of MC1 and αvβ3 integrin receptor-expressing melanoma lesions. Secondly, a potential synergistic effect between the MC1 and αvβ3 integrin receptors could facilitate the binding of the hybrid peptides to receptors by elevating the regional peptide concentration in the proximity of receptors. For instance, the hybrid peptide bound to either MC1 or αvβ3 integrin receptor would be in the close proximity of other available receptors. When the hybrid peptide dissociates from the current receptor, it has more opportunities to bind to other receptors in the close proximity. In our previous report [17], 99mTc-RGD-Lys-(Arg11)CCMSH showed higher M21 melanoma uptake than 99mTc-RAD-Lys-(Arg11)CCMSH or 99mTc-RGD-Lys-(Arg11)CCMSHscramble. The melanoma uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was 2.5 and 2.2 times the tumor uptake of 99mTc-RAD-Lys-(Arg11)CCMSH and 99mTc-RGD-Lys-(Arg11)CCMSHscramble, respectively.
One potential application of the dual receptor-targeting hybrid peptide is to label it with therapeutic radionuclides such as 188Re or 186Re. Both 99mTc and 188Re/186Re share similar coordination chemistry. It is worthwhile to note that the (Arg11)CCMSH motif not only serves as a MC1 receptor binding moiety, but also serves as a site-specific chelating system for direct labeling of 99mTc/188Re/186Re. Specifically, three -SH groups from Cys3, Cys4 and Cys10 and one -NH from Cys4 yield a NS3 chelating system for 99mTc/188Re/186Re. In other words, the radiolabeling and peptide cyclization can be accomplished simultaneously during the radiolabeling process. Meanwhile, the conjugation of Tc and Re metals to the (Arg11)CCMSH motif didn't affect the MC1 receptor binding. Both linear (Arg11)CCMSH and cyclized Re-(Arg11)CCMSH peptides exhibited similar MC1 receptor binding affinities (1.7 vs. 1.9 nM, respectively) [22].
Despite the success of 99mTc-RGD-Lys-(Arg11)CCMSH for human melanoma imaging, 99mTc-RGD-Lys-(Arg11)CCMSH displayed extremely high renal uptake (90.80 ± 19.35% ID/g at 4 h post-injection) in M21 human melanoma-xenografted nude mice [17]. Thus, it is necessary to reduce the non-specific renal uptake to facilitate the potential therapeutic application. In this study, we substituted the Lys linker with Aoc or PEG2 linker to examine whether the linker change could substantially reduce the renal uptake. The substitution of the Lys linker with Aoc or PEG2 linker retained low nanomolar MC1 receptor binding affinity (1.5–1.9 nM), but decreased the αvβ3 integrin receptor binding affinity by 2- to 3-fold. It was likely that the longer hydrocarbon chains from Aoc and PEG2 linker somehow affected the αvβ3 integrin receptor binding. Despite that the length of Aoc linker is as same as the length of PEG2 linker, RGD-Aoc-(Arg11)CCMSH displayed slightly stronger binding affinities to both MC1 and αvβ3 integrin receptor than RGD-PEG2-(Arg11)CCMSH. Thus, we fully determined the melanoma targeting and imaging properties of 99mTc-RGD-Aoc-(Arg11)CCMSH while we only examined the biodistribution property of 99mTc-RGD-PEG2-(Arg11)CCMSH at 2 h post-injection for comparison.
The biodistribution results of 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH in M21 human melanoma xenografts supported our hypothesis. The substitution of the Lys linker with the Aoc and PEG2 linker substantially decreased the renal uptake (Figure 4). The kidney uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH was 31%, 42%, 41% and 51% of the kidney uptake of 99mTc-RGD-Lys-(Arg11)CCMSH at 0.5, 2, 4 and 24 h post-injection respectively. Meanwhile, the tumor uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH was comparable as the tumor uptake of 99mTc-RGD-Lys-(Arg11)CCMSH at 0.5 and 2 h post-injection. It is worthwhile to note that the co-injection of 6.1 nmol (~13 µg) of peptide blockade decreased the tumor uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH by 50%. Generally, approximately 200–300 µg of RGD peptide are used for tumor blocking studies. Thus, it was likely that greater than 6.1 nmol (~13 µg) of peptide blockade would be needed to further block the tumor uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH. Although 99mTc-RGD-PEG2-(Arg11)CCMSH exhibited similar renal uptake as 99mTc-RGD-Aoc-(Arg11)CCMSH, the tumor uptake of 99mTc-RGD-PEG2-(Arg11)CCMSH was significantly (p<0.05) lower than that of 99mTc-RGD-Aoc-(Arg11)CCMSH at 2 h post-injection. As shown in Figure 3, the M21 human melanoma lesions could be clearly visualized by SPECT/CT using 99mTc-RGD-Aoc-(Arg11)CCMSH as an imaging probe.
Figure 4.

The melanoma and renal uptake of 99mTc-RGD-Linker-(Arg11)CCMSH peptides at 2 h post-injection in M21 human melanoma-xenografted nude mice. Data of 99mTc-RGD-Lys-(Arg11)CCMSH are cited for comparison [17].
The receptor binding affinities need to be similar for the dual receptor-targeting peptide to have maximal effect. Despite the success of 99mTc-RGD-Aoc-(Arg11)CCMSH in terms of decreased renal uptake, it is worthwhile to note that the binding affinity of RGD-Aoc-(Arg11)CCMSH for αvβ3 integrin receptor was in low micromolar range. Although the αvβ3 integrin receptor density is 96,555 receptors/cell on M21 human melanoma cells [17], such receptor density might not be sufficient to overcome the weak αvβ3 integrin receptor binding. Thus, it is necessary to improve the receptor binding affinity of the hybrid peptide for αvβ3 integrin receptor to enhance the benefit of dual receptor-targeting peptide. One potential way would be the optimization of the linker length between the RGD and (Arg11)CCMSH moieties. The impact of linker length on receptor binding affinity was reported for bombesin peptides [19–21]. For instance, the DOTA-conjugated bombesin peptides with 5-carbon to 8-carbon hydrocarbon linkers exhibited 0.6–1.7 nM receptor binding affinities, whereas the DOTA-conjugated bombesin peptides with either shorter or longer hydrocarbon linkers displayed much weaker receptor binding by 100-fold. It is worthwhile to note that the substitution of the Lys linker with Aoc linker decreased the αvβ3 integrin receptor binding by 2.8-fold in this report. Obviously, the hydrocarbon chain of Aoc is longer than that of Lys. The impact on the αvβ3 integrin receptor binding indicated that the longer hydrocarbon chain of Aoc somehow influenced the αvβ3 integrin receptor binding. One possible way to restore and improve the αvβ3 integrin receptor binding would be replacing the Aoc linker with Gly so that the linker length would be as same as Lys. It would be interesting to examine this hypothesis in the future.
CONCLUSIONS
The substitution of Lys linker with Aoc and PEG2 linker significantly reduced the renal uptake of 99mTc-RGD-Aoc-(Arg11)CCMSH and 99mTc-RGD-PEG2-(Arg11)CCMSH by 58% and 63% at 2 h post-injection. 99mTc-RGD-Aoc-(Arg11)CCMSH displayed higher tumor uptake than 99mTc-RGD-PEG2-(Arg11)CCMSH in M21 human melanoma xenografted nude mice. The M21 human melanoma lesions could be clearly visualized by SPECT/CT using 99mTc-RGD-Aoc-(Arg11)CCMSH as an imaging probe. The favorable effect of Aoc and PEG2 linker in reducing the renal uptake provided a new insight into the design of novel dual receptor-targeting radiolabeled peptides.
ACKNOWLEDGMENTS
We appreciate Dr. Fabio Gallazzi for his technical assistance. This work was supported by the NIH grant NM-INBRE P20RR016480/P20GM103451. The SPECT/CT image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Giblin MF, Wang N, Hoffman TJ, Jurisson SS, Quinn TP. Design and characterization of alpha-melanotropin peptide analogs cyclized through rhenium and technetium metal coordination. Proc Natl Acad Sci USA. 1998;95:12814–12818. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–1706. [PubMed] [Google Scholar]
- 3.Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled alpha-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- 4.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, et al. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- 5.Miao Y, Figueroa SD, Fisher DR, Moore HA, Testa RF, Hoffman TJ, et al. 203Pb-labeled alpha-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J Nucl Med. 2008;49:823–829. doi: 10.2967/jnumed.107.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao Y, Gallazzi F, Guo H, Quinn TP. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjugate Chem. 2008;19:539–547. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo H, Shenoy N, Gershman BM, Yang J, Sklar LA, Miao Y. Metastatic melanoma imaging with an 111In-labeled lactam bridge-cyclized alpha-melanocyte-stimulating hormone peptide. Nucl Med Biol. 2009;36:267–276. doi: 10.1016/j.nucmedbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for MicroPET imaging of melanocortin 1 receptor expression. Bioconjugate Chem. 2007;18:765–772. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo H, Yang J, Gallazzi F, Miao Y. Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide resulting in enhanced melanoma uptake. J Nucl Med. 2010;51:418–426. doi: 10.2967/jnumed.109.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo H, Yang J, Gallazzi F, Miao Y. Effects of the amino acid linkers on melanoma-targeting and pharmacokinetic properties of Indium-111-labeled lactam bridge-cyclized α-MSH peptides. J Nucl Med. 2011;52:608–616. doi: 10.2967/jnumed.110.086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo H, Miao Y. Cu-64-labeled lactam bridge-cyclized α-MSH peptides for PET imaging of melanoma. Mol Pharm. 2012;9:2322–2330. doi: 10.1021/mp300246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 13.Cheresh DA, Harper JR. Arg-Gly-Asp recognition by a cell adhesion receptor requires its 130-kDa alpha subunit. J Biol Chem. 1987;262:1434–1437. [PubMed] [Google Scholar]
- 14.Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, et al. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 15.Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, et al. Radiolabeled alpha(v)beta(3) integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med. 1999;40:1061–1071. [PubMed] [Google Scholar]
- 16.Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, et al. Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med. 2004;45:892–902. [PubMed] [Google Scholar]
- 17.Yang J, Guo H, Miao Y. Technetium-99m-labeled Arg-Gly-Asp-conjugated alpha-melanocyte stimulating hormone hybrid peptides for human melanoma imaging. Nucl Med Biol. 2010;37:873–883. doi: 10.1016/j.nucmedbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Guo H, Padilla RS, Berwick M, Miao Y. Replacement of the Lys linker with an Arg linker resulting in improved melanoma uptake and reduced renal uptake of Tc-99m-labeled Arg-Gly-Asp-conjugated alpha-melanocyte stimulating hormone hybrid peptide. Bioorg Med Chem. 2010;18:6695–6700. doi: 10.1016/j.bmc.2010.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes DL, Owen NK, et al. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J Nucl Med. 2003;44:823–831. [PubMed] [Google Scholar]
- 20.Smith CJ, Gali H, Sieckman GL, Higginbotham C, Volkert WA, Hoffman TJ. Radiochemical investigations of 99mTc-N3S-X-BBN[7–14]NH2: an in vitro/in vivo structure-activity relationship study where X=0-, 3-, 5-, 8- and 11-carbon tethering moieties. Bioconjug Chem. 2003;14:93–102. doi: 10.1021/bc020034r. [DOI] [PubMed] [Google Scholar]
- 21.Parry JJ, Kelly TS, Andrews R, Rogers BE. In vitro and in vivo evaluation of 64Cu-labeled DOTA-Linker-Bombesin(7–14) analogues containing different amino acid linker moieties. Bioconjug Chem. 2007;18:1110–1117. doi: 10.1021/bc0603788. [DOI] [PubMed] [Google Scholar]
- 22.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. In vivo evaluation of 188Re labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int J Cancer. 2002;101:480–487. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]