Abstract
Accumulation of bile acids is a major mediator of cholestatic liver injury. Recent studies indicate bile acid composition between humans and rodents is dramatically different, as humans have a higher percent of glycine conjugated bile acids and increased chenodeoxycholate content, which increases the hydrophobicity index of bile acids. This increase may lead to direct toxicity that kills hepatocytes, and promotes inflammation. To address this issue, this study assessed how pathophysiological concentrations of bile acids measured in cholestatic patients affected primary human hepatocytes. Individual bile acid levels were determined in serum and bile by UPLC/QTOFMS in patients with extrahepatic cholestasis with, or without, concurrent increases in serum transaminases. Bile acid levels increased in serum of patients with liver injury, while biliary levels decreased, implicating infarction of the biliary tracts. To assess bile acid-induced toxicity in man, primary human hepatocytes were treated with relevant concentrations, derived from patient data, of the model bile acid glycochenodeoxycholic acid (GCDC). Treatment with GCDC resulted in necrosis with no increase in apoptotic parameters. This was recapitulated by treatment with biliary bile acid concentrations, but not serum concentrations. Marked elevations in serum full-length cytokeratin-18, high mobility group box1 protein (HMGB1), and acetylated HMGB1 confirmed inflammatory necrosis in injured patients; only modest elevations in caspase-cleaved cytokeratin-18 were observed. These data suggest human hepatocytes are more resistant to human-relevant bile acids than rodent hepatocytes, and die through necrosis when exposed to bile acids. These mechanisms of cholestasis in humans are fundamentally different to mechanisms observed in rodent models.
Keywords: bile acids, obstructive cholestasis, primary human hepatocytes, biomarkers, inflammation, HMGB1, apoptosis
INTRODUCTION
Bile acids (BAs) are the primary constituent of bile and are known to be cytotoxic to hepatocytes (Malhi et al., 2010). The predominant hypothesis for the development of cholestatic liver injury is that BAs accumulate in hepatocytes, and subsequently in serum, during cholestasis, which exposes hepatocytes to potentially cytotoxic levels of BAs (Spivey et al., 1993; Jaeschke et al., 2002; Giucciardi et al., 2013). This paradigm is supported by well-characterized mechanisms of apoptosis in rat hepatocytes (Malhi et al., 2010; Guicciardi et al., 2013) and human hepatoma lines that have been transfected with the sodium taurocholate cotransporting polypeptide (NTCP) to facilitate BA uptake (Faubion et al., 1999; Rust et al., 2009). A majority of these studies use glycochenodeoxycholic acid (GCDC) as a model hydrophobic bile acid to induce apoptosis, as it is the major BA present in serum of cholestatic patients (Spivey et al., 1993; Trottier et al., 2012). In contrast, studies in the bile duct ligation (BDL) model of cholestasis suggest BA concentrations in mice may not be directly toxic (Zhang et al., 2012). Instead, BAs act as pro-inflammatory signals, which trigger CXC chemokine formation in hepatocytes (Allen et al., 2011). These chemokines together with osteopontin derived from biliary epithelial cells recruit neutrophils to areas of biliary leakage in the liver during obstructive cholestasis (Allen et al., 2011; Yang et al., 2014b). In support of this hypothesis, mice deficient in either intercellular adhesion molecule-1 (ICAM-1) or CD18 are highly protected against both BDL-induced neutrophil recruitment and injury, implicating neutrophils as a major contributor to the pathogenesis (Gujral et al., 2003a; 2004a). Thus, BDL-induced liver injury in mice is caused by a neutrophilic inflammatory response (Woolbright and Jaeschke, 2012); BAs support this mechanism by generating chemotactic mediators in hepatocytes (Copple et al., 2010). However, the relevance of this injury mechanism, identified in the mouse BDL model, for the human pathophysiology of obstructive cholestasis remains unclear.
While there have been a substantial number of studies done in rodent models and transfected hepatoma lines, few studies have been performed to assess how BAs affect primary human hepatocytes. Early studies confirmed BA toxicity in human hepatocytes using GCDC, and protection by ursodeoxycholic acid (UDCA) (Galle et al., 1990). Of note, the concentrations required for even low levels of cell death were substantially higher than what is typically used in rat hepatocyte models (Galle et al., 1990). However, many of the mechanisms of BA toxicity established in rat hepatocytes have yet to be investigated in primary human hepatocytes. In particular, it remains unclear how human hepatocytes respond to pathophysiological concentrations of relevant BAs measured in human patients. Therefore, the objective of the current study was to determine BA composition and concentrations in serum and bile of patients with extrahepatic cholestasis and then expose primary human hepatocytes to these BAs. We hypothesized human hepatocytes would be more resistant to bile acid-induced apoptosis than rodent hepatocytes. Furthermore, our goal was to assess if pathophysiologically relevant concentrations of human BAs measured during obstructive cholestasis could cause direct cytotoxicity, or induce pro-inflammatory mediator formation in primary human hepatocytes.
MATERIALS AND METHODS
Criteria for Cholestatic Patients
Patients admitted to the University of Kansas Hospital were enrolled in an institutional review board (IRB) approved protocol. Inclusion criteria included subjects undergoing planned endoscopic retrograde cholangiopancreatography (ERCP) for medical diagnosis and potential treatment of cholestasis (Supplementary Table 1). Uninjured patients were defined as patients with ALT<50U/L and ALP<110U/L. Injured patients were defined as patients with ALT≥50U/L and ALP≥110U/L and defined cholestasis as evidenced by ERCP. Blood and bile samples were collected during the ERCP procedure. Bile dilution was held to an absolute minimum in this study. Injection of contrast dyes and other agents occurred after the initial suction of bile for collection. We estimate overall dilution would be no greater than 10% in any single sample. This study adhered to the Helsinki Declaration and all studies were done under informed consent.
Isolation and Culture of Human Hepatocytes
Primary human hepatocytes were freshly isolated from liver resections by the Biospecimen Core in the Department of Pharmacology, Toxicology and Therapeutics at the University of Kansas Medical Center. All human tissues were obtained with informed consent from each patient, according to ethical and institutional guidelines. The study was approved by the Institutional Review Board at the University of Kansas Medical Center. Cells were isolated using a multi-step collagenase procedure as described in detail (Xie et al., 2014). Media consisted of Williams’ Medium E (Life Technologies, Grand Island, NY) supplemented with L-glutamine (2 mM) (Life Technologies), HEPES (10 mM), insulin (10−7M), dexamethasone (10−7 M), penicillin (100 U/ml), streptomycin (100 μg/ml) and amphotericin B (0.25 μg/ml). Media was not supplemented with fetal bovine serum as initial experiments did not show an effect on cell death if serum was present or not (data not shown). After an initial 3 h attachment period, cultures were washed with phosphate-buffered saline (PBS) and then culture medium, vehicle or media containing the indicated concentration of the bile acid or inhibitor were added along with the appropriate vehicle.
Isolation and Culture of Rat Hepatocytes
Sprague-Dawley rats (200-230 g body weight) were acquired from Jackson Laboratories (Bar Harbor, ME). A three-step collagenase perfusion method was used to isolate hepatocytes. After the induction of anesthesia, the peritoneal cavity was opened, and a 20G catheter was inserted into the inferior vena cava. The liver was perfused in situ via the inferior vena cava after cutting the portal vein for 10 min with calcium and magnesium free HBSS containing 0.1 mM EGTA followed by a washout step using Calcium and Magnesium free HBSS without EGTA. The final perfusion step consisted of Eagle’s Minimum Essential Medium containing 25 mM HEPES buffer and 0.025 mg/ml of Liberase TM (Roche, Basel, Switzerland) and continued until the liver showed signs of digestion. The remaining portion was cut into smaller pieces with scissors to release remaining cells. The cell suspension was sequentially filtered through nylon gauze and collected in 50 ml conical tubes. The cells were centrifuged for 5 min at 50 × g and 4°C and then resuspended in fresh cold Dulbecco’s Minimum Essential Medium with 25 mM HEPES. This was repeated 3 times in order to isolate the hepatocyte fraction. Hepatocyte viability was assessed using a hemocytometer and the trypan blue exclusion assay. After an initial 3-h attachment period, cultures were washed with PBS and then culture medium (controls) or media containing the indicated concentrations of bile acids were added. Inhibition studies using the pancaspase inhibitor z-VAD-fmk (10 μM) (Enzo Life Sciences, Ann Arbor, MI) were carried out by pretreating for one hour with the indicated concentration of inhibitor and then adding the indicated treatment.
Murine Studies
C57BL/6J mice (20-25 g bodyweight) were purchased from Jackson Laboratories (Bar Harbor, ME). All animals received humane care according to the criteria outlined in Guide for the Care and Use of Laboratory Animals. All experimental protocols were approved by the Animal Use Committees of the University of Kansas Medical Center. Bile duct ligation (BDL) was performed as described in detail (Woolbright et al., 2013). In addition, some mice were also treated with galactosamine and endotoxin for 6 h as described previously (Jaeschke et al., 1998).
Bile Acid Measurements
Bile acid measurements were performed as previously described in detail (Woolbright et al., 2014a). In brief, bile samples were first diluted 1:50 in water, whereas serum samples were used as is. The samples were prepared by mixing 20 ul of serum or bile with 80 μL of methanol and the resulting mixtures were centrifuged at 14,000 × g for 10 minutes to remove protein. The supernatants were injected to UPLC-QTOFMS for analysis.
Chromatographic separation of bile acids was achieved using a 100 mm × 2.1 mm (Acquity 1.7 μm) UPLC BEH C-18 column (Waters, Milford, MA). TOFMS was calibrated with sodium formate and monitored by the intermittent injection of lock mass leucine encephalin in real time. TOFMS was operated in a negative mode with electrospray ionization. The concentration of bile acids was calculated based on corresponding standard curves of six different concentrations ranging from 100 ng/mL to 25 μg/mL.
Western Blotting
Liver sections were homogenized in a CHAPS based buffer, and then centrifuged at 14,000 rpm to remove cellular debris. The BCA assay (Pierce Scientific, Rockford, IL) was used to quantify protein levels. Equal quantities of protein were loaded into an Invitrogen Mini Blot electrophoresis system and transferred onto PVDF paper. The blot was probed using a caspase-3 antibody (Cell Signaling, Danvers, MA) and then visualized using a goat anti-rabbit HRP conjugated antibody (Santa Cruz Biotechnology Santa Cruz, CA).
Cell Death Analysis
Cell death was assessed by lactate dehydrogenase (LDH) release, as described previously in detail (Bajt et al., 2004). Caspase activity was based on z-VAD-fmk inhibitable Ac-DEVD-AMC fluorescence measured as described previously using a SpectraMax fluorescence plate reader (Jaeschke et al., 1998).
Propidium Iodide Staining
Cells were seeded on 60 mm plastic dishes and treated with GCDC for the indicated time. Propidium iodide and Hoechst dye were added for the final five minutes of the treatment, before washing the cells and adding fresh PBS. The live cells were imaged on a Zeiss Axiovert inverted fluorescence microscope through a Texas Red filter to assess PI uptake, or a DAPI filter to visualize Hoechst staining. All fluorescence images were taken at the same exposure and later superimposed on phase contrast images or Hoechst images of the same fields using Image J software.
Real Time PCR Analysis
Gene expression measurements were performed by real-time PCR (RT-PCR) analysis as described (Gujral et al., 2004b). The relative differences in expression between groups were expressed using cycle time (Ct) values generated by the ABI 7600 instrument (Applied Biosystems). Genes were normalized to GAPDH and then expressed as fold difference using the delta-delta CT formula.
Bile Acid Uptake
BA uptake was measured as previously described (Rippin et al., 2001). In brief, hepatocytes were seeded to confluence in 12 well plates and allowed to adhere for 3 h. Uptake buffers either with or without sodium were used as in Jigorel et al. (2006) with 1 μM radioactive taurocholate (Perkin Elmer, Boston, MA). Cells were lysed using 1% Triton-X 100 and counted on a Micro Beta TriLux ß counter (Perkin Elmer, Waltham, MA). Values were normalized to protein levels using the BCA assay.
Measurements of serum biomarkers
Hypo- and hyperacetylated high mobility group box 1 (HMGB1) protein and full length cytokeratin-18 (M65) and the caspase-cleaved form (M30) were measured as previously described in detail (Antoine et al., 2012, 2013).
Statistics
Statistical analysis was performed with Sigmaplot 8.0 (Systat Software, Inc., Chicago, IL). Data were assessed using one way ANOVA followed by Student-Newman-Keul’s post-hoc test for comparisons between means or Dunnett’s post-hoc test for comparisons to a control. For data not normally distributed, we used the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. For direct comparisons, Student’s t-test was used for normal data and the Mann-Whitney Rank Sum Test was used for non-normal data. p < 0.05 was considered significant. All results were expressed as mean ± SE.
RESULTS
Bile acid levels in patients with obstructive cholestasis
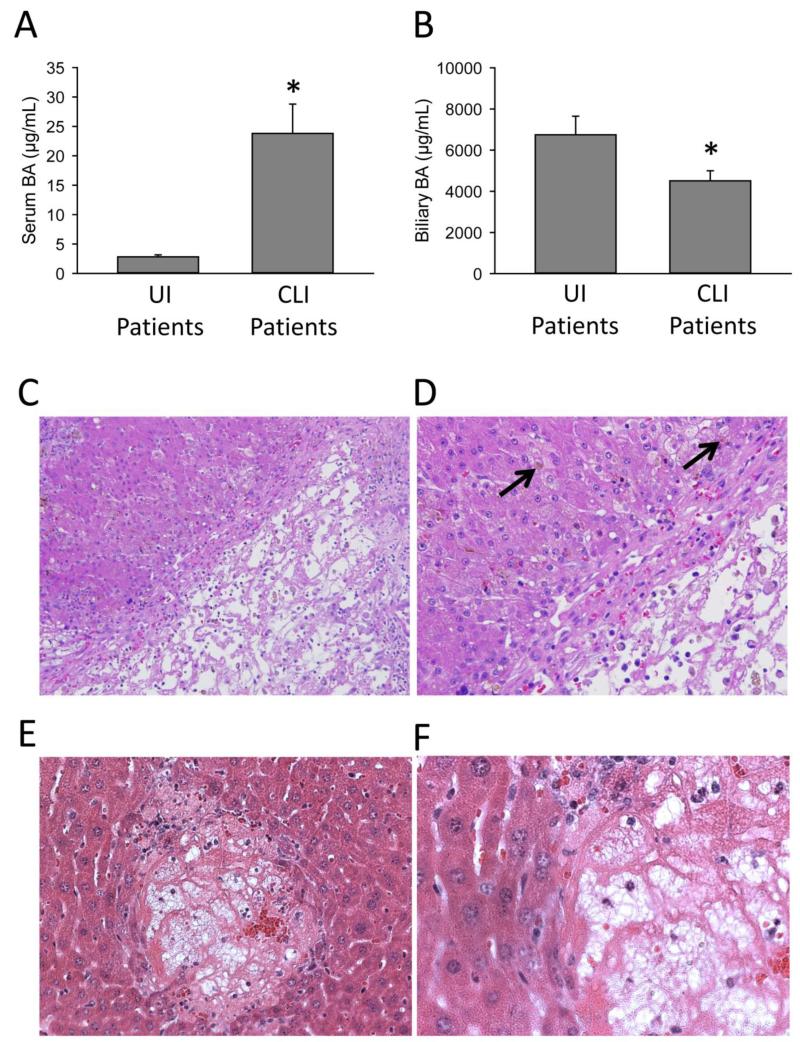
In order to define BA levels in cholestatic patients, BA concentrations were measured in serum and bile of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP). The ERCP procedure was performed for confirmation of presumed extrahepatic cholestasis. Patients were divided into 2 groups, those with cholestatic liver injury (cholestatic liver injury patients or CLI patients) and those without concurrent injury (uninjured patients or UI patients) as defined by clinically elevated ALT (ALT >50 U/L) and ALP (ALP >110 U/L) values (Table 1). Etiologies for patients are listed in Supplementary Table 1. Serum and biliary BA values were measured in both patient groups (Table 2). Glycine and taurine conjugated primary bile acids were most prevalent in serum, and increased to the highest values in serum during cholestasis. Of note, mean GCDC in serum increased to 22 μM (Table 2). A large majority of BAs in both serum and bile were conjugated in both cholestatic and non-cholestatic patients, with only a very small percentage being unconjugated. While total serum BA values increased dramatically in CLI patients as expected (Figure 1A), biliary levels of total BAs decreased significantly in CLI patients (Figure 1B). This was largely mediated by a significant decrease in glycodeoxycholic acid (GDCA) and a trend towards a decrease in glycocholic acid (GCA) and GCDC (Table 2). Histological analysis of livers with significant extrahepatic cholestasis revealed bile infarcts similar to those seen in mice after bile duct ligation, with frank necrosis and significant inflammation (Figure 1C). Extra-biliary bile pools (Figure 1D, arrow), were readily apparent in the hepatic parenchyma around areas of necrosis, suggesting obstructive cholestasis and leakage of bile. Areas surrounding bile displayed characteristic feathery degeneration and flocculation of cytoplasm in both mice and humans, consistent with hepatocellular necrosis. The infarcts seen in human livers were highly similar to infarcts observed after bile duct ligation in mice (Figure 1E,F), which are also characterized by necrosis and inflammation (Gujral et al., 2004b; Fickert et al., 2005; Nalapareddy et al., 2009).
Table 1. Clinical Patient Data.
Patient values for bile acid analysis. Patients with presumed cholestatic liver injury were recruited to the study, whereupon bile was acquired during routine ERCP, and serum acquired before intervention. Clinical chemistry and patient history were obtained by hospital staff. Patients were split into uninjured (UI) and cholestatic liver injury (CLI) groups based on levels of ALT and ALP. Patients with ALT >50 and ALP >110 were placed in the CLI group, and patients with ALT <50 and ALP <110 were placed in the UI group.
| UI Patients | CLI Patients | |
|---|---|---|
| N | 10 | 17 |
| Mean Age | 56 ± 3 | 55 ± 2 |
| % Female | 45 | 55 |
| ALT (U/L) | 18 ± 2 | 188 ± 36* |
| ALP (U/L) | 65 ± 8 | 506 ± 101* |
| Bilirubin (mg/dL) | 0.8 ± 0.1 | 5.6 ± 1.8* |
P<0.05 (versus UI group).
Table 2. Bile and Serum Bile Acid Values in Patients.
Bile acid levels were measured in serum and bile of patients with and without cholestatic liver injury via UPLC/QTOFMS (as described in methods). Patient groups were defined by elevations in ALT and ALP.
| Bile | Serum | |||
|---|---|---|---|---|
| Bile Acid | UI Patient (μM) | CLI Patient (μM) | UI Patient (μM) | CLI Patient (μM) |
| LCA | <10 | <10 | <0.1 | <0.1 |
| UDCA | 67 ± 20 | 71 ± 20 | <0.1 | <0.1 |
| CDCA | <10 | <10 | 0.9 ± 0.3 | 0.4 ± 0.1 |
| DCA | 116 ± 100 | 25 ± 16 | 1.0 ± 0.4 | 0.1 ± 0.1*# |
| CA | <10 | <10 | <0.1 | <0.1 |
| TCA | 2,403 ± 223 | 2,460 ± 295 | 0.4 ± 0.1 | 11.7 ± 4.2* |
| GCDC | 4,325 ± 661 | 2,756 ± 339 | 2.8 ± 0.4 | 22.1 ± 5.3* |
| TCDC | 1,067 ± 217 | 1,058 ± 191 | 0.4 ± 0.1 | 6.8 ± 1.6* |
| GCA | 3,030 ± 600 | 1,530 ± 269 | 0.4 ± 0.1 | 7.6 ± 1.9* |
| GDCA | 3,643 ± 742 | 1,152 ± 355* | 0.8 ± 0.3 | 3.8 ± 1.3* |
| TDCA | 712 ± 202 | 607 ± 320 | 0.3 ± 0.1 | 1.2 ± 0.3* |
P<0.05 (versus UI patients).
some samples not detectable within standard curve.
LCA, lithocholic acid; UDCA, ursodeoxycholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; CA, cholic acid; TCA, taurocholic acid; GCDC, glycochenodeoxycholic acid; TCDC, taurochenodeoxycholic acid; GCA, glycocholic acid; GDCA, glycodeoxycholic acid; TDCA, taurodeoxycholic acid.
Figure 1.
Infarction of the biliary tracts mediates the initial injury in cholestatic human patients. Serum (A) and bile (B) samples obtained from patients thought to have biliary obstruction during routine ERCP. Total and individual bile acid levels were measured in these samples via UPLC/MS. Patient data are presented in Table 1. H&E staining was performed in liver sections from human patients with confirmed extrahepatic cholestasis (C,D) and mice 72 h after bile duct ligation (E,F). Frank necrosis is seen in the image (C) in an area of biliary infarction. Bile (marked by black arrow) leaks into adjacent hepatic parenchyma (D). Bile infarcts in mice subjected to 72 h bile duct ligation show similar histological features (E,F).
GCDC-induced toxicity in primary human hepatocytes
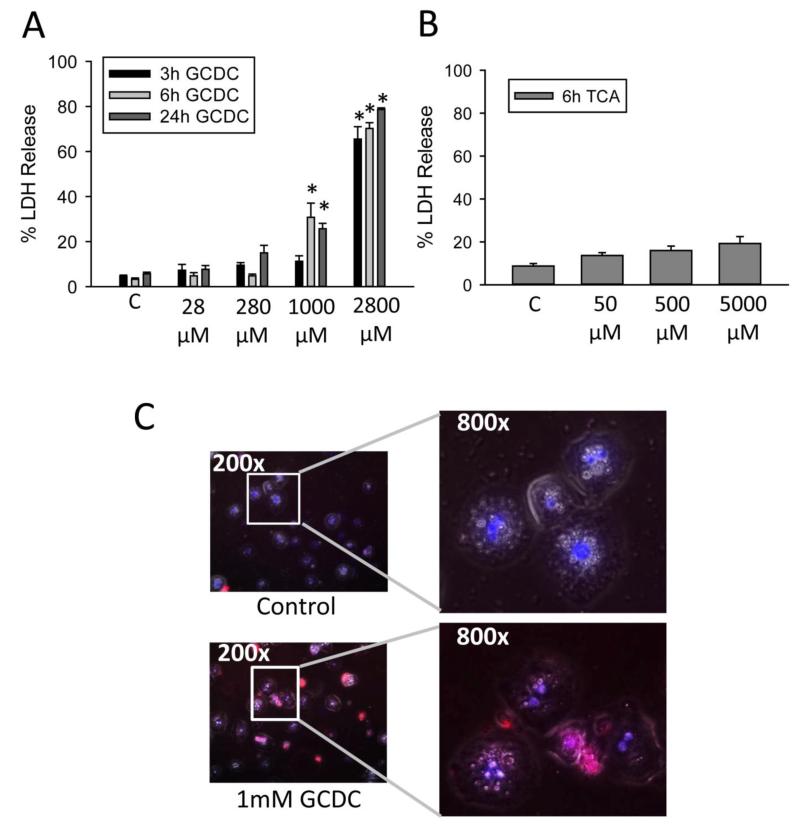
To assess the direct toxicity of BAs, freshly isolated primary human hepatocytes were first exposed to various concentrations of the hydrophobic BA GCDC. Values used were based on maximum concentrations of these bile acid species in the serum of cholestatic patients as measured by us (Table 2) and others (Trottier et al., 2011, 2012). Values of GCDC consistent with high serum concentrations (>20 μM) produced no toxicity after 3, 6 or 24 h of exposure (Figure 2A). Concentrations of 1 mM GCDC and above resulted in significantly increased LDH release at both 6 and 24 h (Figure 2A). While the mM concentrations far exceed serum levels, they are consistent with values seen in bile of cholestatic patients (Table 2; Dilger et al., 2012). In contrast, values of taurocholic acid (TCA) up to 5 mM showed limited toxicity after 6 h of treatment (Figure 2B). To confirm the toxicity, hepatocytes were incubated with 1 mM GCDC for 6 h and then exposed briefly to propidium iodide and Hoechst 33342 dyes. Uptake of propidium iodide was readily apparent in GCDC-treated hepatocytes but not the controls (Figure 2C) or TCA-treated hepatocytes (data not shown). These data confirm acute toxicity after exposure to GCDC in vitro in human hepatocytes.
Figure 2.
Bile acid exposure results in cell death in human hepatocytes. LDH was measured 3, 6 or 24 h after exposure to GCDC (A) or TCA (B). N=4 batches of isolated cells; *P< 0.05 (compared to controls). Propidium iodide and Hoechst stain were applied to hepatocytes 6 h after treatment with 1 mM GCDC (C). Images were overlaid using Image J software.
Primary human hepatocytes were also exposed to increasing concentrations of TCA to see if this would induce pro-inflammatory genes, as BA-induced inflammatory gene induction has been proposed as a mechanism for inflammation in the BDL mouse model (Allen et al., 2011; Zhang et al., 2012; Woolbright and Jaeschke, 2012). However, human hepatocytes exposed to TCA showed little increase in pro-inflammatory genes, especially when compared to values seen in murine hepatocytes (Table 3). A small, but significant, increase was seen in IL-8 expression after TCA treatment. While this study does not preclude other potential inflammatory mediators being upregulated by BAs, this facet of the murine response to BAs in vitro is minimally recapitulated in human hepatocytes.
Table 3. Gene Expression Changes after TCA Treatment.
Human or mouse hepatocytes were treated with the indicated concentration of taurocholic acid (TCA) and then RT-PCR was used to assess gene changes. No changes were found at 50 or 500 μM TCA treatment in human hepatocytes (data not shown). N=3.
|
Murine
Hepatocytes | ||
| Control | 5000μM TCA | |
| mKC | 1.0 ± 0.1 | 25.1 ± 4.6* |
| MIP-2 | 1.0 ± 0.1 | 780.0 ± 122.0* |
| VCAM-1 | 1.0 ± 0.2 | 3.9 ± 0.2* |
| ICAM-1 | 1.0 ± 0.1 | 7.4 ± 0.3* |
| TNF-α | 1.0 ± 0.2 | 2.2 ± 0.4* |
|
Human
Hepatocytes | ||
| Control | 5000μM TCA | |
|
CXCL-1
(mKC) |
1.0 ± 0.4 | 1.3 ± 0.4 |
|
CXCL-2
(MIP-2) |
1.0 ± 0.4 | 0.8 ± 0.1 |
| ICAM-1 | 1.0 ± 0.1 | 1.5 ± 0.4 |
| IL-8 | 1.0 ± 0.2 | 2.4 ± 0.4* |
P<0.05 (versus control).
mKC, mouse keratinocyte derived factor; MIP-2, macrophage inflammatory protein 2; VCAM-1, vascular cellular adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; TNF-α, tumor necrosis factor α; CXCL, CXC chemokine motif ligand; IL-8, interleukin-8.
Bile acid uptake in human hepatocytes
BA uptake has previously been suggested to be a critical aspect of toxicity in mouse models of cholestatic liver injury (Gartung et al., 1996; 1997). Uptake primarily occurs through the sodium taurocholate cotransporting polypeptide (NTCP) but can also be mediated by organic anion transporting polypeptides (OATPs) (Kullak-Ublich and Meier, 2000). BA uptake was measured in human hepatocytes (Supplementary Figure 1A) or rat hepatocytes (Supplementary Figure 1B) via uptake of radio-labelled taurocholate using a sodium- or choline chloride-containing medium, which measure either sodium-dependent (NTCP-mediated) or sodium-independent (OATP-mediated) uptake, respectively. Sodium dependent uptake was predominant in line with previous reports (Szabo et al., 2013) in both cell types. However, human hepatocytes retained their uptake capacity 24 h after the initial plating, suggesting these cells may be usable for longer term experiments in the future. This is pertinent, as rat hepatocytes are known to decrease expression of transporters rapidly when in culture, and lose their native responses to bile acid exposure (Liang et al., 1996).
Mode of cell death in GCDC-treated human hepatocytes
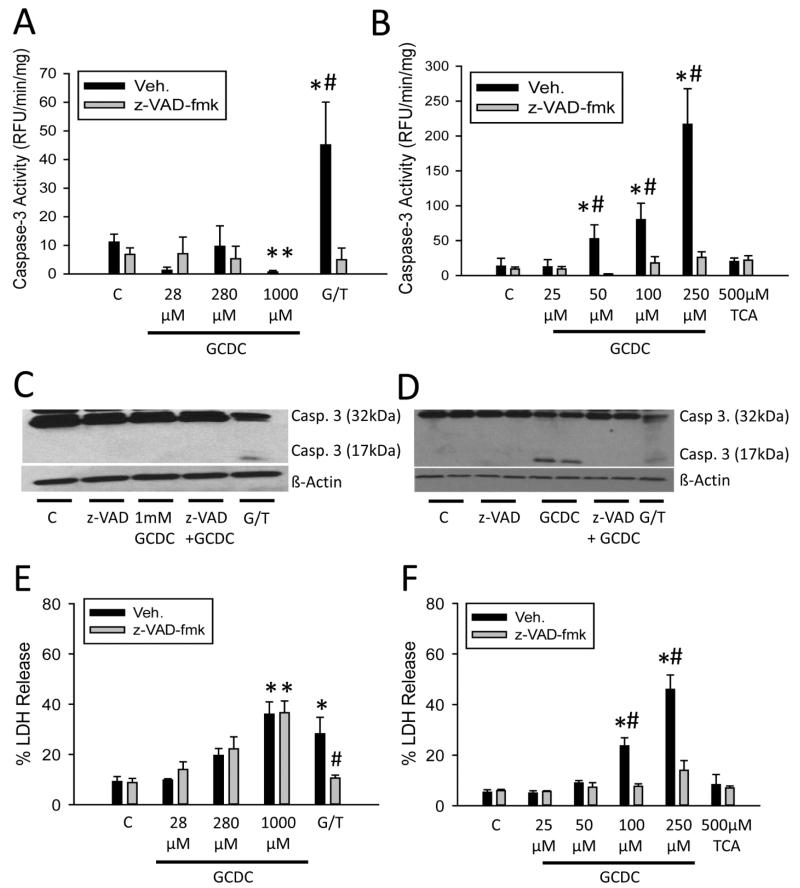
GCDC has been shown to cause apoptosis in hepatoma lines transfected with a functional NTCP and in isolated rat hepatocytes (Rust et al., 2009; Malhi et al., 2010). To assess potential apoptotic cell death after exposure to GCDC in human cells, caspase activity was measured in untreated or z-VAD-fmk-treated primary human hepatocytes exposed to increasing concentrations of GCDC. While there was baseline caspase-3 activity in control primary human hepatocytes, there was no increase in caspase activity after GCDC treatment (Figure 3A). These data were confirmed by Western Blot, as there was no evidence of caspase-3 processing in GCDC-treated hepatocytes (Figure 3C). As a positive control, human hepatocytes were also treated with 5 mM galactosamine and 250 ng/mL recombinant human TNF-α for 12 hours. Gal/TNF treatment caused extensive caspase-3 activation and caspase-3 processing, which was prevented by the pan-caspase inhibitor z-VAD-fmk (Figure 3A,C). These data suggested that GCDC-induced cell death in human hepatocytes is not caused by apoptosis. Rat hepatocytes generally die by apoptosis after GCDC exposure (Malhi et al., 2010). In order to confirm these data, rat hepatocytes were also treated with increasing concentrations of GCDC (Figure 3B). Dramatically increased caspase-3 activity was found starting with a 50 μM concentration, consistent with numerous previous reports. These data were confirmed by Western blotting showing caspase-3 processing and appearance of the active caspase-3 fragments after GCDC in rat hepatocytes (Figure 3D). No increase in caspase activity or caspase processing was seen when rat hepatocytes were treated with TCA, suggesting this effect was specific to the more toxic, hydrophobic bile acids such as GCDC (Figure 3B). Of note, onset of toxicity (LDH release) occurred at concentrations 10-20 fold lower in rat hepatocytes than in human hepatocytes, indicating that human hepatocytes are generally more resistant to BA-induced cell death (Figure 3E,F). To assess whether or not inhibition of caspases could prevent cell death, LDH release was measured in control or z-VAD-fmk-treated human hepatocytes. The pancaspase inhibitor had no effect on cell death in human hepatocytes at any GCDC dose tested but effectively reduced Gal/TNF-induced apoptosis (Figure 3E). However, pretreatment with z-VAD-fmk effectively protected against GCDC-induced apoptosis in rat hepatocytes (Figure 3F). These data indicate that in contrast to rat hepatocyte models, BA-induced cytotoxicity occurs primarily through necrosis in human hepatocytes in vitro.
Figure 3.
Bile acid exposure results in hepatic necrosis in vitro. Caspase-3 activity was measured after exposure to the indicated concentration of GCDC or TCA for 6 h or after 12 h galactosamine/TNF treatment (G/T) in human (A) and rat (B) hepatocytes pretreated with vehicle (DMSO) or z-VAD-fmk. Caspase-3 activation was assessed via western blot in human (C) or rat (D) hepatocytes after GCDC treatment. Galactosamine/TNF-treated human hepatocytes were used as controls. LDH release was measured in untreated cells or after pretreatment with 10μM z-VAD-fmk in human (E) or rat hepatocytes (F). N=5 batches of isolated cells for human hepatocytes, n=3 for rat hepatocytes; *P<0.05 (versus control). #P<0.05 (versus vehicle).
Hepatocyte necrosis in response to biliary concentrations of bile acids
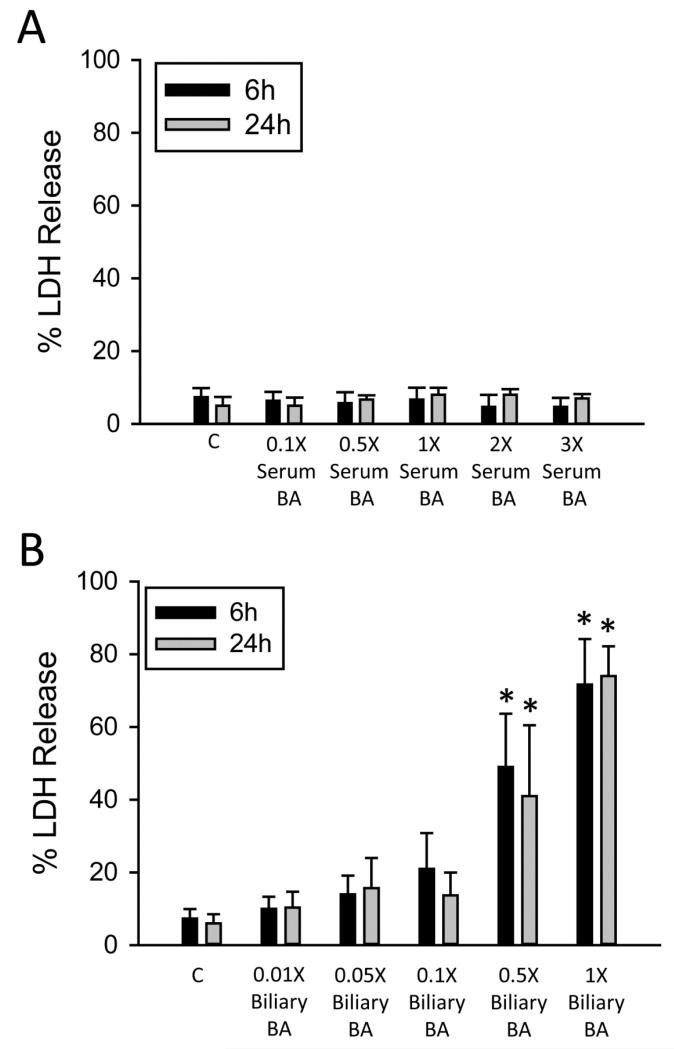
To test the hypothesis that biliary values of BAs were required for cell death, primary human hepatocytes were exposed to a reconstituted BA milieu composed of specific concentrations of BA measured in serum or bile of injured patients in this study. This was achieved by generating a single solution of commercially available BAs containing each individual BA at the measured concentration in Table 2, for both serum values and biliary values, and exposing primary human hepatocytes to these solutions at different concentrations. The 1× concentration corresponds to the values listed in Table 2 for bile and serum respectively, and derivations of this concentration are fold dilutions of the total mixture. Exposure to the serum BA milieu resulted in no increases in LDH release at 6 or 24 hours (Figure 4A), even at exposures of up to three times the total concentration of serum bile acids (3× or three times the values of each individual measured bile acid reconstituted together into a single treatment). Exposure to the biliary BA milieu (Figure 4B) resulted in significant toxicity at a 0.5× dose (0.5× the value of each individual bile acid reconstituted together into a single treatment). Exposure to the complete biliary BA milieu resulted in near total LDH release. Direct exposure to total human bile confirmed this toxicity (data not shown). These data indicate serum BAs are not the likely cause of BA toxicity during obstructive cholestatic liver injury in patients, and that biliary leakage likely accounts for much of the toxic accumulation of BA.
Figure 4.
Biliary concentrations of bile acids and not serum concentrations cause toxicity in human hepatocytes. LDH release was measured in human hepatocytes after exposure to a complete serum BA milieu or a derivation of this concentration (A) or a complete biliary bile acid milieu or a derivation of this concentration for 6 or 24 h (B). N=3 batches of isolated cells;. *P<0.05 (versus control).
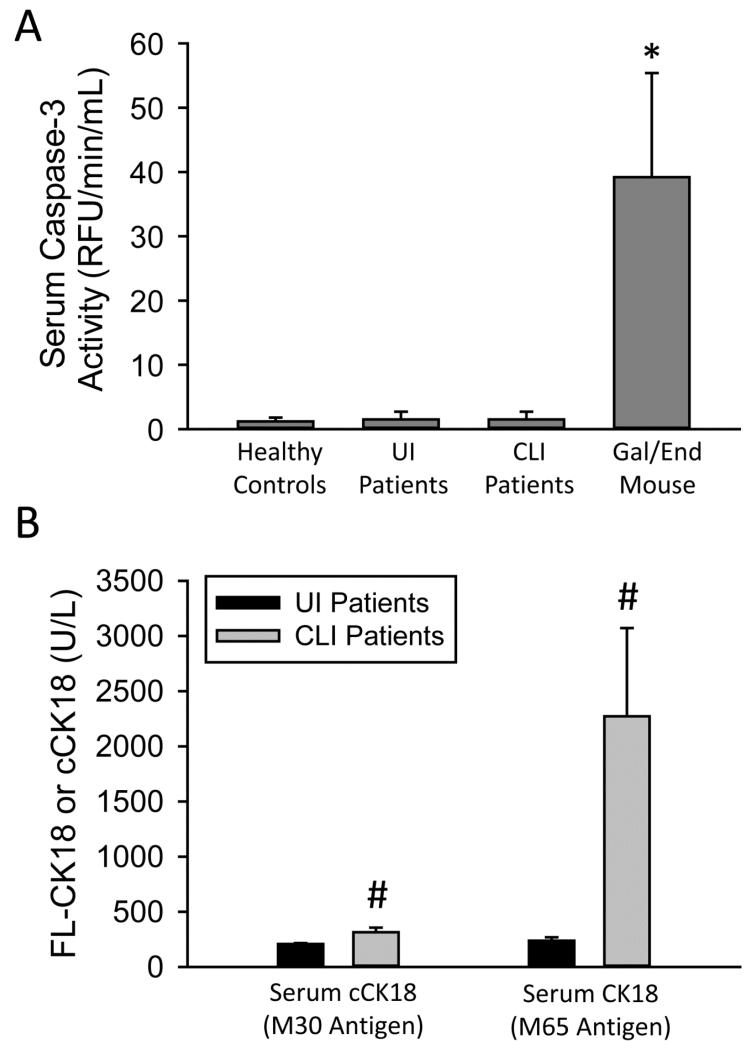
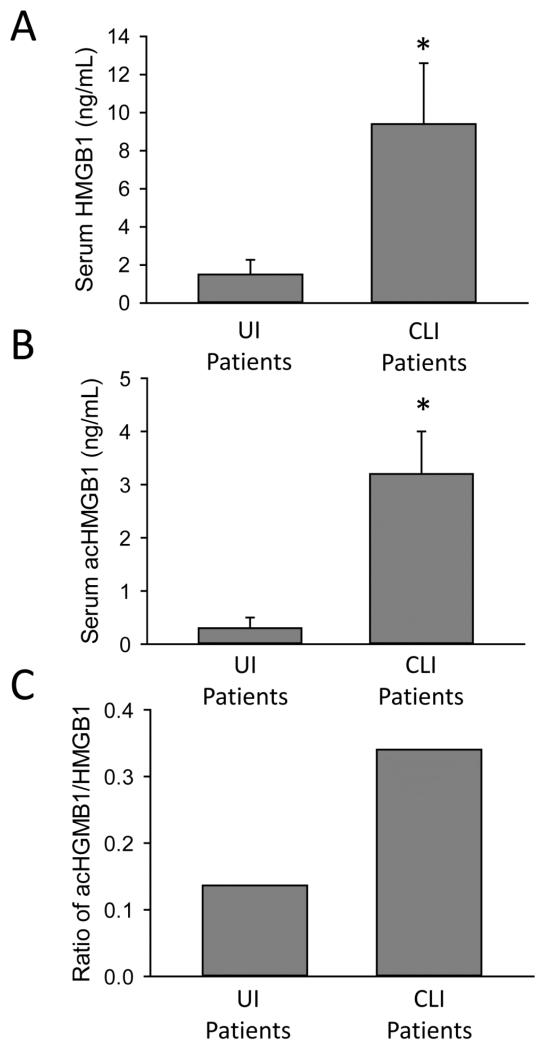
While evidence in this study strongly favors BA-induced necrosis, a previous investigation reported a significant increase of caspase-cleaved cytokeratin-18 in patients with severe intrahepatic cholestasis (Yagmur et al., 2007). To assess potential apoptosis in vivo in our study, we first measured caspase-3 activities in serum of patients with obstructive cholestasis (Figure 5A). There was no significant increase in caspase-3 activity in the injured patient group as compared to control patients or uninjured patients (Figure 5A). In contrast, in an established experimental model of apoptosis, the murine galactosamine/endotoxin model, extensive caspase-3 activities were measured in serum (Figure 5A). Full-length cytokeratin-18 and the caspase-cleaved form are biomarkers for necrosis and apoptosis, respectively (Antoine et al., 2012, 2013). While there was a more than 10-fold increase of full-length cytokeratin-18 measured in serum of cholestatic patients with liver injury, the caspase-cleaved form increased only 1.5 fold (Figure 5B). The caspase-cleaved cytokeratin-18 fragment represented approximately 13% of total circulating cytokeratin-18, indicating predominant necrosis during the injury process. To confirm this conclusion, hypoacetylated high mobility group box-1 (HMGB1), a protein that is passively released during necrotic cell death (Evankovich et al., 2010), was measured in serum of these patients (Antoine et al., 2009; 2012). HMGB1 levels were elevated in the obstructive cholestasis group as compared to the uninjured group confirming necrotic injury (Figure 6A). Additionally, elevated levels of the acetylated form of HMGB1 (acHMGB1) were also found in the cholestatic patient group (Figure 6B). These patients also had a higher mean ratio of acHGMB1 to HMGB1 levels in serum (Figure 6C). The acetylated form is actively secreted by immune cells such as macrophages (Bonaldi et al., 2003). While BAs had only a modest effect on induction of CXC chemokines or adhesion molecules in human hepatocytes (Table 3), passive and active release of HMGB1 during cholestatic necrosis may trigger a sterile innate immune response, which correlates with the obvious inflammatory infiltrate seen in the tissue section (Figure 1C,D). Together, these data indicate that bile acids cause direct necrosis in human hepatocytes, and likely induce a sterile inflammatory response via the release of damage-associated molecular patterns, e.g. HMGB1.
Figure 5.
Necrosis predominates during cholestatic liver injury in man. Serum caspase-3 activities were measured in human patients and in galactosamine/endotoxin-treated mice (A). Full length cytokeratin 18 (FL-CK18) (M65 antigen values) and caspase-cleaved (cCK18) (M30 antigen values) were measured in serum of human patients (B). Patient data are presented in Table 1. *P<0.05 (versus control). #P<0.05 (versus UI patients).
Figure 6.
Elevated serum HMGB1 levels in injured patients during cholestatic liver injury in man. HMGB1 (A) and acHMGB1 levels (B) were measured in serum of human patients. The ratio of acHMGB1 to HMGB1 was also calculated (C). Patient data are presented in Table 1. *P<0.05 (versus UI group).
DISCUSSION
The objective of this investigation was to assess bile acid toxicity and mechanisms of liver injury during obstructive cholestasis in humans. Our data indicate that cholestatic liver injury in humans is caused at least in part by direct cytotoxicity of glycine-conjugated bile acids leaking out of the biliary system back into the parenchyma triggering necrotic cell death. This results in release of DAMPs such as HMGB1 that might initiate a sterile inflammatory response.
Bile acid levels in humans during cholestatic liver injury
In order to assess if direct BA toxicity can play a role in cholestatic liver injury in humans, it was necessary to establish BA levels in serum and bile. Hepatocytes are constantly exposed to BAs in blood and a dramatic increase in these concentrations could trigger cytotoxicity. This is especially relevant because BAs are effectively taken up into hepatocytes and, if the biliary export is impaired, may partially accumulate intracellularly. On the other hand, leakage of bile back into the parenchyma, as has been shown in experimental models of obstructive cholestasis (Fickert et al., 2002), may expose hepatocytes locally to biliary concentrations of these BAs.
The measurement of BA levels in human serum and bile confirmed the known prevalence of glycine-conjugated over taurine-conjugated BAs (Trottier et al., 2011, 2012). Cholestatic liver injury substantially enhanced serum levels of the BAs to μM concentrations but did not change the overall composition. In contrast, the biliary levels of BAs significantly declined in cholestatic patients but still remained in the mM range. Thus, the overall concentrations of various BAs hepatocytes could be exposed to during obstructive cholestasis range from 1 μM to over 20 μM in serum, or up to 1-5 mM in bile. This applies to the conjugated bile acids such as GCDC, TCDC, GCA, TCA, GDCA and TDCA, which are the BAs that make up about 95% of the total bile acid pool in human serum or bile during cholestasis. Thus, any direct cytotoxicity by any of these BAs, or the combination of them, has to occur within these concentration ranges.
Bile acid cytotoxicity in humans during cholestatic liver injury
When isolated primary human hepatocytes were exposed to GCDC, one of the major bile acids in humans, a clear dose-dependent cytotoxicity was observed. The remarkable finding was that no cell death was detected within 24 h when serum levels of GCDC found in cholestatic patients were used. It should be noted that free bile acid concentrations in serum may actually be lower than what is measured here, as albumin present in serum can bind bile acids to various degrees (Roda et al., 1982), further emphasizing the idea that serum bile acid concentrations in vivo are unlikely to cause direct toxicity. In contrast, there was extensive cytotoxicity at ≥ 1,000 μM GCDC, which represent biliary levels. This was confirmed when a mixture of BAs found in bile or serum (Figure 4) was applied instead of a single BA. Together, these findings suggest that even the highest serum levels of human BAs measured in patients with obstructive cholestasis are unable to kill primary human hepatocytes. Instead, biliary levels of BAs are required to directly cause cell death.
Obstructive cholestasis in rodents causes focal necrosis (bile infarcts) with no zonal preference. This indicates that this injury cannot be caused by systemic BA exposure but had to be caused by a more localized insult. After bile duct ligation, it has been shown that bile leaks back into the parenchyma (Fickert et al., 2002) and attracts neutrophils, which are responsible for the focal necrosis (Gujral et al., 2003a). When we evaluated human liver biopsies of cholestatic patients, areas of focal necrosis were also evident (Figure 1). This suggests that liver injury after obstructive cholestasis in humans may be a similar local event as in rodents. However, in contrast to rodent bile, human bile can be directly cytotoxic to human hepatocytes. The lower BA levels in bile of cholestatic patients support the hypothesis of biliary leakage.
Mode of cell death during obstructive cholestasis
One of the prevailing hypotheses of cholestatic liver injury is that 50 μM GCDC, a BA elevated in human serum during cholestasis, causes apoptotic cell death in rat hepatocytes (reviewed in Malhi et al., 2010). On the other hand, the prevalent bile acids in mice, i.e. TCA and unconjugated and taurine-conjugated muricholic acid, are not cytotoxic to mouse hepatocytes up to mM concentration (Allen et al., 2011; Zhang et al., 2012; Woolbright et al., 2014b.). In contrast, our data indicate that only biliary concentrations of GCDC and other cytotoxic BAs cause necrotic cell death in primary human hepatocytes. This conclusion was supported by the extensive LDH release and propidium iodide uptake in the absence of any caspase-3 activation as indicated by measurement of the enzyme activity and processing of procaspase-3. In contrast, treatment with galactosamine/TNF-α as positive control caused extensive caspase-3 activation and apoptosis in primary human hepatocytes. Furthermore, we confirmed that ≥ 50 μM GCDC induced caspase-3 activation and apoptotic cell death in rat hepatocytes, which suggests that there is a true species difference in the susceptibility to BAs. This was further confirmed by treatment with the highly effective pancaspase inhibitor z-VAD-fmk, which only blocked BA-induced caspase-3 activation and cell death in rat hepatocytes. Human hepatocytes are clearly much less susceptible to GCDC and other glycine conjugated bile acids than rat hepatocytes; in addition, human hepatocytes exposed to BAs die by necrosis in vitro.
To evaluate if these in vitro data with primary human hepatocytes have relevance for patients with obstructive cholestasis, several biomarkers were measured in serum of patients with cholestatic liver injury. Full-length cytokeratin-18 and hypoacetylated HMGB1 protein have been used as highly sensitive serum biomarkers for necrotic cell death in animal models of acetaminophen hepatotoxicity (Antoine et al., 2009), hepatic ischemia-reperfusion injury (Yang et al., 2014a) and obstructive cholestasis (Woolbright et al., 2013) and in patients with acetaminophen-induced liver injury (Antoine et al., 2012, 2013). Consistent with these studies and the increase of ALT activities, we observed substantial elevation of both full-length cytokeratin-18, and of hypoacetylated HMGB1 in cholestatic patients. In addition, the selective caspase-cleaved fragment of cytokeratin -18 was only very modestly elevated in these patients. Furthermore, serum caspase-3 activity, which can be used as biomarker for hepatic caspase-3 activity increases in animals (Gujral et al., 2003b) and humans (McGill et al., 2012), was not detectable. These findings strongly suggest that the vast majority of cell death in patients with obstructive cholestasis occurs by necrosis in agreement with the in vitro data.
Bile acids as inducers of inflammatory mediators
Previous studies have demonstrated that cell necrosis in the experimental model of obstructive cholestasis is caused by a neutrophilic inflammatory response (Gujral et al., 2003a, 2004a). Neutrophil recruitment is initiated by cleaved osteopontin from bile (Yang et al., 2014b) and amplified by CXC chemokines generated by hepatocytes after exposure to BAs such as TCA (Allen et al., 2011). However, in contrast to the dramatic induction of pro-inflammatory mediators in murine hepatocytes, especially the CXC chemokine MIP-2, TCA induced only a moderate expression of the human neutrophil chemotactic chemokine IL-8. This would suggest that the non-toxic BAs such as TCA do not induce pro-inflammatory mediators in human hepatocytes to the same degree as in mouse hepatocytes. It should be noted that bile acids also can regulate immune processes through the bile acid receptor G protein-coupled bile acid receptor 1 (GPBAR-1 or TGR5) which is expressed on macrophages and other non-parenchymal cells (Keitel et al., 2007, 2008; Wang et al., 2011). These effects are obviously lost during hepatocytes monoculture, and thus, their loss may mask some effects on inflammatory signaling mediated by bile acids. Given the elevation in serum bile acid concentrations that occurs during cholestasis, liver macrophages may play a central role as a rheostat for immunomodulatory signaling during cholestasis. In this study, hyperacetylated HMGB1 protein, a potent ligand for toll-like receptors such as TLR4 expressed on macrophages, was significantly elevated in patients with obstructive cholestasis and liver injury (Figure 6). These data provide preliminary evidence for the release of damage-associated molecular patterns (DAMPs) during BA-induced necrosis as potential initiators of a sterile inflammatory response in cholestatic patients. While the role of neutrophils in human cholestatic liver injury has not been assessed, the proximity of neutrophils to the site of injury, and the implications for sterile inflammation suggested by the elevated levels of HMGB1 and acHGMB1 in patients with liver injury indicate neutrophils may also play a role during the pathophysiology in human patients. This would be consistent with the role of neutrophils in the murine BDL model (Gujral et al., 2003a, 2004a). However, the presence of neutrophils in the necrotic tissue does not necessarily mean that they aggravate the injury. As shown extensively in acetaminophen hepatotoxicity, evidence in both the murine model and human patients suggest neutrophil involvement in the repair but not in the injury process (Jaeschke et al., 2012; Williams et al., 2014). Thus, more studies in human cholestatic patients are required to assess if neutrophils aggravate the initial BA-induced injury or are recruited for clearing the necrotic cell debris.
Summary and Conclusions
We provided qualitative and quantitative information about BA levels in serum and bile of humans with obstructive cholestasis. Using this information, we demonstrated that even elevated serum levels of the most common human BAs alone or in combination are insufficient to cause cell death in primary human hepatocytes. In contrast, biliary concentrations of BAs can readily cause necrotic cell death. This mode of cell death was confirmed in patients using biomarkers of necrotic and apoptotic cell death. In addition, TCA did only induce modest amounts of IL-8 in primary human hepatocytes; however, DAMPs such as HMGB1 were being released by necrotic cells in vivo. Together, these data suggest that during obstructive cholestasis in patients, bile leaking back into the parenchyma can cause direct BA-induced necrosis, which, through release of DAMPs, can initiate an inflammatory response. This mechanism of obstructive cholestasis in humans is fundamentally different to the previously favored models such as bile duct ligation in mice or GCDC-induced apoptosis in rat hepatocytes. These results may help explain why high doses of bile acids such as UDCA not only fail to provide protection to obstructive cholestatic disorders in some patient populations, but can actually worsen them (Fickert et al., 2002; Lindor et al., 2009). If bile acids given as a drug are excreted in large quantities into the bile where they stagnate, this can increase biliary pressure, which would result in a greater number of infarcts, more inflammation, and increased morbidity (Fickert et al., 2002). Studies targeted at understanding differences in bile acid export in cholestatic patients given UDCA, and studies targeted towards understanding the molecular and physiological mechanisms behind hepatic infarction of the biliary tracts may explain the dramatic differences seen between patients that undergo some level of protection from UDCA treatment and those that experience a greater degree of morbidity.
Supplementary Material
Highlights.
Cholestatic liver injury is due to cytoplasmic bile acid accumulation in hepatocytes.
Primary human hepatocytes are resistant to BA-induced injury compared to rodents.
Primary human hepatocytes undergo largely necrosis in response to BA toxicity.
Cholestatic liver injury in vivo is predominantly necrotic with minor apoptosis.
Rodent models of bile acid toxicity may not recapitulate the injury in man.
ACKNOWLEDGEMENTS
The authors would like to thank Brian Bridges, Dr. Steven Weinman and the Kansas University Medical Center Liver Center for their assistance in patient recruitment. This work was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # UL1TR000001 (formerly #UL1RR033179). In addition, this work was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916 (to H.J.), R01 GM077336 (to B.H.) and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to B.L.W.) from the National Institute of Environmental Health Sciences. Furthermore, this work was supported in part by the UK Medical Research Council (G0700654 to D.J.A, R.E.J. and B.K.P.), the MRC Integrative Toxicology Training Program (D.J.A. and J.I.C.) and the Wellcome Trust (to D.J. A.).
Abbreviations
- LCA
lithocholic acid
- UDCA
ursodeoxycholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- CA
cholic acid
- TCA
taurocholic acid
- GCDC
glycochenodeoxycholic acid
- TCDC
taurochenodeoxycholic acid
- GCA
glycocholic acid
- GDCA
glycodeoxycholic acid
- TDCA
taurodeoxycholic acid
- NTCP
sodium taurocholate cotransporting polypeptide
- ICAM-1
intercellular adhesion molecule
- ERCP
endoscopic retrograde cholangiopancreatography
- BDL
bile duct ligation
- HMGB1
high mobility group box-1
- UPLC/QTOF
ultra-performance liquid chromatography quadrupole time of flight mass spectrometry
- CK18
cytokeratin-18
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
REFERENCES
- Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol. Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin. Liver Dis. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- Dilger K, Hohenester S, Winkler-Budenhofer U, Bastiaansen BA, Schaap FG, Rust C, Beuers U. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J. Hepatol. 2012;57:133–140. doi: 10.1016/j.jhep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L, Klune JR, Zlotnicki J, Billiar T, Tsung A. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol. Chem. 2010;285:39888–39897. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin. Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J. Hepatol. 2005;42:378–385. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, Trauner M. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- Galle PR, Theilmann L, Raedsch R, Otto G, Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990;12:486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- Gartung C, Ananthanarayanan M, Rahman MA, Schuele S, Nundy S, Soroka CJ, Stolz A, Suchy FJ, Boyer JL. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199–209. doi: 10.1053/gast.1996.v110.pm8536857. [DOI] [PubMed] [Google Scholar]
- Gartung C, Schuele S, Schlosser SF, Boyer JL. Expression of the rat liver Na+/taurocholate cotransporter is regulated in vivo by retention of biliary constituents but not their depletion. Hepatology. 1997;25:284–90. doi: 10.1002/hep.510250205. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003a;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Jaeschke H. Oncotic necrosis and caspase-dependent apoptosis during galactosamine-induced liver injury in rats. Toxicol. Appl. Pharmacol. 2003b;190:37–46. doi: 10.1016/s0041-008x(03)00154-6. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2004a;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004b;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J. Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32 doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab. Dispos. 2006;34:1756–1763. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem. Biophys. Res. Commun. 2008;372 doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, Häussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45 doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Meier PJ. Mechanisms of cholestasis. Clin. Liver Dis. 2000;4:357–385. doi: 10.1016/s1089-3261(05)70114-8. [DOI] [PubMed] [Google Scholar]
- Liang D, Hagenbuch B, Stieger B, Meier PJ. Parallel decrease of Na(+)-taurocholate cotransport and its encoding mRNA in primary cultures of rat hepatocytes. Hepatology. 1993;18:1162–1166. [PubMed] [Google Scholar]
- Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50 doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol. Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy PD, Schüngel S, Hong JY, Manns MP, Jaeschke H, Vogel A. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am. J. Pathol. 2009;175:1077–85. doi: 10.2353/ajpath.2009.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippin SJ, Hagenbuch B, Meier PJ, Stieger B. Cholestatic expression pattern of sinusoidal and canalicular organic anion transport systems in primary cultured rat hepatocytes. Hepatology. 2001;33:776–782. doi: 10.1053/jhep.2001.23433. [DOI] [PubMed] [Google Scholar]
- Roda A, Cappelleri G, Aldini R, Roda E, Barbara L. Quantitative aspects of the interaction of bile acids with human serum albumin. J. Lipid Research. 1982;23 [PubMed] [Google Scholar]
- Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J. Biol. Chem. 2009;284:2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
- Spivey JR, Bronk SF, Gores GJ. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J. Clin. Invest. 1993;92:17–24. doi: 10.1172/JCI116546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo M, Veres Z, Baranyai Z, Jakab F, Jemnitz K. Comparison of human hepatoma HepaRG cells with human and rat hepatocytes in uptake transport assays in order to predict a risk of drug induced hepatotoxicity. PLoS One. 2013;8:e59432. doi: 10.1371/journal.pone.0059432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J, Białek A, Caron P, Straka RJ, Milkiewicz P, Barbier O. Profiling circulating and urinary bile acids in patients with biliary obstruction before and after biliary stenting. PLoS One. 2011;6:e22094. doi: 10.1371/journal.pone.0022094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J, Białek A, Caron P, Straka RJ, Heathcote J, Milkiewicz P, Barbier O. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: a pilot study. Dig. Liver Dis. 2012;44:303–310. doi: 10.1016/j.dld.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54 doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol. Appl. Pharmacol. 2014;275 doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol. Appl. Pharmacol. 2013;273:524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J. Gastroenterol. 2012;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, McGill MR, Staggs VS, Winefield RD, Gholami P, Olyaee M, Sharpe MR, Curry SC, Lee WM, Jaeschke H, the Acute Liver Failure Study Group Glycodeoxycholic acid levels as prognostic biomarker in acetaminophen-induced acute liver failure patients. Toxicol. Sci. 2014a;142:436–444. doi: 10.1093/toxsci/kfu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Li F, Xie Y, Farhood A, Fickert P, Trauner M, Jaeschke H. Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol. Lett. 2014b;228:56–66. doi: 10.1016/j.toxlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014;279:266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagmur E, Trautwein C, Leers MP, Gressner AM, Tacke F. Elevated apoptosis-associated cytokeratin 18 fragments (CK18Asp386) in serum of patients with chronic liver diseases indicate hepatic and biliary inflammation. Clin. Biochem. 2007;40:651–655. doi: 10.1016/j.clinbiochem.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Yang M, Antoine DJ, Weemhoff JL, Jenkins RE, Farhood A, Park BK, Jaeschke H. Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2014a;20:1372–82. doi: 10.1002/lt.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol. Lett. 2014b;224:186–95. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.