Abstract
Objective
Age and inflammation are risk factors for cardiovascular disease but the impact of inflammation on cardiovascular risk across the lifespan is not understood. We investigated whether an inflammatory burden is modulated by age in healthy subjects.
Methods
Caucasian and African-American families were recruited from the general population (age range: 6-74 years, n=267). Systemic inflammation was assessed by C-reactive protein (CRP), serum amyloid A (SAA), fibrinogen, haptoglobin and α-acid glycoprotein, and vascular inflammation was assessed by pentraxin-3 (PTX-3), soluble intercellular adhesion molecule-1 and soluble vascular adhesion molecule-1 (sVCAM). To collectively assess systemic or vascular factors across the age spectrum, a composite z-score for each marker category was calculated.
Results
There was a contrasting pattern in systemic versus vascular inflammatory burden over age with an increase in systemic but a decrease in vascular markers in both ethnic groups. The results remained unchanged after adjustments for the covariates and covariance. When looking at individual markers to examine which markers are most contributing factors to the composite scores, CRP and SAA were significantly and positively associated with age, while PTX-3 and sVCAM were significantly and negatively associated with age in both ethnic groups.
Conclusions
The composite z-score for systemic inflammation increased with age, while the composite z-score for vascular inflammation declined with age, irrespective of ethnicity. The findings illustrate a regulatory relationship between age and inflammation, and suggest that a perceived elevation of vascular markers among the very young may be an indication of physiological changes rather than reflecting a disease process.
Keywords: inflammatory biomarkers, ethnicity, general population, composite score
INTRODUCTION
Inflammation plays a key role in the atherosclerotic process1 and studies have demonstrated a positive association between risk for cardiovascular disease (CVD) and levels of inflammatory markers2-7. Some markers [high sensitive C-reactive protein (CRP), serum amyloid A (SAA) or fibrinogen] reflect a more systemic inflammatory burden8-10, whereas others are considered more informative with regard to local injuries such as vascular inflammation and endothelial dysfunction11-17. These two types of biomarkers, systemic versus vascular, tend to correlate weakly with each other, suggesting different underlying pathophysiological mechanisms. Furthermore, there are ethnic differences in inflammatory biomarker levels in adults, potentially related to demographic or lifestyle-related factors18. Previously, we have shown heterogeneity in the relationship of systemic versus vascular inflammatory markers with age among middle-aged Caucasian and African-American adults undergoing coronary angiography19.
Increasing evidence now supports the concept that the roots of chronic diseases, such as CVD, occur early in life with disease progression accelerating gradually through childhood and adolescence leading to CVD in adulthood20. Inflammation, a key feature of CVD, may underlie such processes, resulting in the rise of adult disease manifestations in children. In support of this concept, a low-grade inflammation measured by elevated levels of CRP was present in children with detectable differences between ethnic groups21. However, there is limited information available with regard to the distribution and trend patterns of different inflammatory markers across the lifespan in the general population. It is unclear if the heterogeneity in inflammatory burden between systemic and vascular markers over an age spectrum would be present in healthy individuals. To address these questions, we measured two sets of markers, one set representing general systemic inflammation [CRP, SAA, fibrinogen, haptoglobin (HPTG), and α-acid glycoprotein (AGP)] and another set representing vascular-focused inflammation [pentraxin-3 (PTX-3), soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1)] and assessed the trajectory patterns of these markers across an age spectrum in Caucasian and African-American subjects. In order to attenuate the potential contribution of genetic or environmental factors, we recruited members of the same families across ages, i.e. parents and their children.
MATERIALS AND METHODS
Human subjects
Caucasian or African-American families with minimum of two biological children at least six years old were eligible to participate in the study. Families were recruited from the general population in the city of Sacramento and surrounding California counties by advertisement. Healthy subjects responding to advertisement were invited to the Clinical Research Center of the University of California Davis Clinical and Translational Science Center for a medical history questionnaire, physical examination and blood draws. Being healthy was defined for the purpose of the present study as not having any chronic medical condition under treatment by a pediatrician or a family physician, other than high blood pressure, high lipids or seasonal allergies. There were no subjects with intercurrent infections at the time of observation. Information on demographics and medical history using a standardized questionnaire and anthropometrics were obtained. Non-fasting blood was collected and plasma was stored at −80°C until analyses.
We recruited a total of 82 families (60 Caucasian and 22 African-American) where each family had at least two biological children (≤18 years of age). Race/ethnicity was self-reported for each individual family member with use of a questionnaire as Asian/Pacific Islander, White, Native American/Alaskan Native, African-American, or Hispanic. A few families had an admixture of ethnic backgrounds with some members identifying several ethnicities. Based on the self-selection, we classified subjects into three categories: a) Caucasian; b) African-American; and c) Other. There were a total of 327 subjects, 182 self-identified as Caucasians, 87 as African-Americans, and 58 as Others. Two children (one Caucasian and one African-American) were excluded due to missing blood sample. The present report is based on findings in 181 Caucasians and 86 African-Americans. The study was approved by the Institutional Review Board at the University of California Davis and informed consent was obtained from all subjects. Minors were asked to give their assents (Assent form for 12-17 years old; or Letter of information for 8-11 years old), and one of the parents signed the consent forms for their children.
Clinical and biochemical assessment
Blood pressure (BP) was measured with a random-zero mercury sphygmomanometer. Body mass index (BMI) was calculated as body weight (kg) divided by squares of height (m2). For children and adolescents (six to 20 years), BMI-for-age growth charts for either boys or girls (Center for Disease Control and Prevention) were used to obtain a percentile ranking22. Concentrations of total cholesterol (TC), HDL cholesterol (HDL-C), triglycerides, apolipoprotein (apo) B-100, apoA-1, and glucose were measured using standard procedures. LDL cholesterol (LDL-C) concentrations were calculated with the formula of Friedewald et al.23. Plasma fibrinogen concentration was determined at the University of California Davis chemical laboratory. Concentrations of high-sensitivity CRP, HPTG and AGP were measured using Human CVD Panel 3 (Acute Phase) Magnetic Bead Panel Kit assays with manufacturer’s recommended equipment and setting (#HCVD3MAG-67K, Milliplex Map, EMD Millipore, Billerica, MA). Similarly, concentrations of SAA, sICAM-1, and sVCAM-1 were determined by Human CVD Panel 2 Magnetic Bead Panel Kit (#HCVD2MAG-67K, Milliplex Map, EMD Millipore, Billerica, MA). PTX-3 concentration was assessed by ELISA using a commercially available kit (Enzo Life Sciences. Inc., Plymouth Meeting, PA)19, 24. Analyses were run according to the manufacturers’ specifications in duplicate samples with two different quality controls, which were within the recommended precision for each test. Coefficients of variation of inflammatory markers were consistently less than 7%.
Statistics
Statistical analyses of data were performed with SAS software, version 9.4 (SAS Institute, Cary, NC). Results were expressed as mean ± standard error of mean, or median with interquartile range for non-normally distributed variables. Values of triglycerides, glucose, CRP, fibrinogen, SAA, HPTG, AGP, PTX-3, sICAM-1, and sVCAM-1 were logarithmically transformed to achieve normality for statistical inferential analyses. Proportions were compared between groups using χ2 test or Fisher’s exact test as appropriate. Group means were compared using Student’s t-test. Differences in individual markers for each group by age and race/ethnicity were assessed by an Analysis of Variance (ANOVA). Multiple testing was corrected by the Tukey’s Honestly Significant Difference (HSD) procedure to maintain the family-wise Type I error rate at 0.05. To construct a composite score of multiple markers, we first calculated a z-score for each inflammatory marker [z = (x - x−)/SD, where x is an individual marker value, x− is the mean marker value, and SD is the standard deviation of marker values] as previously described19. Using the individual z-scores, we next calculated a composite z-score for systemic inflammation [i.e., z-score (systemic) = Average (z-CRP, z-fibrinogen, z-SAA, z-HPTG, z-AGP)], and for vascular inflammation [z-score (vascular) = Average (z-PTX-3, z-sICAM-1, z-sVCAM-1].
Mixed-effects regression models were used to estimate patterns of marker scores as a function of age and to test the effects of race/ethnicity and covariates on the patterns of marker scores in relation to age. All core models included fixed effects for race/ethnicity, the linear effect of age, and the interaction between race and age, with covariates for multivariate models. To account for the correlated nature of the data among members from the same family, the mixed-effects models included two random effects for family-specific intercepts and slopes. Two-tailed p-values less than 0.05 were considered statistically significant.
We originally aimed to assess the relationships between age and inflammatory markers, adjusting for covariates. Based on our previously published study of various inflammatory markers and after reviewing various partial correlations between age and composite scores of systemic and vascular inflammation, controlling for covariates (Table 3 of Reference 3), we surmised that the true partial correlations would be likely to be at least 0.35 and 0.24 for systemic and vascular inflammation, respectively. We used an ordinary F test to assess the linear relationship between age and inflammation burden, adjusting for covariates in an attempt to determine how many sample sizes would be required to yield adequate statistical power. The power analysis indicated that study sample sizes of 62 and 135, respectively, would be needed to detect significance with 80% power at alpha=0.05. We also note that with 80 participants, the power is 80% to detect a partial correlation greater than 0.31.
Table 3. Correlations (r) between markers of systemic inflammation with and without adjustments for the covariates*.
| CRP | SAA | Fib | HPTG | AGP | z-score | |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| CRP | 1.000 | 0.644‡ | 0.463‡ | 0.517‡ | 0.419‡ | 0.830‡ |
| SAA | 0.644‡ | 1.000 | 0.363‡ | 0.407‡ | 0.334‡ | 0.749‡ |
| Fib | 0.463‡ | 0.363‡ | 1.000 | 0.361‡ | 0.332‡ | 0.687‡ |
| HPTG | 0.517‡ | 0.407‡ | 0.361‡ | 1.000 | 0.387‡ | 0.729‡ |
| AGP | 0.419‡ | 0.334‡ | 0.332‡ | 0.387‡ | 1.000 | 0.674‡ |
| z-score | 0.830‡ | 0.749‡ | 0.687‡ | 0.729‡ | 0.674‡ | 1.000 |
|
| ||||||
| Adjusted | ||||||
| CRP | 1.000 | 0.492‡ | 0.353‡ | 0.290‡ | 0.279‡ | 0.689‡ |
| SAA | 0.492‡ | 1.000 | 0.212† | 0.254‡ | 0.232† | 0.648‡ |
| Fib | 0.353‡ | 0.212† | 1.000 | 0.267‡ | 0.270‡ | 0.654‡ |
| HPTG | 0.290‡ | 0.254‡ | 0.267‡ | 1.000 | 0.280‡ | 0.645‡ |
| AGP | 0.279‡ | 0.232† | 0.270‡ | 0.280‡ | 1.000 | 0.653‡ |
| z-score | 0.689‡ | 0.648‡ | 0.654‡ | 0.645‡ | 0.653‡ | 1.000 |
All individual inflammatory markers and composite z-scores were logarithmically transformed to achieve normality in distribution before statistical analyses.
Body mass index, systolic blood pressure, diastolic blood pressure, LDL cholesterol, HDL cholesterol, triglycerides and glucose levels. Composite z-scores were calculated as described in the Materials and Methods section.
p<0.001;
p<0.0001
Abbreviations: AGP, α-acyl glycoprotein, CRP, C-reactive protein; Fib, fibrinogen; HPTG, haptoglobin; SAA, serum amyloid A.
RESULTS
Clinical characteristics of study population
The mean age, prevalence of females, body weight, BMI, BP, the levels of TC, LDL-C, HDL-C, apoB-100, apoA-1, and glucose and the apoB/apoA-1 ratio did not differ significantly between Caucasians and African-Americans when assessed within each age group (Table 1). In contrast, both African-American adults and children had significantly lower triglycerides levels compared to their respective Caucasian counterparts. As expected, body weight, BMI, BP, and levels of TC, LDL-C, triglycerides, apoB-100, apoB/apoA-1 ratio and glucose were significantly higher in adults compared to children regardless of ethnicity. HDL-C was significantly lower in adults compared to children in African-Americans (p=0.002), but not in Caucasians, while apoA-1 levels did not differ between adults and children in either ethnic group.
Table 1. Clinical characteristics of study population.
| Caucasians‡ | African-Americans‡ | |||
|---|---|---|---|---|
|
|
||||
| Children | Adults | Children | Adults | |
| Number (n) | 65 | 116 | 36 | 50 |
| Female (n, %) | 25 (38%) | 56 (48%) | 14 (39%) | 25 (50%) |
| Age (year) | 14 ± 0 | 44 ± 1 | 13 ± 1 | 43 ± 2 |
| Body weight (kg) | 53 ± 2 | 88 ± 2 | 53 ± 3 | 91 ± 3 |
| Body mass index (kg/m2) | 20 ± 0.5 | 30 ± 0.6 | 21 ± 0.7 | 31 ± 1.0 |
| Current smokers (n, %) | 0 (0%) | 12 (10%) | 0 (0%) | 7 (14%) |
| Diastolic blood pressure (mmHg) | 67 ± 1 | 79 ± 1 | 67 ± 1 | 81 ± 2 |
| Systolic blood pressure (mmHg) | 111 ± 1 | 126 ± 1 | 112 ± 2 | 130 ± 2 |
| Medication use (n, %) | ||||
| Lipid-lowering | 0 (0%) | 8 (7%) | 0 (0%) | 3 (6%) |
| Antihypertensive | 0 (0%) | 10 (9%) | 0 (0%) | 8 (16%) |
| Antidiabetic | 0 (0%) | 0 (0%) | 0 (0%) | 7 (14%)§ |
| Total cholesterol (mg/dL) | 167 ± 5 | 199 ± 4 | 163 ± 6 | 191 ± 6 |
| LDL cholesterol (mg/dL) | 94 ± 4 | 117 ± 4 | 91 ± 4 | 117 ± 6 |
| HDL cholesterol (mg/dL) | 53 ± 2 | 49 ± 1 | 58 ± 3 | 47 ± 2 |
| Triglycerides (mg/dL) | 85 (70-119) | 149 (96-216) | 64 (48-79)† | 120 (71-150)* |
| ApoB-100 (mg/dL) | 68 ± 2 | 92 ± 2 | 65 ± 3 | 93 ± 4 |
| ApoA-1 (mg/dL) | 143 ± 3 | 147 ± 2 | 152 ± 4 | 149 ± 4 |
| ApoB/ApoA-1 ratio | 0.49 ± 0.02 | 0.64 ± 0.02 | 0.44 ± 0.02 | 0.65 ± 0.03 |
| Glucose (mg/dL) | 87 (80-98) | 96 (86-105) | 78 (87-92) | 91 (81-103) |
Data are expressed as mean ± standard error of mean, except for triglycerides and glucose which are expressed as median with interquartile range. Data for triglycerides and glucose were logarithmically transformed before statistical inferential analyses. Children were defined as ≤18 years of age.
p<0.05 versus Caucasian adults;
p<0.001 versus Caucasian adults;
p<0.001 versus Caucasian children
All variables, excluding HDL cholesterol and apoA-1, were significantly higher in adults versus children. HDL cholesterol levels were significantly lower in adults versus children in African-Americans (p=0.002), but not in Caucasians. ApoA-1 levels did not differ significantly between adults and children in either ethnic group.
Trajectory patterns of systemic and vascular inflammation over age
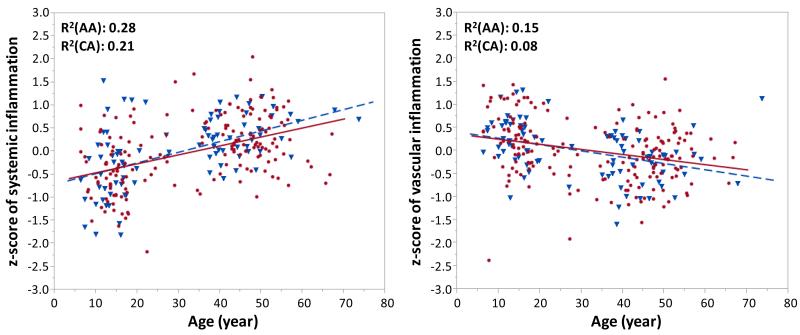
Systemic inflammatory burden was assessed by CRP, SAA, fibrinogen, HPTG, and AGP, and vascular inflammatory burden was assessed by PTX-3, sICAM, and sVCAM. To collectively assess the trend patterns of the relationship between age and inflammatory markers, we constructed a composite z-score for each marker category (systemic or vascular inflammation) based on respective five and three different markers. This approach allows a comparison of individual inflammatory markers within each category at the same scale and provides a standardized way to assess the relative contribution of individual markers to the association with age19. Coefficient estimates for mixed-effects regression models associating age, ethnicity/race, and age-race interaction with the composite z-scores of systemic and vascular inflammation with and without adjustments for covariates are shown in Table 2. Notably, there was a contrasting trend pattern (an opposite direction) in the relationship between age and composite z-scores of systemic versus vascular inflammation in both ethnic groups. The composite z-score for systemic inflammation was significantly and positively associated with age (p<0.001), whereas the composite z-score for vascular inflammation was significantly and negatively associated with age (p<0.001) in both ethnic groups. Adjustments for the covariates (sex, BMI, BP, LDL-C, HDL-C, triglycerides, and glucose) did not alter the trend pattern of the relationship between age and composite z-score of each category (Table 2). As seen in Figure 1, the composite z-score for systemic inflammation increased significantly with age, whereas the composite z-score for vascular inflammation declined significantly with age, irrespective of ethnicity.
Table 2. Coefficient estimates (SE of coefficient estimate) for mixed-effects regression models associating age, ethnicity/race, and age-race interaction with the composite z-scores of systemic and vascular inflammation with and without adjustments for covariates.
| Inflammation |
||
|---|---|---|
| Model term | Systemic | Vascular |
| Unadjusted for covariates | ||
| Intercept | −0.605 (0.109)‡ | 0.372 (0.107)‡ |
| Age | 0.019 (0.002)‡ | −0.012 (0.003)‡ |
| Ethnicity/Race | −0.078 (0.188) | 0.120 (0.177) |
| Age*Ethnicity/Race | 0.004 (0.004) | −0.005 (0.004) |
|
| ||
| Adjusted for covariates | ||
| Intercept | −2.324 (0.669)‡ | −1.250 (0.733) |
| Age | 0.008 (0.003)† | −0.010 (0.003)‡ |
| Race | 0.109 (0.169) | 0.112 (0.177) |
| Age*Ethnicity/Race | −0.003 (0.004) | −0.004 (0.004) |
| Sex | 0.306 (0.06)‡ | −0.082 (0.067) |
| Body mass index | 0.041(0.006)‡ | −0.004 (0.007) |
| Systolic blood pressure | 0.001 (0.004) | −0.0002 (0.0042) |
| Diastolic blood pressure | 0.001 (0.005) | 0.001 (0.005) |
| LDL cholesterol | 0.001 (0.001) | −0.003 (0.001)* |
| HDL cholesterol | −0.003 (0.002) | 0.004 (0.003) |
| Triglycerides | −0.025 (0.065) | 0.005 (0.075) |
| Glucose | 0.192 (0.148) | 0.392 (0.164)* |
p<0.05;
p<0.01;
p<0.001
Figure 1. Relationship between age and composite z-scores of systemic or vascular inflammatory markers.
Composite z-score for each marker category was calculated as described in the Materials and Methods section. Linear regression model was used to describe the trend pattern of inflammatory marker levels over age for each ethnic group. Data for African-Americans are shown with blue triangles and a dotted line, whereas data for Caucasians are given with red circles and a straight line.
Abbreviations: AA, African-Americans; CA, Caucasians
Trajectory patterns of individual systemic and vascular inflammatory markers over age
To assess the individual relative contribution of inflammatory marker level to the relationship between the composite z-score and age across ethnicity, and an age-ethnicity interaction term, we calculated coefficient estimates of the effects of each marker level by fitting mixed-effects regression models with and without adjustments for covariates. Before adjustments, in line with the findings of the composite z-scores, all individual systemic inflammatory markers were significantly and positively associated with age in both ethnic groups (Supplementary Table 1). In contrast, the trend pattern of each individual marker for vascular inflammation over age showed an opposite direction to that of systemic inflammation, confirming the findings of the composite z-scores. PTX-3 and sVCAM were significantly and negatively associated with age, and there was no significant difference in trends between ethnic groups. Similarly, sICAM was negatively associated with age, although the trend was not significant. After multivariate adjustments for the covariates (sex, BMI, BP, LDL-C, HDL-C, triglycerides, and glucose), CRP and SAA remained significantly and positively associated with age, whereas the trend patterns of fibrinogen, HPTG and AGP were no longer associated with age (Supplementary Table 1). In contrast, the results for markers of vascular inflammation were not impacted by these adjustments as PTX-3 and sVCAM levels remained significantly and negatively associated with age independent of ethnicity. The trajectory pattern of each individual inflammatory marker over age is shown in Supplementary Figure 1. Overall, markers for systemic inflammation increased with age, while markers for vascular inflammation declined with age in both ethnic groups.
Systemic and vascular inflammatory marker levels by ethnic and age groups
We next investigated differences in levels of inflammatory markers by ethnicity and age groups. Overall, the levels of systemic inflammatory markers were higher whereas the levels of vascular inflammatory markers were lower in adults compared to children (Supplementary Table 2). A five-fold increase in CRP and a two-fold increase in SAA and HPTG levels were observed in adults compared to children in both ethnic groups (p<0.001 for all). Fibrinogen and AGP levels were modestly elevated in adults versus children and the differences reached statistical significance for fibrinogen in Caucasians (p<0.01). Further, sVCAM levels were significantly lower in adults compared to children in both ethnic groups (p<0.01). Both PTX-3 and sICAM levels were lower in adults than in children, although the differences were statistically significant for PTX-3 only in African-Americans. Another notable observation was that although the difference did not reach significance, several systemic (CRP, SAA, and HPTG) and all vascular markers were elevated in African-Americans compared to Caucasians for both adults and children.
Intercorrelations between markers of inflammation within each category and across categories
Within each marker category, we assessed the intercorrelations between the markers in response to levels with and without adjustments for covariates. All five markers for systemic inflammation significantly and positively correlated with each other (Table 3). Notably, all associations remained significant after multivariate adjustments for the covariates. For vascular inflammation, sVCAM levels were significantly and positively associated with both PTX-3 (p<0.001) and sICAM (p<0.001) levels, whereas there was no significant correlation between PTX-3 and sICAM (Table 4). We next investigated the intercorrelations between the markers crossing over the two categories. We observed consistent negative correlations between the sets of markers of systemic versus vascular inflammation. sVCAM was significantly and negatively correlated with CRP (p<0.001), SAA (p<0.05) and HPTG (p<0.001). sICAM and PTX-3 were negatively correlated with fibrinogen (p<0.05) and HPTG (p<0.01), respectively. Adjustments for the covariates led to disappearance of significant correlations for sVCAM.
Table 4. Correlations (r) between markers of vascular inflammation with and without adjustments for the covariates*.
| PTX-3 | sICAM | sVCAM | z-score | |
|---|---|---|---|---|
| Unadjusted | ||||
| PTX-3 | 1.000 | −0.108 | 0.230‡ | 0.585§ |
| sICAM-1 | −0.108 | 1.000 | 0.218‡ | 0.579§ |
| sVCAM-1 | 0.230‡ | 0.218‡ | 1.000 | 0.755§ |
| z-score | 0.585§ | 0.579§ | 0.755§ | 1.000 |
|
| ||||
| Adjusted | ||||
| PTX-3 | 1.000 | −0.107 | 0.192† | 0.574§ |
| sICAM-1 | −0.107 | 1.000 | 0.216‡ | 0.592§ |
| sVCAM-1 | 0.192† | 0.216‡ | 1.000 | 0.731§ |
| z-score | 0.574§ | 0.592§ | 0.731§ | 1.000 |
All individual inflammatory markers and composite z-scores were logarithmically transformed to achieve normality in distribution before statistical analyses.
Body mass index, systolic blood pressure, diastolic blood pressure, LDL cholesterol, HDL cholesterol, triglycerides and glucose levels. Composite z-scores were calculated as described in the Materials and Methods section.
p<0.01;
p<0.001;
p<0.0001
Abbreviations: PTX, pentraxin-3; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular adhesion molecule-1.
Relationship between individual inflammatory markers and age-related clinical variables
Finally, we investigated the impact of clinical variables known to be influenced by aging on the circulating levels of inflammatory markers. Systemic and vascular inflammatory markers were differentially regulated by clinical variables, including BMI, BP, TC, LDL-C, HDL-C, triglycerides and glucose levels (Supplementary Table 3). Overall, all markers for systemic inflammation were positively associated with BMI, BP, TC, LDL-C, triglycerides and glucose, and negatively with HDL-C levels. Particularly, CRP and SAA levels were consistently and positively associated with all these variables (p<0.0001 for all) except for HDL-C. Among clinical variables, BMI and systolic and diastolic BP were significant predictors of all systemic markers. In contrast, vascular markers were negatively associated with BMI, BP, TC, LDL-C, triglycerides and glucose, and positively with HDL-C levels. Notably, sVCAM was significantly and negatively associated with all these variables (p<0.005) except for glucose.
DISCUSSION
The main novel finding in the current study of healthy Caucasians and African-Americans was a diverging trajectory pattern in systemic versus vascular inflammatory burden over age with an increase in systemic but a decrease in vascular inflammatory markers. The composite z-score, taking into account the relative contribution of individual markers to age at the same scale, increased significantly for systemic inflammation, but declined significantly for vascular inflammation over the age spectrum in both Caucasians and African-Americans. Importantly, age still remained independently associated with the composite z-score for both systemic and vascular inflammation in both ethnic groups after taking the potential confounding effects of covariates into account. The trend pattern of individual markers for systemic inflammation such as CRP and SAA increased with age, whereas trend pattern for markers of vascular inflammation declined with age in both ethnic groups, consistent with the findings of composite z-scores.
Age and inflammation are both considered well-established risk factors for CVD1, 25, yet the relationship between these two factors across the lifespan and various ethnic and health-risk populations is not fully understood. We have previously reported on the extent to which systemic and vascular inflammatory markers are associated with age in a high-cardiovascular-risk population while taking established risk factors into account19. In the latter study of middle-aged (mean age: ≈56 years) Caucasians and African-Americans undergoing coronary angiography, we observed a steady increase in markers for systemic inflammation over age in both ethnicities. Notably, the same trend pattern was found for systemic inflammation in the current study, as circulating levels for all systemic markers increased significantly over the age spectrum, irrespective of ethnicity. In contrast, the trend pattern for vascular inflammatory markers differed between the high-risk and general populations. The levels remained constant over age in the high-risk population, whereas the levels declined over age in the general population with younger individuals having higher levels of PTX-3, sICAM and sVCAM-1 compared to older individuals. The latter findings raise the possibility that these seemingly increased levels may reflect physiological rather than disease-related conditions. It is tempting to speculate that presence of an apparent elevated marker level for vascular inflammation in children versus adults can reflect ongoing vascular remodeling, perhaps as part of a growth process in childhood or adolescence and be a component of the physical development. In a previous study conducted in healthy younger children aged four months to six years old, the median PTX-3 level was 1.54 ng/mL26, which was substantially higher than those in our older children (0.58 ng/mL). A longitudinal study by Nash et al. reported a significant decrease in sVCAM and sICAM concentrations over an age range (9 and 15 years) among normal children27. Further, sICAM levels were significantly higher in healthy preschool children versus school age children28. In another study, sVCAM and sICAM concentrations in healthy children (median age 5 years) were found to be approximately twice as high as that of adults29. These observations corroborate our findings of a relative increase in vascular marker levels at younger ages.
Among U.S. children aged three to 16 years, there was a low-grade inflammation with detectable differences between race/ethnicity21. Notably, when adjusted for social and physical risk factors for inflammation, African-American children had higher CRP levels than Caucasian children21. In line with this observation, although not significantly different, CRP levels were elevated in our African-American children compared to Caucasian children. The mean CRP level in the latter study was 1.2 mg/L, which was close to the levels in our African-American children (≈1.1 mg/dL). The mean fibrinogen level in children aged four to 25 years was 278 mg/dL and associated with physical fitness independent of BMI30. Among our children with a narrower age range (six to 18 years), the concentration was slightly lower.
In view of the complexity of the inflammatory process and the considerable arsenal of markers available, it is recognized that a classification of systemic versus vascular inflammatory markers likely represents a relatively narrow and perhaps somewhat simplistic view. However, in the present study, a number of factors supported our approach to categorize inflammatory markers. First, analyses within each marker category revealed positive intercorrelations between individual markers, whereas cross-analyses of the markers in the two categories revealed an opposite direction of (i.e., negative) correlation. These findings suggest different underlying pathophysiological mechanisms for these two categories of markers and provide a rationale for classification. Second, we observed positive versus negative associations for markers of systemic versus vascular inflammation with traditional cardiovascular risk factors influenced by aging. Finally, trajectory patterns of both systemic and vascular inflammatory markers changed with age but in an opposite direction, with a uniform increase in systemic and a consistent decrease in vascular markers.
We acknowledge limitations of the current study. The cross-sectional study design limits our ability to evaluate the causative and longitudinal effects of age and other factors that might influence levels of inflammatory markers. Subjects for the current study were recruited from the general population residing in greater Sacramento area in California, and were Caucasians and African-Americans. Thus, expanded studies are needed to investigate other geographical and ethnic groups. Unmeasured confounding factors, including socioeconomic status, health consciousness, are other potential limitations. A family-based study setting with a relatively modest sample size may introduce limitations, although it helps to attenuate the potential impact of other genetic as well as environmental factors. Further, our findings of the current and previous studies support the notion that systemic inflammatory burden measured as elevated levels of inflammatory markers is persistently increased by age in both the general and high risk populations; however, additional studies are warranted to verify these findings in a larger scale, prospective setting.
In conclusions, there was a diverging pattern in systemic versus vascular inflammatory burden as a function of age, which was independent of ethnicity, family correlation and the individual metabolic burden in the general population. Overall, the findings suggest an increase in the systemic and a decrease in vascular inflammatory burden with age over a broad age spectrum in healthy Caucasians and African-Americans. These observations underscore: a) the usefulness of separately assessing systemic versus vascular inflammatory markers in their relation to cardiovascular risk over age in the general population; b) that there were no significant differences in the relationship of inflammatory markers with age between the two studied ethnic groups after adjustments for covariates, including traditional risk factors; and c) age was an independent predictor of both systemic and vascular inflammation although with a diverging effect in the general population. Our results confirm and extend previous observations on the influence of aging on inflammatory markers, and suggest that age-related cardiovascular risk may be more influenced by an increased inflammatory burden measured by elevated levels of markers for systemic inflammation in the general community. Taken together, these findings highlight the complexity of assessing an inflammatory process and also illustrate the complex nature of the relationship between age and inflammation. Our findings suggest that a perceived elevation of vascular markers among the very young may results from physiological adjustments as part of the growth process rather than related to a disease process.
Supplementary Material
Supplementary Figure 1. Relationship between age and individual inflammatory marker level
Linear regression model was used to describe the trend pattern of individual inflammatory marker level over age, separately, for each ethnic group. Data for African-Americans is shown with blue triangles and a straight line, whereas data for Caucasians are given with red circles and a dotted line.
Abbreviations: AA, African-Americans; CA, Caucasians
HIGHLIGHTS.
Age and inflammation are both considered as risk factors for cardiovascular diseases.
Inflammatory markers were analyzed in children and their parents.
We observed an increase in systemic and a decrease in vascular inflammatory markers over age.
This pattern was independent of ethnicity, family correlation, and individual metabolic burden.
Higher vascular inflammatory markers at a young age may reflect physiological conditions.
ACKNOWLEDGEMENT
We thank all participants of the UC Davis Family Study. We also thank Tina Fung for her significant contribution to recruiting families.
SOURCES OF FUNDING
These studies were supported by a grant from the National Institutes of Health (NIH) #HL62705 (L.B.) and UC Davis Clinical and Translational Center (CTSC) base operating grant (#TR000002, #RR024146). Dr. Byambaa is a recipient of the UC Davis Highly Innovative Pilot Research Award and the Mentored Clinical and Population Research Program Award from the American Heart Association (#14CRP17930014), and is a current Building Interdisciplinary Research Careers in Women’s Health/K12 training program scholar (#NIH 2K12HD051958-06).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of c-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of c-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The framingham study. JAMA. 1987;258:1183–1186. [PubMed] [Google Scholar]
- 5.Cantin B, Despres JP, Lamarche B, Moorjani S, Lupien PJ, Bogaty P, Bergeron J, Dagenais GR. Association of fibrinogen and lipoprotein(a) as a coronary heart disease risk factor in men (the quebec cardiovascular study) Am J Cardiol. 2002;89:662–666. doi: 10.1016/s0002-9149(01)02336-0. [DOI] [PubMed] [Google Scholar]
- 6.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, c-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 8.Pepys MB, Baltz ML. Acute phase proteins with special reference to c-reactive protein and related proteins (pentaxins) and serum amyloid a protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 9.Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: New insights from an old molecule. QJM. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 10.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43–62. doi: 10.1007/s00281-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 11.Yasunaga T, Ikeda S, Koga S, Nakata T, Yoshida T, Masuda N, Kohno S, Maemura K. Plasma pentraxin 3 is a more potent predictor of endothelial dysfunction than high-sensitive c-reactive protein. Int Heart J. 2014;55:160–164. doi: 10.1536/ihj.13-253. [DOI] [PubMed] [Google Scholar]
- 12.Zamani P, Schwartz GG, Olsson AG, Rifai N, Bao W, Libby P, Ganz P, Kinlay S. Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the miracl study. J Am Heart Assoc. 2013;2:e003103. doi: 10.1161/JAHA.112.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A. Prognostic significance of the long pentraxin ptx3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 14.Wu JT, Wu LL. Association of soluble markers with various stages and major events of atherosclerosis. Ann Clin Lab Sci. 2005;35:240–250. [PubMed] [Google Scholar]
- 15.Rho YH, Chung CP, Oeser A, Solus J, Raggi P, Gebretsadik T, Shintani A, Stein CM. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol. 2008;35:1789–1794. [PMC free article] [PubMed] [Google Scholar]
- 16.Gearing AJ, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, e-selectin, icam-1, and vcam-1: Pathological significance. Ann N Y Acad Sci. 1992;667:324–331. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller MA, Cappuccio FP. Ethnicity and inflammatory pathways - implications for vascular disease, vascular risk and therapeutic intervention. Curr Med Chem. 2007;14:1409–1425. doi: 10.2174/092986707780831131. [DOI] [PubMed] [Google Scholar]
- 19.Anuurad E, Enkhmaa B, Gungor Z, Zhang W, Tracy RP, Pearson TA, Kim K, Berglund L. Age as a modulator of inflammatory cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31:2151–2156. doi: 10.1161/ATVBAHA.111.232348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in u.S. Children aged 3-16 years. Am J Prev Med. 2010;39:314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 cdc growth charts for the united states: Methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Enkhmaa B, Anuurad E, Ozturk Z, Zhang W, Pearson TA, Berglund L. Differential associations of serum amyloid a and pentraxin-3 with allele-specific lipoprotein(a) levels in african americans and caucasians. Transl Res. 2011;158:92–98. doi: 10.1016/j.trsl.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 26.Biagi E, Col M, Migliavacca M, Dell’Oro M, Silvestri D, Montanelli A, Peri G, Mantovani A, Biondi A, Rossi MR. Ptx3 as a potential novel tool for the diagnosis and monitoring of pulmonary fungal infections in immuno-compromised pediatric patients. J Pediatr Hematol Oncol. 2008;30:881–885. doi: 10.1097/MPH.0b013e318180bc1d. [DOI] [PubMed] [Google Scholar]
- 27.Nash MC, Wade AM, Shah V, Dillon MJ. Normal levels of soluble e-selectin, soluble intercellular adhesion molecule-1 (sicam-1), and soluble vascular cell adhesion molecule-1 (svcam-1) decrease with age. Clin Exp Immunol. 1996;103:167–170. doi: 10.1046/j.1365-2249.1996.925616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furukawa S, Imai K, Matsubara T, Yone K, Yachi A, Okumura K, Yabuta K. Increased levels of circulating intercellular adhesion molecule 1 in kawasaki disease. Arthritis Rheum. 1992;35:672–677. doi: 10.1002/art.1780350611. [DOI] [PubMed] [Google Scholar]
- 29.Nash MC, Shah V, Dillon MJ. Soluble cell adhesion molecules and von willebrand factor in children with kawasaki disease. Clin Exp Immunol. 1995;101:13–17. doi: 10.1111/j.1365-2249.1995.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isasi CR, Starc TJ, Tracy RP, Deckelbaum R, Berglund L, Shea S. Inverse association of physical fitness with plasma fibrinogen level in children: The columbia university biomarkers study. Am J Epidemiol. 2000;152:212–218. doi: 10.1093/aje/152.3.212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Relationship between age and individual inflammatory marker level
Linear regression model was used to describe the trend pattern of individual inflammatory marker level over age, separately, for each ethnic group. Data for African-Americans is shown with blue triangles and a straight line, whereas data for Caucasians are given with red circles and a dotted line.
Abbreviations: AA, African-Americans; CA, Caucasians