Abstract
Breast cancer stem cells (BCSCs) are a subset of tumor cells that are believed to be the cells responsible for the establishment and maintenance of tumors. Moreover, BCSCs are suggested to be the main cause of progression to metastasis and recurrence of cancer because of their tumor-initiating abilities and resistance to conventional therapies. Ductal carcinoma in situ (DCIS) is an early precursor in breast carcinogenesis which progresses to invasive ductal carcinoma (IDC). We have previously reported that a vitamin D compound, BXL0124, inhibits the progression of DCIS to IDC. In the present study we sought to determine whether this effect was mediated through an influence on BCSCs. In MCF10DCIS cells treated with vitamin D compounds (1α25(OH)2D3 or BXL0124), the breast cancer stem cell-like population, identified by the CD44+/CD24−/low and CD49f+/CD24−/low subpopulations, was reduced. To determine the effects of vitamin D compounds on cancer stem cell activity, the MCF10DCIS mammosphere cell culture system, which enriches for mammary progenitor cells and putative BCSCs, was utilized. Untreated MCF10DCIS mammospheres showed a disorganized and irregular shape. When MCF10DCIS cells were treated with 1α25(OH)2D3 or BXL0124, the mammospheres that formed exhibited a more organized, symmetrical and circular shape, similar to the appearance of spheres formed by the non-malignant, normal mammary epithelial cell line, MCF10A. The mammosphere forming efficiency (MFE) was significantly decreased upon treatment with 1α25(OH)2D3 or BXL0124, indicating that these compounds have an inhibitory effect on mammosphere development. Treatment with 1α25(OH)2D3 or BXL0124 repressed markers associated with the stem cell-like phenotype, such as CD44, CD49f, c-Notch1, and pNFκB. Furthermore, 1α25(OH)2D3 and BXL0124 reduced the expression of pluripotency markers, OCT4 and KLF-4 in mammospheres. This study suggests that vitamin D compounds repress the breast cancer stem cell-like population, potentially contributing to their inhibition of breast cancer.
Keywords: Vitamin D, Vitamin D analog, Stem Cell, Mammosphere, Breast Cancer
Introduction
Ductal carcinoma in situ (DCIS) is an early, non-malignant lesion of the breast constituting approximately 25% of breast abnormalities (1). The treatment of DCIS typically consists of surgery, radiation, and when necessary, hormonal therapy. Despite the effective treatment regimen and initial response to therapy, approximately 15% of patients with DCIS will relapse (2). Additionally, 30–50% of DCIS cases will progress to invasive ductal carcinoma (IDC) if left untreated (3, 4). Evidence suggests that these processes are regulated through the interaction of the breast cancer stem cells (BCSCs) with the surrounding microenvironment via cell adhesion molecules and receptors (5). Therefore, the progression of DCIS to IDC, along with the recurrence of DCIS or IDC after treatment are two critical events that could be driven by BCSCs and their understanding is crucial in the prevention of breast cancer.
Increasing evidence supports the notion that the initiation and maintenance of breast tumors are sustained by the BCSCs (or tumor initiating cells) (6). These cells have the ability to self-renew through symmetrical division or differentiate through asymmetrical division (7). A tumor can be viewed as a heterogeneous mass of cells comprising cells at various stages of the differentiation process, all originating from an initial BCSC, or TIC (8). Local recurrence and distant metastases demonstrate the resistance of BCSCs to radio- and chemotherapies (9). Studies have shown that cells that remain after chemotherapy are enriched for putative BCSCs and these cells were capable of higher mammosphere forming efficiencies compared to cells tested before treatment (10, 11). The interaction of BCSCs with the microenvironment results in crosstalk of signaling and regulation of BCSCs via growth factors and cytokines from the microenvironment (12). However, it is unclear how BCSC signaling can interact with the surrounding microenvironment, such as the stroma, to influence breast cancer progression. Signaling crosstalk with surrounding cells along with other emerging biological properties of BCSC-like cells provide strategies and targets to effectively develop therapies against the BCSC population. Elimination of the BCSC population may improve the treatment outcomes for breast cancer patients.
Initial studies identified the CD44high/CD24−/low subpopulation of breast cancer cells from breast tumors to be enriched in cancer stem cells (6). Tumor initiation studies with various subpopulations have also been used to assess the tumorigenicity of putative BCSCs (13, 14). Previous studies have shown that 1α25(OH)2D3 promoted the differentiation of colon cancer cells through increased E-cadherin expression and the inhibition of β-catenin (15). Another study demonstrated antiproliferative effects of calcitriol on adult prostate progenitor and stem cells, however these effects have not been proven in BCSC-like cells (16). It has also been found that a Gemini vitamin D analog decreased the expression of CD44, which has been identified as a cancer stem cell marker (17). These studies have stimulated further investigation of the inhibitory effects of vitamin D and its analogs on the putative BCSC population.
Recent studies demonstrated that a cancer stem cell-like population identified within basal-like DCIS has the capacity to drive malignant progression to IDC (18). Previously, we have shown that a Gemini vitamin D analog, BXL0124, can inhibit the transition from DCIS to IDC in vivo (19). We have also demonstrated that BXL0124 was capable of reducing the CD44+/CD24−/low subpopulation of MCF10DCIS cells (17). Recent review on cell culture and animal models of cancer support a role of vitamin D compounds in decreasing cancer development and progression (20). Since BCSCs have the potential to drive DCIS progression, we investigated the effects of vitamin D compounds on the DCIS breast cancer stem cell population, and determined their potential to inhibit stem cell-like properties. We utilized the MCF10DCIS basal-like breast cancer cell line in mammosphere forming assays. These assays have been used in various tissue types for the quantification of stem cell activity and self-renewal (21). The formation of primary mammospheres is a measure of stem cell and early progenitor activity (22). The present study will examine the ability of vitamin D compounds to target the putative BCSCs, which have important implications in the treatment and prevention of breast cancer.
Materials and Methods
Cell Culture and Reagents
1α25(OH)2D3 and a Gemini vitamin D analog (BXL0124; 1α,25-dihydroxy-20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluoro-cholecalciferol, >95% purity) (Fig. 1) were provided by BioXell, Inc. (Nutley, NJ) (23). The MCF10DCIS human breast cancer cells (MCF10DCIS) were provided by Dr. Fred Miller at the Barbara Ann Karmanos Cancer Institute (Detroit, MI). The MCF10DCIS cell line was authenticated by short tandem repeat profiling at American Type Culture Collection (Manassas, VA). MCF10DCIS cells were maintained in DMEM/F12 medium supplemented with 5% horse serum, 1% penicillin/streptomycin, and 1% HEPES solution at 37°C, 5% CO2. MCF10A normal breast epithelial cells were acquired from American Type Culture Collection. MCF10A cells were grown in DMEM/F12 medium, 5% horse serum, 1% penicillin/streptomycin, 10 mM HEPES solution, 500 ng/ml hydrocortisone (Stemcell Technologies, Vancouver, BC), 20 ng/ml epidermal growth factor (EGF) (Sigma Aldrich, St. Louis, MO), 10 μg/ml insulin (Life Technologies, Carlsbad, CA) and 100 ng/ml cholera toxin (Sigma Aldrich, St. Louis, MO).
Figure 1.

The structures of 1α25(OH)2D3 and the Gemini vitamin D analog, BXL0124, are shown.
Flow Cytometry
In monolayer cell culture, MCF10DCIS cells were grown to 50% confluence and were subsequently treated with fresh medium containing 1α25(OH)2D3 (100 nM) or BXL0124 (10 nM) for 24 h. Cells were harvested and processed for further analysis. The detailed procedure was described previously (17). MCF10DCIS cells were stained with antibodies against CD44-FITC (Cat. 555478), CD49f-FITC (Cat. 561893) and CD24-PE (Cat. 561893) from BD Biosciences (San Jose, CA). The stained MCF10DCIS cells were analyzed by flow cytometry using an FC500 Analyzer (Beckman Coulter) to determine the percentage of different CD44/CD24 and CD49f/CD24 subpopulations. The acquisition of ≥ 5,000 cells per treatment was analyzed.
Mammosphere Forming Assay
MCF10A or MCF10DCIS cells were grown to 50–60% confluence and cells were detached with StemPro Accutase (Life Technologies). Cells were then plated at 10,000 cells/mL in 6-well ultra-low attachment plates and maintained in Mammocult serum-free medium supplemented with hydrocortisone and heparin (Stem Cell Technologies). Cells were treated with 1α25(OH)2D3 (100 nM) or BXL0124 (10 nM). For secondary and tertiary mammosphere culture, primary mammospheres were collected and enzymatically dissociated using StemPro Accutase (Life Technologies). Then, cells were re-plated at a density of 5,000 cells/mL for subsequent passages. Photos of mammospheres were taken, and the number of mammospheres was counted to determine the mammosphere forming efficiency (MFE). The MFE was calculated by dividing the number of mammospheres (≥100 μm) formed by the number of single cells seeded. Roundness of spheres was obtained by analysis of photos with ImageJ software (US National Institutes of Health, Bethesda, MD). The formula used to calculate roundness is 4 × ([area]/π[major axis]2). A value of 1.0 represents an object that is perfectly round. A value of 0.0 represents an object that is formless. Experiments were repeated in triplicates.
Annexin-FITC Apoptosis Assay
The procedures have been described previously (24). The apoptosis assay was performed using an apoptosis kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. MCF10DCIS cells were plated at 5,000 cells/mL in 6-well ultra-low attachment plates and treated with 1α25(OH)2D3 (100 nM) or BXL0124 (10 nM) for 5 days. Mammospheres were then trypsinized to form a single cell suspension. Cells were resuspended in binding buffer at a density of 106 cells/mL. Cells were stained with Annexin V-FITC and propidium iodide, and analyzed by flow cytometry.
Western Blot Analysis
The procedures have been described previously (25). Whole-cell lysates (15 μg/lane) were resolved in 4% to 20% SDS-PAGE from Bio-Rad (Hercules, CA). Blots were then probed with the indicated antibodies. Primary antibodies against CD44 (sc-7297, 1:200), which recognizes both CD44s (standard form) and CD44v (variant form), NFκB (sc-372, 1:200) and cyclin D1 (sc-718, 1:200) were from Santa Cruz Biotechnology (Santa Cruz, CA); CD49f (3750, 1:1000), Notch1 (4380, 1:1000), c-Notch1 (4147, 1:1000), and pNFκB (3031, 1:1000) were from Cell Signaling Technology (Beverly, MA); CD24 (555426, 1:200) was from BD Biosciences (San Jose, CA), proliferating cell nuclear antigen (PCNA) (M0879, 1:400) was from Dako (Carpinteria, CA), and β-actin (A1978, 1:2000) was from Sigma-Aldrich (St. Louis, MO). Secondary antibodies were from Santa Cruz Biotechnology.
Quantitative Real-Time Polymerase Chain Reaction Analysis
These procedures have been reported previously (26, 27). The Taqman® probe-based gene expression system from Applied Biosystems (Foster City, CA) was used to detect the genes of interest.
Statistical analysis
Statistical significance was evaluated using the Student’s t-test.
Results
1α25(OH)2D3 and BXL0124 decrease the breast cancer stem cell population in MCF10DCIS cells
MCF10DCIS cells represent a basal-like or claudin-low breast tumor subtype, which typically carries a poor prognosis (28). These cells are primarily CD44+ and CD49f+. We first examined the addition of CD24 as a marker to further define subpopulations within MCF10DCIS cells. Breast cancer stem cells are thought to reside in the CD44+/CD24−/low and CD49f+/CD24−/low subpopulations of cells (29, 30). These populations were decreased with 1α25(OH)2D3 (100 nM) and BXL0124 (10 nM) treatment (Fig. 2). The CD44+/CD24−/low subpopulation was decreased from 68.7% in the control to 42.9% with 1α25(OH)2D3 (p < 0.01) and to 40.3% with BXL0124 treatment (p < 0.01). Similarly, the CD49f+/CD24−/low population was decreased from 69.0% to 37.6% with 1α25(OH)2D3 (p < 0.01) and to 39.7% with BXL0124 treatment (p < 0.05). Concomitantly, the CD44+/CD24high cell fraction was increased from 31.3% to 59.7% and 57.1% with 1α25(OH)2D3 and BXL0124 treatments, respectively, and the CD49f+/CD24high population was increased from 31.0% to 62.4% with 1α25(OH)2D3 and to 60.3% with BXL0124 treatment (Fig. 2). These data indicate that 1α25(OH)2D3 and BXL0124 treatments shift the CD44+/CD24−/low and CD49f+/CD24−/low subpopulations to populations that are more CD44+/CD24high and CD49f+/CD24high.
Figure 2. 1α25(OH)2D3 and BXL0124 repress the breast cancer stem cell population in MCF10DCIS cells.

MCF10DCIS cells were grown in monolayer cell culture and treated with 1α25(OH)2D3 (100 nM) and BXL0124 (10 nM) for 24 h. Cells were stained with combinations of antibodies against CD44 or CD49f and CD24, and then flow cytometry was performed. Representative histograms from flow cytometry of MCF10DCIS cells treated with vitamin D compounds are shown. Different subpopulations based on varying levels of CD24 are highlighted on the histograms with bold rectangles. The average percentage of CD44+/CD24−/low and CD49f+/CD24−/low subpopulations as well as the CD44+/CD24high and CD49f+/CD24high subpopulations from three independent experiments are represented as a bar graph to show the difference between the control and treatment groups. The data are presented as the mean ± S.E.M. * p < 0.05, **p < 0.01.
1α25(OH)2D3 and BXL0124 reduce the mammosphere forming efficiency and alter the mammosphere phenotype of MCF10DCIS cells
In mammosphere cultures, MCF10DCIS cells began to form colonies of 100 μm in size starting at day 3 (data not shown). The mammospheres continued to grow in size and number through days 4 and 5. Between days 5 and 7, the number of mammospheres began to decrease. We analyzed mammospheres at their peak of growth at days 4 and 5, as well as when their number began to decrease at day 7. Spheres that formed from MCF10DCIS cells were irregularly shaped and formless (Fig. 3A). When these spheres were grown in the presence of 1α25(OH)2D3 or BXL0124, they showed a more round and uniform shape (Fig. 3A). Spheres from the normal mammary epithelial cell line, MCF10A, appeared relatively round and uniform, and there was no significant change in the MCF10A mammosphere shape in the presence of 1α25(OH)2D3 or BXL0124 (Fig. 3A). As shown in figure 3B, treatment of MCF10DCIS mammospheres with 1α25(OH)2D3 or BXL0124 for 4, 5, and 7 days resulted in an overall increase in the roundness of spheres. Day 5 showed the most significant difference with an increase of 35.4% with 1α25(OH)2D3 (p < 0.01) and an increase of 35.6% with BXL0124 (p < 0.01). Treatment with 1α25(OH)2D3 or BXL0124 did not show significant changes in the roundness of spheres in MCF10A cells (Fig. 3B). The mammosphere forming efficiency (MFE) of MCF10DCIS was 0.12% and 0.12% at days 4 and 5 respectively, and by day 7 the MFE was reduced to 0.06% in the control. Treatment with 1α25(OH)2D3 significantly reduced the MFE of MCF10DCIS mammospheres at day 4 (38.8% inhibition, p < 0.01), day 5 (58.0% inhibition, p < 0.01), and day 7 (47.1% inhibition, p < 0.05) (Fig. 3C). The treatment with BXL0124 also inhibited the MFE of MCF10DCIS mammospheres at day 4 (40.8% inhibition, p < 0.01), day 5 (53.8% inhibition, p < 0.01), and day 7 (48.6% inhibition, p < 0.01) (Fig. 3C). Although 1α25(OH)2D3 and BXL0124 modestly affected the MFE in MCF10A cells, the changes were not statistically significant (Fig. 3C).
Figure 3. 1α25(OH)2D3 and BXL0124 inhibit the mammosphere formation of MCF10DCIS cells.
A. MCF10DCIS cells were plated at a density of 10,000 cells/mL in ultra-low attachment 6-well plates and grown for 4, 5 and 7 days in the presence of 1α25(OH)2D3 (100 nM) and BXL0124 (10 nM). MCF10A cells were plated at a density of 10,000 cells/mL in ultra-low attachment 6-well plates and growing them for 5 days. Representative pictures of MCF10DCIS and MCF10A mammospheres are shown for phenotypic comparison, scale bar 100 μm. B. Quantification of the roundness of the mammospheres is shown. A value of 1.0 represents an object that is perfectly round. A value of 0.0 represents an object that is formless. At least eight different mammospheres from three separate experiments were quantified to give the average measure of roundness. The data are presented as the mean ± S.E.M. * p < 0.05, **p < 0.01. C. Mammosphere forming efficiency (MFE) is shown. MFE was calculated by dividing the number of mammospheres (≥ 100 μm) formed by the number of cells seeded presenting this as a percentage. The data are presented as the mean ± S.E.M. (n=6). * p < 0.05, **p < 0.01.
1α25(OH)2D3 and BXL0124 inhibit the proliferation and decrease the expression of putative stem cell markers in MCF10DCIS mammospheres
MCF10DCIS mammospheres treated with vitamin D compounds demonstrated decreased cellular proliferation, as indicated by the reduction of protein levels of cyclin D1 and proliferating cell nuclear antigen (PCNA) (Fig. 4A). However, treatment of MCF10DCIS mammospheres with 1α25(OH)2D3 and BXL0124 did not induce apoptosis (Fig. 4B).
Figure 4. 1α25(OH)2D3 and BXL0124 decrease proliferation but do not affect apoptosis in MCF10DCIS mammospheres.
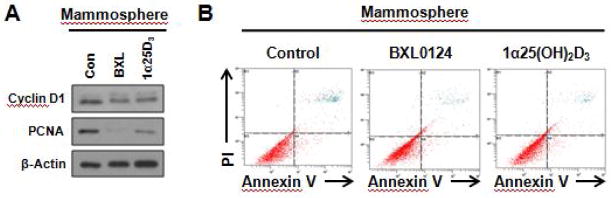
A. MCF10DCIS mammospheres were formed by plating 5,000 cells/mL in ultra-low attachment 6-well plates and growing them for 5 days. Western blot analysis was performed on mammospheres treated with DMSO control (Con), 1α25(OH)2D3 (1α25D3, 100 nM) or BXL0124 (BXL, 10 nM), and analyzed for markers of proliferation. B. MCF10DCIS mammospheres were formed by plating 5,000 cells/mL. Mammospheres were collected after 5 days of treatment with DMSO control, 1α25(OH)2D3 (100 nM), or BXL0124 (10 nM), and analyzed by flow cytometry after staining with Annexin V and propidium iodide (PI). Experiments were performed in duplicate and representative histograms from flow cytometry are shown.
Putative stem cell markers were analyzed to further characterize the effects that were observed in mammospheres. As early as day 4, treatment with vitamin D compounds showed a reduction in the protein levels of markers of stem cell maintenance and stem cell signaling molecules, such as CD44s (standard form) and CD44v (variant form), CD49f, cleaved-Notch1 (c-Notch1; the activated form of Notch1), and phosphorylated NFκB (pNFκB) (Fig. 5A). The reduction of these markers by vitamin D compounds persisted through days 5 and 7 of treatment, with the exception of pNFκB at day 7, which did not change upon treatment. The levels of total Notch1, NFκB, and CD24 were unaffected by treatment with vitamin D compounds (Fig. 5A).
Figure 5. 1α25(OH)2D3 and BXL0124 decrease the level of stem cell markers in MCF10DCIS mammospheres.
A. Western blot analysis was performed on mammospheres collected from 4, 5, and 7 days of treatment with DMSO control (Con), 1α25(OH)2D3 (1α25D3, 100 nM) or BXL0124 (BXL, 10 nM), and analyzed for markers associated with stem cell maintenance. qPCR analysis was performed on mammospheres harvested after 5 days of growth to assess the gene expression of markers associated with the stem cell phenotype (B), stem cell signaling (C), and genes related to pluripotency (D). The data are presented as the mean ± S.E.M. (n=6). *p < 0.05, **p < 0.01. Cycle numbers for qPCR are shown in parenthesis: CD44 (#22), ITGA6 (#23), ITGB6 (#25), LAMA5 (#24), CD24 (#24), NOTCH1 (#27), JAG1 (#26), JAG2 (#28), NFKB1 (#26), OCT4 (#29), GATA3 (#26), KLF4 (#28), SOX2 (#34), and MYC (#24).
The mRNA levels of key markers associated with stem cell maintenance, such as CD44, ITGA6, ITGB6, LAMA5, and CD24, were assessed at day 5. The expression of these markers decreased upon treatment with vitamin D compounds (Fig. 5B). Cell surface receptor CD44 expression was decreased by 65% and 73% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. Integrin ITGA6 expression was decreased by 63% and 72% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. Expression of another integrin, ITGB6, was decreased by 48% and 56% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. LAMA5 expression was decreased by 66% and 70% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. CD24 expression was modestly decreased by 26% and 40% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively.
Treatment with vitamin D compounds also reduced the expression of key molecules involved in stem cell signaling such as NOTCH1, Jagged ligands, and NFKB1 (Fig. 5C). Receptor NOTCH1 expression was decreased by 72% and 84% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. Ligand JAG1 expression was decreased by 54% and 65% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. Another ligand, JAG2 expression was decreased by 69% and 75% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. NFKB1 expression was decreased by 62% and 66% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively.
Genes involved in pluripotency and maintenance of the stem cell population were also suppressed, including OCT4 by 74% and 79% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively (Fig. 5D). Expression of GATA3, a transcription factor regulating luminal-epithelial differentiation, was decreased by 76% and 78% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. Transcription factor KLF4 (Kruppel-like factor 4) expression was decreased by 53% and 53% with 1α25(OH)2D3 (p < 0.01) and BXL0124 (p < 0.01), respectively. The expression of transcription factors SOX2 and MYC were slightly decreased by with 1α25(OH)2D3 and BXL0124.
1α25(OH)2D3 and BXL0124 decrease the self-renewal of MCF10DCIS mammospheres
Control spheres that formed from MCF10DCIS cells showed an irregular and formless shape in primary, secondary and tertiary mammospheres (Fig. 6A). When MCF10DCIS spheres were grown in the presence of 1α25(OH)2D3 (100 nM) or BXL0124 (10 nM), they showed a more round and uniform shape (Fig. 6A). The mammosphere forming efficiency (MFE) of MCF10DCIS primary mammospheres was significantly reduced upon treatment with 1α25(OH)2D3 (37.2% inhibition, p < 0.01) or BXL0124 (48.0% inhibition, p < 0.01) (Fig. 6B). Importantly, the MFE of mammospheres was increased from primary (0.24%) to secondary (0.50%) passages. Similar to the results with primary mammospheres, the MFE of secondary mammospheres was significantly repressed with 1α25(OH)2D3 (52.6% inhibition, p < 0.01) or BXL0124 (50.6% inhibition, p < 0.01). Tertiary mammospheres had a MFE of 0.56% in the control, and MFE was inhibited by 1α25(OH)2D3 (46.5% inhibition, p < 0.01) or BXL0124 (41.7% inhibition, p < 0.01) (Fig. 6B).
Figure 6. 1α25(OH)2D3 and BXL0124 inhibit the self-renewal of MCF10DCIS mammospheres.
A. MCF10DCIS cells were plated at a density of 5,000 cells/mL in ultra-low attachment 6-well plates and grown for 5 days with 1α25(OH)2D3 (100 nM) and BXL0124 (10 nM). Spheres were collected, dissociated, and re-plated at a density of 5,000 cells/mL for secondary and tertiary passages. Representative pictures of MCF10DCIS mammospheres are shown for phenotypic comparison (scale bar 100 μm). B. Mammosphere forming efficiency (MFE) of primary, secondary and tertiary passages of MCF10DCIS mammosphere is shown. MFE was calculated by dividing the number of mammospheres (≥ 100 μm) formed by the number of cells seeded presenting this as a percentage. Experiments were performed in duplicate and the data are presented as the mean ± S.E.M. **p < 0.01.
Discussion
Accumulating evidence has shown that breast cancer stem cells (BCSCs) are responsible for the initiation, maintenance, and progression of breast cancer. Typically fluorescence-activated cell sorting (FACS) has been used to identify populations of cancer cells that contain cancer stem cells (31). Different combinations of cell surface markers such as CD44, CD49f, CD24, and CD29 as well as the activity of certain enzymes such as aldehyde dehydrogenase isoform 1 (ALDH1) have been used to identify BCSCs (9). Mammosphere cell culture, which produces spherical colonies enriched in stem and progenitor cells, have also been widely used to study pathways and properties of stem and progenitor cells (32). Utilizing the cell surface markers, CD44, CD49f, and CD24, along with the mammosphere forming assay, we have shown that vitamin D compounds significantly decreased the putative BCSC population and reduced mammosphere formation. Our data, in part, contribute to the mechanism by which vitamin D compounds reduce breast tumor growth, and point to effects mediated at the level of putative breast cancer stem cells.
In this study, we found that the CD44+/CD24−/low stem cell enriched population of MCF10DCIS cells is shifted to a predominantly CD44+/CD24high population upon treatment with 1α25(OH)2D3 or BXL0124 (Fig. 2). Supporting this finding, the CD49f+/CD24−/low population was shifted to a primarily CD49f+/CD24high population upon treatment with 1α25(OH)2D3 or BXL0124 (Fig. 2). MCF10DCIS falls under the basal-like or claudin-low breast tumor subtype, which consist primarily of a CD44+ population (33–35). For this reason we attempted to utilize EpCAM as a secondary marker to further enrich for BCSCs in the primarily CD44+ and CD49f+ MCF10DCIS cell line. However, the cells were entirely EpCAM positive, so this marker did not further enhance the ability to distinguish a stem cell-like population in this cell line (data not shown). The shift to a CD24high population suggests that treatment with vitamin D compounds has the potential to specifically alter signaling of the putative BCSC subpopulation.
Treatment with vitamin D compounds suppressed mammosphere formation of MCF10DCIS cells (Fig. 3C). Since the mammosphere forming assay enriches for stem and progenitor cells, the reduction of MFE with vitamin D compounds could be partially due to the ability to suppress the putative CD44+/CD24−/low or CD49f+/CD24−/low BCSC population. In addition, treatment with vitamin D compounds reduced cell proliferation but did not induce apoptosis in MCF10DCIS mammospheres (Fig. 4A–B). The data suggest that the reduction of MFE by vitamin D compounds is primarily due to reduced growth of mammospheres and regulation of putative stem cell signaling pathways, rather than the killing of the BCSC-like cells. The irregular shape formed from MCF10DCIS cells grown in mammospheres was altered to a more round appearance by 1α25(OH)2D3 and BXL0124 treatment. This round shape is similar to the appearance of the normal MCF10A cell line. The shapes and MFE of MCF10A were unchanged with treatment from vitamin D compounds supporting the notion that these compounds do not significantly affect normal mammary cells. Although the change from irregular spheres to more round spheres has not traditionally been linked to stem cells phenotype, it has recently been reported with the chemotherapeutic drug, paclitaxel, in MCF-7 mammospheres (36). The overall effects on mammospheres indicate that treatment with vitamin D compounds could alter the characteristics of the stem cell population to that of a less malignant cell type.
To further investigate the role of 1α25(OH)2D3 and BXL0124 in the inhibition of stem cells, we analyzed markers commonly associated with stem cell maintenance and signaling. CD44 is a transmembrane glycoprotein which is involved in malignant progression and metastasis of breast cancer (37). Knockdown of CD44 induces differentiation and drives the BCSC-like population toward a non-BCSC-like phenotype (38). Knock down of α6-intergrin/ITGA6, also known as CD49f, causes a loss of the ability of cells to form mammospheres in the MCF-7 cell line, as well as reduced tumorigenicity in vivo, suggesting that CD49f is essential for the growth and survival of a more tumorigenic subpopulation of tumor cells (39). The Notch proteins are transmembrane receptors which are involved in the developmental fate of tissues and interact with a variety of ligands including jagged 1 and jagged 2 (40). NFκB has been implicated in cancer stem cell proliferation and has demonstrated to play a role in mammosphere formation and maintenance (41–43). Therefore, reduction of these markers by treatment with vitamin D compounds may contribute to the inhibition of tumorigenicity directed by putative stem cells. The basal levels of two markers critical in mammosphere formation, CD44s and c-Notch1, were expressed at higher levels at days 4 and 5 compared to day 7. The reduction of these markers at day 7 correlated with the reduction of the MFE of MCF10DCIS mammospheres at day 7 (Fig. 3C). As expected, sustained levels of signaling molecules associated with stem cells and mammosphere formation were critical in the maintenance of mammospheres over long periods of time.
Secondary and tertiary mammosphere formation was significantly inhibited by vitamin D compounds, suggesting the reduction of self-renewal ability of putative stem cells by the treatment (Fig. 6). We further investigated the role of vitamin D compounds on key transcription factors involved in the self-renewal of stem cells. Oct4 is a transcription factor that forms a heterodimer with Sox2 and regulates stem cell self-renewal capacity, and the knockdown of Oct4 promotes differentiation (44, 45). The transcription factor, Gata3, has been established as a critical regulator of luminal differentiation (46). The repression of pluripotency markers, such as OCT4, KLF-4, and GATA3, indicate that MCF10DCIS mammospheres treated with vitamin D compounds could potentially induce the differentiation of putative BCSCs.
Cancer progression, metastasis, and recurrence are significant problems in managing breast cancer. A significant body of evidence indicates that breast cancer stem cells drive these processes, complicating treatment strategies. A better understanding of how BCSCs drive breast cancer progression will aid in developing targeted therapies toward BCSCs. Our present study suggests a potential treatment strategy to reduce the putative BCSC population, and therefore enhance the effectiveness of breast cancer prevention and treatment through the use of vitamin D compounds.
Highlights.
Identifies a potential stem cell population in basal-like breast cancer
Characterizes breast cancer stem cell markers in mammosphere cell culture
Assesses the effects of vitamin D compounds on putative breast cancer stem cells
Suggests a novel approach to therapeutically target putative cancer stem cells
Acknowledgments
The authors would like to thank Dr. Philip Furmanski for his helpful suggestions. This work was supported in part by the National Institutes of Health National Cancer Institute R01 CA127645, the National Institute of Environmental Health Sciences Grant ES005022 and The Trustees Research Fellowship Program at Rutgers, The State University of New Jersey.
Abbreviations
- 1α
25(OH)2D3, 1α,25-dihydroxyvitamin D3
- BCSC
breast cancer stem cell
- DCIS
ductal carcinoma in situ
- GATA3
GATA binding protein 3
- IDC
invasive ductal carcinoma
- KLF4
Kruppel-like factor 4
- MFE
mammosphere forming efficiency
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- OCT4
octamer-binding transcription factor 4
- PCNA
proliferating cell nuclear antigen
- SOX2
SRY (sex determining region Y)-box 2
Footnotes
Conflicts of Interest: Authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100:1643–8. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowble B, Hanlon AL, Fein DA, Hoffman JP, Sigurdson ER, Patchefsky A, et al. Results of conservative surgery and radiation for mammographically detected ductal carcinoma in situ (DCIS) Int J Radiat Oncol Biol Phys. 1997;38:949–57. doi: 10.1016/s0360-3016(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 3.Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses’ Health Study. Cancer. 2005;103:1778–84. doi: 10.1002/cncr.20979. [DOI] [PubMed] [Google Scholar]
- 4.Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–4. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wicha MS. Targeting self-renewal, an Achilles’ heel of cancer stem cells. Nat Med. 2014;20:14–5. doi: 10.1038/nm.3434. [DOI] [PubMed] [Google Scholar]
- 8.Gangopadhyay S, Nandy A, Hor P, Mukhopadhyay A. Breast cancer stem cells: a novel therapeutic target. Clin Breast Cancer. 2013;13:7–15. doi: 10.1016/j.clbc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Ablett MP, Singh JK, Clarke RB. Stem cells in breast tumours: are they ready for the clinic? Eur J Cancer. 2012;48:2104–16. doi: 10.1016/j.ejca.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 11.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 12.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–9. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 14.Vaillant F, Asselin-Labat ML, Shackleton M, Lindeman GJ, Visvader JE. The emerging picture of the mouse mammary stem cell. Stem Cell Rev. 2007;3:114–23. doi: 10.1007/s12015-007-0018-2. [DOI] [PubMed] [Google Scholar]
- 15.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maund SL, Barclay WW, Hover LD, Axanova LS, Sui G, Hipp JD, et al. Interleukin-1alpha mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer Res. 2011;71:5276–86. doi: 10.1158/0008-5472.CAN-10-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, et al. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol. 2011;79:360–7. doi: 10.1124/mol.110.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Yao Y, Eades G, Liu Z, Zhang Y, Zhou Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2014;33:2589–600. doi: 10.1038/onc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahler J, So JY, Kim YC, Liu F, Maehr H, Uskokovic M, et al. Inhibition of the transition of ductal carcinoma in situ to invasive ductal carcinoma by a Gemini vitamin D analog. Cancer Prev Res (Phila) 2014;7:617–26. doi: 10.1158/1940-6207.CAPR-13-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 21.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 22.Rota LM, Lazzarino DA, Ziegler AN, LeRoith D, Wood TL. Determining mammosphere-forming potential: application of the limiting dilution analysis. J Mammary Gland Biol Neoplasia. 2012;17:119–23. doi: 10.1007/s10911-012-9258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maehr H, Lee HJ, Perry B, Suh N, Uskokovic MR. Calcitriol derivatives with two different side chains at C-20. V. Potent inhibitors of mammary carcinogenesis and inducers of leukemia differentiation. J Med Chem. 2009;52:5505–19. doi: 10.1021/jm900780q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So JY, Lin JJ, Wahler J, Liby KT, Sporn MB, Suh N. A Synthetic Triterpenoid CDDO-Im Inhibits Tumorsphere Formation by Regulating Stem Cell Signaling Pathways in Triple-Negative Breast Cancer. PLoS One. 2014;9:e107616. doi: 10.1371/journal.pone.0107616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, et al. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin Cancer Res. 2009;15:4242–9. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, et al. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72:332–43. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 27.So JY, Smolarek AK, Salerno DM, Maehr H, Uskokovic M, Liu F, et al. Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PloS one. 2013;8:e54020. doi: 10.1371/journal.pone.0054020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–81. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson RL, Yang WT, Rosen DG, Landis MD, Wong H, Lewis MT, et al. Cancer stem cell markers are enriched in normal tissue adjacent to triple negative breast cancer and inversely correlated with DNA repair deficiency. Breast Cancer Res. 2013;15:R77. doi: 10.1186/bcr3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer MJ, Fleming JM, Lin AF, Hussnain SA, Ginsburg E, Vonderhaar BK. CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer Res. 2010;70:4624–33. doi: 10.1158/0008-5472.CAN-09-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67:93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 32.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricardo S, Vieira AF, Gerhard R, Leitao D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937–46. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- 36.de la Mare JA, Sterrenberg JN, Sukhthankar MG, Chiwakata MT, Beukes DR, Blatch GL, et al. Assessment of potential anti-cancer stem cell activity of marine algal compounds using an in vitro mammosphere assay. Cancer Cell Int. 2013;13:39. doi: 10.1186/1475-2867-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 38.Pham PV, Phan NL, Nguyen NT, Truong NH, Duong TT, Le DV, et al. Differentiation of breast cancer stem cells by knockdown of CD44: promising differentiation therapy. J Transl Med. 2011;9:209. doi: 10.1186/1479-5876-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cariati M, Naderi A, Brown JP, Smalley MJ, Pinder SE, Caldas C, et al. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int J Cancer. 2008;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- 40.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 (Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shostak K, Chariot A. NF-kappaB, stem cells and breast cancer: the links get stronger. Breast Cancer Res. 2011;13:214. doi: 10.1186/bcr2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cioce M, Gherardi S, Viglietto G, Strano S, Blandino G, Muti P, et al. Mammosphere-forming cells from breast cancer cell lines as a tool for the identification of CSC-like- and early progenitor-targeting drugs. Cell Cycle. 2010;9:2878–87. [PubMed] [Google Scholar]
- 43.Liu M, Sakamaki T, Casimiro MC, Willmarth NE, Quong AA, Ju X, et al. The canonical NF-kappaB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res. 2010;70:10464–73. doi: 10.1158/0008-5472.CAN-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]
- 45.Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–7. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 46.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]





