Abstract
Background: The health impacts of climate change are an issue of growing concern in the Pacific region. Prior to 2010, no formal, structured, evidence-based approach had been used to identify the most significant health risks posed by climate change in Pacific island countries. During 2010 and 2011, the World Health Organization supported the Federated States of Micronesia (FSM) in performing a climate change and health vulnerability and adaptation assessment. This paper summarizes the priority climate-sensitive health risks in FSM, with a focus on diarrheal disease, its link with climatic variables and the implications of climate change.
Methods: The vulnerability and adaptation assessment process included a review of the literature, extensive stakeholder consultations, ranking of climate-sensitive health risks, and analysis of the available long-term data on climate and climate-sensitive infectious diseases in FSM, which involved examination of health information data from the four state hospitals in FSM between 2000 and 2010; along with each state’s rainfall, temperature and El Niño-Southern Oscillation data. Generalized linear Poisson regression models were used to demonstrate associations between monthly climate variables and cases of climate-sensitive diseases at differing temporal lags.
Results: Infectious diseases were among the highest priority climate-sensitive health risks identified in FSM, particularly diarrheal diseases, vector-borne diseases and leptospirosis. Correlation with climate data demonstrated significant associations between monthly maximum temperature and monthly outpatient cases of diarrheal disease in Pohnpei and Kosrae at a lag of one month and 0 to 3 months, respectively; no such associations were observed in Chuuk or Yap. Significant correlations between disease incidence and El Niño-Southern Oscillation cycles were demonstrated in Kosrae state.
Conclusions: Analysis of the available data demonstrated significant associations between climate variables and climate-sensitive infectious diseases. This information should prove useful in implementing health system and community adaptation strategies to avoid the most serious impacts of climate change on health in FSM.
Keywords: infectious diseases, climate, Federated States of Micronesia
Introduction
Pacific island countries (PICs) are among the most vulnerable in the world to the effects of climate change, including the likely detrimental impacts on human health [1, 2]. These impacts are significant, measurable and far-reaching: it is estimated that over the last decade, between 100,000 and 200,000 deaths annually worldwide were attributable to the effects of climate change [3]. In the Pacific region, growing concern about climate change and health led to the formulation of the Regional Framework for Action to Protect Human Health from Effects of Climate Change in the Asia-Pacific Region by the World Health Organization (WHO) in 2008 [4] and prompted the Pacific island Health Ministers to prioritize action on climate change and health at their biennial meeting in 2009 [5]. These regional mandates provided the impetus for an ambitious program of work, led by the WHO South Pacific office, with support from the WHO Western Pacific Regional Office and funding from the governments of the Republic of Korea and Japan, to assess the vulnerability of PICs to the impact of climate change on health and plan appropriate adaptation strategies to minimize these risks.
The Federated States of Micronesia (FSM) was one of eleven countries involved in this WHO-supported climate change and health project in the Pacific. FSM is a small island developing state in the northern Pacific, comprised of four states – Yap, Chuuk, Pohnpei and Kosrae (see Map 1).
Map 1.
Federated States of Micronesia (source: http://www.fsmgov.org/info/maplg.gif)
A summary of key population and health indicators for FSM is provided in Table 1.
Table 1.
Key population and health indicators for FSM
| Indicator | Total |
|---|---|
| Land areaa (square kilometres) | 704.6 - Chuuk: 127 - Kosrae: 110 - Pohnpei: 345 - Yap: 118 |
| Population – total and distributionb | 102 624 - Chuuk: 49% - Kosrae: 8% - Pohnpei: 32% - Yap: 11% |
| Key health indicatorsb - life expectancy (at birth) - infant mortality rate - under 5 mortality rate |
69 13.5/1000 live births 39/1000 live births |
| Leading causes of morbidity (inpatient)b | Hypertension Diarrhea/gastroenteritis Diabetes mellitus Skin disorders Urinary tract infection |
| Leading causes of mortalityb | Myocardial infarction Diabetes mellitus Chronic obstructive pulmonary disease Cerebrovascular accident |
| Top three communicable disease categories (burden of disease, by incidence)b | Acute upper respiratory infections Influenza-like illness Diarrhea/gastroenteritis |
| Top three non-communicable diseases (burden of disease, by prevalence)b | Hypertension Diabetes mellitus Cardiovascular disease |
Sources: a) FSM Government website (http://www.fsmgov.org/info/geog.html)
b) WHO Country Health Information Profile for FSM (2011) (http://www.wpro.who.int/countries/fsm/17MICtab2011_finaldraft.pdf?ua=1)
The key climate change phenomena expected to occur in FSM include [6]: accelerating sea-level rise and ocean acidification; increasing air and sea-surface temperatures; more very hot days; altered rainfall patterns (with more extreme rainfall events and decreased drought frequency); and possibly more severe typhoons.
In FSM, prior to the commencement of the WHO project, climate change and health considerations had been included in several key high-level national policy frameworks, including the Nationwide Climate Change Policy (2009), the Second National Communication to the United Nations Framework Convention on Climate Change (UNFCCC), and the National Strategic Development Plan for 2003–2023. This previous work noted that climate variability and change, including sea-level rise, are important determinants of health and are of growing concern in FSM (as is the case in all Pacific Island countries), with the impacts expected to be mostly adverse. However, these preceding efforts toward health vulnerability assessments lacked formal health sector and expert technical input.
Thus, the purpose of this project was to assess more formally the key climate-sensitive health risks for FSM, based on a review of the relevant literature, in-country consultations and analysis of available climate and health data, and to provide an evidence-based framework for climate change and health adaptation, as the health sector’s contribution towards national adaptation planning (or HNAP).
This paper summarizes the methodology and results of this climate change and health vulnerability assessment for FSM, with a focus on climate-sensitive infectious diseases, which were ranked as the highest priority climate-sensitive health risks in FSM as a result of this assessment process. The paper also provides an insight into the scientific basis for implementation of adaptation strategies to reduce or avoid the most serious impacts of climate change on the burden of these diseases in FSM.
Methods
The process for assessing FSM’s vulnerabilities and planning adaptation strategies related to the health impacts of climate change broadly followed the guidelines set out by WHO and others [7–11]. These steps are summarized in Box 1.
Box 1.
Steps in assessing vulnerability and adaptation (Source: Kovats et al., 2003 [11]).
| 1. | Determine the scope of the assessment |
| 2. | Describe the current distribution and burden of climate-sensitive diseases |
| 3. | Identify and describe current strategies, policies and measures that reduce the burden of climate-sensitive diseases |
| 4. | Review the health implications of the potential impact of climate variability and change on other sectors |
| 5. | Estimate the future potential health impact using scenarios of future climate change, population growth and other factors and describe the uncertainty |
| 6. | Synthesise the results and draft a scientific assessment report |
| 7. | Identify additional adaptation policies and measures to reduce potential negative health effects, including procedures for evaluation after implementation |
In FSM, this process incorporated both qualitative and quantitative elements. These included stakeholder consultations, community surveys, expert consensus and analysis of the available climate and health data to describe, in some detail, the relationships between climate variables and climate-sensitive diseases in each country.
The climate change and health vulnerability and adaptation assessment process in FSM commenced in 2010, with a project—led by the Department of Health and Social Affairs and supported by WHO—aimed at improving understanding of the relationship between climate and disease in the four States of FSM and compiling a National Climate Change and Health Action Plan (NCCHAP). This project involved a WHO team assisting the Department of Health and Social Affairs over three distinct phases of work between 2010 and 2011, with the participation of multiple in-country partners including, inter alia, the Office for Environment and Emergency Management (OEEM), the Environmental Protection Agency (EPA) and the Weather Service Office (WSO).
The first phase of the project was a regional plenary meeting, conducted in Pohnpei in early 2010, which included representatives from the neighbouring countries of Palau and the Republic of the Marshall Islands who were conducting similar WHO-supported national vulnerability and adaptation assessment projects.
In the first and second phases of the project, a review of health sector reports and data, combined with extensive consultation with stakeholders in FSM and the guidance of the WHO team of experts, revealed a list of priority climate-sensitive health risks of concern in the country. These climate-sensitive health risks were then ranked according to a “likelihood versus impact” matrix, which has proved useful in environmental health impact assessments elsewhere, including in the context of climate change and health [12, 13]—see Table 2 below.
Table 2.
Matrix used to assess climate-sensitive health risks in FSM, in terms of their likelihood and impact
| Likelihood | Impact (Considering consequence and coping
capacity) |
||||
|---|---|---|---|---|---|
| Insignificant | Minor | Moderate | Major | Catastrophic | |
| Almost Certain | Medium | Medium | High | Extreme | Extreme |
| Likely | Low | Medium | High | High | Extreme |
| Possible | Low | Medium | Medium | High | High |
| Unlikely | Low | Low | Medium | Medium | Medium |
| Rare | Low | Low | Low | Low | Medium |
The actors involved in the participatory action process of consensus-building regarding the priority climate-sensitive health risks in FSM are listed in Table 3.
Table 3.
Actors involved in participatory decision-making process in FSM
| Actors | FSM |
|---|---|
| Coordination | Office for the Environment and Emergency Management Department of Health and Social Affairs WHO |
| Participation | Environmental Protection Agency Weather Service Office Department of Resources and Development Department of Agriculture State health and environment services Island Food Community* |
* Non-governmental organization (NGO)
The process of prioritization of climate-sensitive health risks of concern in FSM placed an emphasis on infectious diseases, which were thus the focus of the quantitative analysis that followed.
The climate-sensitive disease data from the four State hospital records (inpatient and outpatient) between 2003 and 2010 were collected from the Health Information Department. Hospital records include sex, age and diagnosis coded by the International Classification of Diseases, version 10 (ICD-10). These records represent the most complete health datasets available on a routinely collected basis in FSM, apart from a complementary, Pacific-wide syndromic surveillance system (specific to for four categories of communicable disease) overseen by WHO. Thus it is assumed that these represent close to all of the reported cases; the proportion of unreported cases is unknown.
Weather data were collected from the WSO. The individual patient data were collated into daily all-cause and cause-specific counts and combined with daily weather data, with this study focusing on the aforementioned priority climate-sensitive infectious diseases.
Time series distribution of monthly average of the daily number of inpatients and outpatients in each state were plotted along with weather data. Monthly averages of daily maximum temperatures were computed; these and total monthly rainfall were used for the subsequent analyses. Time series analysis of the three climate-sensitive infectious diseases deemed to be the highest risk were then performed [dengue fever (ICD-10: A90-A91), diarrheal illness (ICD-10: A00-A09) and leptospirosis (ICD-10: A27)].
The association with the El Niño-Southern Oscillation (ENSO), a source of inter-annual climate variability, was also examined for each disease category. The strength of the ENSO was measured by sea-surface temperature anomalies in the Niño 3 region (NINO3) in the Pacific Ocean, which were derived from NOAA Climate Prediction Center data (http://www.cpc.ncep.noaa.gov).
Generalized linear Poisson regression models allowing for over-dispersion were used to examine the relationship between weather variables (temperature and rainfall) and NINO3 variability and the number of cause-specific patient presentations at different monthly lags (0, 1, 2 and 3 months), with a focus on outpatients. This analytical technique was selected based on historical and scientific precedents for its use in comparable studies [14]. To identify the broad shape of any association, we fitted natural cubic splines (3df) to the weather variables and NINO3. The temperature, rainfall and NINO3 terms were separately incorporated into the model. As there was no clear seasonal trends observed in disease incidence, seasonality was not controlled in the model. Overall association for each disease-weather pattern was tested using Wald test. Any missing data was treated as missing; no interpolation has been conducted to fill the missing values. All statistical analyses were carried out using Stata 10.0 (Stata Corporation, College Station, Texas).
The results of the vulnerability assessment were then used to compile a hierarchy of adaptation strategies for the health sector, and all of this information was collated into the FSM National Climate Change and Health Action Plan (NCCHAP), which was presented at the inaugural FSM Climate Change and Health Symposium in Pohnpei in December 2011.
The key findings and recommendations from the FSM NCCHAP and the companion documents for the other ten PICs included in the WHO-led project have subsequently been synthesized into a forthcoming WHO report on climate change and health in the Pacific region, which will be launched in late 2014.
Results
Review of the relevant data and extensive consultation with stakeholders, primarily from government departments, in FSM between 2010 and 2011, in combination with a review of the literature (the specific methodology and results of which are not shown here) and the expert opinions of the WHO consultant team, yielded the following table of climate-sensitive health vulnerabilities (Table 4), ranked according to their risk (in terms of likelihood versus impact—see Table 2 above).
Table 4.
List of climate change and health vulnerabilities in FSM
| Climate-sensitive disease | Risk (likelihood versus impact) |
|---|---|
| Diarrheal diseases (water- and food-borne) | High |
| Vector-borne diseases (principally arboviruses such as dengue fever)* | High |
| Zoonoses (primarily leptospirosis) | High |
| Malnutrition | High |
| Non-communicable diseases | Medium |
| Mental health | Medium |
| Respiratory diseases | Medium |
| Skin disease | Medium |
| Poverty and socio-economic disadvantage | Medium |
| Traumatic injuries and deaths | Low |
| Ciguatera** | Low |
* Lymphatic filariasis and malaria were also considered under the heading of vector-borne diseases, but were deemed to represent significantly lower risks than arboviruses in the context of climate change in FSM (see below).
** Ciguatera is a toxidrome caused by a dinoflagellate organism which bio-accumulates in the marine food chain. Humans typically contract ciguatera through consumption of contaminated reef fish.
While allowing for the fact that the list in Table 4 is based on a combination of health information review, consultation and expert consensus, this nevertheless indicates that the predominant climate-sensitive health risks of concern in FSM are likely to be infective in nature. The process of quantitative analysis therefore focused on three categories of climate-sensitive infectious diseases: diarrheal illness, vector-borne diseases and leptospirosis. This analysis was attempted despite the paucity of relevant health data, as this was the express mandate of the climate change and health vulnerability assessment project, as well as being the preferred methodological approach of WHO and the project partners in FSM.
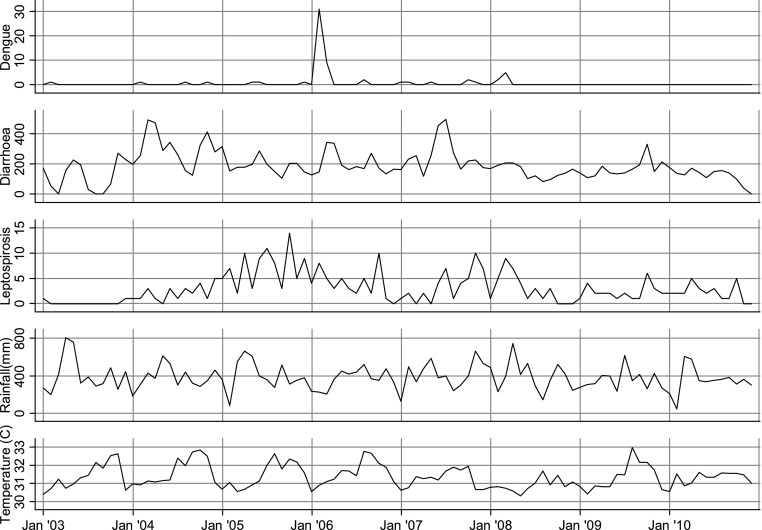
Time series of monthly average of daily dengue, diarrhea and leptospirosis inpatients showed no obvious trend or seasonality (the results for Pohnpei state are shown in Fig. 1).
Fig. 1.
Number of dengue, diarrhea and leptospirosis outpatients per month and weather variables (total rainfall and average temperature) in Pohnpei
As can be seen from Figure 1, there were substantial gaps in the data for all three disease categories, as was the case for the other three states. This apparently reflects intermittent lapses in health information capacity within the Department of Health and Social Affairs in each of the states over the period.
There were also generally low rates of dengue fever and leptospirosis in all four states, with less than 0.5 cases occurring on average per day (i.e. approximately <15 cases per month) in each state. It should be noted that, while diarrheal disease and leptospirosis are considered endemic in FSM, dengue fever typically occurs in infrequent but severe epidemics [15, 16]. Given these very small numerators, along with the infeasibility of aggregating all the cases for correlation with climate variables given the significantly asynchronous meteorological patterns between states, no further environmental epidemiological analysis of dengue fever and leptospirosis was undertaken in this study.
There may be an apparent threshold effect for increased cases of diarrheal illness in Pohnpei at a lag of one month following monthly maximum temperatures of ≥ 32–33°C (see Fig. 2b).
Fig. 2.
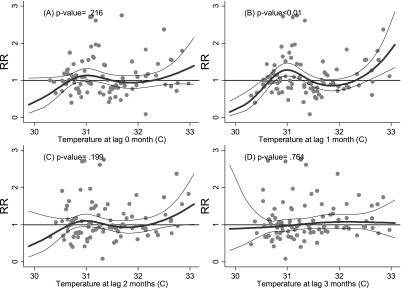
Relationship between relative risk (RR) of diarrhea scaled to the mean monthly number of outpatients in Ponhpei and maximum temperature (shown as a 3 d.f. natural cubic spline) at lags of 0, 1, 2 and 3 months. The center line in each graph shows the estimated spline curve, and the upper and lower lines represent the 95% confidence limits. P-values represent the level of significance of the association between diarrhea and temperature.
The corresponding analysis for Kosrae state showed a similar effect of high temperature (> 32°C) at lags of 0 and 1 month, although the relationship was weaker than that observed for Pohnpei. In addition, a negative relationship between temperature and diarrhea cases was observed in Kosrae below 31°C (see Fig. 3). It is possible that different pathogens contribute to the two curves or slopes of this apparently U-shaped relationship.
Fig. 3.
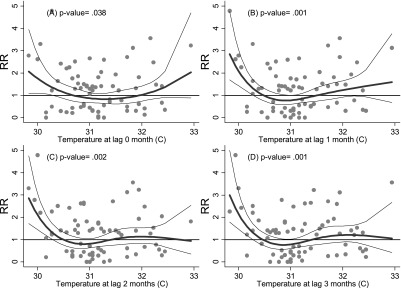
Relationship between relative risk (RR) of diarrhea scaled to the mean monthly number of outpatients in Kosrae and maximum temperature (shown as a 3 d.f. natural cubic spline) at lags of 0, 1, 2 and 3 months. The center line in each graph shows the estimated spline curve, and the upper and lower lines represent the 95% confidence limits. P-values represent the level of significance of the association between diarrhea and temperature.
The analysis was repeated for rainfall, but no significant relationship was found in any of the four states (results not shown).
Diarrheal illness was also correlated with NINO3 at different monthly lags, with an apparently statistically significant, roughly U-shaped relationship demonstrated for Kosrae (Fig. 4), but no statistically significant results were found for the other three states.
Fig. 4.
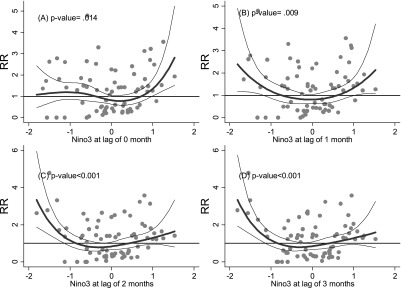
Relationship between relative risk (RR) of diarrhea scaled to the mean monthly number of outpatients in Kosrae and Nino3 (shown as a 3 d.f. natural cubic spline) at lags of 0, 1, 2 and 3 months. The center line in each graph shows the estimated spline curve, and the upper and lower lines represent the 95% confidence limits. P-values represent the level of significance of the association between diarrhea and Nino3.
Discussion
This study revealed that the principal health risks posed by climate change in FSM include a number of climate-sensitive infectious diseases. Of these, diarrheal disease has been shown to be associated with climatic factors such as temperature and the ENSO index in at least two of the states of FSM.
The following discussion therefore focuses on climate-sensitive infectious diseases, particularly diarrheal disease, given the high level of priority given to these issues in the climate change and health vulnerability assessment for FSM.
Some important notes on the abovementioned categories of “climate-sensitive health risks” are as follows: with respect to vector-borne diseases, the only long-term data available for analysis was for dengue fever, which has been known to exist in FSM since at least the early 1990s [15], despite the fact that, at least in recent years, FSM has been plagued by other arboviruses including Zika virus [17] and chikungunya. FSM has also long been considered endemic for lymphatic filariasis, although the burden of this disease is decreasing, as elsewhere in the Pacific, due to mass drug administration and vector control programs [18]. FSM is not currently one of the PICs considered endemic for malaria; while the possibility remains that will climate change will affect the geographic range of the malaria vector, causing intrusion into non-endemic countries, this is currently considered to be a relatively low risk for FSM.
Secondly, “diarrheal illness” is a broad category of disease which obviously is not limited to infectious pathogens; nor are the infectious aetiologies limited to those transmitted via food and water (i.e. the modes of transmission considered most likely to be sensitive to environmental perturbations). Nevertheless, given the significant burden of disease due to diarrheal illness in FSM, particularly in children under five [19] and the strong evidence linking diarrheal illness to climatic factors such as temperature, rainfall, ENSO cycles and hydrometeorological disasters in the Pacific region and elsewhere in the world [20–25], it was considered justifiable to aggregate diarrheal illnesses for the purposes of this analysis.
As a final note, the category of “respiratory disease” was not included in this study focusing on climate-sensitive infectious diseases due to the fact that, while it may be assumed that this category includes respiratory infections (both acute illness like influenza and pneumonia, and chronic infections such as tuberculosis), it also includes non-infectious illnesses such as asthma and chronic obstructive airways disease. The latter constitute a significant cause of morbidity and mortality in FSM, particularly in adults [19], and while obstructive airways diseases, including asthma, may certainly be considered sensitive to changes in climate [26–28], as a non-communicable disease (NCD) it has not been included in this infectious disease-focused paper. The same principle applies to skin diseases: it was not deemed feasible or useful to attempt to differentiate infectious and non-infectious skin disorders for the purposes of this paper.
The outcomes of the climate change and health vulnerability assessment in FSM are broadly consistent with those of other PICs [12, 29, 30], with relatively high priorities given to climate-sensitive infectious diseases, but concern was also raised regarding the prospect of climate change-induced impacts on NCDs, malnutrition, ciguatera, mental health, the health consequences of extreme weather events and disruptions to health and social services.
A summary of the overall climate change and health vulnerability and adaptation assessment process and key findings for FSM and thirteen other PICs can be found in a forthcoming WHO report entitled “Human Health and Climate Change in Pacific Small Island States”, to be launched in late 2014.
With respect to climate-sensitive infectious diseases and their relationship with climate in the context of FSM, the paucity of relevant disease data limited opportunities for the analysis described above and efforts to demonstrate statistically significant associations between climate variables and the burden of the pre-eminent diseases of concern in FSM (diarrheal illness, vector-borne diseases and leptospirosis).
Nevertheless, there is abundant evidence from elsewhere in the region and around the world supporting the “climate-sensitivity” of these diseases and vindicating their inclusion among the highest priority climate-sensitive health risks in FSM, despite the fact that dengue fever and leptospirosis currently represent relatively small burdens of disease in the country.
Vector-borne diseases in general, and dengue fever in particular, have been shown to be exquisitely sensitive to hydrometeorological phenomena, including temperature, rainfall, humidity and ENSO [31–37], including in the Pacific region [38, 39], where recent attention has shifted towards the potential for climate-based early warning systems to minimize the impact of dengue fever epidemics [40].
In the case of leptospirosis, the links with ecological and meteorological factors are also relatively well-established [41–43], the burden of disease in FSM is becoming more clear [44], and the potential for early warning systems is gaining attention in the Pacific.
There is a similarly strong case to be made for the climate-sensitivity of diarrheal illness, as pointed out above. Although the pathways by which factors such as temperature, rainfall, ENSO and extreme events may affect the multiple pathogens causing infectious diarrhea create a complex aetiological picture [20, 24, 45–49], as shown by our results, a significant association can be observed between climatic factors such as temperature and the incidence of diarrheal disease, at least in Pohnpei and Kosrae states. This is relevant in FSM, and neighbouring Micronesian countries where both food- and water-borne pathogens have been known to cause large outbreaks of diarrheal illness in recent years [50, 51].
The lack of robust, long-term data on these three categories of climate-sensitive infectious diseases limited the extent to which detailed “exposure-response” models could be constructed for each of the four states. Additionally, the heterogeneity of the climate-disease relationships precluded, at least in part, the potential for aggregation and/or averaging at the national level. Nevertheless, it was still deemed useful to consider, at least in a general, qualitative sense, the current and likely increased future climate change-attributable burden of these climate-sensitive infectious diseases in FSM, with respect to the opportunity for implementation of various adaptation strategies at the local, state and national levels.
The recommendations for health sector adaptation in relation to these three high-priority climate-sensitive infectious diseases in FSM include:
community education and health promotion campaigns (e.g. on preventive behaviours such as protection against mosquito bites or contact with contaminated water and soil, including the risk inherent in cultural practices such as communal consumption of sakau [kava]);
distribution of household equipment such as mosquito nets, safe water storage containers and water testing and treatment kits;
increased recruitment and training of public and environmental health officers in the areas of water and food safety, animal health, vector surveillance and outbreak response;
expansion of public and environmental health surveillance and control activities to outer islands (currently neglected due to lack of sufficient resources);
policy, legislative and regulatory measures targeting water and food safety, mosquito control (particularly habitat eradication) and improved hygiene and management of domestic livestock (particularly pigs);
scale-up of diagnostic capacity, including improved microbiological capabilities, and increased use of rapid test kits for dengue fever and leptospirosis;
health professional capacity-building in the fields of diagnosis, management and prevention of these climate-sensitive infectious diseases, as well as in applied environmental epidemiological techniques and the use of environmental health indicators in relation to climate and health [52];
increased research on the epidemiology, burden of disease and climate-sensitivity of infectious diseases in FSM and elsewhere in Micronesia and the wider Pacific region; and
consideration of the use of climate-based early warning systems for infectious diseases in FSM.
The latter recommendation regarding climate-based early warning systems (CBEWS) is common in the literature on climate change and health adaptation [53–57]. In FSM, this process is clearly impeded by the abovementioned data and model constraints. However, even with the limited data and models available for infectious diseases in FSM, it may be possible to construct a CBEWS for diarrheal disease based on the analysis and results described in this paper.
With reference to Figure 4, for example, it can be seen that the relative risk (RR) of diarrheal incidence in Pohnpei appears to increase beyond a temperature threshold of approximately 32.5 degrees Celsius in the previous month. It thus could prove feasible for a collaboration between the WSO and Pohnpei Department of Health Services to establish a mechanism for the issuing of alerts when the average maximum temperature in a given month, or four-week sliding window, reaches 32.5 degrees, which triggers a “surge” response of public and environmental health interventions targeting, for example, water and food safety and community health promotion. The efficacy of such interventions could then be analyzed epidemiologically, and the exposure-response models updated, as the time-series of climate and disease data is extended over time.
Apropos of the latter recommendation, it should also be pointed out that all of the analyses and models discussed above could and should be updated over time, and the NCCHAP—including the theory and assumptions contained within it—should undergo similar reiterations to incorporate contemporary data and improved knowledge of the associations and implications of climate change and the high-priority climate-sensitive infectious diseases in FSM.
Conclusions
Infectious diseases were identified as among the highest priority climate-sensitive health risks of concern in FSM as part of the national climate change and health vulnerability assessment and adaptation planning process. Specifically, diarrheal disease, dengue fever (and other vector-borne diseases) and leptospirosis were considered to represent high risks with respect to future climate change-attributable burdens of disease in FSM.
Analysis of the available data on historical climate and cases of infectious diseases, although limited, yielded some potentially useful associations between climate variables and diarrheal disease in particular, which may have application in the context of a climate-based early warning system and the potential for public and environmental health interventions to limit the impact of near-term epidemics.
Adaptation strategies recommended in the FSM National Climate Change and Health Action Plan similarly prioritize climate-sensitive infectious diseases; successful implementation of any number of these measures may reduce or avert the most severe detrimental effects of climate change on these and other infectious diseases and their impact on the health of communities in FSM and the wider Micronesia and Pacific regions.
Acknowledgements
The authors wish to acknowledge the invaluable contributions of Mr Kamal Khatri and Dr Vita Skilling, and the support from the Governments of Japan and Korea in providing the funding for this project.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Woodward A, Hales S, Weinstein P. Climate change and human health in the Asia Pacific region: who will be most vulnerable? Clim Res 1998; 11: 31–38. [Google Scholar]
- 2.Ebi KL, Lewis ND, Corvalan C. Climate Variability and Change and Their Potential Health Effects in Small Island States: Information for Adaptation Planning in the Health Sector. Environ Health Perspect 2006; 114: 1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patz Ja, Campbell-Lendrum D, Holloway T, et al. . Impact of regional climate change on human health. Nature 2005; 438: 310–317. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization, Western Pacific Regional Office, Manila P. Regional Framework for Action to Protect Human Health from Effects of Climate Change in the Asia-Pacific Region. Manila, Philippines: Western Pacific Regional Office; 2007. pp. 1–4. [Google Scholar]
- 5.WHO and Secretariat for the Pacific Community. Madang Commitment 2009; 13–14. [Google Scholar]
- 6.Program PCCS. Climate Change in the Pacific: Scientific Assessment and New Research - Country Report for Kiribati. 2011, Two. [Google Scholar]
- 7.Ebi KL, Kovats RS, Menne B. An Approach for Assessing Human Health Vulnerability and Public Health Interventions to Adapt to Climate Change. Environ Health Perspect 2006; 1930–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardekker JA, de Jong A, van Bree L, et al. . Health risks of climate change: An assessment of uncertainties and its implications for adaptation policies. Environ Health 2012; 11: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patz J, Campbell-Lendrum D, Gibbs H, et al. . Health impact assessment of global climate change: expanding on comparative risk assessment approaches for policy making. Annu Rev Public Health 2008; 29: 27–39. [DOI] [PubMed] [Google Scholar]
- 10.Haines A, Kovats RS, Campbell-Lendrum D, et al. . Climate change and human health: impacts, vulnerability and public health. Public Health 2006; 120: 585–596. [DOI] [PubMed] [Google Scholar]
- 11.Kovats R, Ebi K, Menne B. Methods of assessing human health vulnerability and public health adaptation to climate change. Pan Am Health 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spickett JT, Katscherian D, McIver L. Health Impacts of Climate Change in Vanuatu: An Assessment and Adaptation Action Plan. Glob J Health Sci 2013; 5: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spickett JT, Brown HL, Katscherian D. Adaptation strategies for health impacts of climate change in Western Australia: Application of a Health Impact Assessment framework. Environ Impact Assess Rev 2011; 31: 297–300. [Google Scholar]
- 14.Bhaskaran K, Gasparrini A, Hajat S, et al. . Time series regression studies in environmental epidemiology. Int J Epidemiol 2013; 42: 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage HM, Fritz CL, Rutstein D, et al. . Epidemic of dengue-4 virus in Yap State, Federated States of Micronesia, and implication of Aedes hensilli as an epidemic vector. Am J Trop Med Hyg 1998; 58: 519–524. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Dengue Outbreak—Federated States of Micronesia, 2012–2013. MMWR Morb Mortal Wkly Rep 2013; 62: 570–573. [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy MR, Chen T-H, Hancock WT, et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360: 2536–2543. [DOI] [PubMed] [Google Scholar]
- 18.Burkot T, Ichimori K. The PacELF programme: will mass drug administration be enough? Trends Parasitol 2002; 18: 109–115. [DOI] [PubMed] [Google Scholar]
- 19.Samo M, Elymore A. Mortality analysis of registered deaths in the FSM from 1990–2003. Pac Health Dialog 2010; 16: 115–122. [PubMed] [Google Scholar]
- 20.Wu J, Yunus M, Streatfield PK, et al. . Association of climate variability and childhood diarrheal disease in rural Bangladesh, 2000–2006. Epidemiol Infect 2014; 142: 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashizume M, Armstrong B, Hajat S, et al. . Association between climate variability and hospital visits for non-cholera diarrhea in Bangladesh: effects and vulnerable groups. Int J Epidemiol 2007; 36: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 22.Singh RB, Hales S, de Wet N, et al. . The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environ Health Perspect 2001; 109: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leclerc H, Schwartzbrod L, Dei-Cas E. Microbial agents associated with waterborne diseases. Crit Rev Microbiol 2002; 28: 371–409. [DOI] [PubMed] [Google Scholar]
- 24.Hashizume M, Wagatsuma Y, Faruque ASG, et al. . Factors determining vulnerability to diarrhea during and after severe floods in Bangladesh. J Water Health 2008; 6: 323–332. [DOI] [PubMed] [Google Scholar]
- 25.Checkley W, Epstein LD, Gilman RH, et al. . Effects of El Niño and ambient temperature on hospital admissions for diarrheal diseases in Peruvian children. Lancet 2000; 355: 442–450. [DOI] [PubMed] [Google Scholar]
- 26.Jouret J. Respiratory implications of a changing climate. Lancet Respir Med 2013; 1(3): 196. [DOI] [PubMed] [Google Scholar]
- 27.Beggs PJ, Bambrick HJ. Is the Global Rise of Asthma an Early Impact of Anthropogenic Climate Change? Environ Health Perspect 2005; 113: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaro TK, Knowlton K, Balmes JR. Climate change and respiratory health: current evidence and knowledge gaps. Expert Rev Respir Med 2013; 7: 349–361. [DOI] [PubMed] [Google Scholar]
- 29.Mciver L, Woodward A, Davies S, et al. . Assessment of the Health Impacts of Climate Change in Kiribati. Int J Environ Res Public Health 2014; 11(5): 5224–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIver L. Climate change and health in the Pacific: cause for concern; opportunities for adaptation. Inform’Action 2012; 36: 3–6. [Google Scholar]
- 31.Hii YL, Zhu H, Ng N, et al. . Forecast of Dengue Incidence Using Temperature and Rainfall. PLoS Negl Trop Dis 2012; 6: e1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto E, Coelho M, Oliver L, et al. . The influence of climate variables on dengue in Singapore. Int J Environ Health Res 2011; 21: 415–426. [DOI] [PubMed] [Google Scholar]
- 33.Morin CW, Comrie AC, Ernst K. Climate and Dengue Transmission: Evidence and Implications. Environ Health Perspect 2013; 121: 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheong Y, Burkart K, Leitão P, et al. . Assessing Weather Effects on Dengue Disease in Malaysia. Int J Environ Res Public Health 2013; 10: 6319–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H.-Y., Fu X, Lee LKH, et al. . Statistical Modeling Reveals the Effect of Absolute Humidity on Dengue in Singapore. PLoS Negl Trop Dis 2014; 8: e2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosa-Freitas MG, Schreiber KV, Tsouris P, et al. . Associations between dengue and combinations of weather factors in a city in the Brazilian Amazon. Rev Panam Salud Publica 2006; 20: 256–267. [DOI] [PubMed] [Google Scholar]
- 37.Vu HH, Okumura J, Hashizume M, et al. . Regional Differences in the Growing Incidence of Dengue Fever in Vietnam Explained by Weather Variability. Trop Med Health 2014; 42: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banu S, Hu W, Hurst C, et al. . Dengue transmission in the Asia-Pacific region: impact of climate change and socio-environmental factors. Trop Med Int Heal 2011; 16: 598–607. [DOI] [PubMed] [Google Scholar]
- 39.Hales S, Weinstein P, Souares Y, et al. . El Niño and the dynamics of vectorborne disease transmission. Environ Health Perspect 1999; 107: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Descloux E, Mangeas M, Menkes CE, et al. . Climate-based models for understanding and forecasting dengue epidemics. PLoS Negl Trop Dis 2012; 6: e1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desvars A, Jégo S, Chiroleu F, et al. . Seasonality of human leptospirosis in Reunion Island (Indian Ocean) and its association with meteorological data. PLoS One 2011; 6: e20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau CL, Dobson AJ, Smythe LD, et al. . Leptospirosis in American Samoa 2010: epidemiology, environmental drivers, and the management of emergence. Am J Trop Med Hyg 2012; 86: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanova S, Herbreteau V, Blasdell K, et al. . Leptospira and rodents in Cambodia: environmental determinants of infection. Am J Trop Med Hyg 2012; 86: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colt S, Pavlin BI, Kool JL, et al. . Human leptospirosis in The Federated States of Micronesia: a hospital-based febrile illness survey. BMC Infect Dis 2014; 14: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braks M, Husman Ade R. Dimensions of Effects of Climate Change on Water-Transmitted Infectious Diseases. Air Water Borne Dis 2013; 2: 1–8. [Google Scholar]
- 46.Cann KF, Thomas DR, Salmon RL, et al. . Extreme water-related weather events and waterborne disease. Epidemiol Infect 2013; 141: 671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown L, Murray V. Examining the relationship between infectious diseases and flooding in Europe: A systematic literature review and summary of possible public health interventions. Disaster Heal 2013; 1(2): 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashizume M, Chaves LF, Faruque ASG, et al. . A Differential Effect of Indian Ocean Dipole and El Niño on Cholera Dynamics in Bangladesh. PLoS One 2013; 8: e60001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashizume M, Armstrong B, Hajat S, et al. . The Effect of Rainfall on the Incidence of Cholera in Bangladesh. Epidemiology 2008; 19: 103–110. [DOI] [PubMed] [Google Scholar]
- 50.Johnson E, Jim R, Pavlin BI. Hepatitis A in Pohnpei State, Federated States of Micronesia, 2008–2009. Pac Health Dialog 2010; 16: 91–97. [PubMed] [Google Scholar]
- 51.Thein C, Trinidad R, Pavlin B. A Large Foodborne Outbreak on a Small Pacific Island. Pacific Heal Dialogue 2010; 16(1): 75–80. [PubMed] [Google Scholar]
- 52.Hambling T, Weinstein P, Slaney D. A review of frameworks for developing environmental health indicators for climate change and health. Int J Environ Res Public Health 2011; 8: 2854–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaves LF, Pascual M. Comparing models for early warning systems of neglected tropical diseases. PLoS Negl Trop Dis 2007; 1: e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lowe R, Bailey TC, Stephenson DB, et al. . The development of an early warning system for climate-sensitive disease risk with a focus on dengue epidemics in Southeast Brazil. Stat Med 2013; 32: 864–883. [DOI] [PubMed] [Google Scholar]
- 55.Ebi KL, Lindgren E, Suk JE, et al. . Adaptation to the infectious disease impacts of climate change. Clim Change 2012; 118: 355–365. [Google Scholar]
- 56.World Health Organization Protecting health from climate change: Global research priorities. World Heal Organ Public Heal. Available: http://www.who.int/globalchange/publications/9789241598187/en/
- 57.Ebi K, Rocklov J. Climate change and health modeling: horses for courses. Glob Health Action 2014; 7: 24154. [DOI] [PMC free article] [PubMed] [Google Scholar]