Abstract
Repeated systemic administration of the mitochondrial complex I inhibitor rotenone produces a rodent model of Parkinson disease (PD). Mechanisms of relatively selective rotenone-induced damage to nigrostriatal dopaminergic neurons remain incompletely understood. According to the “catecholaldehyde hypothesis,” buildup of the autotoxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL) contributes to PD pathogenesis. Vesicular uptake blockade increases DOPAL levels, and DOPAL is detoxified mainly by aldehyde dehydrogenase (ALDH). We tested whether rotenone interferes with vesicular uptake and intracellular ALDH activity. Endogenous and F-labeled catechols were measured in PC12 cells incubated with rotenone (0-1000 nM, 180 minutes), without or with F-dopamine (2 μM) to track vesicular uptake and catecholamine metabolism. Rotenone dose-dependently increased DOPAL, F-DOPAL, and 3,4-dihydroxyphenylethanol (DOPET) levels while decreasing dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) levels and the ratio of dopamine to the sum of its deaminated metabolites. In test tubes, rotenone did not affect conversion of DOPAL to DOPAC by ALDH when NAD+ was supplied, whereas the direct-acting ALDH inhibitor benomyl markedly increased DOPAL and decreased DOPAC concentrations in the reaction mixtures. We propose that rotenone builds up intracellular DOPAL by decreasing ALDH activity and attenuating vesicular sequestration of cytoplasmic catecholamines. The results provide a novel mechanism for selective rotenone-induced toxicity in dopaminergic neurons.
Keywords: Rotenone, Dihydroxyphenylacetaldehyde, DOPAL, Aldehyde dehydrogenase, Parkinson disease, Vesicular monoamine transporter
INTRODUCTION
Parkinson disease (PD) is characterized by drastic depletion of the catecholamine, dopamine (DA), in the putamen (Kish et al. 1988), neuronal loss in the mesencephalic substantia nigra (Halliday et al. 1990), and Lewy bodies (Lees et al. 2008), which contain abundant alpha-synuclein (Spillantini et al. 1997). Increasing epidemiologic evidence points to a contribution of exposure to pesticides in the pathogenesis of PD (Fitzmaurice et al. 2014), including the mitochondrial complex I inhibitor, rotenone (Tanner et al. 2011). Repeated systemic administration of rotenone to rodents evokes neurobehavioral abnormalities resembling those in PD, accompanied by striatal DA depletion, nigral neuronal loss, and alpha-synuclein deposition (Sherer et al. 2003, Betarbet et al. 2000).
Systemically injected rotenone is selectively toxic to the nigrostriatal DA system (Ferrante et al. 1997). Since rotenone readily traverses cell membranes and inhibits mitochondrial complex I in all cells, the question arises as to how systemic rotenone administration results in relatively specific nigrostriatal damage. This study explored a potential answer—an injurious interaction between rotenone and products of oxidation of DA in dopaminergic cells (Wu & Johnson 2011, Sai et al. 2008, Liu et al. 2005, Dukes et al. 2005).
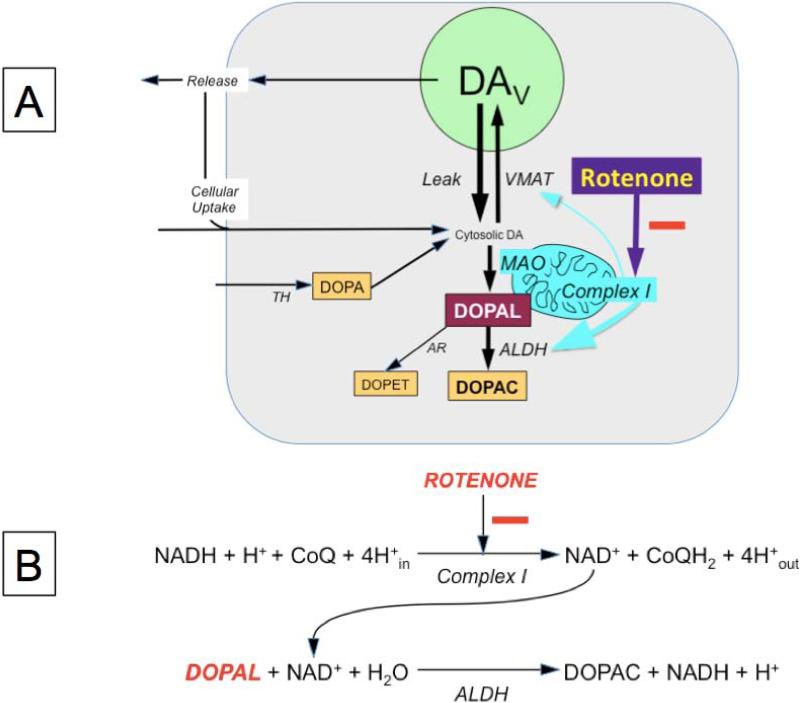
Cytoplasmic DA within neurons undergoes oxidative deamination catalyzed by monoamine oxidase-A (MAO-A, EC 1.4.3.4) to form the catecholaldehyde, 3,4-dihydroxyphenylacetaldehyde (DOPAL, Figure 1). DOPAL is cytotoxic, both when administered exogenously (Panneton et al. 2010, Burke et al. 2003) and when produced intracellularly (Goldstein et al. 2012), at least partly via protein cross-linking and oxidation to harmful quinones (Anderson et al. 2011). DOPAL also potently oligomerizes alpha-synuclein (Burke et al. 2008, Jinsmaa et al. 2014), and alpha-synuclein oligomers are thought to be pathogenic in PD (Kazantsev & Kolchinsky 2008).
Figure 1. Diagram of proposed mechanism for intracellular 3,4-dihydroxyphenylacetaldehyde (DOPAL) buildup.
Several factors might affect DOPAL levels, including release and reuptake of endogenous dopamine (DA), effectively translocating DA from storage vesicles into the cytoplasm; tyrosine hydroxylase (TH), the rate-limiting enzyme in DA synthesis; aldehyde/aldose reductase (AR), aldehyde dehydrogenase (ALDH), cellular uptake of DA from the extracellular fluid and vesicular sequestration of cytoplasmic DA via the vesicular monoamine transporter (VMAT). Based on the present results, the main mechanism of rotenone-induced DOPAL buildup is decreased ALDH activity. This fits with rotenone inhibiting Complex I and thereby reducing the availability of NAD+, a required co-factor for ALDH. Abbreviations: DAV=vesicular dopamine; DOPAC=3,4-dihydroxyphenylacetic acid; DOPET=3,4-dihydroxyphenylethanol; MAO=monoamine oxidase.
Studies reported by Lamensdorf and colleagues provided the first evidence of a link between DOPAL and rotenone-induced cytotoxicity (Lamensdorf et al. 2000a). In PC12 cells, rotenone increased endogenous DOPAL content, and blockade of DOPAL production attenuated rotenone-evoked cell death (Lamensdorf et al. 2000b); however, the mechanisms by which rotenone builds up endogenous DOPAL were not examined. Elucidating these mechanisms was the main purpose of the present study.
In general, there are two determinants of DOPAL levels—net leakage of DA from vesicles into the cytoplasm, where the DA is deaminated to form DOPAL, and decreased metabolism of DOPAL by aldehyde dehydrogenase (ALDH, Figure 1A). There is evidence suggesting that rotenone both interferes with the type 2 vesicular monoamine transporter (VMAT2) and attenuates ALDH activity. In cultured human neuroblastoma cells, rotenone inhibits VMAT2 and redistributes DA from the vesicles into the cytoplasm (Watabe & Nakaki 2008, Watabe & Nakaki 2007). This shift would be expected to increase availability of DA as a precursor for DOPAL production. Via blockade of mitochondrial complex I and thereby of NAD+ generation, rotenone could decrease intracellular ALDH activity indirectly, because NAD+ is a required co-factor for ALDH (Figure 1B). Decreased NAD+ availability is thought to be the mechanism by which rotenone interferes with metabolism of the aldehyde lipid peroxidation product, trans-4-hydroxy-2-nonenal (Leiphon & Picklo 2007, Meyer et al. 2004).
Simultaneous measurements of levels of catechols provide means to assess specific aspects of the synthesis and disposition of catecholamines, including vesicular sequestration of cytoplasmic dopamine and metabolism of DOPAL by ALDH. Given that essentially all of intracellular DA is within the vesicles, that vesicular uptake offsets substantial passive leakage of vesicular cytoplasmic catecholamines, and that the main alternative fate of cytoplasmic DA besides vesicular uptake is enzymatic deamination by monoamine oxidase, if rotenone inhibited vesicular uptake then cellular DA content would decrease, the sum of its deaminated metabolites would increase and the ratio of DA:(DOPAC+DOPAL+DOPET) would decrease (Goldstein et al. 2012). Since NE is synthesized within the vesicles, decreased vesicular sequestration also would be expected to decrease NE content (Taylor et al. 2014) and increase the ratio of the cytoplasmic metabolite 3,4-dihydroxyphenylglycol (DHPG) to NE (Goldstein et al. 2014b). If vesicular uptake were inhibited by rotenone, then upon incubation of the cells with F-DA, cell contents of F-DA would be decreased, in a manner similar to that produced by the classic VMAT inhibitor reserpine (Goldstein et al. 2012).
As shown in Figure 1, since ALDH converts DOPAL to DOPAC, if rotenone inhibited ALDH, then DOPAC levels would be decreased, DOPAL levels would be increased, and the ratio of DOPAC:DOPAL would be decreased substantially (Goldstein et al. 2013), both in cells and medium. Another approach for evaluating ALDH activity is based on incubation of the cells with F-labeled DA, which allows tracking of catecholamine metabolism. Upon incubation with F-labeled DA, FDOPAC:F-DOPAL ratios would be decreased if ALDH activity were decreased. Because of decreased metabolism of DOPAL to DOPAC, levels of the alternative DOPAL metabolite via aldose reductase (Figure 1), 3,4-dihydroxyphenylethanol (DOPET) would be expected to increase, assuming no concurrent inhibition of aldehyde/aldose reductase (Goldstein et al. 2012).
Although studying PC12 cells offers advantages in that the cells synthesize, store, and metabolize catecholamines and contain endogenous DOPAL, a disadvantage is that if rotenone affected multiple steps concurrently (e.g., simultaneously decreased cellular uptake of catecholamines from the medium, vesicular uptake of catecholamines from the cytoplasm, and ALDH activity), this would complicate interpretation of the neurochemical results. To evaluate specifically whether rotenone inhibits intracellular ALDH, we therefore also studied cultured human glioblastoma cells, as these cells do not store catecholamines in vesicles but do express ALDH (Casida et al. 2014). The human genome contains 19 ALDH genes, and we did not find published information on relative expression rates of these genes in rat PC12 cells or human glioblastoma cells. If rotenone inhibited intracellular ALDH, then during incubation of glioblastoma cells with DOPAL, cellular contents of DOPAC would decrease as a function of the concentration of rotenone added to the medium. We compared rotenone with benomyl, which is known to inhibit ALDH directly (Fitzmaurice et al. 2013), in terms of the extents of decline in glioblastoma cellular DOPAC content and the DOPAC:DOPAL ratio.
To test whether rotenone inhibits ALDH indirectly, via rotenone's effects on complex-1 and consequently decreased availability of the essential co-factor NAD+, we conducted a test-tube study in which we measured DOPAC production following incubation DOPAL with ALDH2, NAD, and a range of concentrations of rotenone or benomyl.
MATERIALS AND METHODS
Institutional approval of the research reported here was obtained from the Division of Intramural Research of the NINDS.
Materials
Rat pheochromocytoma PC12 cells and human glioblastoma cells were from the American Type Culture Collection (ATCC, Manassas, VA; PC12 cells catalog no. CRL-1721, glioblastoma cells catalog no. HTB-15). The cell culture media, F12K and DMEM, were from Gibco Life Technologies (Grand Islands, NY); tolcapone (to block catechol-O-methyltransferase) from Orion Pharma (Espoo, Finland); and dopamine, norepinephrine, DOPAC, DOPET, and DHPG from Sigma. 6F-Dopamine was obtained in solution (about 1.35 mM) from the NIH PET Department after having passed quality control for i.v. administration to humans. DOPAL standard was synthesized in the laboratory of and provided by Dr. Kenneth L. Kirk (NIDDK).
PC12 and Glioblastoma Cell Cultures
PC12 cells were kept frozen in liquid nitrogen until passaged for experiments. The experiments reported here involved non-adherent, non-differentiated cells. Non-adherent PC12 cells were grown in F12K medium with 15% horse serum and 2.5% fetal bovine serum. The cells were incubated at 37 ©C in an atmosphere of 5% carbon dioxide. Media were changed several times per week and cells passaged once per week.
At 24 hours prior to plating for experiments, the cells were centrifuged, and media was replaced with media containing 10 μM tolcapone and 40 ng/ml epidermal growth factor (EGF). Since PC12 cells express catechol-O-methyltransferase (EC 2.1.1.6), whereas dopaminergic neurons do not, the cells in this study were always pre-incubated for 24 hours in medium containing the catechol-O-methyltransferase inhibitor, tolcapone. After the 24 hours, the cells were collected, suspended in the same media, counted, and the suspensions diluted with medium to provide 500,000 cells/mL.
Human glioblastoma cells were cultured in DMEM medium containing 10% fetal bovine serum, and 24 hours prior to experiments the cells were transferred into wells (500,000 cells/well) containing 10 mM tolcapone. Experiments began after 24 hours of incubation with tolcapone-containing medium (“baseline”).
Rotenone and F-Dopamine Incubation
For dose-response experiments in PC12 cells, effects of rotenone on levels of endogenous and F-labeled catechols in cells and medium were examined after 24 hours of incubation with tolcapone. Into 3 wells, 6.0 μL (final concentration 0.6% vol:vol) of dimethylsulfoxide vehicle was added. In another 3 wells, dimethylsulfoxide vehicle was added along with F-DA (final concentration 2 μM). In a total of 12 other wells, dimethylsulfoxide vehicle containing rotenone (1, 10, 100, and 1000 nM, 3 wells at each concentration) was added, along with F-DA. For all 18 wells, at 180 minutes the cells and medium were separated and frozen until assayed for endogenous and F-catechols, as described below.
For time-response measurements, the medium was removed at 10, 20, 30, 60, 120, and 180 minutes after addition of rotenone in dimethylsulfoxide (final concentration 100 nM, N=7 observations at each time point). The medium was centrifuged, the supernatant removed, and the sedimented cells taken up in 400 μL of a 20:80 mixture of 40 mM phosphoric acid and 200 mM acetic acid. The separated medium and the disrupted cell solutions were frozen until assayed for catechol contents, as described below.
Human Glioblastoma Cells
To confirm an inhibitory effect of rotenone on ALDH activity, we conducted an experiment in human cultured glioblastoma cells, in which rotenone (1, 10, 100, 1000 nM) was co-incubated with DOPAL (3 μM) for 180 minutes (3 wells at each rotenone concentration, total 12 wells). For comparison with rotenone, benomyl was added to 12 other wells (3 wells each at 1, 10, 100, and 1000 nM). DOPAL alone was added to 3 other wells, for a total of 27 wells in the experiment.
Direct ALDH Inhibition
To compare rotenone with benomyl in terms of direct ALDH inhibition when NAD+ was available, we conducted a test tube experiment. DOPAL (10 μM) in 100 mM Tris-HCl buffer (pH 7.4) was incubated with human recombinant ALDH2 (from Sigma-Aldrich, lot numbers 9B136332 and 9H226332, enzyme activity >0.012-0.024 U/mL., 1 mM NAD+, and rotenone or benomyl at 0.01, 0.1, 1, 10 μM (2 observations at each concentration) for 2 hours at 37 °C. The chosen amount of ALDH2 for the reaction was based on a preliminary experiment in which 50-60% of DOPAL was converted into DOPAC. The reaction was stopped by adding a 1:10 dilution of a 20:80 mixture of 40 mM phosphoric acid and 200 mM acetic acid. The reaction mixtures were frozen and stored at -80 °C until assayed for catechol contents.
Catechol Assays
Endogenous and F-labeled catechols were assayed by liquid chromatography with electrochemical detection after batch alumina extraction (Holmes et al. 1994, Goldstein et al. 2012). The analyses were done on matching liquid chromatographic/electrochemical detection systems. Each chromatographic system included a Waters model 515 pump and 717 Plus refrigerated autosampler (Waters, Inc., Milford, MA). The mobile phase recipe was: 1 L Type I water, 13.8 g NaH2PO4, 50 mg EDTA, 59 mg octanesulfonic acid, acetonitrile approximately 2.5%, pH approximately 3.2 (acetonitrile and pH adjusted to optimize chromatographic separations). The column was a Thomson BioAdvantage Basic C18 5 μM 250 x 4.6 mm (part no. BA400-046250 (Thomson Instrument Co., Clear Brook, VA). The post-column electrochemical detector system included an ESA (now Thermo) Coulochem III controller and a series of a Conditioning Cell (part no. 70-6088) and then an Analytical Cell (part no. 70-5561). The Conditioning Cell electrode potential was set at +350 mV, and the Analytical Cell electrodes were set at +150 mV and -350 mV.
Cell contents of catechols were assayed from 100-200 μL aliquots of the 400 μL of disrupted cells. Concentrations of endogenous and F-labeled catechols were expressed in units of nmol/L. Cell counts were measured using a Cellometer device (Nexcelom Bioscience, Lawrence, MA).
Data Analysis and Statistics
Neurochemical data were displayed using KaleidaGraph 4.01 (Synergy Software, Reading, PA). Dose-response data were analyzed by independent means t-tests comparing the 0 nM vs. 1000 nM concentrations. A p value less than 0.05 defined statistical significance.
RESULTS
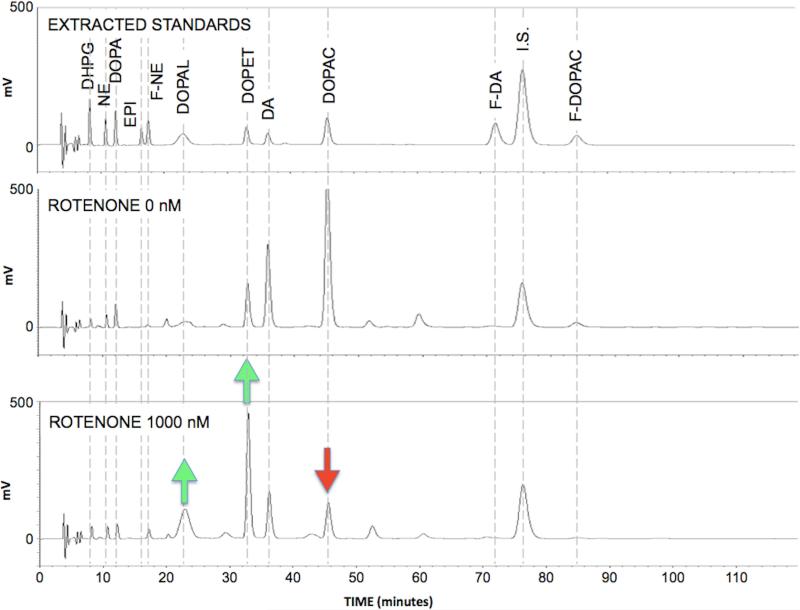
As illustrated in Figure 2, the liquid chromatographic-electrochemical method used in this study successfully baseline-resolved 8 endogenous catechols, the internal standard, F-DA, F-DOPAC, and F-NE simultaneously. Because of the wide range of catechol concentrations in cells and medium, from 0.08 nmol/L (cellular DHPG) to more than 10,000 nmol/L (cellular DA), for each sample multiple volumes were assayed and alumina eluates injected, and each chromatographic run lasted a relatively long time (typically 2 hours). Of note, the DOPAL peak stood out from others in being short, broad, and often irregular, which is a characteristic feature of this aldehyde (Goldstein et al. 2012).
Figure 2. Chromatographs of (A) catechol standards, (B) medium catechols at a rotenone concentration of 0 nM, and (C) medium catechols at a rotenone concentrations of 1000 nM.
Green arrows placed to emphasize increases in DOPAL and DOPET chromatographic peak heights and red arrow to emphasize decrease in DOPAC peak height.
The chromatographs in Figure 2 also demonstrate key findings from the study, that rotenone increases endogenous DOPAL and DOPET levels while decreasing endogenous DOPAC levels, a pattern that indicates decreased metabolism of DOPAL by ALDH.
Endogenous DA and Its Deaminated Metabolites
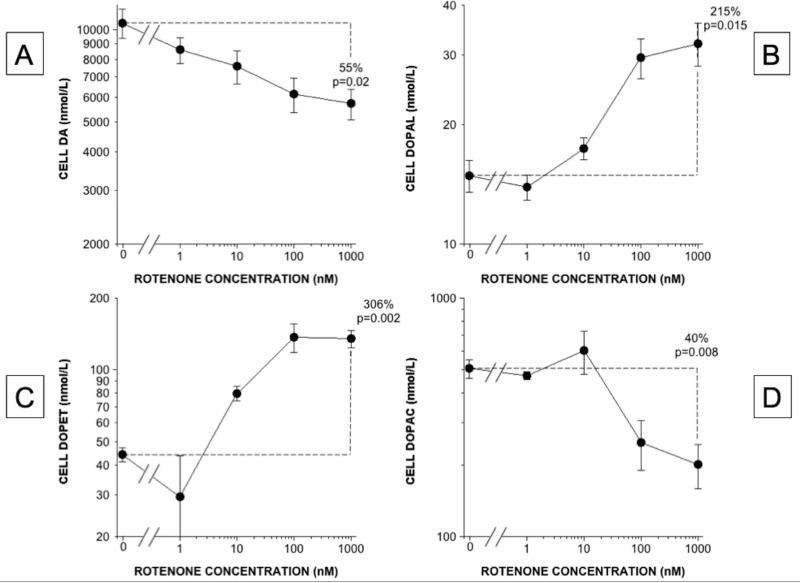
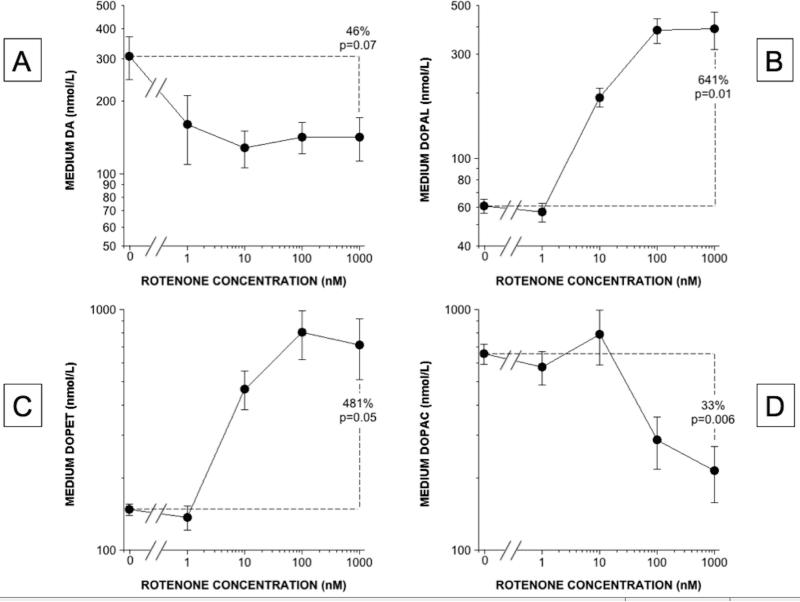
Rotenone incubation produced dose-related (Figure 3) and time-related (Figure 4) increases in cellular DOPAL content. DOPAL and DOPET contents increased, while DA and DOPAC decreased (Figure 3). At a rotenone concentration of 100 nM, cellular DOPAL, DOPET, and DOPAC contents increased progressively over 180 minutes (Figure 4). In the medium the same pattern of rotenone-induced neurochemical changes was found as in the cells (Figures 2 and 5).
Figure 3. Rotenone effects on cell contents of DA and its deaminated metabolites in PC12 cells.
(A) DA; (B) DOPAL; (C) DOPET; (D) DOPAC. Mean (±SEM) concentrations are expressed as functions of rotenone concentrations on log-log scales. DA content decreased slightly across rotenone doses, contents of the deaminated metabolites DOPAL and DOPET increased exponentially, and DOPAC content changed bi-phasically, decreasing at the higher rotenone concentrations.
Figure 4. Rotenone (100 nM) effects on cell levels of catechols as a function of time of incubation.
(A) DA; (B) NE; (C) DOPAL; (D) DOPAC; (E) DOPET; (F) DHPG. Whereas cell contents of DA and NE were little changed, contents of the deaminated metabolites DOPAL, DOPAC, DOPET, and DHPG all increased progressively.
Figure 5. Rotenone effects on medium contents of DA and its deaminated metabolites.
(A) DA; (B) DOPAL; (C) DOPET; (D) DOPAC. Mean (±SEM) concentrations are expressed as functions of rotenone concentrations on log-log scales. DA content decreased across rotenone doses, contents of the deaminated metabolites DOPAL and DOPET increased exponentially, and DOPAC content changed biphasically, decreasing at the higher rotenone concentrations.
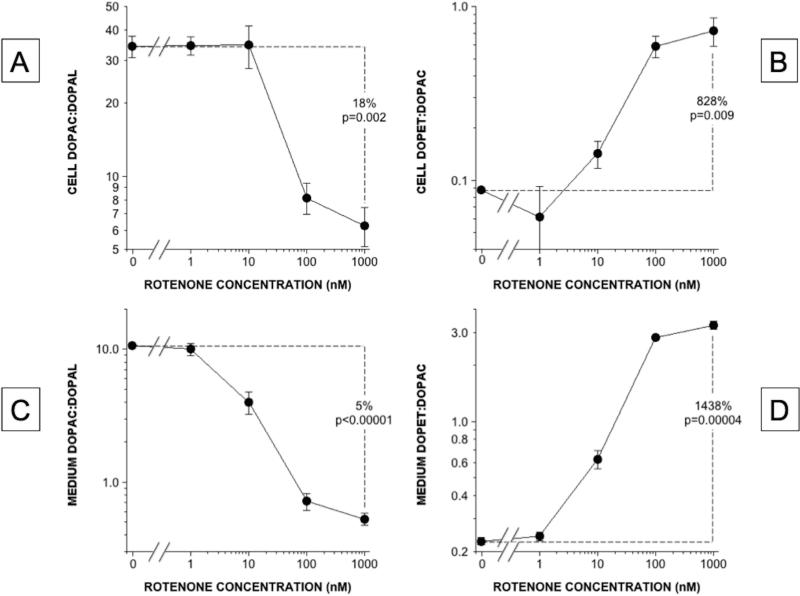
The ratio of DOPAC:DOPAL, a sensitive index of ALDH activity, decreased substantially as a function of the rotenone concentration, in both the cells and the medium (Figure 6). As expected if there were decreased ALDH activity without a decrease in aldehyde/aldose reductase activity, the ratio of DOPET:DOPAC increased in both the cells and the medium.
Figure 6. Rotenone effects on catechol ratios in PC12 cells.
(A, C) DOPAC:DOPAL in cells and medium; (B, D) DOPET:DOPAC in cells and medium. DOPAC:DOPAL ratios decreased markedly, indicating decreased ALDH activity, and DOPET:DOPAC ratios increased markedly, indicating a shift from ALDH to aldehyde reductase in the intracellular metabolism of DOPAL.
The ratio of DA:(DOPAC+DOPAL+DOPET) provides an index of vesicular sequestration vs. oxidative deamination of cytoplasmic DA. At a rotenone concentration of 10 nM, the mean value for this index (11.1 ± 1.0) in the cells was decreased from that in cells not incubated with rotenone (18.5 ± 0.4, p=0.03). At higher rotenone concentrations, the mean value for this index was not decreased. In the medium, mean DA:(DOPAC+DOPAL+DOPET) was decreased, between no rotenone and 10 nM (0.35 ± 0.05 vs. 0.09 ± 0.01, p=0.005), 100 nM (0.10 ± 0.01, p=0.006), and 1000 nM (0.11 ± 0.01, p=0.007).
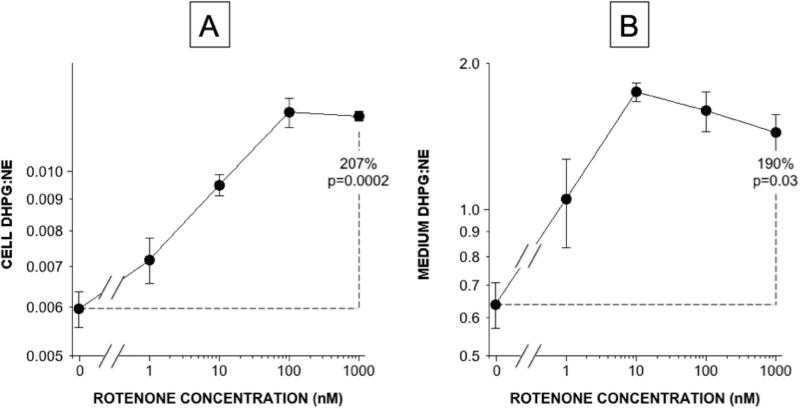
Another neurochemical index of a vesicular sequestration-to-oxidative deamination shift is the ratio of DHPG:NE (Goldstein et al. 2014b). As shown in Figure 7, the mean value for DHPG:NE increased progressively as a function of rotenone concentrations, both in cells (Figure 7A) and in medium (Figure 7B).
Figure 7.
Rotenone effects on cell (A) and medium (B) DHPG:NE ratios.
F-Labeled Catechols
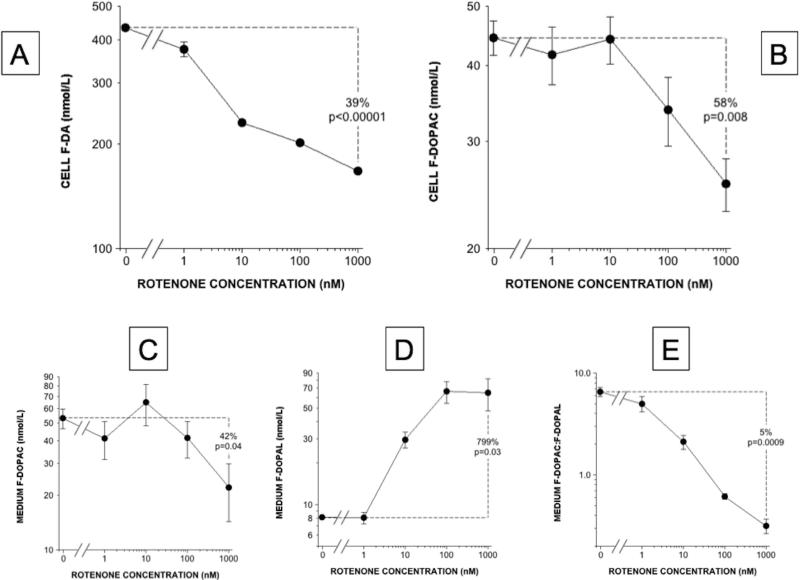
Rotenone dose-dependently decreased cell F-DA content (Figure 6A), which is affected by cellular uptake and vesicular storage. F-DOPAC levels decreased in both cells and medium (Figure 6B and 6C), while F-DOPAL in the medium increased progressively (Figure 6D). F-DOPAL was not detected in the cells. The mean FDOPAC:F-DOPAL ratio in the medium decreased substantially as a function of rotenone concentrations (Figure 6E).
Cell and medium concentrations of both endogenous and F-labeled DOPAC seemed to change bi-phasically as a function of rotenone concentrations, with increases at 10 nM rotenone and decreases at 1000 nM rotenone (Figures 3D, 5D, 8B, and 8C).
Figure 8. Rotenone effects on cell and medium contents of F-labeled compounds.
(A) Cell F-DA, (B) Cell F-DOPAC, (C) Medium F-DOPAC, (D) Medium F-DOPAL, (E) Medium F-DOPAC:F-DOPAL. Rotenone decreased cell F-DA and F-DOPAC while increasing medium F-DOPAL and decreasing medium F-DOPAC:F-DOPAL.
Glial Cells
Incubation of glial cells with DOPAL and rotenone or benomyl dose-dependently decreased DOPAC levels (Figure 9A and 9B) and DOPAC:DOPAL ratios (Figures 9C and 9D).
Figure 9. Comparison of rotenone (A, C) with benomyl (B, D) effects on DOPAC:DOPAL ratios in human glial cells.
(A, B) Cells incubated with 1 μM DOPAL; (C, D) 3 μM DOPAL. Rotenone and benomyl both decreased DOPAC:DOPAL ratios. Without rotenone or benomyl, DOPAL at 3 μM was associated with a lower DOPAC:DOPAL than at 1 μM.
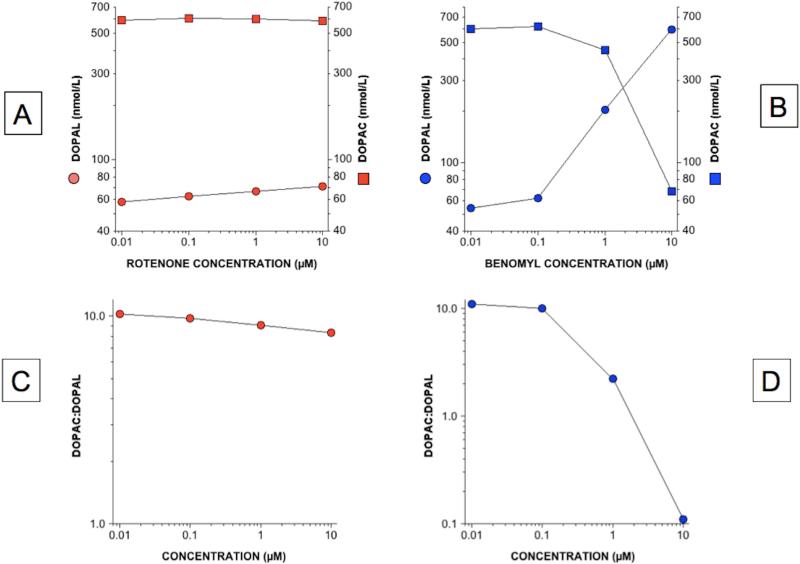
Test-Tube Study
When DOPAL, NAD+, and rotenone at increasing concentrations were incubated in test tubes, the contents of DOPAL and DOPAC in the reaction mixtures changed by relatively little (N=2 at each concentration; Figure 10A). In contrast, incubation with benomyl markedly increased DOPAL and decreased DOPAC concentrations in the reaction mixtures, in a dose-related manner (Figure 10B). As expected, the DOPAC:DOPAL ratio changed by relatively little as a function of the rotenone concentrations while decreasing drastically as a function of the benomyl concentrations (Figure 10C and 10D).
Figure 10. Comparison of rotenone (red, A) with benomyl (blue, B) effects on DOPAL (circles) and DOPAC (squares) levels and DOPAC:DOPAL ratios (C) in test tubes after incubation with ALDH2 and NAD+.
Benomyl potently inhibited conversion of DOPAL to DOPAC, whereas rotenone exerted only a mild effect.
DISCUSSION
In this study, levels of endogenous and F-labeled catechols in cells and medium were used to examine potential mechanisms of rotenone-induced buildup of the cytotoxic DA metabolite DOPAL. As explained below, we obtained evidence in PC12 cells for rotenone-induced decreases in both vesicular uptake of cytoplasmic catecholamines and metabolism of DOPAL by ALDH, the latter effect probably reflecting decreased NAD+ availability due to mitochondrial complex I inhibition by the pesticide. The combination of a vesicular sequestration-to-oxidative deamination shift in the fate of cytoplasmic catecholamines and decreased ALDH activity produced by rotenone mimics the pattern of neurochemical abnormalities found in Parkinson disease (Goldstein et al. 2013, Goldstein et al. 2014b) and therefore provides support for the validity of the rotenone animal model (Sherer et al. 2003, Hoglinger et al. 2003).
Rotenone Increases DOPAL Production
Rotenone produced dose- and time-related increases in DOPAL contents in cells and medium. Increased cellular DOPAL contents were evident within 1 hour of incubation in medium containing 100 nM rotenone. These results replicated those reported previously (Lamensdorf et al. 2000a). Rotenone also increased medium FDOPAL content upon incubation of PC12 cells with F-DA.
Rotenone Inhibits Metabolism of DOPAL by ALDH
Multiple results from the present study indicate that rotenone inhibits intracellular ALDH activity. Rotenone decreased DOPAC levels while increasing DOPAL levels and decreased the ratio of DOPAC:DOPAL, a sensitive index of ALDH activity (Goldstein et al. 2013). Highly analogous results were obtained from medium F-DOPAC:F-DOPAL ratios during incubation of PC12 cells with rotenone and F-DA. Meanwhile, levels of DOPET, an alternative metabolite of DOPAL, increased markedly in cells and medium, and the DOPET:DOPAC ratio, an index of the shift from ALDH to aldehyde reductase in the metabolism of DOPAL, was substantially increased. Human glial cells do not possess storage vesicles, and thus this model enables assessment of rotenone effects on ALDH activity without the complicating aspect of concurrent effects on vesicular sequestration. When human glioblastoma cells were incubated with DOPAL, rotenone decreased cellular DOPAC content and DOPAC:DOPAL ratios, confirming the inhibitory effect of rotenone on intracellular ALDH activity.
A test-tube experiment showed that rotenone exerts little or no direct inhibitory effect on ALDH if NAD is added to the test tube. In contrast, benomyl, which directly inhibits ALDH, produced mirror image increases in DOPAL and decreases in DOPAC concentrations in the reaction mixture. Therefore, the results suggest that most of the decrease in ALDH activity exerted by rotenone in PC12 cells is mediated by decreased NAD+ availability, probably due to the well known interference by rotenone with mitochondrial complex I. Although the data from the test tube experiment show that rotenone does not inhibit ALDH importantly when NAD is supplied, we did not test in cells whether decreased NAD+ availability explains the decreased ALDH activity. Increased reactive oxygen species could be another—and not mutually exclusive—explanation.
A previous study reported a larger decrease in DOPAC content than in DA content after incubation of PC12 cells with rotenone (Dukes et al. 2005), a pattern consistent with decreased MAO or ALDH activity. The authors excluded decreased MAO activity, but, since DOPAL was not detected, they could not evaluate ALDH activity.
In cellular and animal models of rotenone-induced Parkinsonism, ALDH2 activation is neuroprotective (Chiu et al. 2015). Relative efficiencies of ALDH isoforms in metabolizing DOPAL have not yet been reported. If the mechanism of decreased ALDH activity were NAD+ unavailability, then theoretically rotenone would decrease activity of all isoforms of ALDH.
Rotenone Inhibits Vesicular Storage
Vesicular uptake of cytoplasmic catecholamines is an energy-requiring process, and one might expect that rotenone would interfere with this process because of mitochondrial toxicity, which would decrease ATP availability. In PC12 cells vesicular uptake is mediated by the type 1 VMAT (Tao-Cheng & Eiden 1998), and in brain dopaminergic and sympathetic noradrenergic neurons vesicular uptake is mediated by the type 2 VMAT (Eiden & Weihe 2011). Rotenone inhibits expression of VMAT2 (Watabe & Nakaki 2008) and redistributes DA from vesicles to cytoplasm in human neuroblastoma SH-SY5Y cells (Watabe & Nakaki 2007). We predicted that the pattern of levels of catechols upon incubation of PC12 cells with rotenone would resemble that after incubation with reserpine, a classic inhibitor of vesicular uptake by both forms of VMAT (Goldstein et al. 2012).
Reserpine treatment of PC12 cells results in marked, rapid depletion of cell DA content—by about 50% at 20 minutes and 90% at 180 minutes (Goldstein et al. 2012). In the present study, rotenone produced more modest but still significant decreases in cell DA content, in agreement with a previous report (Dukes et al. 2005). If there were a shift from vesicular uptake to oxidative deamination of cytoplasmic DA, then the ratio of DA to the sum of its deaminated metabolites—i.e., DA:(DOPAC+DOPET+DOPAL)—would be decreased, and this was the case in both cells and medium at a rotenone dose of 10 nM. Rotenone also increased the DHPG:NE ratio in both cells and medium, which would be consistent with a vesicular sequestration-oxidative deamination shift in the fate of endogenous NE (Goldstein et al. 2014b). Taken together, the results suggest that rotenone interferes with vesicular storage of cytoplasmic catecholamines.
Levels of DOPAC and F-DOPAC in cells and medium seemed to be related biphasically to rotenone concentrations. A decrease in vesicular uptake of cytoplasmic DA at low rotenone concentrations and a decrease in ALDH activity at high concentrations could explain the bi-phasic concentration-response curves.
This study involved a few limitations. We did not identify the isoforms of ALDH expressed in the rat PC12 or human glioblastoma cell lines. Although the data from our test tube experiment showed that rotenone does not inhibit ALDH importantly when NAD+ is supplied, we did not test in cells whether decreased NAD+ availability explains the decreased ALDH activity. We also did not assess whether rotenone decreases the ratio of NAD+:NADH in the cytoplasm of either rat PC12 or human glioblastoma cells.
In summary, in this study we obtained evidence that rotenone decreases intracellular ALDH activity and attenuates vesicular storage of cytoplasmic catecholamines. This combination seems to account for the buildup of DOPAL. The present findings provide a mechanism for how rotenone-induced cytotoxicity in dopaminergic cells reflects oxidative injury from altered DA metabolism (Sai et al. 2008).
Since DOPAL potently oligomerizes alpha-synuclein (Burke et al. 2008, Jinsmaa et al. 2014), rotenone-induced DOPAL buildup might also account for production of cytoplasmic inclusions resembling Lewy bodies in dopaminergic neurons of rodents treated with systemic rotenone injections. DOPAL and alpha-synuclein may be nodes in a pathophysiologic nexus (Goldstein et al. 2014a) that interact complexly to threaten the integrity of nigrostriatal dopaminergic neurons.
ACKNOWLEDGEMENTS
The research reported here was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
Abbreviations
- ALDH
aldehyde dehydrogenase
- AR
aldehyde/aldose reductase
- DA
dopamine
- DHPG
3,4-dihydroxyphenylglycol
- DOPAC
3,4-dihydroxyphenylacetic acid
- 6F-DOPAC
6F-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- 6F-DOPAL
3,4-dihydroxyphenylacetaldehyde
- 6FDOPET
3,4-dihydroxyphenylethanol
- MAO-A
monoamine oxidase-A
- NE
norepinephrine
- 6F-NE
6F-norepinephrine
- PD
Parkinson disease
- VMAT2
type 2 vesicular monoamine transporter
Footnotes
Authorship Credit:
David S. Goldstein: Conception and design, data analysis, data interpretation, drafting the article, final approval
Patti Sullivan: Data acquisition, data analysis
Adele Cooney: Data acquisition, drafting the article, data analysis
Yunden Jinsmaa: Data acquisition, data analysis, conception and design, drafting the article, revising the article
Irwin J. Kopin: Conception and design, data analysis, drafting the article, revising the article critically for important intellectual content
Yehonatan Sharabi: Conception and design, data analysis, drafting the article, revising the article critically for important intellectual content
The authors have no conflicts of interest to disclose.
REFERENCES
- Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J. Biol. Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Kumar VB, Pandey N, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson's disease pathogenesis. Brain Res. 2003;989:205–213. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, Goldstein DS. Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson's disease. Chem. Res. Toxicol. 2014 doi: 10.1021/tx5002223. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Yeh TH, Lai SC, et al. Neuroprotective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp Neurol. 2015;263:244–253. doi: 10.1016/j.expneurol.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes AA, Korwek KM, Hastings TG. The effect of endogenous dopamine in rotenone-induced toxicity in PC12 cells. Antioxid. Redox Signal. 2005;7:630–638. doi: 10.1089/ars.2005.7.630. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N.Y. Acad. Sci. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Schulz JB, Kowall NW, Beal MF. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997;753:157–162. doi: 10.1016/s0006-8993(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014;82:419–426. doi: 10.1212/WNL.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Lulla A, et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:636–641. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014a;144:268–282. doi: 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Sullivan R, Gross DJ, Holmes C, Kopin IJ, Sharabi Y. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 Cells: Relevance to the pathogenesis of Parkinson disease. J. Neurochem. 2012;123:932–943. doi: 10.1111/j.1471-4159.2012.07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson's disease. J. Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Sharabi Y, Kopin IJ. A vesicular sequestration to oxidative deamination shift in myocardial sympathetic nerves in Parkinson's disease. J. Neurochem. 2014b doi: 10.1111/jnc.12766. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson's disease. Ann. Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 2003;84:491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B Biomed. Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, Sullivan P, Gross D, Cooney A, Sharabi Y, Goldstein DS. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci. Lett. 2014;569:27–32. doi: 10.1016/j.neulet.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Kolchinsky AM. Central role of alpha-synuclein oligomers in neurodegeneration in Parkinson disease. Arch. Neurol. 2008;65:1577–1581. doi: 10.1001/archneur.65.12.1577. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Eisenhofer G, Harvey-White J, Hayakawa Y, Kirk K, Kopin IJ. Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. J. Neurosci. Res. 2000a;60:552–558. doi: 10.1002/(SICI)1097-4547(20000515)60:4<552::AID-JNR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Eisenhofer G, Harvey-White J, Nechustan A, Kirk K, Kopin IJ. 3,4-Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res. 2000b;868:191–201. doi: 10.1016/s0006-8993(00)02309-x. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Selikhova M, Andrade LA, Duyckaerts C. The black stuff and Konstantin Nikolaevich Tretiakoff. Mov. Disord. 2008;23:777–783. doi: 10.1002/mds.21855. [DOI] [PubMed] [Google Scholar]
- Leiphon LJ, Picklo MJ., Sr. Inhibition of aldehyde detoxification in CNS mitochondria by fungicides. Neurotoxicology. 2007;28:143–149. doi: 10.1016/j.neuro.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Liu HQ, Zhu XZ, Weng EQ. Intracellular dopamine oxidation mediates rotenone-induced apoptosis in PC12 cells. Acta Pharmacol. Sin. 2005;26:17–26. doi: 10.1111/j.1745-7254.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- Meyer MJ, Mosely DE, Amarnath V, Picklo MJ., Sr. Metabolism of 4-hydroxy-trans-2-nonenal by central nervous system mitochondria is dependent on age and NAD+ availability. Chem. Res. Toxicol. 2004;17:1272–1279. doi: 10.1021/tx049843k. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One. 2010;5:e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai Y, Wu Q, Le W, Ye F, Li Y, Dong Z. Rotenone-induced PC12 cell toxicity is caused by oxidative stress resulting from altered dopamine metabolism. Toxicol. In Vitro. 2008;22:1461–1468. doi: 10.1016/j.tiv.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Eiden LE. The vesicular monoamine transporter VMAT2 and vesicular acetylcholine transporter VAChT are sorted to separate vesicle populations in PC12 cells. Adv. Pharmacol. 1998;42:250–253. doi: 10.1016/s1054-3589(08)60740-1. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW. Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology. 2014;76(Pt A):97–105. doi: 10.1016/j.neuropharm.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone-elicited dopamine redistribution from vesicles to cytosol in human dopaminergic SH-SY5Y cells. J. Pharmacol. Exp. Ther. 2007;323:499–507. doi: 10.1124/jpet.107.127597. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SH-SY5Y cells. Mol. Pharmacol. 2008;74:933–940. doi: 10.1124/mol.108.048546. [DOI] [PubMed] [Google Scholar]
- Wu YN, Johnson SW. Dopamine oxidation facilitates rotenone-dependent potentiation of N-methyl-D-aspartate currents in rat substantia nigra dopamine neurons. Neuroscience. 2011;195:138–144. doi: 10.1016/j.neuroscience.2011.08.041. [DOI] [PubMed] [Google Scholar]