Abstract
BACKGROUND
Escalation of consumption is a hallmark of cocaine addiction. Many animal models reveal escalation by increasing the duration of drug access (e.g., 6 to 24 h/day) after longer histories of self-administration. We recently developed a method that reveals escalation early post-acquisition under shorter access conditions. However, whether or not rats will escalate cocaine consumption both early post-acquisition under short access (2 h/day) conditions, and later under long access (6 h/day) conditions, has not been demonstrated.
METHODS
All rats acquired cocaine self-administration (0.8 mg/kg, i.v.) under 2 h conditions, and then continued 2 h self-administration for an additional 13 sessions. Then, rats were assigned either to 2 or 6 h conditions, and self-administered cocaine (0.8 mg/kg, i.v.) for an additional 19 sessions. In addition, four cocaine-induced locomotor activity measurements were taken for each rat: before cocaine exposure, after non-contingent cocaine administration, and after escalation in the short and long access experimental phases.
RESULTS
Following acquisition, rats displayed a robust escalation of intake during 2 h sessions. Rats that self-administered cocaine in continued 2 h sessions exhibited stable intake, whereas rats that self-administered cocaine in 6 h sessions further escalated intake. Despite the second escalation in 6 h rats, cocaine-induced locomotor activity did not differ between 2 and 6 h rats.
CONCLUSIONS
Escalation of cocaine self-administration can occur in the same rats both early post-acquisition, and later under long access conditions. Importantly, this early post-acquisition period provides a new opportunity to determine the mechanisms first involved in the escalation phenomenon.
Keywords: Addiction, Cocaine self-administration, Escalation, Sensitization, Animal model
1. INTRODUCTION
The development of cocaine addiction is highly variable: for example, only 10% to 15% of intranasal cocaine users are estimated to develop addiction (Gawin, 1991). Addicted individuals, however, play a significant role in the danger that cocaine poses to public health. Although heavy cocaine users have been estimated to represent 20% of the cocaine using population, they consume two-thirds of the domestic supply of the drug (Rydell and Everingham, 1994). Thus, identifying factors that contribute to the transition from recreational use to addiction is critical for understanding this devastating disease.
One hallmark of the transition to addiction is an escalation in cocaine consumption. To date, several investigators have modeled increases in cocaine consumption in animals (e.g., Ahmed and Koob, 1998; Carroll et al., 1989; Fitch and Roberts, 1993; Mantsch et al., 2001; Tornatzky and Miczek, 2000). In these models, rats trained to stably self-administer cocaine are switched to a condition in which cocaine is available for extended periods of time (e.g., from 6 to 24 h/day). During these long access sessions, rats are typically restricted to low to moderate doses of cocaine (Carroll et al., 1989); or when high doses are available, the number of infusions per hour is restricted (Fitch and Roberts, 1993). In the most widely used model, rats gradually escalate their intake of 0.75 mg/kg/i.v. infusion cocaine over time when permitted to self-administer cocaine in daily 6 h (“long access” or LgA) sessions. In contrast to LgA rats, rats that self-administer the same dose of cocaine under short access conditions (i.e., 1 h sessions; ShA) tend to display lower, stable patterns of drug intake over many sessions (Ahmed and Koob, 1998).
We have recently developed a model of escalation in cocaine consumption that reveals escalation in 2 h sessions very early after acquisition of self-administration (Mandt et al., 2012a). In this model, rats are trained to self-administer a moderate dose of cocaine (0.6 mg/kg/i.v. infusion under our conditions) under a fixed ratio 1 (FR1) schedule of reinforcement until they meet an intake-based acquisition criterion (three consecutive days of at least 4 mg/kg intake). Immediately after acquisition, the self-administered cocaine dose is increased to a relatively high dose (1.2 mg/kg/i.v. infusion under our conditions). Using this paradigm, rats that undergo a “dose-switch” show an escalation of both intake on a FR1 schedule of reinforcement and break points (BPs) on a progressive ratio (PR) schedule of reinforcement; in contrast, rats that continue to self-administer the training dose (0.6 mg/kg/i.v. infusion) show neither of these effects (Mandt et al., 2012a). Interestingly, our model also reveals escalation early after acquisition in the absence of a “dose-switch”, when the dose of cocaine that is self-administered during the acquisition phase is sufficiently high (e.g., 0.8 mg/kg/infusion; Mandt et al., 2012b; Simmons et al., 2013). Thus, it appears that higher-dose cocaine self-administration results in escalation of cocaine consumption very early in the drug-taking process, and may be conflated with “acquisition” (and not reported) in most studies.
In addition to escalation, cocaine sensitization (e.g., locomotor sensitization) is also thought to contribute to the transition to addiction. Although behavioral sensitization is a well-documented phenomenon (e.g., Kalivas and Duffy, 1993; Post, 1980; Sabeti et al., 2003), the relationship of behavioral sensitization and self-administration (i.e., contingent cocaine exposure) is less clear. In particular, studies of escalation in cocaine self-administration and sensitization have contradictory findings. Whereas some studies found that animals develop sensitization regardless of whether or not escalation occurs, other studies found that escalation under various schedules of reinforcement produces tolerance to cocaine-induced locomotor activity (Ahmed and Cador, 2006; Calipari et al., 2014; Lack et al., 2008).
The escalation revealed with the LgA procedure occurs well after animals have acquired self-administration, and data from the acquisition phase are rarely reported. We have argued previously that the escalation of higher dose cocaine self-administration early post-acquisition may be conflated with acquisition in traditional escalation studies. We have not yet determined, however, whether or not escalation can occur in the same animals both early post-acquisition, and later in self-administration when the duration of access in increased. Thus, the present study sought to determine whether or not escalation revealed early post-acquisition during short access 2 h sessions precludes escalation that develops during long access 6 h sessions. Additionally, we sought to determine the effect of long access self-administration, relative to short access self-administration, on established cocaine locomotor sensitization. Specifically, we used our new method for analyzing escalation under 2 h conditions (Mandt et al., 2012b) and a traditional 6 h model of escalation (Ahmed and Koob, 1998) to test the hypothesis that escalation can occur both early and late in an animal's self-administration history.
2. MATERIALS AND METHODS
2.1. Animals
Outbred male Sprague–Dawley rats (n = 24) weighing between 225 and 250 g were purchased from Harlan Industries (Indianapolis, IN, USA). Rats were housed individually with ad libitum access to food and water in an animal care facility at the University of Colorado Denver – Downtown Campus (CU Denver). Rats were housed on a 12-h light/dark cycle (lights on at 0700 hours), and all testing was conducted during the light cycle. All animal care and use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the CU Denver Institutional Animal Care and Use Committee.
2.2. Catheter Construction and Placement
Intravenous catheters were constructed in the laboratory and surgically implanted into the right jugular vein under ketamine (100 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.) anesthesia using established procedures (Thomsen and Caine, 2005). Rats received carprofen as an analgesic (5 mg/kg, s.c.) 24 h pre- and 48 h post-surgery. Rats recovered from surgery for at least one week before testing began. Catheters were flushed with 0.3 ml of bacteriostatic 0.9% sodium chloride containing heparin (33 U/ml) before and after each self-administration session. Catheter patency was verified with methohexital sodium (10 mg/kg, i.v.) as necessary throughout the experiments and at the end of the study.
2.3. Cocaine-induced locomotor activity
Cocaine-induced locomotor activity testing was conducted as previously described (e.g., Mandt et al., 2012b). Briefly, rats were taken to the behavioral testing room in their home cages and allowed to habituate for 60 min. At the start of the behavioral recording session, rats were placed in open field activity chambers consisting of Plexiglas boxes (43.2 × 43.2 × 43.2 cm) fitted with photobeam frames (16 beams per dimension; MED Associates, St. Albans, VT). After acclimation to the novel environment for 90 min, rats were injected with cocaine (10 mg/kg, i.p.) and returned to the chamber for an additional 30 min. Locomotor activity was quantified by using the automated consecutive horizontal photobeam interruptions converted to distance traveled (cm) and summed into 5-min bins. Cocaine-induced locomotor activity was defined as the sum of distance traveled over the 30-min post-cocaine period. Cocaine-induced locomotor activity was tested on four separate occasions: 1) prior to catheter surgery (i.e., in a drug naïve state), 2) 72 h following five once-daily non-contingent cocaine infusions (0.8 mg/kg, i.v.) in the self-administration context, 3) 48 - 72 h after 10 days of active cocaine-self-administration, and 4) 48 - 72 h after the final self-administration session.
2.4. Cocaine in the Self-Administration Context
Non-contingent (i.e., experimenter delivered) and contingent (i.e., self-administration) cocaine administration both occurred in 16 Plexiglas and metal operant conditioning chambers (29 × 24 × 21 cm; MED Associates) housed within sound-attenuating cabinets. Each chamber had two retractable levers on the front wall with stimulus lights positioned 6 cm above each lever. A tone presentation speaker (Sonalert Tone Generator, 2900 Hz; MED Associates) and a white noise speaker (90 dB) were mounted 12 cm above the floor on the wall opposite the levers. A house light (100 mA) was mounted 6 cm above the tone speaker, and a computer-controlled syringe pump delivered all cocaine infusions. All behavioral events were monitored and controlled by a personal computer using MED-PC for Windows software (MED Associates). All sessions began with the extension of the retractable levers, white-noise activation, and stimulus-light illumination on the right side of the chamber.
One-second after non-contingent session initiation, an i.v. cocaine infusion (0.8 mg/kg in a volume of 0.2 ml delivered over 5–7 s, based on the weight of the rat) was delivered coinciding with lever retraction, stimulus-light extinction, and 15-s tone-house light stimulus complex presentation. Non-contingent cocaine sessions were 30 min in duration, and the second locomotor activity test was conducted 72 h after the fifth session.
Self-administration training began 48 h after the second locomotor activity test. One-second after session initiation, a cocaine priming infusion was delivered (0.8 mg/kg/i.v. infusion in a volume of 0.2 ml over 5–7 s, based on the weight of the rat). During this priming infusion and all subsequent self-administered infusions (0.8 mg/kg/i.v. infusion in a volume of 0.2 ml over 5–7 s, based on the weight of the rat), the stimulus light over the active lever was turned off, and the tone-house light stimulus complex was activated for the 15 s coinciding with a “timeout” period.
Acquisition sessions were 2 h in duration, and testing was conducted 5 days a week. Responses on the active lever were reinforced with a cocaine infusion according to a FR1 schedule of reinforcement. Responses emitted on the active lever during cocaine infusion and stimulus complex presentation (i.e., timeout) were not reinforced and were recorded separately from reinforced responses. Responses on the inactive lever were recorded, but had no programmed consequence. Acquisition in these experiments was defined as the first of three consecutive sessions during which a rat consumed at least 4 mg/kg cocaine; the first of the three sessions is designated x, the day of acquisition (Mandt et al., 2012a, 2012b; Simmons et al., 2013). In our work, rats that meet these criteria have clearly acquired self-administration; and these criteria are similar to intake-based acquisition criterion used by other laboratories (e.g., Carroll and Lac, 1997; Mantsch et al., 2001). Immediately following acquisition (i.e., x+2), rats were given an additional thirteen 2 h FR1 sessions (i.e., x+3 to x+15).
At the completion of this initial phase, rats were assigned to either a 2 or a 6 h group. Group assignments were matched based on acquisition day and cocaine intake during the initial self-administration phase. Rats in the 2 and 6 h groups continued to self-administer cocaine (0.8 mg/kg/i.v. infusion) according to a FR1 schedule for an additional 19 sessions. The only testing difference between groups was session duration (i.e., 2 or 6 h).
2.5. Exclusions and Exceptions
Seven of the 24 rats used in this study were excluded from final data analyses. Six rats were excluded from all analyses: one rat had catheter patency failure during the acquisition phase; two rats did not acquire self-administration; and three rats had catheter patency failure during the 2 h access escalation phase. An additional rat (2 h group) was excluded only from the 6 h access escalation and locomotor sensitization analyses, because his responding was unstable only during this final escalation phase of the study.
2.6. Data Analysis
Statistical analyses were conducted with PASW Statistics, version 21.0 (IBM Corp., Somers, NY, USA) and GraphPad Prism, version 5.0f (GraphPad Software, Inc., La Jolla, CA, USA). Cocaine intake during phase one (i.e., 2 h sessions) was analyzed with two-way repeated measures ANOVA (RMANOVA). Subsequent access condition (2 or 6 h) and session (within subjects variable) were treated as independent variables, and intake was treated as the dependent measure. Cocaine intake during phase two (i.e., either 2 and 6 h sessions) was analyzed with separate one-way RMANOVAs; intake during the first 2 h was analyzed with two-way RMANOVA. Cocaine-induced locomotor activity on the four separate tests was compared by two-way RMANOVA. Access condition (2 or 6 h) and test (within subjects variable) were treated as independent variables, and the 30-min sum of cocaine-induced locomotor activity was treated as the dependent measure. Sensitization scores (ratio of subsequent test activity to initial activity) and escalation scores (ratio of final intake to initial intake) were compared with linear regression and correlational analysis. When main or interaction effects were revealed, one-way RMANOVA with either Dunnett's or Bonferroni's multiple comparison tests were used for post-hoc analyses. When the assumption of sphericity was violated for a particular repeated-measures analysis, as revealed by Mauchly's test statistic, tests of significance were based on the more conservative Huynh-Feldt corrected degrees of freedom. The symbol, a, indicates Huynh-Feldt corrected values throughout the text. Data are presented as mean values ± SEM and the level of statistical significance was set at p < 0.05.
2.7. Drugs
The National Institute on Drug Abuse (Bethesda, MD) generously provided the (−)cocaine hydrochloride used in these studies. For i.p. injections, cocaine was dissolved in sterile saline (0.9% sodium chloride) at a concentration of 10 mg/ml and administered in a volume of 1 ml/kg. For i.v. infusions, cocaine also was dissolved in sterile saline. Methohexital sodium (10 mg/kg; Sigma-Aldrich, St. Louis, MO) was administered intravenously. Ketamine was purchased from MWI (Boise, ID, USA), and xylazine was purchased from Sigma-Aldrich. Drug weights refer to the salt.
3. RESULTS
3.1. Acquisition
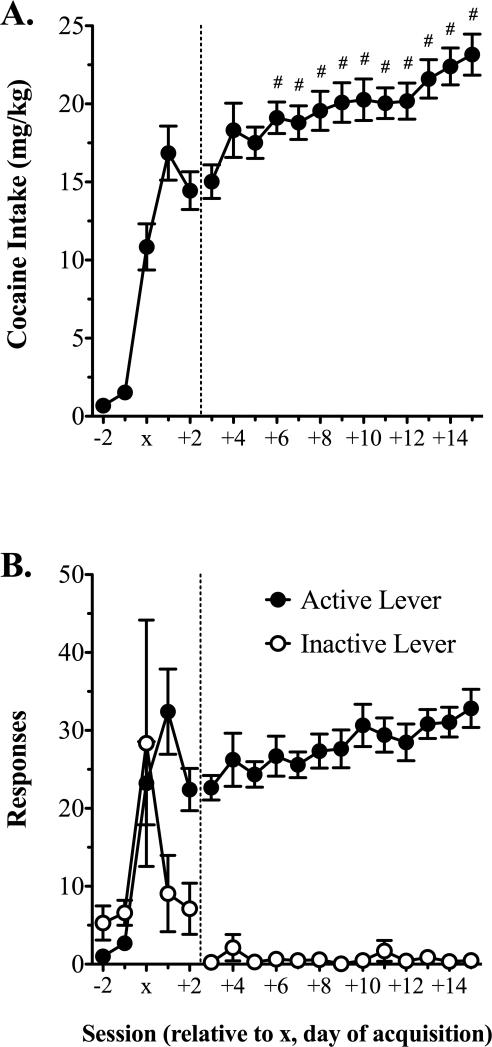
Over 20 acquisition sessions, 21/23 rats acquired self-administration in an average of 3.2 ± 0.6 days. Average days of acquisition for rats subsequently separated into the 2 or 6 h access conditions were 4.0 ± 1.4 and 3.1 ± 0.9, respectively (p = 0.607). Analysis of intake during acquisition with two-way RMANOVA revealed a main effect of session [F(3, 24) = 25.3, p < 0.001], but no other significant effects. Relative to the session prior to acquisition (i.e., x–1), rats consumed significantly more cocaine on sessions x to x+2 (Fig. 1A, left panel). It is noteworthy that this statistically significant difference was revealed despite the fact that 11 rats were dropped by the statistical analysis of acquisition intake, because they acquired on the first session and did not have intake values for session x–1. Whereas active to inactive lever responding was equivalent prior to acquisition, rats displayed an 83.7% ± 5.9% preference for the active to inactive lever upon acquisition (i.e., session x+2; Fig. 1B, left panel).
Figure 1.
Cocaine intake during acquisition and escalation during 2 h sessions. A) Cocaine (0.8 mg/kg/i.v. infusion) intake during the two 2 h FR1 sessions prior to acquisition of cocaine self-administration (x-2 and x-1), the three sessions used to define acquisition (x to x+2), and the thirteen post-acquisition self-administration sessions. B) Active and inactive lever responding over the same sessions shown in panel (A). The vertical dotted line in (A) and (B) designates the acquisition (left panel) and escalation (right panel) phases of 2 h self-administration. Data are mean values ± SEM. # p < 0.05 vs. session x+3. X refers to the day of acquisition. n = 18.
3.2. 2 h Access Escalation
Immediately following acquisition, all rats were given 13 additional 2 h self-administration sessions. Analysis of intake in rats subsequently assigned to either 2 or 6 h conditions with two-way RMANOVA revealed a main effect of session [aF(12, 180) = 5.8, p < 0.001], but no other significant effects. Dunnett's multiple comparisons test revealed that relative to the first of these sessions (x+3), rats consumed significantly more cocaine from the fourth session onward (i.e., x+6 to x+15), and increased intake 35% by the end of testing (Fig. 1A, right panel).
3.3. 6 h Access Escalation
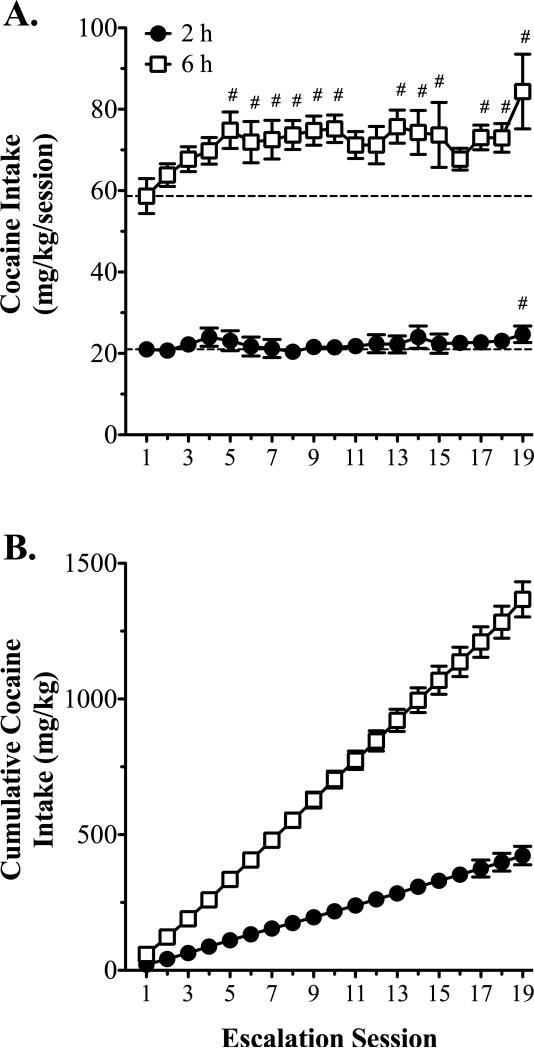
At the completion of the 2 h access testing, rats were divided into either a 2 or a 6 h access group. Rats then self-administered cocaine under 2 or 6 h conditions for an additional 19 sessions. In the 2 h group, analysis of intake with one-way RMANOVA revealed a main effect of session [F(18, 126) = 1.8, p = 0.038]. Dunnett's multiple comparisons test revealed that relative to the first session, cocaine intake was significantly greater only in the last session (Fig. 2A). In the 6 h group, analysis of intake with one-way RMANOVA revealed a main effect of session [F(18, 144) = 2.7, p = 0.001]. Dunnett's multiple comparisons test revealed that cocaine intake in the 6 h group was significantly greater than the first session beginning with the fifth session, and increased 31% by the end of testing (Fig. 2A). Additionally, cumulative cocaine intake analysis revealed clear differences in the slope of the intake curves for the 2 and 6 h rats (slope = 22.2 ± 0.8 vs. 72.9 ± 1.5 mg/kg/session for the 2 and 6 h rats, respectively; 95% confidence = 20.6 to 23.8 vs. 70.0 to 75.8 mg/kg/session for 2 and 6 h rats, respectively; Fig. 2B). Analysis of cumulative cocaine intake during the first 2 h with two-way RMANOVA revealed an interaction [F(18, 270) = 4.7, p < 0.001]. Pairwise comparisons revealed that with the exception of session 17, cumulative intake during the first 2 h was significantly greater in 6 h than in 2 h rats from session 10 onward (data not shown).
Figure 2.
Cocaine intake during either short or long access self-administration. A) Cocaine (0.8 mg/kg/i.v. infusion) intake over nineteen 2 or 6 h self-administration sessions. B) Cumulative cocaine intake over the same sessions shown in (A). The dashed lines in (A) represent the mean intake levels on the first session for the 2 h (n = 8) and the 6 h (n = 9) groups. Data are mean values ± SEM. # p < 0.05, intake vs. session 1.
3.4. Cocaine-induced Locomotor Activity
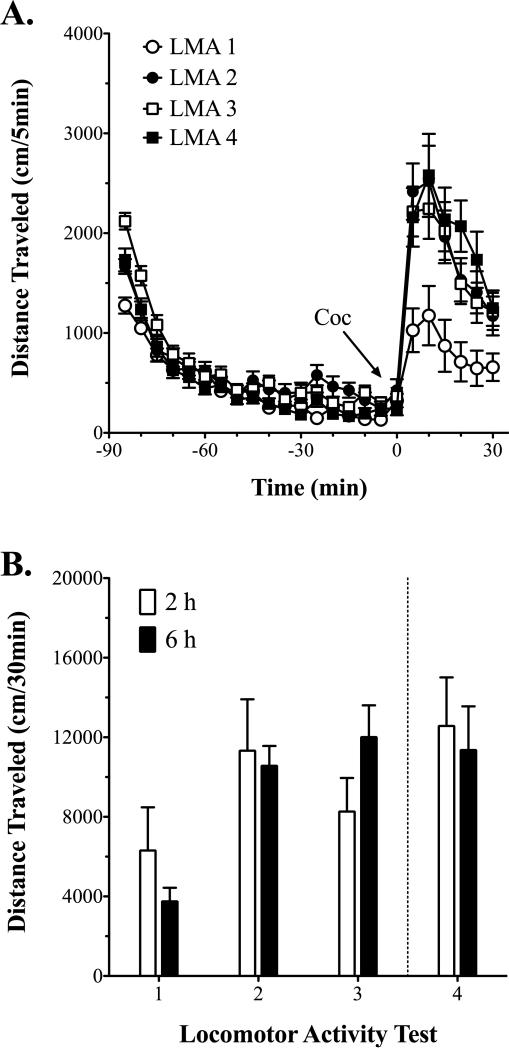
Cocaine-induced (10 mg/kg, i.p.) locomotor activity was tested on four separate occasions: 1) in a drug naïve state, 2) following five once-daily non-contingent cocaine infusions (0.8 mg/kg, i.v.) in the self-administration context, 3) after 10 active cocaine self-administration sessions (i.e., x+10), and 4) at the end of the study (Figs. 3A and B). Analysis of cocaine-induced locomotor activity with two-way RMANOVA revealed a main effect of test [aF(3, 45) = 6.9, p = 0.001], but no other significant effects. Bonferroni's multiple comparisons test revealed that relative to the initial cocaine-induced locomotor activity test, rats exhibited significantly greater cocaine-induced locomotor activity on every subsequent test; there were no significant differences, however, between locomotor activity tests 2 thru 4 (Fig. 3B). Furthermore, correlational analysis did not reveal significant relationships between the magnitude of cocaine-induced locomotor sensitization and escalation of consumption under either 2 or 6 h conditions: sensitization scores at session x+12 (test 3) vs. escalation scores at session x+12, Pearson's r = 0.418, r2 = 0.175, p = 0.107; final sensitization scores (test 4) vs. escalation scores at escalation session 19, Pearson's r = 0.080, r2 = 0.006, p = 0.836.
Figure 3.
Cocaine-induced locomotor sensitization. A) Locomotor activity over the 90-min pre- and 30-min post-cocaine (10 mg/kg, i.p.) injection in rats on each of the four separate locomotor activity tests. Because there were no effects of access condition (i.e., 2 h vs. 6 h), locomotor activity is shown collapsed across groups for clarity. B) Cocaine-induced (10 mg/kg, i.p.) locomotor activity over the first 30-min post-injection on each of the four separate locomotor activity tests in rats subsequently assigned either to 2 h (n = 9, except for test 4 where n = 8) or 6 h (n = 9) conditions. Test 4 (right of dashed line) is the only test where self-administration condition differed between groups. LMA 1: drug naïve; LMA 2: 72 h post non-contingent cocaine infusions (0.8 mg/kg/i.v. infusion); LMA 3: 48 – 72 h after 10 active cocaine self-administration sessions (i.e., x+12); LMA 4: 48 – 72 h after the final self-administration session.
4. DISCUSSION
In the present study, we determined the effect of an escalation of cocaine consumption early post-acquisition under short access conditions on subsequent escalation under long access conditions. Similar to our previous reports (Mandt et al., 2012a, 2012b; Simmons et al., 2013), again we observed an escalation of cocaine consumption very early after acquisition of higher dose cocaine self-administration. Following this initial escalation, rats allowed to self-administer cocaine in 6 h sessions displayed a second escalation of consumption. In contrast to 6 h sessions, rats that self-administered cocaine in continued 2 h sessions did not display further increases in cocaine intake. Despite the second escalation of consumption and high levels of cocaine exposure in 6 h rats, cocaine-induced locomotor activity did not differ between 2 and 6 h rats. Thus, these findings demonstrate that escalation can occur both early and late in an animal's self-administration history, and highlight this early post-acquisition period as a new target for investigating the escalation phenomenon.
Similar to our previous studies (Mandt et al., 2012b; Simmons et al., 2013), we observed an escalation of intake early after acquisition when rats self-administered a higher cocaine dose (0.8 mg/kg/i.v. infusion; Fig. 1A). Although we define acquisition as a relatively short period of time (i.e., three active self-administration sessions; x to x+2), we are confident that rats meeting our criteria have truly learned to self-administer cocaine. Despite dose-dependent differences in the number of responses required to satisfy our intake-based criteria, rats show clear lever discrimination, even when the number of responses required is as low as four (e.g., 1.2 mg/kg/infusion; Mandt et al., 2012b). This also was the case in the present study: upon meeting acquisition criterion (i.e., session x+2) rats displayed an 83.7% preference for the active to inactive lever, and averaged a 97.7% preference for the active to inactive lever over the duration of the 2 h testing phase (i.e. session x+3 to x+15; Fig. 1B). It is clear that this early escalation of intake is not simply part of the acquisition process, as rats clearly discriminate the active from inactive lever before escalation occurs, and escalation does not occur with lower cocaine doses (e.g., 0.6 mg/kg, i.v.; Mandt et al., 2012a).
To date, the LgA model is the most widely used model of escalation (Ahmed and Koob, 1998). Typically, rats given access to cocaine (0.75 mg/kg/i.v. infusion) for 6 h display a gradual escalation of cocaine consumption over time, but rats that continue to self-administer cocaine in 1 h sessions do not (e.g., Ahmed and Cador, 2006; Ahmed and Koob, 1998; Ben-Shahar et al., 2004). However, these studies do not present acquisition data or do not distinguish acquisition from an early escalation during “training”. It is notable that the 0.75 mg/kg/i.v. infusion dose of cocaine typically used with the LgA model is similar to the dose of cocaine used here (0.8 mg/kg/i.v. infusion), with which we again observed escalation early post-acquisition; however, we had not previously tested whether or not the same rats would demonstrate both an early escalation under short access conditions, and further escalation under long access conditions. In agreement with other reports, rats in the current study given access to cocaine in daily 6 h sessions escalated intake over the course of 19 additional sessions, whereas rats self-administering cocaine during 2 h sessions did not (Fig. 2A). It is worth restating, however, that all rats exhibited a robust escalation of intake during the initial phase of the study (i.e., sessions x+3 to x+15; Fig. 1A) prior to assignment to 2 or 6 h conditions. Thus, it appears that escalation early post-acquisition under short access conditions and escalation under long access conditions are not mutually exclusive phenomenon. Furthermore, and most importantly, escalation early post-acquisition provides an opportunity to determine the mechanisms that first regulate changes in drug-taking behavior not possible with the extensive training of the LgA procedure.
Our study is not the first, however, to suggest that escalation can occur under short access conditions. Indeed, escalation in cocaine intake (0.1 or 0.25 mg/kg/infusion) has been revealed during 1 h sessions when rats trained to self-administer cocaine in 10 min sessions are given access to cocaine for 60 min (Beckmann et al., 2012). That study also found that escalation only occurred when 6 h sessions were signaled by discrete cues, suggesting that escalation under those conditions was a learned effect. Together, these findings led the authors to conclude that escalation resulting from a switch in duration of access actually reflects “normal discrimination learning rather than dysregulated, addiction-like behavior” (Beckmann et al., 2012). Although our current study and the Beckmann et al. (2012) study appear similar (i.e., escalation under short and long access conditions), they are actually quite distinct. First, the short access escalation revealed here occurred very early post-acquisition without any additional manipulations; in contrast, the Beckmann et al. (2012) study still increased duration of access (i.e., 10 to 60 min) to reveal short access escalation, and did so only later in training (e.g., 12 acquisition sessions prior to 14 escalation sessions; Beckmann et al., 2012). Second, although the Beckmann et al. (2012) study assessed the effects of cues on escalation within the same animals, rats still only escalated under 6 h conditions; we demonstrate that escalation can occur in the same animals under short, and then, long access conditions. Thus, we believe that the early post-acquisition escalation under short access conditions observed here, and previously (Mandt et al., 2012a, 2012b; Simmons et al., 2013), is unique from escalation revealed by increasing duration of access.
Regardless of when it occurs (i.e., early or late), the mechanisms underlying escalation (e.g., sensitization and/or tolerance to some effect of cocaine, etc.) are debatable. For example, sensitization to cocaine's pharmacological effects might be predicted to reduce, rather than increase, self-administration. Many studies report that well-trained animals titrate intake when the cocaine dose is increased or decreased (Gerber and Wise, 1989; Lynch et al., 1998; Panlilio et al., 2003; Pickens and Thompson, 1968). Thus, heightened sensitivity to cocaine's pharmacological effects would be the equivalent of increasing dose, resulting in decreased intake; conversely, tolerance to cocaine's pharmacological effects would be expected to increase intake (i.e., escalation). Sensitization also develops to the “motivational” effects of cocaine, however, and animals that are more motivated to consume cocaine also could be expected to increase intake (Lack et al., 2008; Mandt et al., 2012a; Morgan et al., 2006). Indeed, this premise underlies the incentive-sensitization theory of addiction, which postulates that addiction arises from increased “drug-wanting”, without a change, or even a decrease, in “drug-liking” (e.g., Robinson and Berridge, 1993, 2001, 2008); parsing these effects in animal models, however, can be difficult.
The vast majority of escalation studies, including the present study, measure escalation in cocaine consumption with simple FR1 schedules of reinforcement. Data obtained under these conditions alone do not allow for conclusions about the reinforcing effectiveness of cocaine and the underlying mechanisms that drive escalation: increased intake could arise from sensitization to the “motivational” effects of cocaine, tolerance to the pharmacological effects of cocaine, a combination of both, or something entirely different. The original study with the LgA escalation procedure concluded that LgA animals display an upward shift in the cocaine dose-response curve (i.e., neither sensitization nor tolerance, but a shift in “hedonic set point”; Ahmed and Koob, 1998). Previously, however, we demonstrated that self-administration of high dose cocaine (1.2 mg/kg, i.v. infusion) immediately post-acquisition not only results in escalation of intake on a FR1 schedule of reinforcement, but also escalation of BPs on a PR schedule of reinforcement, suggesting sensitization to the “motivational” effects of cocaine (Mandt et al., 2012a). The similar time-course of increases in intake on the FR schedule and BPs on the PR schedule (i.e., over the first five post-acquisition sessions) suggested that the escalation in intake likely was not the result of tolerance to the reinforcing effects of cocaine (Mandt et al., 2012a). Further supporting this conclusion, rats that escalated intake on the FR schedule exhibited increased BPs when subsequently tested on the PR schedule (Mandt et al., 2012a). Without conducting post-escalation assessments (e.g., dose-consumption testing, PR testing), however, our current ability to directly compare the behavioral mechanisms underlying an early escalation and later long access escalation in the same rats is limited, leaving these questions to be addressed by future studies.
Studies that have directly assessed the development of cocaine locomotor sensitization following escalation have inconsistent findings. For example, studies have found a lack of differences in locomotor sensitization between ShA and LgA rats (Ahmed and Cador, 2006; Knackstedt and Kalivas, 2007), greater locomotor sensitization in LgA than ShA rats (Ferrario et al., 2005), or no locomotor sensitization in LgA rats (Ben-Shahar et al., 2004). The primary conclusion of these studies is that locomotor sensitization is not associated with the “transition to addiction” represented by an escalation of drug intake. However, in each of these cases, locomotor sensitization was observed in ShA rats. As previously stated, studies with the LgA model typically train their rats to self-administer cocaine until “stable” responding develops. Thus, it is possible that locomotor sensitization is associated with an escalation of intake that occurs earlier in self-administration (e.g., during training in these studies). Because we induced sensitization prior to self-administration, the current study cannot determine whether or not an early escalation results in locomotor sensitization, unfortunately, and additional studies are needed to fully address this possibility.
It is also possible that inducing locomotor sensitization prior to self-administration produced a ceiling effect that masked any further increase in locomotor activity resulting from self-administration; we believe this is unlikely, however. For example, mean cocaine-induced locomotor activity for all rats was ~11,000 cm/30-min on each of the different sensitization tests. However, maximal activity detected in individual rat's on each of those tests was ~23,000 cm/30-min. Thus, rats were capable of emitting, and our system was capable of detecting, greater cocaine-induced locomotor activity than the mean activity levels on each of those tests. To thoroughly rule out the possibility of a ceiling effect, however, future studies will need to include groups of rats in which cocaine locomotor sensitization is not induced prior to assessing escalation of cocaine self-administration.
In contrast to sensitization, other studies report tolerance to the locomotor stimulating effects of cocaine following escalation of either cocaine intake or BPs for cocaine (Calipari et al., 2014; Lack et al., 2008). In the study investigating escalation of drug intake (Calipari et al., 2014), however, rats were exposed to high levels of cocaine from the very beginning of training (e.g., ~300 mg/kg over the first 5 days). It is therefore possible that the high level of initial exposure, and not escalation per se, was responsible for the tolerance revealed in that study. We opted to induce locomotor sensitization prior to self-administration in this study to control for initial exposure: a variable that is under the animal's control during self-administration unless infusions are restricted. Here we found that relative to continued 2 h self-administration, 6 h escalation did not attenuate rats’ cocaine-induced locomotor activity, despite the high levels of cocaine exposure during long access. We cannot rule out the possibility, however, that cocaine self-administration alone decreased the magnitude of locomotor sensitization that would have developed if the rats had not self-administered cocaine (e.g., compared to rats undergoing abstinence following induction of locomotor sensitization). However, because there were not differences in cocaine-induced locomotor activity between rats self-administering cocaine under 2 or 6 h conditions, we can conclude that escalation of drug intake under 6 h access conditions is not always associated with tolerance to the locomotor stimulatory effects of cocaine (e.g., Calipari et al., 2014).
We believe the current study demonstrates three main findings: 1) escalation of cocaine consumption occurs early post-acquisition when the cocaine dose is sufficiently high, 2) early escalation of consumption does not preclude a second escalation of consumption when the duration of drug access is increased, and 3) long access escalation does not always result in tolerance to cocaine's locomotor stimulatory effects. However, this study does have limitations that we plan to address in the future. In particular, we are interested to know whether or not locomotor sensitization develops under the brief exposure of the acquisition phase (i.e., x to x+2) as it does with low non-contingent cocaine exposure (e.g., once-daily infusions), and the direct effects of an early escalation of consumption on locomotor sensitization. Most importantly, though, these data further demonstrate that escalation can occur early in the self-administration process, and suggest that this early post-acquisition period provides a new opportunity to investigate the behavioral and neurobiological changes first associated with an escalation of cocaine consumption.
Highlights for review.
Escalation of cocaine consumption is observed in a short access self-administration procedure.
Escalation during short access self-administration does not preclude escalation during traditional long access self-administration.
Relative to self-administration under short access conditions, escalation under long access conditions does not attenuate established cocaine locomotor sensitization.
Acknowledgments
Role of Funding Source: This work was supported by National Institutes of Health Grants R01 DA004216 and K05 DA015050.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Bruce H. Mandt participated in research design, data collection, data analysis, and writing of the manuscript. Richard M. Allen participated in research design, data analysis, and writing of the manuscript. Leland I. Copenhagen participated in data collection and data analysis, and Nancy R. Zahniser contributed to writing of the manuscript.
Conflict of Interest: No conflict declared.
REFERENCES
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich JA, Bardo MT. Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl.) 2012;222:257–267. doi: 10.1007/s00213-012-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J. Neurochem. 2014;128:224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl.) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology (Berl.) 1989;97:23–29. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol. Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fitch TE, Roberts DC. The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend. 1993;33:119–128. doi: 10.1016/0376-8716(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol. Biochem. Behav. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J. Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J. Pharmacol. Exp. Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Lack CM, Jones SR, Roberts DC. Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology (Berl.) 2008;195:517–525. doi: 10.1007/s00213-007-0919-4. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, LaBounty LP, Carroll ME. A novel paradigm to investigate regulation of drug intake in rats self-administering cocaine or heroin intravenously. Exp. Clin. Psychopharmacol. 1998;6:22–31. doi: 10.1037//1064-1297.6.1.22. [DOI] [PubMed] [Google Scholar]
- Mandt BH, Gomez E, Johnston NL, Zahniser NR, Allen RM. Cocaine dose and self-administration history, but not initial cocaine locomotor responsiveness, affects sensitization to the motivational effects of cocaine in rats. J. Pharmacol. Exp. Ther. 2012a;342:214–221. doi: 10.1124/jpet.112.194092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Johnston NL, Zahniser NR, Allen RM. Acquisition of cocaine self-administration in male Sprague-Dawley rats: effects of cocaine dose but not initial locomotor response to cocaine. Psychopharmacology (Berl.) 2012b;219:1089–1097. doi: 10.1007/s00213-011-2438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl.) 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Morgan D, Liu Y, Roberts DC. Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology. 2006;31:121–128. doi: 10.1038/sj.npp.1300773. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology (Berl.) 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J. Pharmacol. Exp. Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydell CP, Everingham SS. Controlling Cocaine: Supply Versus Demand Programs. Rand; Santa Monica: 1994. [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J. Pharmacol. Exp. Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Mandt BH, Ng CM, Richards TL, Yamamoto DJ, Zahniser NR, Allen RM. Low- and high-cocaine locomotor responding rats differ in reinstatement of cocaine seeking and striatal mGluR5 protein expression. Neuropharmacology. 2013;75:347–355. doi: 10.1016/j.neuropharm.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr. Protoc. Neurosci. 2005 doi: 10.1002/0471142301.ns0920s32. Chapter 9, Unit 9 20. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology (Berl.) 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]