Abstract
Aims
To investigate if consumption of pulses was associated with a reduced risk of developing abnormal glucose metabolism, increases in body weight and increases in waist circumference in a multi-ethnic cohort in Mauritius.
Methods
Population-based surveys were performed in Mauritius in 1992 and in 1998. Pulse consumption was estimated from a food frequency questionnaire in 1992 and outcomes were measured in 1998. At both time points, anthropometry was undertaken and an oral glucose tolerance test was performed.
Results
Mauritian women with the highest consumption of pulses (highest tertile) had a reduced risk of developing abnormal glucose metabolism [odds ratio 0.52; 95% CI 0.27, 0.99) compared with those with the lowest consumption, and also after multivariable adjustments. In women, a high consumption of pulses was associated with a smaller increase in BMI.
Conclusions
High consumption of pulses was associated with a reduced risk of abnormal glucose metabolism and a smaller increase in BMI in Mauritian women. Promotion of pulse consumption could be an important dietary intervention for the prevention of Type 2 diabetes and obesity in Mauritius and should be examined in other populations and in clinical trials.
Introduction
Mauritius is an economically developing country in the Indian ocean with a multi-ethnic population comprising mainly people of South-Asian or African origin. The prevalence of diabetes in Mauritius increased by 62% between 1987 and 2009, when 24% of Mauritian adults had diabetes [1,2]. Westernization of lifestyles, including dietary westernization (more energy-dense diets) and sedentary behaviour, accelerated in the 1980s and 1990s in Mauritius, starting among the more wealthy, the young and among men [3].
Pulses, such as lentils, chickpeas, beans and peas, have a high nutritional value [4]. Components of pulses have been shown to beneficially alter energy expenditure, substrate trafficking and fat oxidation as well as adipose deposition [5]. Bioactive components in pulses may induce satiety, both by prolonged gastric emptying and effects on hormones [6], and may also beneficially affect blood glucose and insulin responses [7].
Consumption of beans was associated with lower body weight and smaller waist circumference in the cross-sectional American National Health and Nutrition Examination Survey among more than 8000 adults [8]. Prospective studies examining the consumption of pulses and risk of diabetes are scarce. In the prospective Shanghai Women's Health Study, a high consumption of pulses was associated with a decreased risk of Type 2 diabetes [9]. Some studies have examined the associations between soy consumption and risk of Type 2 diabetes, with inconsistent results [10,11]. A large population-based cross-sectional study from India found an inverse association between daily consumption of pulses and Type 2 diabetes in women and a nonsignificant inverse association in men [12,13].
The objective of the present study was to investigate whether consumption of pulses was associated with a reduction in risk of developing abnormal glucose metabolism (defined as Type 2 diabetes, impaired glucose tolerance or impaired fasting glucose), as well as increases in BMI and waist circumference, in a well characterized cohort of men and women in Mauritius.
Subjects and Methods
The population in Mauritius is 68% South-Asian, 27% African, 3% Chinese and 2% Franco-Mauritian in origin [14]. As part of a series of population-based longitudinal risk factor surveys [2,15], a sub-sample of the 1992 sample completed a dietary survey. Follow-up was conducted in 1998.
In 1992, 2059 people aged 30–64 years were randomly chosen (stratified for age, sex and ethnicity) from the risk factor survey (n=6616) to answer a 44-item food frequency questionnaire, which included pictures to estimate portion size of the food items. Of these people, 1956 participated in the dietary survey (95% of those invited). They also participated in a 24-h dietary recall interview, which enabled energy intake to be estimated.
To exclude the most evident under- and over-reporters, people with energy intake outside the ranges of 500–3500 kcal for women and 800–4000 kcal for men in 1992 were excluded from further analysis (n=33) [16]. People of Chinese origin were excluded because of their small number (n=3).
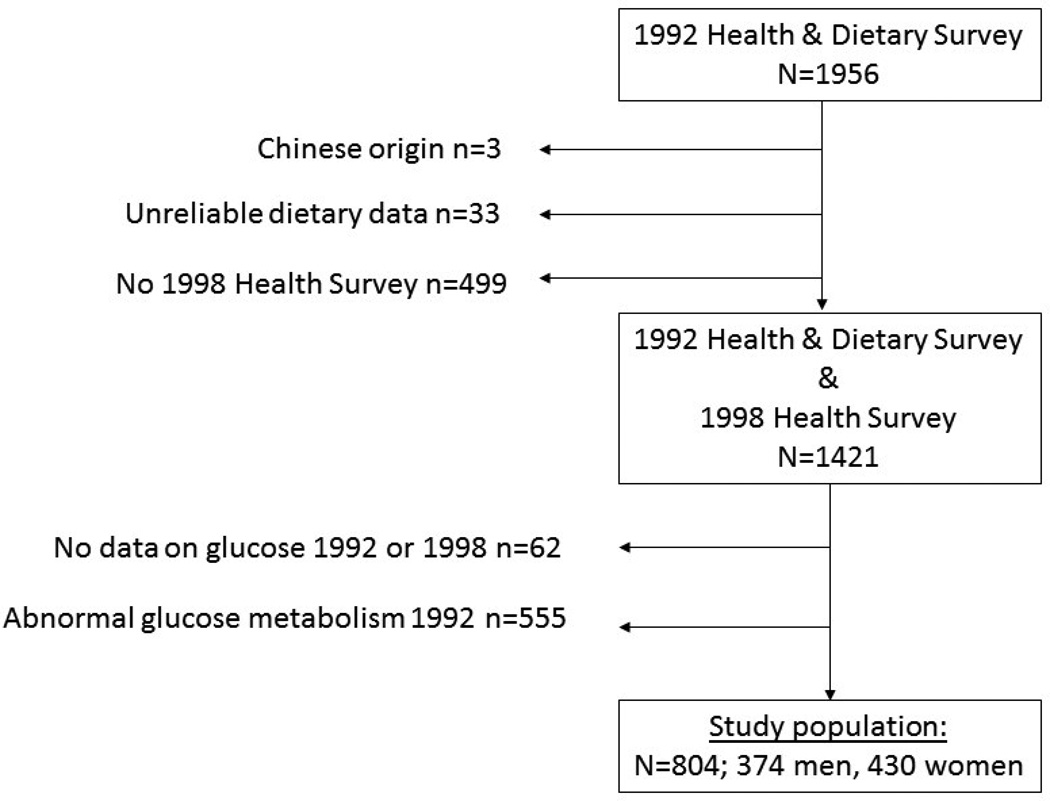
A total of 1421 individuals (650 men and 771 women) had reliable dietary data at baseline in 1992 and any outcome data (glucose tolerance status, BMI or waist circumference) at follow-up in 1998. Comparisons between those with dietary data who came back for the follow-up survey (responders) and those who did not (non-responders, excluding those who had died) showed that responders had a higher educational level, were less likely to be smokers, had a higher prevalence of family history of diabetes and a higher BMI at baseline. Of those participating in the dietary survey in 1992 with dietary data, and any outcome data from the follow-up, 62 individuals had missing data and could not be classified for glucose tolerance status. Those with abnormal glucose metabolism at baseline were excluded (n=555), leaving 804 individuals in the analysis (Fig. 1).
FIGURE 1.
Description of selection and exclusion of study participants, from the 1992 Health Survey and Dietary survey and the 1998 Health Survey, in Mauritius.
Food frequency questionnaire
Participants were asked, in the food frequency questionnaire, how many times they usually ate selected foods during the previous 3 months, using a 14-answer scale ranging from never to ≥4 times daily. All records were converted to daily consumption frequencies, by dividing the daily, weekly or monthly frequencies by one, seven or 30, respectively [17].
Estimation of intake of pulses
There was one question about the consumption of pulses in the 1992 food frequency questionnaire. Intake in g/day of pulses was computed by multiplying the portion size in grams with daily intake frequency of pulses. To estimate the average portion size of each food item, the interviewee could choose the most appropriate portion size from models prepared from non-perishable materials on standard-sized plates in use in Mauritius. Intake of pulses was adjusted for total energy intake estimated from the 24-h dietary recall, by dividing intake in g/day with total energy intake to g/MJ.
All interviewers (nurses or midwives) were trained by a nutritionist in the interviewing techniques for the 24-h dietary recall and food frequency questionnaire [3].
There was a significant correlation between intake of pulses according to the food frequency questionnaire and the 24-h dietary recall (Spearman's rank correlation = 0.30; P<0.001), supporting the validity of the estimation of pulse intake.
Anthropometric data at baseline and follow-up
Anthropometric data such as height, weight and waist circumference were measured at baseline and follow-up as previously described [18]. Data on gender, ethnicity (South-Asian, African, Chinese), family history of diabetes (yes or no/not known), treatment of diabetes (no/diet/herbal or medication), educational level (none/primary or high school/higher), leisure time physical activity and occupational physical activity at baseline were collected by trained interviewers. Participants were asked to report their leisure time physical activity and occupational physical activity as sedentary, light, moderate or heavy. Definitions of leisure time physical activity levels were given as follows: sedentary, e.g. housebound; light, e.g. gardening, walking; moderate, e.g. aerobic sport 1–2 days/week; and heavy, e.g. aerobic sport ≥ 3 days/week. Definitions of occupational physical activity levels were given as follows: sedentary, e.g. office, unemployed; light, e.g. sales, sewing, housework; moderate, e.g. trades worker; heavy, e.g. labourer. Energy-adjusted intake (g/MJ) of all food items was calculated from the baseline dietary data.
There were 11 and 16 individuals with missing data on covariates used in the analyses of abnormal glucose metabolism or models of changes in BMI or waist circumference, respectively.
Blood glucose and diabetes diagnosis at baseline and follow-up
An oral glucose tolerance test was performed in all participants (75 g glucose) unless they reported using hypoglycaemic agents to treat their diabetes. Diabetes was classified based on fasting plasma glucose ≥ 7.0 mmol/l or 2-h plasma glucose ≥ 11.1 mmol/l, or current treatment with insulin or oral hypoglycaemic medication. Fasting plasma glucose <7.0 mmol/l and 2-h plasma glucose ≥7.8 mmol/l but <11.1 mmol/l indicated impaired glucose tolerance, and fasting plasma glucose 6.1–6.9 mmol/l and 2-h plasma glucose <7.8 mmol/l indicated impaired fasting glucose. A fasting plasma glucose <6.1 mmol/l, together with a 2-h plasma glucose <7.8 mmol/l, indicated normal glucose tolerance [18].
Outcomes
The study outcomes were abnormal glucose metabolism, change in BMI and waist circumference. Abnormal glucose metabolism at follow-up was defined as Type 2 diabetes, impaired glucose tolerance or impaired fasting glucose at follow-up. Changes in BMI and waist circumference were calculated from 1992 to 1998.
Statistical analyses
Baseline variables were compared in tertiles of energy-adjusted pulse consumption using Pearson's chi-squared test or the Kruskal–Wallis non-parametric test, as appropriate. Spearman's correlation coefficients were calculated between energy-adjusted intake of pulses and energy-adjusted intake of other food items.
The association between pulse intake and abnormal glucose metabolism was estimated by logistic regression and reported as odds ratios (ORs) and 95% CIs. Participants were classified in tertiles of energy-adjusted consumption of pulses, calculated from all those with data on pulse consumption in 1992. Modelling was undertaken with the lowest tertile of energy-adjusted pulse consumption as the reference group.
Logistic regression analysis on associations between pulse consumption at baseline and abnormal glucose metabolism at follow-up were performed for all participants and for men and women separately. No significant interaction was found between consumption of pulses and sex (P=0.206), but because previous studies on associations between consumption of pulses and diabetes were performed only on women [9] or found stronger associations in women [12,13], we performed analysis stratified for sex. Multivariable adjustments were performed with baseline variables associated with pulse consumption at baseline and/or abnormal glucose metabolism at follow-up and included model 1: age, and model 2: ethnicity, occupational physical activity (as ordinal variable in all statistical analysis), waist circumference, smoking, energy-adjusted consumption of vegetables, energy-adjusted consumption of fibre (associated with consumption of pulses; Table 1), BMI and family history of diabetes (associated with abnormal glucose metabolism at follow-up). Tertiles of energy-adjusted consumption of food items associated with energy-adjusted consumption of pulses (Rs ≥ ± 0.15), and associated with abnormal glucose metabolism at follow-up (canned fish, potatoes and beef), were included in the multivariable model for abnormal glucose metabolism. In additional analyses, changes from baseline to follow-up in weight or waist circumference, respectively, were added to the multivariate model.
Table 1.
Baseline characteristics of men and women in Mauritius, free of diabetes or abnormal glucose metabolism in 1992 and with data on abnormal glucose metabolism status in 1998, according to tertile of energy-adjusted pulse consumption
| Men | women | |||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1: ≤6.14 g/MJ |
Tertile 2: 6.15–11.1 g/MJ |
Tertile 3 ≥11.2 g/MJ |
Tertile 1 ≤6.14 g/MJ |
Tertile 2 6.15–11.1 g/MJ |
Tertile 3 ≥11.2 g/MJ |
|||
| N | N | |||||||
| Mean (sd) age, years | 374 | 42.4 (9.22) | 44.2 (9.11) | 43.8 (9.76) | 430 | 44.7 (10.2) | 43.8 (10.3) | 45.4 (9.41) |
| Ethnicity, % South-Asian | 374 | 53.9* | 66.4 | 76.6 | 430 | 48.4* | 67.9 | 76.2 |
| Median (25th – 75th percentile) intake of pulses, g/day | 374 | 31.4 (15.7–47.1)* | 66.8 (62.9–94.3) | 141 (94.3–210) | 430 | 27.1 (15.7–31.4)* | 60.8 (47.1–62.9) | 136 (94.3–157) |
| Median (25th–75th percentile) intake of pulses, g/MJ | 374 | 3.70 (2.32–4.79)* | 7.96 (6.92–9.38) | 15.4 (12.8–20.7) | 430 | 3.93 (3.08–4.89)* | 8.34 (7.16–9.64) | 16.7 (13.3–21.9) |
| Mean (sd) energy intake, kcal | 374 | 2252 (562) | 2211 (609) | 2157 (594) | 430 | 1748 (466) | 1732 (441) | 1683 (472) |
| Family history of diabetes, % | 374 | 21.9 | 23.8 | 21.8 | 430 | 22.8 | 15.7 | 20.2 |
| ≥ Moderate leisure time physical activity, % | 373 | 20.5 | 21.3 | 21.8 | 429 | 0.83 | 4.29 | 2.98 |
| ≥ Moderate occupational physical activity, % | 374 | 48.4 | 53.3 | 50.8 | 429 | 15.7* | 15.7 | 20.2 |
| ≥ Secondary school, % | 374 | 39.1 | 42.6 | 47.6 | 429 | 28.1 | 22.1 | 20.8 |
| Smoking,% | 374 | 57.8 | 45.1 | 46.0 | 425 | 9.17* | 2.88 | 3.61 |
| Mean (sd) waist circumference, cm | 374 | 84.2 (8.72)* | 86.9 (7.78) | 84.9 (7.50) | 423 | 82.4 (9.30) | 81.6 (10.0) | 80.8 (10.5) |
| Mean (sd) BMI, kg/m2 | 374 | 23.7 (3.64) | 24.5 (3.53) | 23.6 (3.22) | 423 | 25.6 (4.19) | 25.6 (4.54) | 25.1 (5.13) |
| Median (25th–75th percentile) fruit intake, g/MJ | 374 | 5.19 (2.50–8.86) | 5.47 (2.95–8.58) | 5.77 (2.83–7.87) | 430 | 6.49 (3.00–13.1) | 5.60 (3.18–9.44) | 6.71 (3.90–9.28) |
| Median (25th–75th percentile) vegetable intake, g/MJ | 374 | 3.59 (2.38–6.25)* | 4.52 (2.99–6.85) | 6.76 (4.23–9.72) | 430 | 5.27 (3.24–8.76)* | 5.36 (3.51–8.72) | 7.27 (4.68–10.0) |
| Median (25th–75th percentile) fibre intake, g/MJ | 374 | 1.73 (1.51–2.07)* | 1.91 (1.58–2.33) | 2.02 (1.68–2.43) | 430 | 1.82 (1.55–2.29)* | 1.91 (1.67–2.42) | 2.12 (1.76–2.60) |
Significant difference (P<0.05) among tertiles (Kruskal–Wallis non-parametric test or Pearson's chi-squared test).
The associations between pulse consumption and change in BMI or waist circumference were estimated using a general linear model, adjusted for age in model 1 and adjusted for the baseline variables associated with energy-adjusted pulse consumption and/or the respective outcome in model 2. For change in BMI analyses, ethnicity, occupational physical activity, smoking, energy-adjusted consumption of vegetables, fibre and educational level were added, and for waist circumference analyses, ethnicity, occupational physical activity, smoking, energy-adjusted intake of vegetables and fibre were added in model 2. Food items, which correlated with energy-adjusted consumption of pulses and were associated with the change in BMI and/or change in waist circumference (energy-adjusted consumption of beef for both outcomes and potatoes and canned fish for change in waist circumference), were also included in the multivariable models. spss version 20.0 was used for the statistical analyses. A P value <0.05 was taken to indicate statistical significance.
Informed consent was obtained from all participants and the study was approved by the ethics committees of the Ministry of Health and Quality of Life, Port Louis, Mauritius, and of the International Diabetes Institute, Melbourne, Australia.
Results
Table 1 shows baseline characteristics according to tertiles of energy-adjusted pulse consumption for those with normal glucose metabolism at baseline. People of South-Asian descent had a higher consumption of pulses compared with those of African descent (median 9.65 g/MJ vs 6.78 g/MJ, P ≤ 0.001). The same proportion of Mauritians of South-Asian and African origin developed abnormal glucose metabolism over time (26%). Women with a high intake of pulses were more likely to undertake a heavy level of occupational physical activity at baseline compared with those with low consumption, and women in the lowest tertile of pulse consumption were more likely to be smokers than those who consumed more pulses. In men, a higher consumption of pulses was associated with higher waist circumference at baseline. High consumption of pulses was associated with energy-adjusted consumption of vegetables and fibre in both men and women. Men had a higher absolute intake of pulses (g/day; P=0.017), whilst women had a higher energy-adjusted intake of pulses (g/MJ; P=0.013).
Energy-adjusted intake of pulses (g/MJ) was positively correlated (all Rs ≥ 0.15) with energy-adjusted intake of total fibre (men Rs=0.18, P<0.001; women Rs=0.16, P=0.001), vegetables (men Rs=0.28, P<0.001; women Rs=0.20, P<0.001), Faratha (Indian bread; men Rs=0.16, P=0.002; women Rs=0.27, P<0.001) and canned fish (men Rs=0.17, P=0.001; women Rs=0.17, P=0.001) in both men and women. In addition, energy-adjusted intake of pulses correlated positively with energy-adjusted intake of potatoes (Rs=0.22; P<0.001), fried eggs (Rs=0.17; P=0.001) and negatively with energy-adjusted intake of beef (Rs=−0.23; P<0.001) in women. Among the energy-adjusted food items, canned fish and potatoes were negatively associated with abnormal glucose metabolism, beef was positively associated with both increase in BMI and waist circumference and potatoes and canned fish were negatively associated with increase in waist circumference.
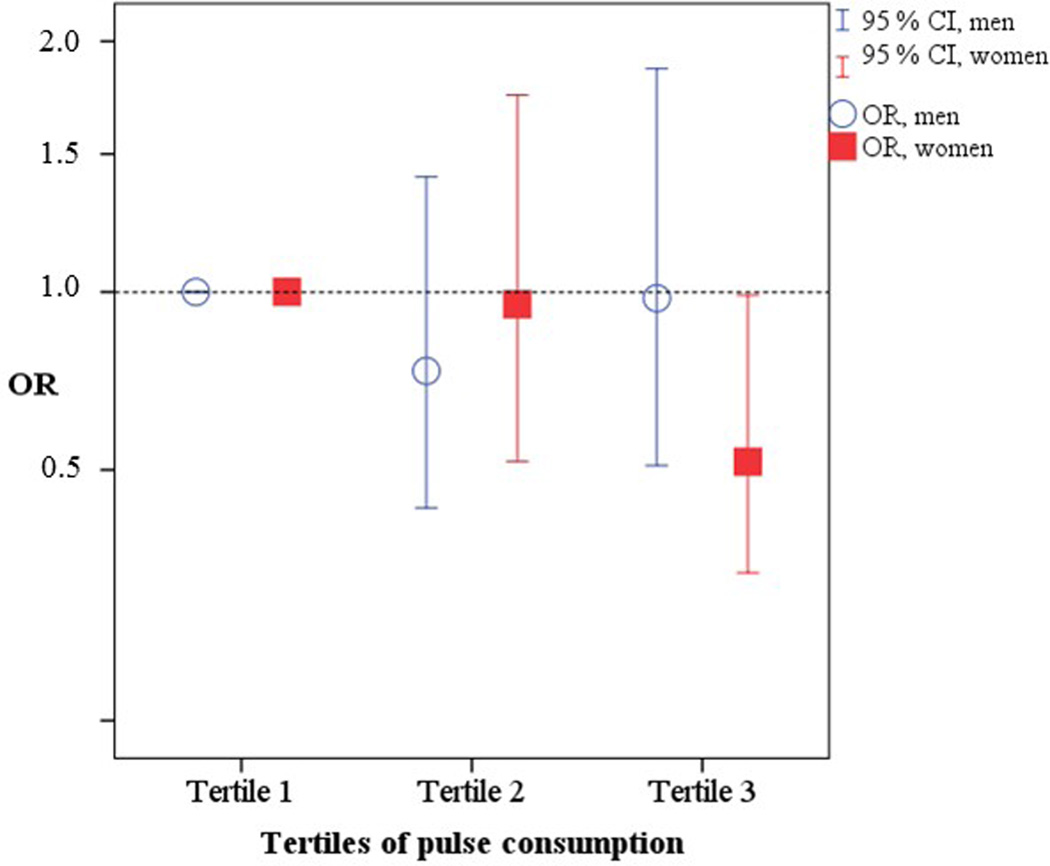
Of the 804 people eligible for the analysis on abnormal glucose metabolism in 1998, 209 (97 men and 112 women) developed abnormal glucose metabolism. Women with a high consumption of pulses at baseline, defined as the highest tertile, had a reduced risk of developing abnormal glucose metabolism at follow-up, after adjustment for age, ethnicity, waist circumference, smoking, occupational physical activity, energy-adjusted consumption of vegetables, fibre, canned fish, potatoes and beef, family history of diabetes and BMI, compared with the lowest tertile (OR 0.52; 95% CI 0.27, 0.99). In men, pulse consumption at baseline was not associated with a reduced risk of developing abnormal glucose metabolism (Table 2; Fig. 2). In the multivariable model, women in the highest tertile of potato consumption had a decreased risk of future abnormal glucose metabolism (OR 0.46; 95 % CI 0.24, 0.85).
Table 2.
Risk of developing abnormal glucose metabolism at follow-up, according to baseline energy-adjusted pulse consumption, in 804 people with normal glucose metabolism at baseline in Mauritius
| Odds ratio (95% CI) | ||||
|---|---|---|---|---|
| Cases/N | Tertile 1 ≤ 6.14 g/MJ |
Tertile 2 6.15–11.1 g/MJ |
Tertile 3 ≥ 11.2 g/MJ |
|
| Model 1* | ||||
| All | 209/804 | 1.00 | 0.93 (0.63, 1.38) | 0.70 (0.47, 1.03) |
| Men | 97/374 | 1.00 | 0.85 (0.47, 1.50) | 0.91 (0.51, 1.59) |
| Women | 112/430 | 1.00 | 0.99 (0.58, 1.69) | 0.56 (0.32, 0.96)† |
| Model 2‡ | ||||
| All | 206/793 | 1.00 | 0.88 (0.58, 1.33) | 0.69 (0.44, 1.08) |
| Men | 97/374 | 1.00 | 0.76 (0.41, 1.41) | 0.98 (0.51, 1.87) |
| Women | 109/419 | 1.00 | 0.96 (0.52, 1.75) | 0.52 (0.27, 0.99)† |
Adjusted for age at baseline.
Statistically significant association (P<0.05).
Adjustments in model 1 plus ethnicity, baseline occupational physical activity, waist circumference, smoking, energy-adjusted consumption of vegetables, fibre, canned fish, potatoes and beef, BMI and family history of diabetes.
FIGURE 2.
Odds ratio (OR) of abnormal glucose metabolism by tertile of energy-adjusted pulse consumption in 374 men and 419 women, adjusted for age, ethnicity, occupational physical activity, smoking, family history of diabetes, BMI, waist circumference, and energy-adjusted consumption of vegetables, fibre, potatoes, canned fish and beef at baseline.
In an additional analysis, using quartiles, women in the fourth quartile of pulse consumption were protected from developing abnormal glucose metabolism (model 2: OR 0.47; 95% CI 0.23, 0.96) and, when modelled in quintiles, women in the fifth quintile of pulse consumption also had a trend towards reduced risk of developing abnormal glucose metabolism (model 2: OR 0.47; 95% CI 0.21, 1.04).
The addition of changes in BMI or waist circumference between baseline and follow-up slightly attenuated the protective association of high pulse consumption with abnormal glucose metabolism found in women [addition of change in BMI resulted in ORT3 0.54 (95% CI 0.28, 1.04) and addition of change in waist circumference resulted in ORT3 0.54 (95% CI 0.28, 1.03)].
Men and women in all tertiles of pulse consumption had an increase in BMI from baseline to follow-up; however, women with the highest consumption of pulses at baseline had a smaller increase in BMI than did those with lower consumption (P<0.05;Table 3).
Table 3.
Change in BMI from baseline to follow-up, in Mauritian men and women, by tertile of pulse consumption at baseline
| Change (95% CI) in BMI, kg/m2 | ||||
|---|---|---|---|---|
| N | Tertile 1 | Tertile 2 | Tertile 3 | |
| Model 1 | ||||
| All | 794 | 0.9 (0.6, 1.1) | 1.0 (0.7, 1.2) | 0.5 (0.3, 0.8)* |
| Men | 374 | 0.7 (0.4, 1.0) | 0.7 (0.4, 1.0) | 0.5 (0.2, 0.8) |
| Women | 420 | 1.1 (0.7, 1.4) | 1.2 (0.9, 1.6) | 0.5 (0.2, 0.9)* |
| Model 2 | ||||
| All | 788 | 0.9 (0.6, 1.1) | 1.0 (0.7, 1.2) | 0.6 (0.3, 0.8)† |
| Men | 373 | 0.7 (0.4, 1.0) | 0.7 (0.4, 1.0) | 0.6 (0.2, 0.9) |
| Women | 415 | 1.0 (0.6, 1.4) | 1.2 (0.9, 1.6) | 0.6 (0.2, 0.9)† |
Model 1 adjusted for age at baseline, and model 2 adjusted for age at baseline plus variables associated with energy-adjusted consumption of pulses at baseline and/or the follow-up outcome in men and/or women (ethnicity, baseline occupational physical activity, smoking, educational level, energy-adjusted consumption of vegetables, fibre and beef).
Significantly different from tertiles 1 and 2 (P<0.05).
Significantly different from tertile 2 (P<0.05).
Waist circumference increased in men in all tertiles, with no difference between levels of consumption. In contrast, waist circumference did not increase in women, and those with the highest consumption of pulses even decreased their waist circumference (P<0.05). This difference was attenuated in the fully adjusted model (Table 4).
Table 4.
Change in waist circumference from baseline to follow-up, in Mauritian men and women, by tertile of pulse consumption at baseline
| Change (95% CI) in waist circumference, cm | ||||
|---|---|---|---|---|
| N | Tertile 1 | Tertile 2 | Tertile 3 | |
| Model 1 | ||||
| All | 794 | 1.0 (0.3, 1.7) | 0.4 (−0.2, 1.1) | −0.3 (−1.0, 0.3)‡ |
| Men | 374 | 2.2 (1.3, 3.1) | 1.2 (0.2, 2.1) | 1.3 (0.4, 2.2) |
| Women | 420 | −0.2 (−1.2, 0.8) | −0.2 (−1.2, 0.7) | −1.6 (−2.4, −0.7)* |
| Model 2 | ||||
| All | 788 | 0.8 (0.1, 1.5) | 0.4 (−0.2, 1.1) | −0.1 (−0.8, 0.5) |
| Men | 373 | 2.1 (1.1, 3.0) | 1.1 (0.2, 2.1) | 1.5 (0.5, 2.4) |
| Women | 415 | −0.3 (−1.4, 0.7) | −0.2 (−1.2, 0.7) | −1.5 (−2.3, −0.6) |
Model 1 adjusted for age at baseline, and model 2 for age at baseline plus variables associated with energy-adjusted consumption of pulses at baseline and/or the follow-up outcome in men and/or women (ethnicity, baseline occupational physical activity, smoking, energy-adjusted consumption of vegetables, fiber, potatoes, canned fish and beef).
Significantly different from tertile 1 (P<0.05).
Significantly different from tertiles 1 and 2 (P<0.05).
In the multivariable models, energy-adjusted consumption of beef was positively associated with both increasing BMI (P=0.001) and waist circumference (P=0.006), whereas energy-adjusted consumption of vegetables (P=0.022) and potatoes (P=0.039) were negatively associated with increasing waist circumference.
The effect of pulse consumption on changes in BMI or waist circumference was also evaluated in all 1421 participants with data on pulse consumption at baseline and outcome at follow-up (that is, including also those with abnormal glucose metabolism at baseline) and these analysis produced results similar to those of the analysis that was restricted to people with normal glucose metabolism at baseline (data not shown).
Discussion
We found a significant association between high consumption of pulses and reduced risk of abnormal glucose metabolism in Mauritian women, but not in men. We also found a protective association of pulse consumption against increase in BMI and waist circumference, in women, but for waist circumference the association was no longer statistically significant after multivariable adjustments.
The inclusion of change in weight or waist circumference during follow-up only slightly attenuated the protective association between high consumption of pulses and abnormal glucose metabolism in women. This indicates that the association is not simply explained by a beneficial effect on weight or fat distribution by pulse consumption.
The protective association found in women between pulse consumption and abnormal glucose metabolism is in accordance with a previous large cross-sectional study in an Indian population, where a protective association between daily consumption of pulses and lower prevalence of diabetes was found in women (OR 0.71; 95% CI 0.58, 0.86), while the association in men was not statistically significant (OR 0.78; 95% CI 0.61, 1.01) [12]. Also, a prospective study conducted on women in Shanghai found a protective association between high consumption of legumes and soy beans and lower risk of Type 2 diabetes (highest compared with lowest quintile: OR 0.62; 95% CI 0.51, 0.74) [9]. A protective association was found for the second quintile compared with the first quintile in the Shanghai study, while in the present study the protective association in women was evident in the highest tertile of pulse consumption. The consumption of pulses was higher in the present study, with a median consumption of 136 g/day in the highest tertile compared with 65 g/day in the highest quintile in the Shanghai study.
Possible mechanisms explaining the protective association between pulse consumption and decreased risk of abnormal glucose metabolism, could be the blood glucose stabilizing effect of pulses [7,19]. Modulation of hepatic trafficking of carbohydrates by increasing glycogen storage and decreasing glycolysis, caused by butyric acid [20,21] could also explain this association; however, these mechanisms provide no explanation for the gender-related difference.
Beneficial effects on fat distribution by pulse consumption are supported by previous studies in animals and humans [22–24]. A suggested mechanism is the oestrogen effect of the phyto-estrogens in pulses [25] which is expected to be greater in women than men. This could, at least in part, explain the gender difference found in the present study, in which women with the highest consumption of pulses decreased their waist circumference from baseline to follow-up, which was not the case for women with a lower consumption of pulses.
Another reason that may explain the gender differences could be related to awareness of food consumption. Women are usually more involved in preparation of foods and more accurate diet reporting by women has been observed in previous research [26,27].
The protective association between high consumption of pulses and abnormal glucose metabolism found in women could be attributable to confounding factors such as a correlation between pulse consumption and high consumption of other protective food items or avoidance of other harmful food items. The negative association between high consumption of pulses and abnormal glucose metabolism found in women remained after the addition of food items associated with consumption of pulses and the outcome (energy-adjusted consumption of vegetables, potatoes, canned fish and beef). Consumption of pulses has previously been associated with a traditional lifestyle in Mauritius [17], and there may be other components in a traditional lifestyle, protective against abnormal glucose metabolism, which we have not been able to account for in this study. Furthermore, data collection of physical activity levels is generally crude.
A limitation of the present study is that one single 24-h diet recall was available for estimation of energy intake and several 24-h recalls would have increased the precision; however, it has been previously found that the intra-individual variability of energy intake is lower in less westernized countries [28].
Other limitations were related to our inability to reliably study changes in diet between baseline and follow-up as the food frequency questionnaires used were modified over time, the number of food items collected increasing from 44 in 1992 to 102 in 1998. We cannot exclude the possibility that participants changed their patterns of consumption of food items during follow-up. This may have attenuated the associations between pulse consumption and outcomes. Strengths of the present study include its prospective design, its 6-year follow-up and the use of an oral glucose tolerance test to ascertain diabetes status.
The present study indicates that increased consumption of pulses could be a goal for a dietary intervention to curb the rising prevalence of abnormal glucose metabolism and obesity in Mauritius. Given that the ethnicities represented in Mauritius account for a significant proportion of the world's diabetes population, the data presented here could be relevant for prevention of Type 2 diabetes in many other settings.
In conclusion, in the present prospective study in Mauritius, we found an association between high consumption of pulses and lower risk of abnormal glucose metabolism in women. There were also associations between high pulse consumption and a smaller increase in BMI in women. Promotion of pulse consumption could be one important dietary intervention to decrease the prevalence of Type 2 diabetes and obesity in Mauritius. Clinical trials should be carried out in both men and women to confirm the association between pulse consumption and risk of Type 2 diabetes and obesity.
What's new?
Mauritian women with a high consumption of pulses had a reduced risk of abnormal glucose metabolism.
In Mauritian women, a high consumption of pulses was associated with a smaller increase in BMI from baseline to follow-up, compared with a lower consumption.
These results indicate that promotion of pulse consumption could be one important dietary intervention for the prevention of Type 2 diabetes and obesity in Mauritius.
Clinical trials should be carried out in both men and women to confirm the association between pulse consumption and risk of Type 2 diabetes and obesity.
Acknowledgements
We are grateful to the many people involved in organizing and conducting of the Mauritius studies, and to the participants for volunteering their valuable time. We also thank Marie Eriksson, Umeå University, Sweden, for valuable statistical advice.
Funding sources
The surveys were undertaken with the support and collaboration of the Ministry of Health and Quality of Life (Mauritius), the WHO (Geneva, Switzerland), the Baker IDI Heart and Diabetes Institute (formerly the International Institute, Melbourne, Australia), the University of Newcastle upon Tyne (UK), and the National Public Health Institute (Helsinki, Finland). The study was partially funded by US National Institutes of Health Grant DK-25446 and the Academy of Finland. The work was supported by the Victorian Government's OIS Program. M.W. was supported by the Swedish Research Council for Health, Working Life and Welfare, S.S was supported by grants from the Västerbotten County Council (ALF), J.E.S. was supported by a National Health and Medical Research Council fellowship (586623).
Footnotes
Competing interests
None declared.
References
- 1.Magliano DJ, Soderberg S, Zimmet PZ, Chen L, Joonas N, Kowlessur S, et al. Explaining the increase of diabetes prevalence and plasma glucose in Mauritius. Diabetes Care. 2012;35:87–91. doi: 10.2337/dc11-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P, et al. High incidence of type 2 diabetes and increasing conversion rates from impaired fasting glucose and impaired glucose tolerance to diabetes in Mauritius. J Intern Med. 2004;256:37–47. doi: 10.1111/j.1365-2796.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 3.Uusitalo U. Doctoral dissertation. Ithaca, NY, USA: Cornell University; 2003. Dietary Westernization and body mass index in Mauritius. [Google Scholar]
- 4.Leterme P. Recommendations by health organizations for pulse consumption. Br J Nutr. 2002;88(Suppl. 3):S239–S242. doi: 10.1079/BJN2002712. [DOI] [PubMed] [Google Scholar]
- 5.Marinangeli CP, Jones PJ. Pulse grain consumption and obesity: effects on energy expenditure, substrate oxidation, body composition, fat deposition and satiety. Br J Nutr. 2012;108(Suppl. 1):S46–S51. doi: 10.1017/S0007114512000773. [DOI] [PubMed] [Google Scholar]
- 6.Degen L, Matzinger D, Drewe J, Beglinger C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides. 2001;22:1265–1269. doi: 10.1016/s0196-9781(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson A, Johansson E, Ekstrom L, Bjorck I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: a randomized cross-over study. PloS One. 2013;8:e59985. doi: 10.1371/journal.pone.0059985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papanikolaou Y, Fulgoni VL., 3rd Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: results from the National Health and Nutrition Examination Survey 1999–2002. J Am Coll Nutr. 2008;27:569–576. doi: 10.1080/07315724.2008.10719740. [DOI] [PubMed] [Google Scholar]
- 9.Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, et al. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr. 2008;87:162–167. doi: 10.1093/ajcn/87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto Y, Steinbrecher A, Kolonel LN, Maskarinec G. Soy consumption is not protective against diabetes in Hawaii: the Multiethnic Cohort. Eur J Clin Nutr. 2011;65:279–282. doi: 10.1038/ejcn.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, et al. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected] Eur J Nutr. 2012;51:1033–1040. doi: 10.1007/s00394-011-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal S, Ebrahim S. Prevalence and risk factors for self-reported diabetes among adult men and women in India: findings from a national cross-sectional survey. Public Health Nutr. 2012;15:1065–1077. doi: 10.1017/S1368980011002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal S, Ebrahim S. Association between legume intake and self-reported diabetes among adult men and women in India. BMC Public Health. 2013;13:706. doi: 10.1186/1471-2458-13-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(CIA) CIA. The World Factbook 2013 Washington DC2013. [updated July 8, 2013]; Available at: https://www.cia.gov/library/publications/the-world-factbook/geos/mp.html. Last accessed.
- 15.Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P, et al. Increasing prevalence of Type 2 diabetes mellitus in all ethnic groups in Mauritius. Diabet Med. 2005;22:61–68. doi: 10.1111/j.1464-5491.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 16.Willett W. Nutritional Epidemiology. New York: Oxford University Press; 2013. [Google Scholar]
- 17.Uusitalo U, Sobal J, Moothoosamy L, Chitson P, Shaw J, Zimmet P, et al. Dietary Westernisation: conceptualisation and measurement in Mauritius. Public Health Nutr. 2005;8:608–619. doi: 10.1079/phn2004716. [DOI] [PubMed] [Google Scholar]
- 18.Magliano DJ, Soderberg S, Zimmet PZ, Cartensen B, Balkau B, Pauvaday V, et al. Mortality, all-cause and cardiovascular disease, over 15 years in multiethnic mauritius: impact of diabetes and intermediate forms of glucose tolerance. Diabetes Care. 2010;33:1983–1989. doi: 10.2337/dc10-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santangelo A, Peracchi M, Conte D, Fraquelli M, Porrini M. Physical state of meal affects gastric emptying, cholecystokinin release and satiety. Br J Nutr. 1998;80:521–527. doi: 10.1017/s0007114598001615. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JW, Bridges SR. Short-chain fatty acid fermentation products of plant fiber affect glucose metabolism of isolated rat hepatocytes. Proc Soc Exp Biol Med. 1984;177:372–376. doi: 10.3181/00379727-177-41958. [DOI] [PubMed] [Google Scholar]
- 21.Beauvieux MC, Roumes H, Robert N, Gin H, Rigalleau V, Gallis JL. Butyrate ingestion improves hepatic glycogen storage in the re-fed rat. BMC Physiol. 2008;8:19. doi: 10.1186/1472-6793-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Zhou L, Gu Y, Zhang Y, Tang J, Li F, et al. Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. Br J Nutr. 2007;98:720–726. doi: 10.1017/S0007114507750870. [DOI] [PubMed] [Google Scholar]
- 23.Morris KL, Zemel MB. Effect of dietary carbohydrate source on the development of obesity in agouti transgenic mice. Obes Res. 2005;13:21–35. doi: 10.1038/oby.2005.5. [DOI] [PubMed] [Google Scholar]
- 24.Marinangeli CP, Jones PJ. Whole and fractionated yellow pea flours reduce fasting insulin and insulin resistance in hypercholesterolaemic and overweight human subjects. Br J Nutr. 2011;105:110–117. doi: 10.1017/S0007114510003156. [DOI] [PubMed] [Google Scholar]
- 25.Pallottini V, Bulzomi P, Galluzzo P, Martini C, Marino M. Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infect Disord Drug Targets. 2008;8:52–60. doi: 10.2174/187152608784139631. [DOI] [PubMed] [Google Scholar]
- 26.Yuhas JA, Bolland JE, Bolland TW. The impact of training, food type, gender, and container size on the estimation of food portion sizes. J Am Diet Assoc. 1989;89:1473–1477. [PubMed] [Google Scholar]
- 27.Karvetti RL, Knuts LR. Validity of the 24-hour dietary recall. J Am Diet Assoc. 1985;85:1437–1442. [PubMed] [Google Scholar]
- 28.Persson V, Winkvist A, Ninuk T, Hartini S, Greiner T, Hakimi M, et al. Variability in nutrient intakes among pregnant women in Indonesia: implications for the design of epidemiological studies using the 24-h recall method. J Nutr. 2001;131:325–330. doi: 10.1093/jn/131.2.325. [DOI] [PubMed] [Google Scholar]