Abstract
Background
Low-Level Light Therapy (LLLT) is used to stimulate healing, reduce pain and inflammation, and preserve tissue from dying. LLLT has been shown to protect cells in culture from dying after various cytotoxic insults, and LLLT is known to increase the cellular ATP content. Previous studies have demonstrated that maintaining a sufficiently high ATP level is necessary for the efficient induction and execution of apoptosis steps after photodynamic therapy (PDT).
Methods
We asked whether LLLT would protect cells from cytotoxicity due to PDT, or conversely whether LLLT would enhance the efficacy of PDT mediated by mono-L-aspartyl chlorin(e6) (NPe6). Increased ATP could lead to enhanced cell uptake of NPe6 by the energy dependent process of endocytosis, and also to more efficient apoptosis. In this study, human osteosarcoma cell line MG-63 was subjected to 1.5 J/cm2 of 810 nm near infrared radiation (NIR) followed by addition of 10 μM NPe6 and after 2 h incubation by 1.5 J/cm2 of 652 nm red light for PDT.
Results
PDT combined with LLLT led to higher cell death and increased intracellular reactive oxygen species compared to PDT alone. The uptake of NPe6 was moderately increased by LLLT, and cellular ATP was increased. The mitochondrial respiratory chain inhibitor antimycin A abrogated the LLLT-induced increase in cytotoxicity.
Conclusions
Taken together, these results demonstrate that LLLT potentiates NPe6-mediated PDT via increased ATP synthesis and is a potentially promising strategy that could be applied in clinical PDT.
Keywords: Photodynamic therapy, Low level light therapy, Photobiomodulation, Mono-L-aspartyl chlorin(e6) Lysosomal uptake, Adenosine triphosphate
Introduction
Photodynamic therapy (PDT) uses three key components: a photosensitizer (PS), a light source of the correct wavelength to be absorbed by the PS, and tissue oxygen. PDT is used in cancer treatment, microbial destruction, and in a wide range of other medical applications [1]. Cells or pathologic tissues can selectively take up the PS, and the specific wavelength of the light excites the PS to produce reactive oxygen species to damage biomolecules and kill cells [2]. The activated PS can undergo two kinds of reaction. First, it can form free radicals through electron transfer (type I reaction) with oxygen or biomolecules. Alternatively, it can transfer its energy directly to ground state triplet oxygen to form highly reactive, excited state singlet oxygen (type II reaction) [3]. However, the cytotoxicity of PDT in vitro often does not correlate with the in vivo situation as tumor response is primarily determined by the tumor accumulation and biodistribution, because the lipoproteins and albumin interact strongly with PS after systemic administration [4]. Therefore the mechanisms of PS uptake into cells have an important role in the selectivity and efficacy of PDT [5, 6].
Mono-L-aspartyl chlorin(e6) (NPe6) is a lysosomal localizing PS that can lead to cellular apoptosis and/or necrosis after illumination. NPe6-PDT results in lysosomal disruption, followed by the release of cathepsins that then cleave BH3-interacting-domain death agonist (Bid) and activate caspases which eventually leads to release of cytochrome C, and cell apoptosis [7]. The uptake of NPe6 into lysosomes is mediated by the process of endocytosis which is an energy-dependent process that is powered by cellular ATP.
Several important physiological processes and biochemical reactions are affected by photobiomodulation. Previous studies have shown that the primary mechanisms of low-level light therapy (LLLT) involve light absorption in mitochondria or other organelles and lead to modulation of signaling pathways, via production of ROS, ATP, Ca2+, and NO [8]. Secondary effects include changes in stress signaling, cell metabolism, cytoskeleton, proliferation, homeostasis, and increased cell survival [9–11]. Several studies have shown that exposing cultured cells in vitro to LLLT using varying wavelengths and doses can protect the cells from dying after they have been treated with different cytotoxic mediators. Eells and Wong-Riley’s laboratory have shown the cytoprotective effect of LLLT applied to cultured neurons that had been treated with cyanide [12], tetrodotoxin [13], rotenone and MPP+ [14]. Da Xing demonstrated LLLT-induced cytoprotection and reduced apoptosis in neurons exposed to amyloid-beta peptide [15, 16].
Previous studies showed that the antitumor effect of hematoporphyrin monomethyl ether-mediated PDT markedly inhibited the proliferation of sarcoma cell lines (LM8, MG63, Saos-2, SW1353, TC71, and RD) through a caspase-dependent apoptosis pathway, both in cells and in a mouse sarcoma model [17]. Importantly, this report concluded that PDT could be a minimally invasive and highly selective treatment to decrease the local recurrence rate of malignant bone tumors during limb salvage procedure. Maintaining a sufficiently high ATP level enhanced the caspase-3 activity and nuclear fragmentation after PDT for cells treated with glucose [18].
In our present study, we asked whether the human osteosarcoma cell line MG-63 treated with 810 nm NIR laser followed by NPe6-PDT would show increased survival after PDT via a cytoprotective mechanism, or alternatively if the cells would show enhanced efficacy of PDT. Enhanced PDT cytotoxicity could be mediated either by increased uptake of NPe6, or by enhanced apoptosis; both effects could in principle be caused by increased ATP production.
Material and methods
Light sources
A Ga-Al-As diode laser (Acculaser Inc., USA) was used for LLLT, 810 nm, continuous wave, irradiance of 20 mW/cm2 appropriately [19]. A non-coherent filtered lamp (LumaCare, USA) (652+15 nm, irradiance of 20 mW/cm2) was used for PDT. The power densities of both light sources were measured with a power meter (Thorlab, USA). After preliminary experiments the fluences of both the 810 nm laser and the 652 nm lamp was set at 1.5 J/cm2 achieved after an exposure duration of 1.2 minutes for both.
Cell culture
A human osteosarcoma cell line MG-63 (ATCC, Mannassas, VA) was cultured in RPMI 1640 medium (Invitrogen, USA) supplemented with 10% fetal bovine serum (Invitrogen, USA), 1% penicillin-streptomycin (Invitrogen, USA) at 37°C and 5% CO2 in an incubator.
Cell viability and PDT treatment
MG-63 cells were seeded at a density of 5 × 103 per well in 96-well culture plates (Fisher Scientific, USA) for overnight culture. At the start of the experiment, 1.5 J/cm2 of 810 nm NIR was delivered, and then the medium was replaced by a new medium with various concentrations of NPe6 (Light Science Corp., USA) for a 2 h-incubation.
Before PDT treatment, the cells were washed with PBS and replaced by a new medium without fetal bovine serum, then exposed to 1.5 J/cm2 of red light At 24 h after PDT treatment, the viability of MG-63 cells was quantitatively measured by a Prestoblue cell assay (Invitrogen, USA). The cells were incubated in cell permeable resazurin-based solution for 1 h at 37°C. Cell viability was quantitatively measured by fluorescent reader (Molecular Devices, USA) with excitation at 560nm and emission at 590nm. Survival of cells was normalized to control cells without NPe6 treatment and laser irradiation.
For each sample, the cellular viability was calculated from the data of 3 wells (n=3) and expressed as a percentage, compared with the untreated cells (100%). Comparison of the mean optical density between the untreated (100%) and treated cells 24 h after PDT allowed the evaluation of the cytotoxicity. Each experiment was repeated 3 times.
Cellular uptake of NPe6
MG-63 cells (1×105 cells) were seeded in 6-well culture plates and incubated at 37°C, 5% CO2 overnight. Before incubation, MG-63 cells were treated with or without 1.5 J/cm2 of 810 nm NIR. The medium in the cell culture well were replaced with medium containing 5 or 10 μM NPe6 and incubated for 2 hours.
After the MG-63 cells were washed with PBS, they were dissolved in 300 μl cell lysis buffer. 150 μl lysates were then transferred to luminescence 96-well plates (Corning, USA) and the amount of NPe6 was fluorometrically determined with an excitation wavelength of 400 nm and emission at 660nm by a fluorescent plate reader. Cell protein was quantitatively measured by Bradford Reagent (Sigma, USA), which allowed the amount of NPe6 per unit of cell protein to be calculated.
Measurement of ROS generation induced by NIR combined with PDT
MG-63 cells (1×105 units) were seeded in 6-well culture plates and incubated at 37°C, 5% CO2 overnight. To monitor ROS generated by NPe6 mediated-PDT, we pretreated MG-63 cells with 1.5 J/cm2 of 810 nm NIR and incubated with 5 and 10 μM NPe6 for 2 h. After the MG-63 cells were washed with PBS and replaced by a medium without fetal bovine serum, they were exposed to 1.5 J/cm2 of 652 nm red light for PDT, and then stained with CM-H2DCFDA (Invitrogen, USA) immediately for 30 minutes to measure the level of ROS by flow cytometry (BD Biosciences, USA).
Cell Localization of NPe6 via Confocal Microscopy
To determine NPe6 intracellular localization, we used confocal microscopy (Olympus FV1000, Japan) to observe NPe6 in conjunction with fluorescent probes. Cells were plated at a density of 5×103/well in a 96-well black walled plate with clear bottoms (Corning, USA) overnight.
MG-63 cells were treated with or without 1.5 J/cm2 of 810 nm NIR. After 2 h of incubation with 10 μM of NPe6, cells were washed with PBS and stained with fluorescent probes specific for different cellular organelles (Invitrogen, USA). Mitochondria were stained with MitoTracker-Green FM (100 nM, 30 min, 37°C), lysosomes with LysoSensor-Red DND-99 (200 nM, 30 min, 37°C) and the nucleus with Hoechst 33342 (5 μg, 30 min, 37°C). Cells were then examined under a confocal microscope. Excitation wavelength and detection filter settings for NPe6 were 400 nm and recorded through a 665-nm IR band-pass filter, respectively. MitoTracker-Green FM was excited at 488 nm with an argon ion laser and emitted light was recorded through a 516-nm band-pass filter.
LysoSensor-Red DND-99 was excited at 559 nm with an argon ion laser and emitted light was recorded through a 590-nm band-pass filter. Hoechst 33342 was excited with a 405 nm laser and emission was recorded through a 461-nm band-pass filter. Images were acquired using FV10-ASW 2.0 viewer (Olympus, JAPAN) software.
ATP activity assay
MG-63 cells (5×104 units) were seeded in 12-well culture plates and incubated the cells at 37°C, 5% CO2 overnight. MG-63 cells were treated with or without 1.5 J/cm2 of 810 nm NIR, and ATP was measured at each time point following NIR. To produce ATP release, 200 μl cell lysis buffer was added and plates were placed on shaker for 5 minutes. 100 μl lysates were then transferred to luminescence 96-well plates (Corning, USA). 100 μl of Cell-Titer Glo Assay mix (Promega, USA) was added into each well to evaluate ATP level. After shaking for 5 minutes, the culture plate was incubated for 10 minutes at room temperature to stabilize the luminescence signal. The luminescence signal was determined by a plate reader.
Pretreatment with mitochondrial respiratory chain inhibitor Antimycin A
MG-63 cells (5×103 units) were seeded in 96-well plates and incubated overnight at 37°C, 5% CO2. The next day antimycin A was added to the wells at 1 nM final concentration for 1 hour incubation, then fresh medium was added and the cells were treated with 1.5 J/cm2 of 810 nm NIR. Next NPe6 was added for a 2-hour incubation, and then cells were washed with PBS and fresh medium added prior to delivery of 1.5 J/cm2 of 652 nm red light for PDT The cell viability was measured at 24 h as described above.
Statistical Analysis
All results are expressed as the mean ± standard deviation. The Student’s t test was performed for statistical analysis, and differences between groups were considered significant at a p value <0.05.
Results
NIR-LLLT enhanced PDT effectiveness
To determine whether NIR-induced increases or decreases in PDT-mediated cytotoxicity, MG-63 cells were pre-treated with or without 1.5 J/cm2 of 810 nm NIR, before incubation with NPe6 at concentration of 10 μM for 2 h. At the end of NPe6 incubation, cells were washed with PBS and supplied with medium without FBS. After exposure to 1.5 J/cm2 of 652 nm red light for PDT, cells were incubated for 24 hours followed by a cell viability assay as shown in Figure 1.
Figure 1. Cell viability of MG-63 cells pretreated with NIR-LLLT followed by PDT.
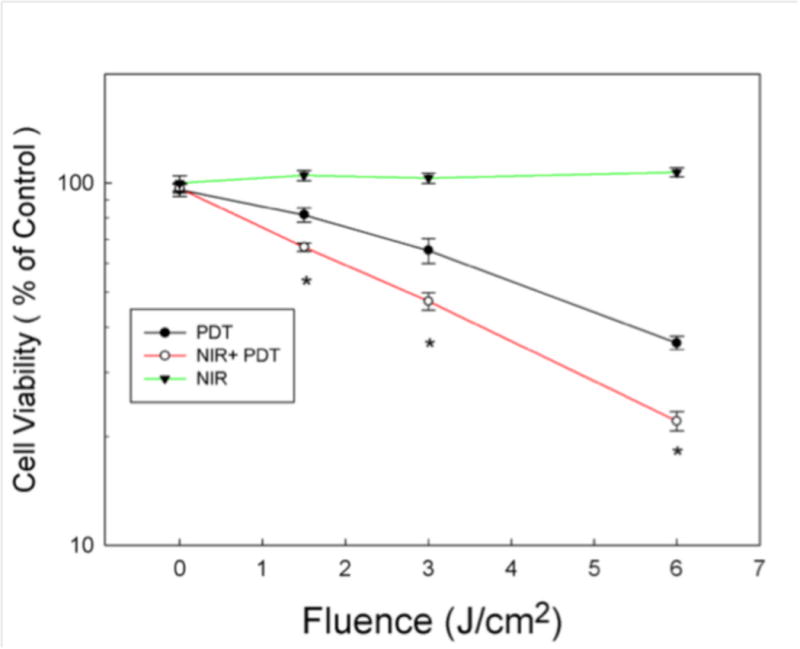
MG-63 cells were treated with or without NIR-LLLT (fluence 1.5 J/cm2) before 10 μM NPe6 incubation, and then treated with PDT at different fluences 0, 1.5, 3 and 6 J/cm2. (Open circle and closed circle). Additionally, MG-63 cells were treated with NIR-LLLT alone at various fluences 0, 1.5, 3 and 6 J/cm2, cell viability was measured at 24 hours (Closed triangle). Cell viability was measured at 24 hours after PDT or NIR
Data are the mean ± SD. An asterisk represents p value < 0.05 as a significant difference between NIR + PDT and PDT alone. All results in this figure are representative of experiments performed in triplicate.
MG-63 cells pre-treated with NIR showed significantly higher cytotoxicity after NPe6-mediated PDT in cell viability assays. Furthermore, the data also showed that NIR alone at various fluences without PDT had no effect on the cell viability.
Cellular uptake of NPe6 after NIR-LLLT
We measured the level of cellular uptake of NPe6 after zero or 1.5 J/cm2 of 810 nm NIR by fluorescence plate reader. Fluorescence intensity of NPe6 was moderately (5–10%) increased by pre-treatment with LLLT with both 5 and 10 μM NPe6 concentration and this increase reached statistical significance at 10 μM (Figure 2).
Figure 2. Quantification of NIR enhances cell uptake of NPe6 in MG-63.
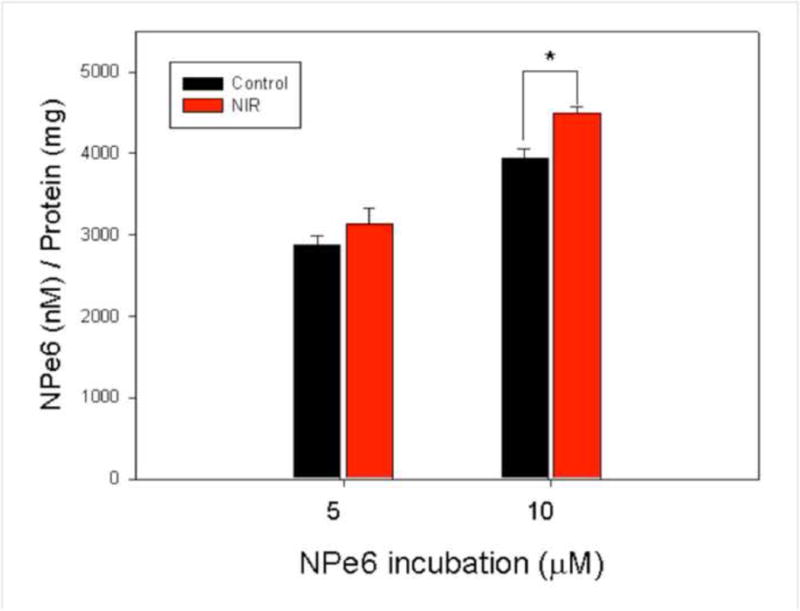
MG-63 cells were treated with or without 1.5 J/cm2 of 810 nm NIR-LLLT before NPe6 incubation for 2 hours. NPe6 was extracted, corrected for total protein concentration and fluorescence level determined in each cell lysate.
Data are the mean ± SD of the fluorescent signal. An asterisk represents the p value < 0.05 as a significant difference between control and NIR-LLLT cells. All results in this figure are representative of experiments performed in triplicate.
NIR-LLLT increases PDT-induced ROS generation
To monitor ROS production form NPe6-mediated PDT, the fluorescent molecular probe H2DCFDA was used to determine ROS in the cells after PDT by flow cytometry. We treated MG-63 cells with or without 1.5 J/cm2 of 810 nm NIR and then cells were incubated with 10 μM NPe6 for 2 hours. After exposure to 1.5J/cm2 of 652 nm light for PDT, cells were immediately incubated in medium containing H2DCFDA for flow cytometry analysis. The data showed that the pre-treatment with NIR slightly (but significantly at 10 uM) increased PDT-induced ROS generation in MG-63 cells compared to PDT cells without LLLT as shown in Figure 3.
Figure 3. NIR-LLLT combined with PDT induced ROS generation determined by flow cytometry.
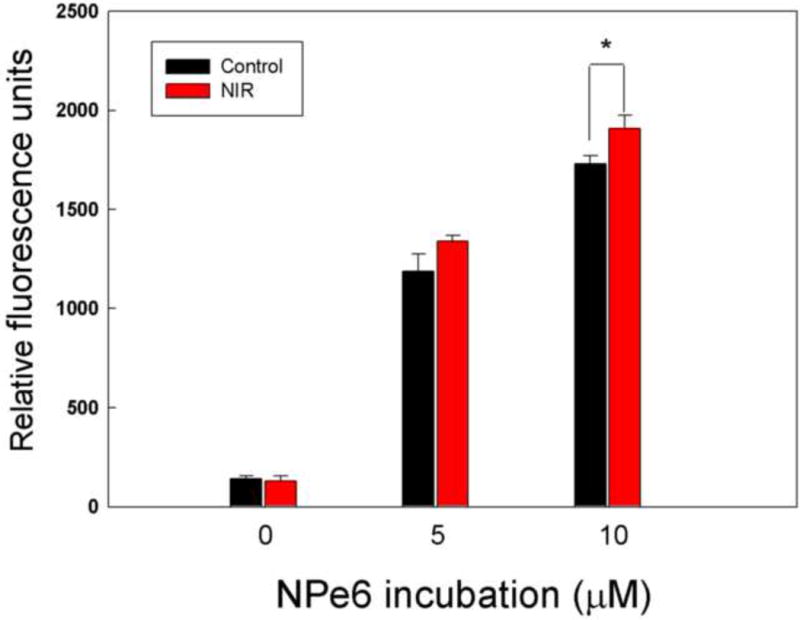
MG-63 cells were pretreated with 1.5 J/cm2 of 810 nm NIR-LLLT and incubated with NPe6 for 2 h, exposed to 1.5 J/cm2 652 nm red light, and then stained with H2DCFDA for 30 minutes to measure the level of ROS by flow cytometry.
Data are the mean ± SD of the fluorescent signal. An asterisk represents the p value < 0.05 as a significant difference between control and NIR-treated cells. All results in this figure are representative of experiments performed in triplicate.
Intracellular localization of NPe6 in MG-63 cells
To determine whether pre-treatment with 1.5 J/cm2 of 810 nm NIR made any difference in the subcellular localization of NPe6 we used fluorescent molecular probes specific for different cellular organelles and confocal microscopy. Figure 4 shows the fluorescence microscope pictures of MG-63 cells incubated with NPe6 (red fluorescence), mitochondria (green fluorescence), nucleus (blue fluorescence), and lysosome (yellow fluorescence). The merged picture indicates that the majority of NPe6 was located in lysosomes with a minor amount detected in mitochondria. Although there appeared to be more NPe6 red fluorescence visible in NIR treated cells compared to control cells, the localization did not appear to be different.
Figure 4. Subcellular localization of the NPe6 in MG-63 cells.
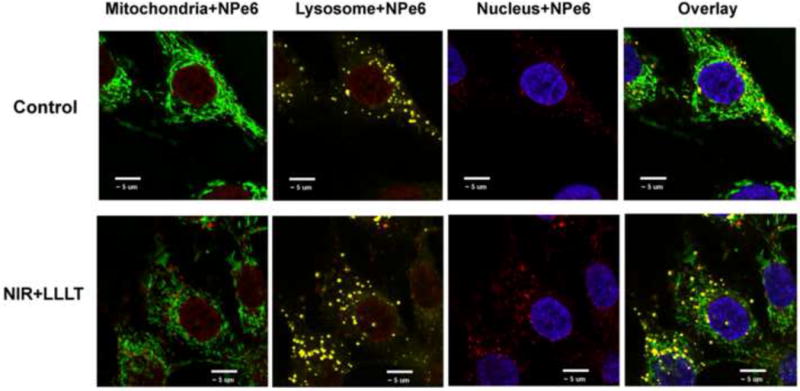
Confocal fluorescence images of subcellular localization of the MG-63 cells with or without 1.5 J/cm2 of 810 nm NIR-LLLT and 2 h of incubation with 10 μM NPe6. Cells were stained with organelle-specific fluorescent probes. NPe6 is red, mitochondria are green, lysosomes are yellow, and nucleus is blue, respectively. Scale bar indicates 10 μm.
Stimulation of ATP production by NIR-LLLT
To determine the effect of NIR on cellular ATP production, ATP levels were measured after exposure to 1.5 J/cm2 of 810 nm NIR at intervals of 5 minutes and 2 h. The results indicated that the ATP level significantly increased more than two-fold 5 min after LLLT and was still significantly increased (about 70%) after an interval of 2 h as shown in Figure 5.
Figure 5. NIR-LLLT stimulates ATP synthesis in MG-63 cells.
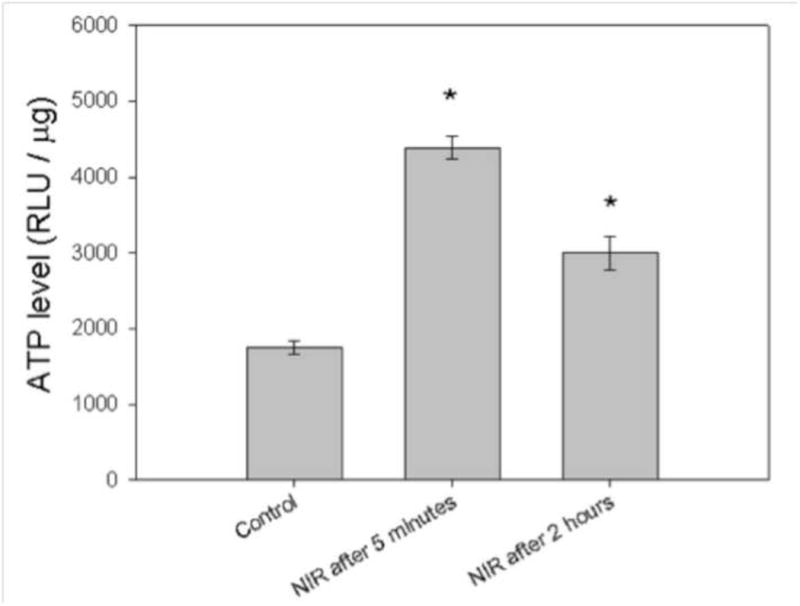
MG-63 cells were treated with 1.5 J/cm2 of 810 nm NIR-LLLT. After 5 min and 2 h, cells were lysed and Cell-Titer Glo Assay mix used to determine ATP levels. Data are the mean ± SD. An asterisk represents the p value < 0.05 as a significant difference between control and NIR-treated cells. All results in this figure are representative of experiments performed in triplicate.
NIR-LLLT potentiation of NPe6 PDT cytotoxicity is abrogated by antimycin A, an inhibitor of ATP synthesis
A previous study demonstrated that 660 nm LLLT-induced expression of M1-related cytokines and chemokines (CCL2) in the human monocyte cell line THP-1 was inhibited by antimycin [20]. To examine if NIR-mediated increase in NPe6 PDT-induced cytotoxicity depends on ATP synthesis, we treated MG-63 cells with or without 1.5 J/cm2 of 810 nm NIR, with and without NPe6-PDT, with and without the mitochondrial respiration chain inhibitor antimycin A. There were six combinations: (1) control; (2) antimycin A alone; (3) PDT alone; (4) NIR + PDT; (5) antimycin A + PDT; (6) antimycin A + NIR + PDT. It should be noted that antimycin A is expected to have a certain level of cytotoxicity in its own right [21]. Figure 6 shows that antimycin A alone produced 15% killing and this was significant. PDT alone produced 22% killing, and the PDT-killing after pretreatment with NIR was 33% (a significant increase over PDT alone as reported above). The combination of two different cytotoxic modalities (antimycin A and PDT) produced an additive effect giving 51% killing. Importantly, when NIR was added after antimycin A and then followed by PDT, the 50% killing was not significantly different from that found with antimycin A plus PDT alone. In other words the potentiation of killing found by combining NIR and PDT has been abrogated by use of antimycin A, an inhibitor of mitochondrial respiration.
Figure 6. NIR-LLLT potentiation of NPe6-PDT is abrogated by antimycin A.
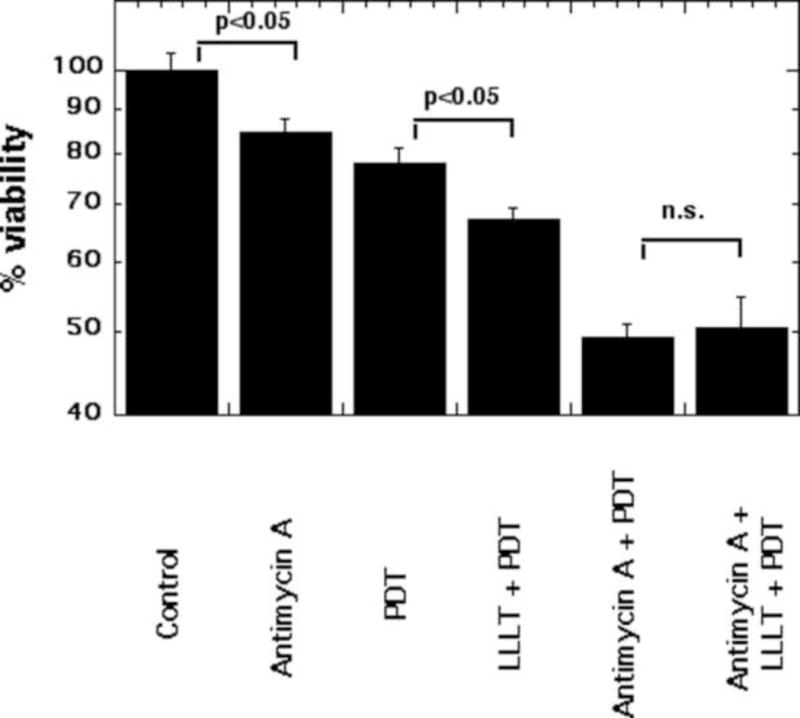
After 1 hour incubation with 1 nM antimycin A, MG-63 cells were treated with or without 1.5 J/cm2 of 810 nm NIR-LLLT before 2 hour NPe6 incubation, and then exposed to 1.5 J/cm2 652 nm red light for PDT.
Cell viability was measured at 24 h after PDT. Data are the mean ± SD of the fluorescent signal. p value < 0.05 showed a significant difference between the indicated groups. All results in this figure are representative of experiments performed in triplicate.
Discussion
In this study we asked an intriguing question: does the pretreatment of cancer cells with NIR-LLLT, protect the cancer cells from the cytotoxic effects of subsequent PDT, or conversely does it increase the cell killing? If the cell killing is increased is this because the uptake of NPe6 in increased, or is it because the increase in cellular ATP allows the cells to die more efficiently?
PDT mediated by NPe6 combined with 810 nm NIR laser led to higher (not lower) cytotoxicity against MG-63 cells (Figure 1). Furthermore, NIR alone without PDT had only a relatively slight effect on the cell viability. We initially tested the hypothesis that the NIR-induced increase in mitochondrial activity and consequent ATP production would enhance cellular uptake of the NPe6.
It is known that NPe6 is largely taken up into cells by endocytosis, where the NPe6 first goes into early endosomes, that then turn into late endosomes, and finally into lysosomes [22]. Roberts and Berns examined [23] the effect of reducing the incubation temperature to 2°C of cells treated with NPe6, and found that at low temperatures the uptake and consequent PDT-induced cytotoxicity was markedly reduced. This observation suggested that an active cellular uptake process was occurring, such as endocytosis, which requires consumption of cellular energy to power the uptake of NPe6. It is further known that NPe6 binds to serum proteins particularly high density lipoprotein and albumin [24], and it may be hypothesized that protein bound PS such as albumin-NPe6 are more likely to be taken up by endocytosis. The significant but rather limited increase in NPe6 uptake after LLLT suggested that a mechanism was induced by NIR that either increased endocytosis of NPe6, or interacted with some other pathway that affected uptake (Figure 2). To our knowledge there have been no reports of LLLT being used to increase uptake of any PS.
To compare PDT-induced ROS generation with or without NIR treatment, the samples were stained with H2DCFDA immediately after PDT treatment, and a higher ROS level was observed after NIR combined with PDT (Figure 3). It should be noted that adding H2DCFDA after the PDT light has been switched off, is likely to primarily measure the ROS produced inside the cells due to damage to mitochondria or other organelles, rather than the ROS directly produced by photochemical activation of the NPe6. This is because of the extremely short lifetime of the major photochemical ROS, singlet oxygen and hydroxyl radicals. Therefore this observation is likely to show increased intracellular damage with PDT after LLLT, consistent with reduced cell survival.
Localization of the NPe6 in lysosomes is correlated to cell death mechanisms through disruption of lysosomal membrane and release of enzymes such as cathepsins to activate apoptotic pathways in the cytosol including the cleavage of pro-apoptotic protein Bid, and the release of cytochrome C from the mitochondria [25]. Our results show that more NPe6 was accumulated in lysosomes and other cellular compartments after NIR combined with PDT (Figure 4).
Previous studies indicated that 632.8 nm He-Ne laser induced an increase in cellular ATP level through activation of the electron flow in the respiratory chain of irradiated HeLa cells [26]. 810 nm laser can increase cellular ATP levels through up-regulation of mitochondrial respiration, and leads to ROS production that can activate NF-kB in mouse embryonic fibroblasts, and LLLT-induced ATP synthesis could be inhibited by the mitochondrial respiration chain inhibitor antimycin A [27].
Our results also showed that ATP rapidly increased after exposure to 810 nm LLLT and the increase lasted for 2 h after irradiation (Figure 5). However, the NIR-induced increase in cytotoxicity was abrogated in the presence of antimycin A, indicating that the mitochondrial respiratory chain acts as a key regulator in the NIR-induced increase of NPe6 uptake and cytotoxicity (Figure 6).
A sufficient supply of cellular energy is required for efficient apoptotic cell death in general [28], and specifically for efficient apoptosis after PDT [18]. Human epidermoid carcinoma cell line A431 can be killed with chloroaluminum(III) phthalocyanine tetrasulfonate mediated PDT [29]. An appropriate level of ATP is critical during the apoptotic cascade. A higher light dose led to a rapid decline in mitochondrial activity and cellular ATP content, and resulted in necrotic cell death, rather than apoptotic cell death. The intracellular ATP level of hypoxic cardiac myocytes was also a critical determinant of the form of cell death whether by apoptosis or necrosis [30]. A recent report from Abraham et al [31] described a new cancer treatment approach that combined LLLT, hyperthermia and ionizing radiation. Although these authors did not investigate the mechanism in any detail, it is reasonable to hypothesize that the role of LLLT in the triple combination is to elevate cellular ATP providing more cellular energy for efficient cell death.
It should be noted that our results only apply to the particular sequence of LLLT and PDT that we chose to investigate. The LLLT could have been applied much longer before PDT (for instance 12 or 24 hours), or after the NPe6 incubation so that PS uptake was not affected by LLLT. Alternatively the LLLT could have been delivered after the red PDT light, in which case perhaps we might have observed cytoprotection rather than potentiation of killing.
LLLT stimulates mitochondrial respiration and ATP production to improve cell proliferation, migration, and adhesion [32]. In this study, we found that pre-treatment with LLLT increased rather than decreased the PDT cell killing. We also clarified that NIR-induced ATP synthesis is involved in the efficacy of PDT mediated by NPe6. These findings suggest that LLLT-induced an increase of cellular ATP and potentiates both drug uptake and cell apoptosis. In future, LLLT could be applied to potentiate not only PDT, but many other cytotoxic cancer therapies that require drug uptake and efficient apoptosis.
Acknowledgments
This work was supported by the US National Institutes of Health (R01AI050875). Shang-Ru Tsai was sponsored by the National Science Council of Taiwan. Legends to the figures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 2.Allison RR, Moghissi K. Photodynamic Therapy (PDT): PDT Mechanisms. Clinical endoscopy. 2013;46:24–9. doi: 10.5946/ce.2013.46.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis and Photodynamic Therapy. 2004;1:279–93. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenkranz AA, Jans DA, Sobolev AS. Targeted intracellular delivery of photosensitizers to enhance photodynamic efficiency. Immunol Cell Biol. 2000;78:452–64. doi: 10.1046/j.1440-1711.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 5.Anselmo AC, Mitragotri S. An Overview of Clinical and Commercial Impact of Drug Delivery Systems. Journal of controlled release: official journal of the Controlled Release Society. 2014 doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Miyoshi H, Nakamura M. Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer. 2007;120:2527–37. doi: 10.1002/ijc.22709. [DOI] [PubMed] [Google Scholar]
- 7.Reiners JJ, Jr, Caruso JA, Mathieu P, et al. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell death and differentiation. 2002;9:934–44. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karu TI. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochemistry and photobiology. 2008;84:1091–9. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 9.Calles C, Schneider M, Macaluso F, et al. Infrared A radiation influences the skin fibroblast transcriptome: mechanisms and consequences. J Invest Dermatol. 2010;130:1524–36. doi: 10.1038/jid.2010.9. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro MG, Homma K, Villarreal S, et al. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012;3:736. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochemical Pharmacology. 2013;86:447–57. doi: 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Liang HL, Whelan HT, Eells JT, et al. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639–49. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 13.Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. The Journal of biological chemistry. 2005;280:4761–71. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 14.Ying R, Liang HL, Whelan HT, et al. Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain research. 2008;1243:167–73. doi: 10.1016/j.brainres.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Wu S, Xing D. Inhibition of Abeta(25–35)-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cellular signalling. 2012;24:224–32. doi: 10.1016/j.cellsig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Xing D, Zhu D, Chen Q. Low-power laser irradiation inhibiting Abeta25–35-induced PC12 cell apoptosis via PKC activation. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2008;22:215–22. doi: 10.1159/000149799. [DOI] [PubMed] [Google Scholar]
- 17.Zeng H, Sun M, Zhou C, et al. Hematoporphyrin Monomethyl Ether-Mediated Photodynamic Therapy Selectively Kills Sarcomas by Inducing Apoptosis. PloS one. 2013;8:e77727. doi: 10.1371/journal.pone.0077727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberdanner CB, Kiesslich T, Krammer B, Plaetzer K. Glucose is required to maintain high ATP-levels for the energy-utilizing steps during PDT-induced apoptosis. Photochemistry and photobiology. 2002;76:695–703. doi: 10.1562/0031-8655(2002)076<0695:GIRTMH>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Chung H, Dai T, Sharma S, et al. The Nuts and Bolts of Low-level Laser (Light) Therapy. Annals of Biomedical Engineering. 2012;40:516–33. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CH, Wang CZ, Wang YH, et al. Effects of low-level laser therapy on M1-related cytokine expression in monocytes via histone modification. Mediators of inflammation. 2014;2014:625048. doi: 10.1155/2014/625048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi EM, Lee YS. Paeoniflorin isolated from Paeonia lactiflora attenuates osteoblast cytotoxicity induced by antimycin A. Food & function. 2013;4:1332–8. doi: 10.1039/c3fo60147a. [DOI] [PubMed] [Google Scholar]
- 22.Roberts WG, Shiau FY, Nelson JS, et al. In vitro characterization of monoaspartyl chlorin e6 and diaspartyl chlorin e6 for photodynamic therapy. Journal of the National Cancer Institute. 1988;80:330–6. doi: 10.1093/jnci/80.5.330. [DOI] [PubMed] [Google Scholar]
- 23.Roberts WG, Berns MW. In vitro photosensitization I. Cellular uptake and subcellular localization of mono-L-aspartyl chlorin e6, chloro-aluminum sulfonated phthalocyanine, and photofrin II. Lasers in surgery and medicine. 1989;9:90–101. doi: 10.1002/lsm.1900090203. [DOI] [PubMed] [Google Scholar]
- 24.Kessel D, Whitcomb KL, Schulz V. Lipoprotein-mediated distribution of N-aspartyl chlorin-E6 in the mouse. Photochemistry and photobiology. 1992;56:51–6. doi: 10.1111/j.1751-1097.1992.tb09601.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Zhang Z, Xing D. Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage. Free Radical Biology and Medicine. 2011;51:53–68. doi: 10.1016/j.freeradbiomed.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 26.Karu T, Pyatibrat L, Kalendo G. Irradiation with He □ Ne laser increases ATP level in cells cultivated in vitro. Journal of Photochemistry and Photobiology B: Biology. 1995;27:219–23. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen AC, Arany PR, Huang YY, et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PloS one. 2011;6:e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicotera P, Leist M, Fava E, et al. Energy requirement for caspase activation and neuronal cell death. Brain pathology (Zurich, Switzerland) 2000;10:276–82. doi: 10.1111/j.1750-3639.2000.tb00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plaetzer K, Kiesslich T, Krammer B, Hammerl P. Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology. 2002;1:172–7. doi: 10.1039/b108816e. [DOI] [PubMed] [Google Scholar]
- 30.Tatsumi T, Shiraishi J, Keira N, et al. Intracellular ATP is required for mitochondrial apoptotic pathways in isolated hypoxic rat cardiac myocytes. Cardiovascular research. 2003;59:428–40. doi: 10.1016/s0008-6363(03)00391-2. [DOI] [PubMed] [Google Scholar]
- 31.Abraham EH, Woo VH, Harlin-Jones C, et al. Application and possible mechanisms of combining LLLT (low level laser therapy), infrared hyperthermia and ionizing radiation in the treatment of cancer. Proc SPIE. 2014;8932:893202–19. [Google Scholar]
- 32.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–83. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]


