Abstract
Past studies have suggested Stroop interference increases with age; however the robustness of this effect after controlling for processing speed has been questioned. Both working memory and the congruency of the immediately preceding trial have also been shown to moderate the magnitude of Stroop interference. Specifically, interference is smaller both for individuals with higher working memory capacity and following an incongruent trial. At present, it is unclear whether and how these three variables (age, working memory and previous congruency) interact to predict interference effects in the standard Stroop color naming task. We present analyses of Stroop interference in a large database of Stroop color naming trials from a life-span sample of well screened, cognitively healthy, older adults. Our results indicated age-related increases in interference (after controlling for processing speed) that were exaggerated for individuals with low working memory. This relationship between age and working memory occurred primarily when the immediately preceding trial was congruent. Following an incongruent trial, interference increased consistently with age regardless of working memory. Taken together, these results support previous accounts of multiple mechanisms underlying control in the Stroop task and provide insight into how each component is jointly affected by age, working memory and trial history.
Keywords: Aging, Attention, Working Memory, Congruency Sequence Effect
Attentional control is a multi-component construct which underlies our capacity to direct attention toward relevant and away from irrelevant stimuli in the environment. One of the most widely used tasks to study attentional control is Stroop color naming (MacLeod, 1991; Stroop, 1935). In the Stroop paradigm, individuals name the ink color that color words are printed in (e.g., the word RED printed in blue ink) and therefore must attend to the color (i.e., blue) while ignoring the irrelevant, but highly salient, word information (i.e., red). Responses are slower and often less accurate when the color and word information are incongruent (RED in blue ink) compared to when they are congruent (RED in red ink), and the magnitude of this interference is often used as an index of attentional control. Importantly, attentional control is hypothesized to decrease in healthy aging and indeed Stroop interference (SI) has been reported to be larger in older adults (e.g., Bugg, DeLosh, Davalos, & Davis, 2007; Jackson & Balota, 2013; Spieler, Balota, & Faust, 1996) but the existence of this effect after controlling for general slowing has been questioned (Verhaeghen & De Meersman, 1998).
Given the debate regarding age effects in the Stroop task, it is important to consider additional factors which may contribute to the observed magnitude of SI. For example, working memory (WM) has been shown to moderate SI, particularly when demands on goal maintenance are high (Hutchison, 2011; Kane & Engle, 2003; Long & Prat, 2002). Goal maintenance refers to the ability to maintain the task set (e.g., “name the color”) across an experiment and is typically examined by manipulating the proportion of congruent stimuli within the list. Since frequent congruent items may encourage a word reading strategy, stronger demands are placed on internal goal representations to maintain the color naming task set when the proportion of congruent trials is relatively high. Furthermore, WM differences primarily manifest under conditions of frequent congruent items which has led some researchers to argue that WM is a key index of goal maintenance ability in the Stroop task (e.g., Kane & Engle, 2003).
A second factor that moderates SI is the degree to which attentional control is dynamically adjusted on a trial by trial basis. Specifically, characteristics of the stimulus on trial N-1 may trigger a tightening or relaxing of the attentional control parameters and these new settings will carry over to influence the response dynamics on trial N. If the updated control parameters are inappropriate for trial N, they can be updated yet again for trial N+1. This constant updating of the control system allows the responder to find the optimal settings to best complete the task. Such trial level changes are typically measured using the congruency sequence effect (CSE), or the “Gratton effect” (Gratton, Coles, & Donchin, 1992), which is the finding that interference is reduced following incongruent trials relative to congruent trials. More specifically, responses to congruent stimuli are faster when preceded by another congruent stimulus (“CC” trials) compared to when preceded by an incongruent stimulus (“IC” trials). Similarly, incongruent responses are faster when preceded by an incongruent trial (“II” trials) than when preceded by a congruent trial (“CI” trials).
Several competing theories of this phenomenon have been proposed and the most prominent appeals to a conflict monitoring mechanism (Botvinick, Braver, Barch, Carter, & Cohen, 2001). The conflict monitoring account proposes that attentional control is dynamically adjusted in reaction to response conflict (e.g., the word information conflicts with the color information). Within this framework, researchers have argued that the amount of conflict on a given trial is detected by the anterior cingulate cortex which then signals pre-frontal brain regions to trigger an appropriate adjustment in control by increasing or decreasing the relative activation of the color and word response pathways. Thus, for example, an incongruent trial will result in a high level of response conflict which then leads to an increase in control which in turn reduces interference on the subsequent trial. In this way, the conflict monitoring framework can accommodate the CSE and indeed has been successful in accounting for a wide variety of other experimental phenomena (Botvinick, Cohen, & Carter, 2004). Importantly, activity in the anterior cingulate cortex (the proposed conflict monitor) has been linked to the reduction in Stroop interference on the subsequent trial lending further support to the conflict monitoring account (Kerns et al. 2004, but see Mayr & Awh, 2009).
Despite the success and intuitive appeal of conflict monitoring, several alternative theories have questioned the need for a dedicated conflict monitoring mechanism, at least in accounting for the CSE. For example, in many interference paradigms such as the Simon or flanker tasks there are only two types of stimuli (e.g., a left or right facing arrow in a Simon type task). Thus, when the congruency repeats (CC or II trials), there is a .5 probability that the target and distracters will be exactly the same as on the previous trial. Therefore, the relative speeding of CC and II trials might potentially be due to simple repetition priming. Indeed, several studies have shown that when such repetitions are removed prior to analysis, the CSE is greatly reduced or eliminated (Mayr, Awh & Laurey, 2003; Mayr & Awh, 2009). However, other studies have found evidence for the CSE even after controlling for cross-trial feature overlap (e.g., Meier & Kane, 2013; Notebaert, Gevers, Verbruggen & Liefooghe, 2006; Ullsperger, Bylsma & Botvinick, 2005), suggesting that feature repetition does not completely account for these effects at least in certain paradigms.
In addition to the contribution of stimulus repetition, other mechanisms have also been proposed to play a role in the CSE including joint adjustment of visuomotor activation gain and output threshold (Schlaghecken & Martini, 2012), expectation adjustment and response slowing (Lamers & Roelofs, 2011) and contingency learning (Schmidt, 2013). Thus, while the presence of a CSE indicates that adjustments are being made to the underlying response dynamics on a trial level basis, the trigger for these adjustments remains under debate.
More importantly, it is critical to determine if such trial level control adjustments contribute to age or WM differences in performance on conflict tasks. A priori, one may expect individuals with better control systems (i.e., higher WM and younger age) to exhibit larger cross trial effects because a highly tuned attentional control system should be better able to flexibly adapt to local trial characteristics in order to maximize performance. For example, an individual with a relatively compromised control system might be less able to increase their level of control after responding to a stimulus that produces a high degree of conflict (i.e., an incongruent stimulus). An increase in control would result in the increase of activation along the color pathway and a decrease of activation in the word pathway, which as described above will reduce SI producing the CSE. However, if control is not adjusted in this manner, the magnitude of the CSE will be minimal.
We are aware of two studies that have examined age differences on the CSE. West and Moore (2005) utilized a modified key-press Stroop task that included a switching component. Specifically, 75% of the trials were color identification trials and the remaining 25% were word identification trials. Importantly, the age by current trial by previous trial interaction was not significant (p > .22), indicating similar CSEs in both younger and older adults. However, the sample size was relatively small (N = 12 per age group), and indeed the pattern of means indicated the CSE was larger in older adults (CSE = 120 ms) than in younger adults (CSE = 68 ms). Similarly, Puccioni and Vallesi (2012) used a manual Stroop task and specifically controlled for repetition priming effects by ensuring that no physical characteristics of stimuli were repeated in immediately adjacent trials. Interestingly, these authors also failed to find any evidence of age differences in the CSE, however it should be noted that they also did not obtain a reliable CSE overall.
Several studies have also examined the relationship between working memory capacity and the CSE with mixed results. Two studies found no relationship between WM and the CSE in a young adult sample, either in the Stroop task (Meier & Kane, 2013; Unsworth, Redick, Spillers & Brewer, 2012) or in a flanker task (Unsworth et al., 2012). On the other hand, Keye, Wilhelm, Oberauer and Ravenzwaaij (2009) used structural equation modeling to specify IC and CI trials as indicators of a latent “context” factor for both a flanker and a Simon task. They then correlated levels of WM with this latent factor and found a negative correlation between WM and the CSE indicating that lower WM individuals produced larger CSEs. However, this relationship was statistically significant only for the Simon task (r = −.22, p < .05) and not the flanker task (r = −.18, p > .05). Using a Stroop paradigm, Hutchison (2011) found a three way interaction among WM, previous trial and proportion congruency in predicting Stroop interference. This complex interaction indicated that low WM participants showed the standard CSE in the context of frequent incongruent items (as might be expected), however these same participants exhibited greater SI following incongruent trials in the context of frequent congruent items. In contrast, for high WM individuals, the standard CSE was obtained in the frequent congruent context but was eliminated in the frequent incongruent context.
Thus, at present it appears there is little evidence to indicate that age moderates the CSE and the evidence regarding the effect of WM is equivocal suggesting that the moderating effect of WM may be specific to certain tasks or contexts. With that in mind, it is important to note that the studies reviewed here typically use “non-standard” variants on the interference tasks. For example, a proportion congruency manipulation is often included (e.g., Hutchison, 2011; Meier & Kane, 2013) or a task-switching component might be introduced (e.g., West & Moore, 2005). It is unclear how these manipulations might influence the relationship between the CSE and measures of individual differences. Furthermore, we are not aware of any study that has looked at the joint relationship among age, WM and trial history in a single study. Thus, the present study was designed to examine the interplay among these three variables in predicting SI in a large sample of well-characterized, cognitively healthy, older adults. Because of the complexity of the design, it is critical to have sufficient sample size to examine these issues, which the current study employed (N = 435). Importantly, we used a standard, vocal Stroop task that is commonly used to investigate age differences in SI.
The present research was motivated by two major questions. First, are age-related changes in global SI moderated by WM? There is reason to predict this because, as mentioned, WM is hypothesized to reflect the ability to maintain task goals throughout the experiment (Kane & Engle, 2003), and to the extent that goals are better maintained, SI will be reduced. Therefore, a relatively high level of WM might offset age-related deficits in attentional control. Second, are age and/or working memory differences in SI moderated by the congruency of the immediately preceding trial? Based on the conflict monitoring hypothesis, one might expect individuals with relatively poor attentional control, as reflected by older age and/ or lower working memory, would produce smaller dynamic adjustments across trials. In other words, they may be less able to adjust control over the word and color pathways in response to recently presented items. However, as noted above, the available evidence regarding this hypothesis is mixed at best.
To address these questions, we conducted analyses on a large sample of Stroop color naming trials as a function of age, WM and trial history using linear mixed effects models (LME). LMEs are quickly becoming preferred to more traditional analyses of variance and are particularly useful for the present design. Specifically, LMEs allow for the modeling of continuous covariates such as age and WM with experimental factors (current and previous congruency), and are robust to unbalanced data that arises due to the random intermixing of trial types (i.e., current by previous congruency was not explicitly controlled). In addition, they allow for modeling of the variance that arises from the specific sample and color words that we employed (Kliegl, R., Wei, P., Dambacher, M., Yan, M., & Zhou, X., 2011).
Method
Participants
Four hundred and thirty-five healthy adults participated in this study. All participants were screened for dementia by highly trained physicians at the Charles F. and Joanne Knight Alzheimer Disease Research Center at Washington University using the sensitive Clinical Dementia Rating Scale (CDR: Morris, 1993). This research group is able to detect the very earliest stages of dementia with a high degree of accuracy (93%, Berg et al., 1998). This is important because the Stroop task has been shown to be particularly sensitive to this early stage of Alzheimer dementia (Balota et al., 2010; Spieler et al., 1996) and we want to insure our participants were all cognitively healthy. Indeed, all participants in the present study were rated as CDR 0 indicating absence of cognitive impairment (furthermore the mean Mini-Mental State Exam score was 29 out of 30). The participants were drawn from a community based life-span sample (mean age = 67, SD = 11, range = 30–96), who completed a battery of tests designed to assess various aspects of attention and memory. The present study reports data from participants’ first time completing this battery.
Stroop Task and Working Memory Assessment
The Stroop task was based on the version of the task used in Spieler et al. (1996) and consisted of four color words printed in four different colors (red, green, blue and yellow) with four neutral words (bad, legal, poor and deep) included as a baseline. The list included 36 congruent trials (each word printed in the corresponding color nine times), 36 incongruent trials (each word printed in each of the three non-matching colors three times) and 32 neutral trials (the words, “bad”, “legal”, “poor” and “deep”, each presented twice in each of the four colors) which were randomly intermixed. Participants were instructed to name the ink color of the stimulus aloud into a microphone which triggered the computer to record response latencies to the nearest millisecond. On each trial the following events occurred: a) fixation for 700 ms; b) 50 ms blank screen; c) presentation of the stimulus; d) participant produced the response which triggered the offset of the stimulus; e) the experimenter recorded the response via a keypress or noted the response as a microphone error (e.g., stutters, false starts, responses that were too soft to trigger the microphone etc.); and f) 1750 ms inter-trial interval. Participants were given a break halfway through the experimental block.
The working memory measure was the computation span task used in McCabe, Roediger, McDaniel, Balota, and Hambrick (2010). In this task, participants verified the accuracy of equations (e.g., 7 – 4 = 3) and were required to remember the middle digit. After a set number of equations (beginning with one and then increasing by one after every three correct recall trials), they were asked to recall the center digits in order. The number of equations administered before recall increased until either 2 out of 3 recall trials were in error or a maximum of 7 equations (e.g., a span score of 7) was reached. The DV used in the present study was the total span score which refers to the number of trials correctly recalled in the last block for which the participant achieved at least two correct recall trials (i.e., the same block that determined the span score).
Analysis
To avoid the undue influence of extreme outliers, each individual’s data were screened in the following manner. First, RTs faster than 200 ms and slower than 4000 ms were removed. Second, a mean and standard deviation was calculated from the remaining trials for each participant and any RTs that exceeded 3 standard deviations from the participant mean were also removed. A small subset of our participants had a relatively high number of RTs removed due to microphone errors (33 subjects had more than 10% of their trials removed). It is possible that high numbers of such trials is indicative of impending cognitive decline (e.g., Alzheimer dementia, see Balota et al. 2010), therefore we removed these subjects before conducting further analysis. Of the remaining subjects, our trimming procedure eliminated 3.6% of the total trials1. After this trimming procedure, we eliminated incorrect responses, the first trial (since the first trial does not have a previous congruency), and trials that occurred after an error (to avoid post-error slowing differences). After removing all of these invalid trials, there was an average of 10–11 correct RTs in each current congruency by previous congruency cell.
Furthermore, it is important to also consider exact and partial repetition of stimuli characteristics when examining the CSE by either removing such trials prior to analysis or explicitly controlling them in the experimental design (c.f. Mayr et al., 2003). Because we are interested in the influence of working memory and age on the SI in a traditional Stroop paradigm, we first present analyses of all trials (including stimuli repetitions). We then report follow-up analyses of post-congruent trials only (i.e., trials following incongruent stimuli were excluded) with stimuli repetitions removed. Post-incongruent trials were not examined due to the large number of trials that would be removed due to feature overlap. This strategy allowed us to be sure that any obtained effects were not due entirely to repetition priming while still retaining a sufficient number of trials for accurate estimates of the effects of interest.
For our primary analyses, trial level, correct, raw RTs2 were analyzed using the lme4 package in R (Bates, Machler, Bolker & Walker, 2014). We first determined the random effects structure by sequentially adding random intercepts for participants and items and random slopes for condition and previous condition, and assessing the increment in model fit after the inclusion of each additional random effect. Model fit increased significantly at each step and thus all random effects were included in the final model.
Next, we entered the following variables into a single model: mean RT to neutral stimuli3 (to control for differences in processing speed), trial number (to control for practice effects), age, working memory, condition and previous condition as well as all the two, three and four way interactions among the four primary variables. Age and WM were standardized and entered as continuous covariates while condition and previous condition were coded as + 0.5 / − 0.5 contrasts. We used mean naming latencies on neutral trials as a measure of processing speed and thus neutral trials themselves were excluded from the present analysis. Finally, there is some uncertainty about how to calculate degrees of freedom in LME and as such, we relied on the procedure of a t-value greater than 2.0 to indicate statistical significance (see Kliegl, Masson, & Richter, 2010). The use of this criterion led to identical inferences as when degrees of freedom were estimated using the Satterthwaite approximation, the results of which are also provided (Kuznetsova, Brockhoff, & Christensen, 2014; Manor & Zucker, 2004)
Results
Response Latencies
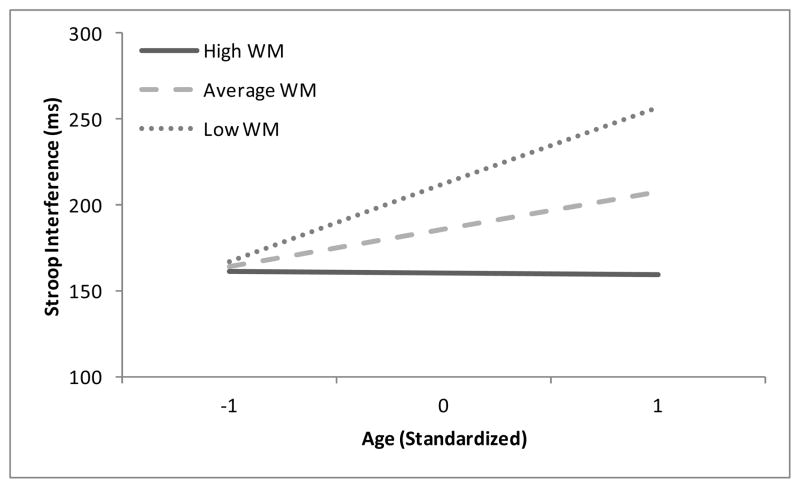
Estimates of the fixed effects from our final model are presented in Table 1. There are several important points to note here. First, we observed an interaction among age, working memory and condition, indicating that age-related differences in the Stroop effect were moderated by working memory. As shown in Figure 1, age-related differences in SI were particularly magnified when WM was low (β = 33.4, t = 5.0, p < .0001). Conversely, when WM was relatively high (one standard deviation above the mean), there was no significant effect of age (β = 7.7, t = 1.2 p = .25), suggesting that high WM can offset more general age-related deficits in Stroop performance.
Table 1.
Estimates of Fixed Effects on Response Latencies
| Effect | Estimate | t-value | p-value |
|---|---|---|---|
| Intercept | 827 | 89.9 | <.0001 |
| Neutral RT | 137 | 43.9 | <.0001 |
| Trial Number | −0.4 | −7.8 | <.0001 |
| Age | −5.3 | −1.7 | .0909 |
| Working Memory (WM) | −1.6 | −0.5 | .5896 |
| Condition (C) | 166 | 9.5 | <.0001 |
| Previous Condition (PC) | 10.3 | 3.1 | .0024 |
| Age*WM | 1.2 | 0.4 | .6610 |
| Age*C | 20.5 | 4.3 | <.0001 |
| Age*PC | 2.3 | 0.7 | .6020 |
| WM*C | −15.6 | −3.2 | .0014 |
| WM*PC | −4.5 | −1.3 | .1878 |
| C*PC | −43.1 | −7.5 | <.0001 |
| Age*WM*C | −12.9 | −2.8 | .0051 |
| Age*WM*PC | −0.2 | −0.05 | .9599 |
| Age*C*PC | −2.1 | −0.36 | .7167 |
| WM*C*PC | 12.4 | 2.1 | .0336 |
| Age*WM*C*PC | 19.1 | 3.5 | .0006 |
Figure 1.
Regression lines of Stroop Interference (Incongruent RT – Congruent RT) as a function of age and working memory capacity.
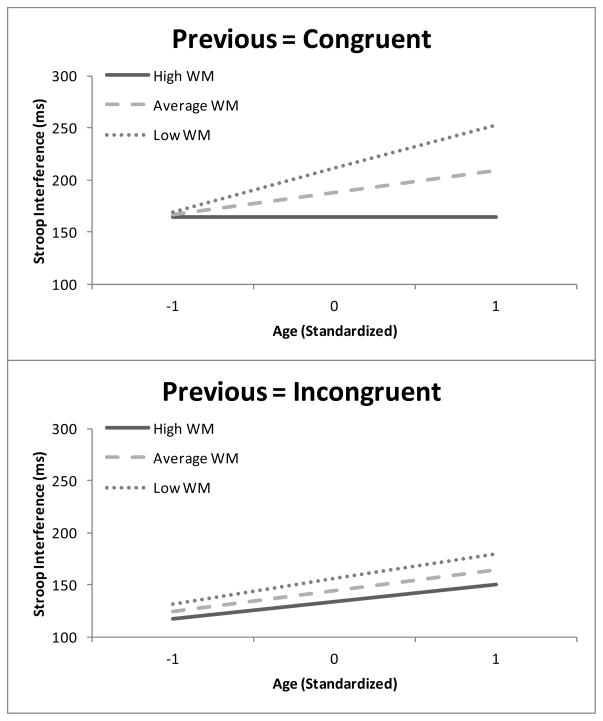
Of primary interest is whether these targeted variables produced higher-order interactions. Indeed, there was a significant four-way interaction amongst age, WM, current condition and previous condition. This interaction indicates that both the age and WM related differences in SI were critically moderated by the congruency of the immediately preceding trial. This interaction is plotted in Figure 2 and depicts the age-related increase in SI as a function of WM and split by previous congruent trials (top panel) and previous incongruent trials (bottom panel). As shown, large age and working memory differences in SI occurred following congruent stimuli, yet these same effects were greatly reduced following incongruent stimuli. Considering just the top panel, for previous congruent trials there was a highly reliable three way interaction among age, WM and condition (t = −4.3, p < .0001). Specifically, there was no age-related increase in SI following congruent stimuli when WM was high (β = −0.8, t = −0.11), but robust increases were observed at average (β = 21.6, t = 3.9, p = .0001) and low WM (β = 44.0, t = 5.7, p < .0001). Turning to the SI following incongruent trials in the bottom panel, the three way interaction among age, WM and condition was not reliable (t = −0.6, p = .54), indicating that SI increased consistently with age regardless of WM.
Figure 2.
Regression lines of Stroop Interference (Incongruent RT – Congruent RT) as a function of age and working memory capacity and split by previous trial type. Top panel = previous congruent and bottom panel = previous incongruent.
In order to insure our obtained effects were not due to repetition priming effects, we analyzed post-congruent trials after removing exact and partial repetitions, which eliminated 36% of the data. Post-incongruent trials (i.e., II and IC trials) were not analyzed for reasons mentioned above. Importantly, even with this smaller number of trials4, we obtained a reliable age by WM by condition interaction (β = −23.0, t = −3.5, p = .0005). This interaction is plotted in Figure 3 and displays the same pattern that was previously noted. Specifically, SI increased significantly with age at low levels of WM (β = 45.0, t = 4.7, p <.0001) and average WM (β = 22.0, t = 3.2, p = .001) but not high WM (β = − 1.0, t = −0.11, p = .91).
Figure 3.
Regression lines of Stroop Interference (Incongruent RT – Congruent RT) as a function of age and working memory capacity after removing stimuli repetitions. Data are from trials following a congruent stimulus (i.e., CC and CI trials) only.
Accuracy Analysis
Although the primary interest in the present study was response latencies, we also explored the same effects on accuracy. Specifically, we modeled the probability of producing an incorrect response as a function of current condition, previous condition, age and WM with random intercepts across subjects. Errors due to stutters, false starts or microphone errors were excluded prior to analysis. Briefly, as shown in Table 2, our results indicated a main effect of condition (p < .001) and a main effect of WM (p = .007). The main effect of condition reflects a greater number of errors produced in the incongruent condition (3%) than in the congruent condition (0%). The main effect of WM indicates the probability of making an error decreased significantly with higher WM as would be expected. No other main effects or higher order interactions were significant. Note that we failed to find evidence for a CSE in our accuracy measure (i.e., the condition by previous condition interaction was not reliable, p = .08), probably due to the floor effects in the congruent condition.
Table 2.
Estimates of fixed effects in the analysis of accuracy.
| Effect | Estimate | z-value | p-value |
|---|---|---|---|
| Intercept | −6.0 | −23.6 | <.0001 |
| Age | 0.31 | 1.37 | .1714 |
| Working Memory (WM) | −0.69 | −2.7 | .007 |
| Condition (C) | 3.33 | 7.7 | <.0001 |
| Previous Condition (PC) | 0.60 | 1.4 | .1604 |
| Age*WM | −0.03 | −0.15 | .8776 |
| Age*C | −0.05 | −0.11 | .9141 |
| Age*PC | −0.17 | −0.40 | .6899 |
| WM*C | 0.16 | 0.34 | .7330 |
| WM*PC | 0.41 | 0.84 | .4007 |
| C*PC | −1.48 | −1.73 | .087 |
| Age*WM*C | −0.07 | −0.17 | .868 |
| Age*WM*PC | −0.09 | −.22 | .8269 |
| Age*C*PC | .26 | 0.29 | .7667 |
| WM*C*PC | −0.73 | −0.76 | .4456 |
| Age*WM*C*PC | −.19 | −0.24 | .80994 |
Discussion
To date, the available literature has been mixed regarding Stroop interference and age which suggests that there are additional factors which potentially moderate age differences in SI. We have highlighted two of these factors in the present report. First, we demonstrated that WM had a moderating influence on age differences in SI. Specifically, SI increased with age at both average and low levels of WM, but not when WM was relatively high. This finding can be accommodated by accounts of Stroop performance that contain two different processes, goal maintenance and conflict resolution (Kane & Engle, 2003). WM is hypothesized to influence goal maintenance (i.e., name the color, not the word) which allows for sustained attention away from the distracting dimension of the stimulus. This is typically examined by manipulating the relative frequency of congruent items in the list. As mentioned in the introduction, frequent congruent items place greater demands on goal maintenance. However, the effect of age on Stroop interference may not depend critically on this maintenance mechanism as evidenced by a lack of age differences in the magnitude of interference as a function of the stimulus to response interval (Jackson & Balota, 2013). Hence, it is possible that the observed moderating influence of working memory on the age by SI interaction may reflect a link between working memory measures and the quality of the task set parameters.
Although the mechanisms by which WM influences Stroop performance is not entirely clear, our data indicate that a high level of WM can offset changes in SI that are due to age. For example, if the effect of age is to diminish control over the prepotent word dimension (Spieler et al. 1996), a high WM could compensate for this by proactively maintaining attention away from that dimension before the stimulus is even presented. This would lead to overall less influence of the word pathway, thereby diminishing SI.
A second critical factor is the congruency of the immediately preceding trial. We replicated the well-established phenomenon that SI is reduced following an incongruent trial relative to following a congruent trial, a finding that has typically been accommodated by assuming the attentional control system can be dynamically adjusted on a trial level basis. A priori, one would expect that those individuals who have relatively good attentional control systems (e.g., younger adults with high working memory ability) would show the largest cross-trial changes. More specifically, high working memory and younger individuals should be better able to dynamically adjust their control system to the ongoing demands of the task. However, precisely the opposite pattern was found in these data. Specifically, it was the individuals with relatively poor attentional control systems (i.e., lower WM and older age) who exhibited the largest trial level changes in interference. Indeed, a greater CSE for individuals with worse control systems is more consistent with the extant literature (see for example West & Moore, 2005).
Interestingly, age differences in SI were particularly magnified following congruent trials. These are trials which cause relatively little response conflict, suggesting that something other than magnitude of response conflict on trial N-1 seems to be increasing Stroop interference on trial N, particularly for older adults, which is inconsistent with the conflict monitoring account. These findings are similar to those of Lamers and Reolofs (2011) who showed neutral trials trigger a similarly sized CSE as incongruent trials whereas congruent trials lead to a significantly larger CSE. Importantly, only incongruent trials cause a relatively large degree of response conflict which again suggests the biggest differences in cross-trial adjustments should occur after these trials, which was not the case in these data. Thus alternative mechanisms are likely to underlie the CSE in the Stroop task.
We have already discussed several such alternative theories. However, we propose the present results may be best accommodated by an additional mechanism which we refer to as pathway priming. This account relies on the notion that there are two pathways (color and word) to making a response in the Stroop task, along with a control system that biases the use of one pathway over the other (e.g., Cohen, Dunbar, & McClelland, 1990). Typically, one must exert control over the faster and prepotent word pathway in order to make a color response. The pathway priming account extends this framework by assuming the relative contribution of each pathway can be moderated by the utility of that pathway in making a response on the immediately preceding trial. Specifically, when trial N-1 is incongruent, the color pathway is primed because color is the selected aspect of an incongruent stimulus. This priming of the color pathway would benefit subsequent incongruent trials, wherein color is again the most relevant dimension, and potentially slow subsequent congruent trials, wherein the word pathway could be beneficial on some percentage of trials (see MacLeod, 1991). Thus, the result of priming the color pathway on incongruent N-1 trials would be a net decrease in the Stroop effect on trial N.
In contrast, when trial N-1 is a congruent trial, the word pathway is primed due to the utility of the fast word pathway in making a correct response. This priming of the word pathway would benefit subsequent congruent trials wherein the word dimension is again useful in making the correct response, and slow down the subsequent incongruent trials when the highly activated word pathway needs to be controlled to produce the correct color response. Thus, the SI effect would be increased following congruent trials, precisely as observed in the present data. Indeed, such a priming mechanism was proposed by Spieler, Balota, and Faust (2000) to account for the unique effect of congruent stimuli at the level of the response time distribution. Spieler et al. further suggested that reliance on the word dimension could be modulated by task demands (e.g., proportion congruency manipulations) and the present results indicate these effects can occur even after a single trial. Thus, age and WM differences in SI as a function of previous congruency can be attributed to a reduced ability to control cross-trial pathway priming which results in greater interference.
It is important to note that the pathway priming account nicely accommodates the observation that both age and working memory modulate the size of this effect in the observed direction. Specifically, the participants with relatively poor attentional control systems (i.e., older adults with low working memory ability) produced the largest CSE following congruent trials. This is predicted because the irrelevant word pathway is more accessible following congruent trials which demands greater control to produce the correct response. Thus, individuals with less attentional control should have particular difficulty with subsequent incongruent trials.
Such a priming perspective places a key role on pathway utility rather than response conflict in determining trial level response dynamics, which is a subtle but important distinction from the conflict monitoring account. Critically, both mechanisms predict reduced SI following incongruent trials but for different reasons. Pathway priming predicts reduced SI because there is no additional priming from the color pathway that must be controlled. Conflict monitoring on the other hand, predicts this because the high level of response conflict from the stimulus on trial N-1 signals an increase of control (i.e., increase color pathway activation and reduce word pathway activation). Due to the similarity in the predicted outcomes, it is critical to examine additional variables, such as individual difference measures (e.g., age and working memory), that may adjudicate between these theories which is a key contribution of the present work.
Other accounts have similarly extended the basic Cohen, Dunbar and McClelland (1990) architecture in accounting for the CSE. Specifically, Mayr and Awh (2009) suggested that because it takes more time to respond to an incongruent stimulus, the control system, which is constantly feeding activation into the relevant pathway, will have more time to operate to increase the activation in the color pathway. Thus, after responding to an incongruent stimulus, the system will be primed to utilize the color pathway on subsequent trials. It is unclear however if this mechanism can accommodate the patterns uncovered here. Specifically, if the critical variable is the degree to which the control system can influence the two pathways per unit of time, one might expect individuals with stronger control (and thus a greater influx of activity to the relevant pathway from the control system) would benefit more from a previous incongruent trial and thus show a larger CSE. Future studies should seek to differentiate between these accounts.
Although there are a number of strengths of the present study, including the large sample of well-characterized participants, which afforded the opportunity to model the effect of multiple individual difference measures, there were also a few differences from past studies which may limit generalization. First, the present study included approximately equal proportions of congruent, incongruent and neutral stimuli in our task. Often, the proportion congruency is manipulated and it is possible that cross trial effects are moderated by the relative probabilities of each stimulus type. However, it is important to note here that a recent study found no relationship between proportion congruency and the magnitude of the CSE (Meier & Kane, 2013), so this may not be a strong limitation of the present study. A second limitation is that although our sample encompassed much of the adult lifespan, the ages were somewhat skewed towards older participants. Both of the previous studies regarding age and the CSE (Puccioni & Vallesi, 2012; West & Moore, 2005) included a group of true young adults as a comparison group. It is possible that the differences we obtained here may not be observed when comparing extreme groups.
Finally, as mentioned in the introduction, it is critically important to consider feature repetitions when investigating the CSE. Due to the number of trials in our database, we were only able to eliminate repetitions following congruent trials. The presence of a significant interaction allows us to infer that modulation of the Stroop effect after congruent trials is not due entirely to feature overlap. However, this does not directly speak to the magnitude of the CSE. Furthermore, it is possible that the relatively low number of trials included in our design contributed to the observed magnitude of the CSE. Specifically, Mayr and Awh (2009) showed the largest CSE to occur within the first 170 trials of the experiment with relatively little CSE occurring after that. However, 170 trials would be well on the upper end of the number of trials that might be administered in a standard Stroop task.
In summary, we observed robust age-related changes in SI indicating deficient attentional control processes in older individuals. Importantly, this relationship was moderated both by levels of WM and also by the congruency of the preceding trial. Specifically, WM moderated age-related changes in SI primarily after congruent stimuli. This suggests different processes are brought online based on trial history and these processes are subsequently influenced differentially by WM. These findings have potential implications for the assessment of attentional control of older adults using the Stroop task. Specifically, age differences in SI may be exaggerated if there are unequal proportions of congruent and incongruent stimuli tested. However, the CSE may be tapping a fundamentally different aspect of the attentional control system, and whether it differs from other measures (such as Stroop errors) in predicting cognitive outcomes (e.g., cognitive decline, Balota et al. 2010 or sensitivity to AD-related biomarkers, Duchek et al., 2013), remains to be determined. More importantly, however, these results provide useful insight into attentional control systems across the adult lifespan and the potential mechanisms underlying the CSE.
Acknowledgments
This research was supported by the National Institute on Aging grants T32 AG000030, P01-AG03991 and P01-AG26276. We thank John Morris and the Clinical Core of the Knight Alzheimer Disease Research Center for the careful description of the research participants.
Footnotes
We repeated our analyses on the full sample of participants, including those with a higher number of outlier trials. Importantly, the results were the same even in the larger sample.
It is standard practice to transform the trial level RTs when conducting LMEs in order to better approximate a normal distribution. Although such transformations can yield misleading inferences (Balota, Aschenbrenner, & Yap, 2013) when we repeated these analyses using log-transformed RTs, the critical 4-way interaction remained significant
To further ensure our effects were not due entirely to simple speed differences we conducted identical analyses using proportional RT (RT / neutral RT) and within subject z-scored RT as the dependent measure rather than raw RT. Again, the 4-way interaction remained reliable even in the transformed data. Furthermore, we tested for a correlation between the random intercept and random slope of the condition effect which was not significant after including neutral RTs in the model (p > .11).
Although a large number of post-incongruent trials are removed due to feature overlap, we also examined the CSE after removing repetition trials. Importantly, even with a smaller number of trials, we obtained a smaller but still significant condition by previous condition interaction (β = −26.9, t = −2.9, p = .004). The higher order interactions including age and WM were also still reliable (ps < .05).
References
- Balota DA, Aschenbrenner AJ, Yap MJ. Additive effects of word frequency and stimulus quality: The influence of trial history and data transformations. Journal of Experimental Psychology: Learning, Memory and Cognition. 2013;39:1563–1571. doi: 10.1037/a0032186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Tse CS, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: The power of errors in Stroop color naming. Psychology and Aging. 2010;25:208–218. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7. 2014 http://CRAN.R-project.org/package=lme4>.
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: Relation of histologic markers to dementia severity. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bugg JM, DeLosh EL, Davalos DB, Davis HP. Age differences in Stroop interference: Contributions of general slowing and task-specific deficits. Aging, Neuropsychology, and Cognition. 2007;14(2):155–167. doi: 10.1080/138255891007065. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Thomas JB, Snyder AZ, Rich P, Benzinger TL, Ances BM. Relationship between Stroop performance and resting state functional connectivity in cognitively normal older adults. Neuropsychology. 2013;27:516–528. doi: 10.1037//a0033402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Hutchison KA. The interactive effects of listwide control, item-based control, and working memory capacity on Stroop performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37(4):851–860. doi: 10.1037/a0023437. [DOI] [PubMed] [Google Scholar]
- Jackson JD, Balota DA. Age-related changes in attentional selection: Quality of task set or degradation of task set across time? Psychology and Aging. 2013;28(3):744–753. doi: 10.1037/a0033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Keye D, Wilhelm O, Oberauer K, van Ravenzwaaij D. Individual differences in conflict-monitoring: Testing means and covariance hypothesis about the Simon and the Eriksen flanker task. Psychological Research. 2009;73:762–776. doi: 10.1007/s00426-008-0188-9. [DOI] [PubMed] [Google Scholar]
- Kliegl R, Masson MEJ, Richter EM. A linear mixed model analysis of masked repetition priming. Visual Cognition. 2010;18(5):655–681. doi: 10.1080/13506280902986058. [DOI] [Google Scholar]
- Kliegl R, Wei P, Dambacher M, Yan M, Zhou X. Experimental effects and individual differences in linear mixed models: Estimating the relationship between spatial, object, and attraction effects in visual attention. Frontiers in Psychology. 2011;1:1–12. doi: 10.3389/fpsyg.2010.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests for random and fixed effects for linear mixed effects models (lmer objects of lme4 package) R package version 2.0-11. 2014 http://CRAN.R-project.org/package=lmerTest.
- Lamers MJM, Roelofs A. Attentional control adjustments in Eriksen and Stroop task performance can be independent of response conflict. The Quarterly Journal of Experimental Psychology. 2011;64(6):1056–1081. doi: 10.1080/17470218.2010.523792. [DOI] [PubMed] [Google Scholar]
- Long DL, Prat CS. Working memory and Stroop interference: An individual differences investigation. Memory & Cognition. 2002;30(2):294–301. doi: 10.3758/bf03195290. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Manor O, Zucker DM. Small sample inference for the fixed effects in the mixed linear model. Computational Statistics & Data Analysis. 2004;46(4):801–817. doi: 10.1016/j.csda.2003.10.005. [DOI] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E. The elusive link between conflict and conflict adaptation. Psychological Research. 2009;73:794–802. doi: 10.1007/s00426-008-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology. 2010;24(2):222–243. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier ME, Kane MJ. Working memory capacity and Stroop interference: Global versus local indices of executive control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2013;39(3):748–759. doi: 10.1037/a0029200. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Notebaert W, Gevers W, Verbruggen F, Liefooghe B. Top-down and bottom-up sequential modulations of congruency effects. Psychonomic Bulletin & Review. 2006;13:112–117. doi: 10.3758/bf03193821. [DOI] [PubMed] [Google Scholar]
- Puccioni O, Vallesi A. Conflict resolution and adaptation in normal aging: The role of verbal intelligence and cognitive reserve. Psychology and Aging. 2012;27(4):1018–1026. doi: 10.1037/a0029106. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F, Martini P. Context, not conflict, drives cognitive control. Journal of Experimental Psychology: Human Perception and Performance. 2012;38(2):272–278. doi: 10.1037/a0025791. [DOI] [PubMed] [Google Scholar]
- Schmidt JR. The Parallel Episodic Processing (PEP) model: Dissociating contingency and conflict adaptation in the item-specific proportion congruent paradigm. Acta Psychologica. 2013;142(1):119–126. doi: 10.1016/j.actpsy.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22(2):461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Levels of selective attention revealed through analyses of response time distributions. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(2):506–526. doi: 10.1037//0096-1523.26.2.506. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: It’s not just priming. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Spillers GJ, Brewer GA. Variation in working memory capacity and cognitive control: Goal maintenance and microadjustments of control. The Quarterly Journal of Experimental Psychology. 2012;65(2):326–355. doi: 10.1080/17470218.2011.597865. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, De Meersman L. Aging and the Stroop effect: A meta-analysis. Psychology and Aging. 1998;13(1):120–126. doi: 10.1037//0882-7974.13.1.120. [DOI] [PubMed] [Google Scholar]
- West R, Moore K. Adjustments of cognitive control in younger and older adults. Cortex. 2005;41:570–581. doi: 10.1016/s0010-9452(08)70197-7. [DOI] [PubMed] [Google Scholar]