Abstract
Immunotherapy has emerged as a promising treatment strategy for the control of HPV-associated malignancies. Various therapeutic HPV vaccines have elicited potent antigen-specific CD8+ T cell mediated antitumor immune responses in preclinical models and are currently being tested in several clinical trials. Recent evidence indicates the importance of local immune activation, and higher number of immune cells in the site of lesion correlates with positive prognosis. Granulocyte macrophage colony-stimulating factor (GMCSF) has been reported to posses the ability to induce migration of antigen presentation cells and CD8+ T cells. Therefore, in the current study, we employ a combination of systemic therapeutic HPV DNA vaccination with local GMCSF application in the TC-1 tumor model. We show that intramuscular vaccination with CRT/E7 DNA followed by GMCSF intravaginal administration effectively controls cervicovaginal TC-1 tumors in mice. Furthermore, we observe an increase in the accumulation of E7-specific CD8+ T cells and dendritic cells in vaginal tumors following the combination treatment. In addition, we show that GMCSF induces activation and maturation in dendritic cells and promotes antigen cross-presentation. Our results support the clinical translation of the combination treatment of systemic therapeutic vaccination followed by local GMCSF administration as an effective strategy for tumor treatment.
Keywords: immunotherapy, GM-CSF, CRT/E7, dendritic cell
Introduction
Human papillomavirus (HPV) is identified as the primary etiologic agent of cervical cancer, subsets of vaginal, vulvar, anal, and head and neck cancers. The HPV 16 subtype is responsible for approximately 90% of the vaginal, vulvar, and anal HPV- associated cancers [1]. Many therapeutic techniques such as chemotherapy, radiotherapy, and surgery have been implemented to treat cervical cancer, but with limited ability to improve the 5-year survival rate for advanced cervical cancer. In addition, while surgical treatment seems to be effective for precancer and early cancer of the cervix, surgical treatment performed on certain subsets of patients or on subsets of vaginal, vulvar, and anal intraepithelial lesions is associated with significant detrimental effects[2],[3],[4]. Therefore, there is an urgent demand for effective alternative therapeutic techniques to treat HPV-associated cancers.
Immunotherapy has emerged as a promising treatment strategy against HPV-associated disease. Therapeutic vaccines can elicit antigen-specific immune response targeting precancer and cancer cells while not affecting uninfected normal cells[5]. Previously, we have developed a DNA vaccine encoding HPV-16 E7 linked to a heat shock-related chaperone protein calreticulin (CRT/E7), which enhances antigen processing and presentation by MHC class I[6]. Intramuscular (IM) administration of CRT/E7 DNA generates significant E7-specific CD8+ T cell-mediated immune responses and antitumor effect in mice challenged with an E7-expressing murine tumor model, TC-1 [7, 8]. The clinical grade version of this vaccine, pNGVL4a-CRT/E7 (detox) is currently being tested in patients with HPV16+ high grade cervical intraepithelial neoplasia (CIN2/3) in a clinical trial.
It has been reported in a phase I trial of therapeutic HPV vaccine that systemic administration does not lead to enhanced tumor regression[9]. Instead, local responses at the site of lesion have been associated with the clearance of disease, suggesting the importance of targeting the elicited HPV immune response to the lesion site[10]. Therapeutic HPV vaccine can be used in combination with other therapeutic agents. Previously, we have showed that imiquimod application in the cervicovaginal tract triggered local innate inflammatory activation, leading to accumulation of antigen-specific immune cells in cervicovaginal tumor and promoted a favorable immune microenvironment within the lesion[11]. Granulocyte macrophage colony stimulating factor (GMCSF) is a leukocyte growth factor approved for use in leukopenic cancer patients and has been incorporated into numerous tumor vaccines[12]. The therapeutic potential of GMCSF has been documented by a significant body of pre-clinical studies[13]-[14]. Furthermore, using GMCSF as an adjuvant for treatment against melanoma led to improved therapeutic outcomes[15]. In addition, GMCSF application is reported to enhance the migration of antigen presenting cells and cytotoxic T-lymphocytes into tumor lesions.
In the current study, we evaluate the therapeutic potential of an HPV DNA vaccine in combination with local GMCSF application in the TC-1 tumor model. We show that intramuscular vaccination with CRT/E7 DNA followed by intravaginal (IVAG) administration of GMCSF lead to enhanced antitumor effect and prolonged survival in tumor-bearing mice. Furthermore, we observe an increase in the number of E7-specific CD8+ T cells in the cervicovaginal tract. Finally, we show that the combination treatment induces maturation and activation in local dendritic cells (DC) and enhances antigen cross-presentation. Our result indicates that therapeutic DNA vaccination followed by local application of GMCSF is an effective therapeutic strategy for clinical translation.
Materials and Methods
Mice
Six- to eight-week-old female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). All animal procedures were performed according to approved protocols by the Johns Hopkins Institutional Animal Care and Committee and in accordance with recommendations for the proper use and care of laboratory animals.
Cells
TC-1 cells, an E7-expressing murine tumor model, were generated by co-transformation of primary C57BL/6 mouse lung epithelial cells with HPV-16 E6 and E7 and an activated Ras oncogene as previously described [16]. E7 (aa49–57)-specific T cell lines have also been previously described [17]. These cell lines were cultured in vitro in PRMI10 (RPMI 1640 supplemented with 10% fetal bovine serum, 50 units/ml of penicillin/streptomycin, 2 mML-glutamine, 1 mM sodium pyruvate, and 2 mM nonessentialamino acids) and grown at 37°C with 5% CO2.
DNA constructs
The construction of the CRT/E7 plasmid DNA vaccine (pNGVL4a-CRT/E7(detox)) used in this study has been described previously. Briefly, the plasmid backbone, pNGVL-4a, obtained from the NIH National Gene Vector Laboratory is a human approved vector, which encodes a kanamycin resistance gene, a transcription unit consisting of a CMV promoter, multi-cloning site, and a poly-A tail. In addition, the plasmid includes two immunestimulatory sequences consisting of tandem repeats of a CpG dinucleotide. Human calreticulin (CRT) and HPV-16 E7 oncogenic protein DNA sequences were cloned into this plasmid, with amino acid substitutions at positions 24 (cysteine to glycine) and 26 (glutamic acid to glycine) of E7, which ablates the Retinoblastoma protein binding site and, thus, prevents malignant transformation of transfected cells.
Reagents
Recombinant Human GM-CSF was purchased from Peprotech.
Progestin was purchased from Depo-Provera; Pharmacia Corp., Peapack, New Jersey, USA.
In vivo tumor treatment with DNA vaccination and GMCSF administration
For the in vivo tumor treatment experiments, 3 mg progestin in 100 μL PBS was injected into 6- to 8-week-old C57BL/6 mice (five per group) subcutaneously (S.C.) 5 days before tumor challenge. 2×10^4 TC-1 tumor cells in 20 μL PBS/mice were injected into the vaginal cavity. Tumor growth was confirmed by IVIS2000 system on three days after tumor injection. Four days after tumor challenge, mice were vaccinated with 40 μg of pNGVL4a-CRT/E7(detox) via intramuscular injection. Mice received a booster vaccination at three-day intervals for a total of three vaccinations. Ten days after tumor challenge, mice were injected with 100ng GMCSF via intravaginal injection 3 times at 2-day intervals. The luminescence intensity of tumor was measured once a week with IVIS imaging machine.
Preparation of single-cell suspensions from Spleen, draining lymph node, and TC-1 tumors
21 days after tumor challenge, spleen, draining lymph node, and TC-1 tumors from the tumor bearing mice treated with various treatment regimens were surgically excised using sterile technique, placed in RPMI-1640 medium containing 100U/ml penicillin and 100 μg/ml streptomycin and washed with PBS. The spleen and lymph node were ground and filtered through 70-μm nylon filter container to remove undigested tissue fragments, and then 2ml of ammonium-chloride-potassium (ACK) lysis buffer (Sigma-Aldrich) was used to lyse the red blood cells. The solid tumors were then minced into 1- to 2-mm pieces and immersed in serum-free RPMI-1640 medium containing 0.05 mg/ml collagenase I, 0.05 mg/ml collagenase IV, 0.025 mg/ml hyaluronidase IV, 0.25 mg/ml DNase I, 100 U/ml penicillin, and 100 μg/mlstreptomycin and incubated at 37 °C with periodic agitation. The tumor digest was then filtered through a 70-μm nylon filter mesh to remove undigested tissue fragments. The resultant single spleen, lymph node, or tumor cell suspensions and tumor-infiltrating lymphocytes were washed twice in Hank's buffered salt solution (HBSS) (400g for 10 min), and viable cells were determined using trypan blue dye exclusion.
Tetramer analysis of E7-specific CD8+ T cells in mouse organ
The cells harvested from the spleen, lymph node, and TC-1 tumors were stained with phycoerythrin (PE)-conjugated HPV16 H-2D-RAHYNIVTF tetramer combined with surface staining using APC-Alexa Fluor-conjugated anti-CD8 (BD Pharmingen). Cells were analyzed on a BD FACSCalibur collecting 500,000 events.
Intracellular cytokine staining and flow cytometry analysis
The cells harvested from the TC-1 tumors were incubated for 24 hours with 1 μg/mL of the E7 peptide containing an MHC class I epitope (amino acids 49–57, RAHYNIVTF) in the presence of GolgiPlug (BD Pharmingen, San Jose, CA). The stimulated cells were then washed once with FACScan buffer and stained with APC-Alexa Fluor-conjugated anti-CD8. Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer's instruction (BD Pharmingen). Intracellular IFN-y was stained with FITC-conjugated anti-mouse IFN-y. All antibodies were purchased from BD Pharmigen. Flow cytometry analysis was done using FACSCalibur with CellQuest software (BD Bioscience).
Flow cytometry analysis and cell surface staining to detect accumulation and maturation of dendritic cell in TC-1 tumor
3 mg progestin in 100 μL PBS was injected into 6- to 8-week-old C57BL/6 mice (five per group) subcutaneously 5 days before tumor challenge. 2×10^4 TC-1 tumor cells in 20 μL PBS/mice were injected into the vaginal cavity. 10 days after tumor challenge, mice were injected with 100ng GMCSF via intravaginal injection 3 times at 2-day intervals. The luminescence intensity of tumor was measured once a week with IVIS imaging machine. 21 days after tumor challenge, TC-1 tumors from the tumor bearing mice were harvested, processed into single cells as mentioned above, stained with APC-labeled CD11c (BD Pharmingen) and FITC-CD45, together with either PE-CD40, PE-CD80, PE-CD86, or PE-ICAM-1, and analyzed by flow cytometry.
In vitro CD8+ T cell activation assay using isolated CD11c+ dendritic cell
3 mg progestin in 100 μL PBS was injected into 6- to 8-week-old C57BL/6 mice (5 per group) subcutaneously 5 days before tumor challenge. 2×10^4 TC-1 tumor cells in 20 μL PBS/mice were injected into the vaginal cavity. 10 days after tumor challenge, mice were injected with 100ng GMCSF via intravaginal injection 3 times at 2 day intervals. The luminescence intensity of tumor was measured once a week with IVIS imaging machine. 21 days after tumor challenge, pelvic lymph nodes were harvested from the tumor bearing mice and processed into single cells as mentioned above. CD11c+ dendritic cells were purified from the suspension by positive selection (CD11c Kit; Miltenyi Biotec). 2 × 10^5 isolated dendritic cells were cultured together with 2×10^5 E7-specific CD8 T cells treated with GolgiPlug as described above for 12 hours and stained with FITC-conjugated IFN-y and APC-conjugated CD8. Flow cytometry analysis was done using FACSCalibur with CellQuest software (BD Bioscience).
Statistical Analysis
All data presented in this study are expressed as mean ± SD. At least 3 samples per group were included in each of these experiments. Flow cytometry data and results of tumor treatment experiments were evaluated by analysis of variance (1-way ANOVA) and the Tukey-Kramer test. Individual data points were compared by student's t-test. Event-time distributions for mice were compared by the Kaplan-Meier method and the logrank test. All p values < 0.05 were considered significant.
Results
Intravaginal administration of GMCSF following systemic CRT/E7 DNA vaccination improves antitumor immune responses
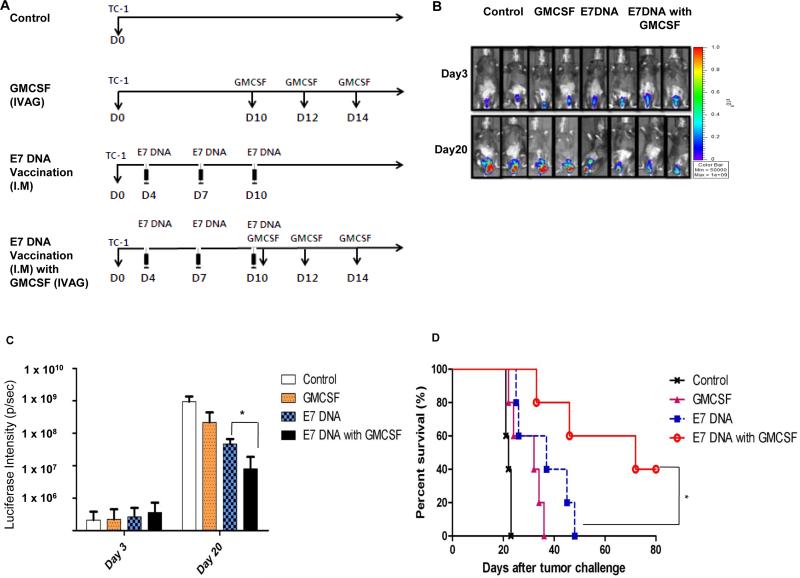
First, we assessed the antitumor effect generated by IMCRT/E7 DNA vaccination followed by IVAG GMCSF injection in the HPV-16 E7-expressing TC-1 tumor model. C57BL/6 mice (5 per group) were challenged with 2×10^4 TC-1 cells, then vaccinated IM with or without CRT/E7 DNA 4 days after for 3 times with a 3 day interval. On day 10, mice were injected with GMCSF IVAG for 3 times with a 2-day interval. As shown in Figure 1B-C, mice treated the DNA vaccine and GMCSF combination showed significantly reduced tumor growth as measured by bioluminescence intensity compared to mice treated with CRT/E7 DNA vaccination alone or GMCSF alone. Importantly, 40% of the mice vaccinated with CRT/E7 DNA and followed by GMCSF injection survived for at least 80 days after tumor challenge, while all the mice in other treatment groups died within 50 days after tumor challenge (Figure 1D). Taken together, our data indicated that the combination of systemic E7 DNA vaccination followed by local GMCSF administration lead to effective tumor control.
Figure 1. Characterization of antitumor effect in tumor bearing mice treated with intramuscular CRT/E7 DNA vaccination followed by GMCSF intravaginal injection.
C57BL/6 mice (5 per group) were injected with 2 × 10^4 TC-1/luc cells intravaginally then vaccinated intramuscularly with or without 40μg CRT/E7 DNA starting 4 days after tumor challenge for a total of 3 times with 3-day interval. 10 days after tumor challenge, mice were injected with or without 100ng GMCSF intravaginally for 3 times with 2-day interval. (A) Schematic diagram of treatment regimens. (B) Bioluminescence images of the representative TC-1/luc tumor-bearing mice at 3 and 20 days after tumor injection. (C) Bar graph depicting the mean luminescence intensity of tumor-bearing mice receiving various treatments on day 3 and 20. (D) Kaplan-Meier survival analysis of TC-1/luc tumor-bearing mice receiving various treatments.* indicates p < 0.05, ** indicates p < 0.01.
Intravaginal administration of GMCSF following systemic CRT/E7 DNA vaccination leads to generation of systemic E7-speific CD8+ T cells
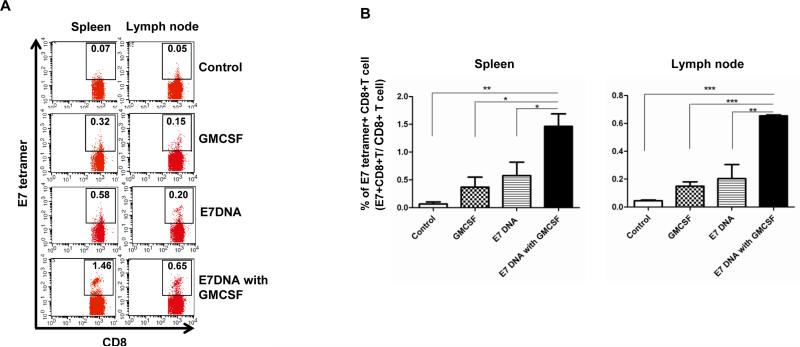
We next evaluated the potential of IVAG GMCSF injection following IM CRT/E7 DNA vaccination in generating antigen-specific adaptive immune responses. C57BL/6 mice (5 per group) were challenged with 2×10^4 TC-1 cells, then vaccinated IM with or without CRT/E7 DNA 4 days after for 3 times with a 3-day interval. On day 10, mice were injected with GMCSF IVAG for 3 times with a 2-day interval. Spleen and lymph node of the tumor bearing mice were harvested 21 days after tumor challenge and analyzed by flow cytometry for E7-specific CD8 T cells. As shown in Figure 2A and B, mice treated with GMCSF alone or with CRT/E7 vaccine alone generated a greater percentage of E7-specific CD8+ T cells compared to untreated mice. Furthermore, the combination of CRT/E7 vaccination and GMCSF injection resulted in a significantly higher percentage of E7-specific CD8+ T cells in both the spleen and lymph node compared with other treatment groups. These data indicate that GMCSF injection following CRT/E7 DNA vaccination can induce potent systemic E7-specific CD8+ T cell generation.
Figure 2. Characterization of E7-specific CD8+ T cells in spleen and lymph node in tumor bearing mice.
C57BL/6 mice (5 per group) were injected with 2 × 10^4 TC-1/luc cells intravaginally then vaccinated intramuscularly with or without 40μg CRT/E7 DNA starting 4 days after tumor challenge for a total of 3 times with 3-day interval. 10 days after tumor challenge, mice were injected with or without 100ng GMCSF intravaginally for 3 times with 2-day interval. 21 days after tumor challenge, spleen and lymph node were harvested and stained with PE-conjugated HPV16 H-2 Db-RAHYNIVTF tetramer and APC-conjugated CD8 monoclonal antibody followed by flow cytometry analysis. (A) Representative flow cytometry showing the percentage of E7-specific CD8+ T cells in spleen and lymph node of various groups. (B) Bar graph depicting the percentage of E7-specific CD8+ T cells in all CD8+ T cells in pooled spleen and lymph node of various groups (mean ± s.d.). * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001.
Intravaginal administration of GMCSF following systemic CRT/E7 DNA vaccination lead to greater local accumulation of E7-specific CD8+ T cells and CD8+ T cell activation
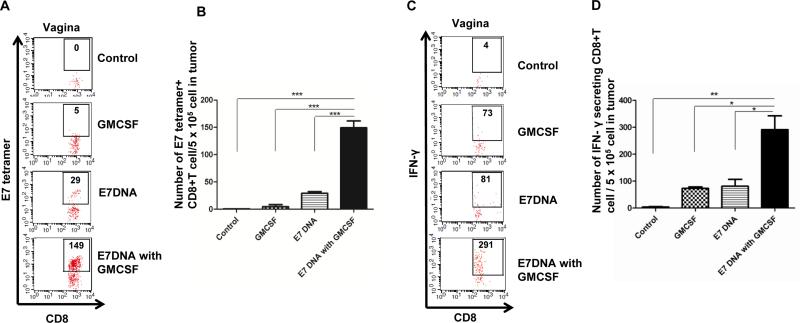
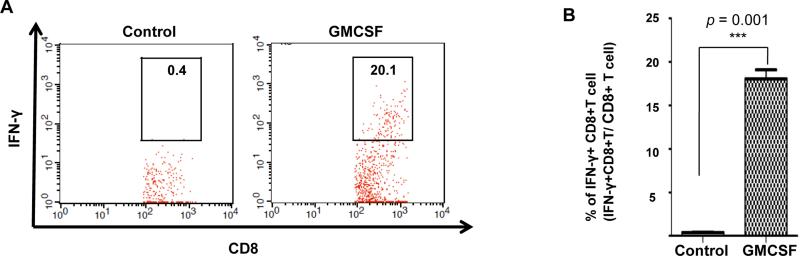
We continue to evaluate the potential of GMCSF injection following CRT/E7 DNA vaccination in generating antigen-specific immune responses. C57BL/6 mice (5 per group) were treated with various regimens described above, and the vaginal tumors were harvested 21 days after tumor challenge. Isolated tumor cells were stained with E7 tetramer, or stimulated with E7 peptide followed by IFNy staining and analyzed by flow cytometry. As shown in Figure 3A-B, mice treated with CRT/E7 DNA alone or GMCSF alone generated more local E7-specific CD8+ T cells in the vaginal tumor compared to untreated mice. Furthermore, mice treated with both CRT/E7 and GMCSF generated the highest amount of local E7-specific CD8+ T cells in the vaginal tumor compared to other groups. In addition, mice treated with CRT/E7 and GMCSF generated the highest number of IFNy secreting E7-specific CD8+ T cells compared to other treatment groups (Figure 3C-D). These results suggest that systemic DNA vaccination followed by local GMCSF administration can induce local accumulation of antigen-specific CD8+ T cells in the tumor.
Figure 3. Characterization of E7-specific CD8+ T cells and activated CD8+ T cells in vaginal tumor.
C57BL/6 mice (5 per group) were injected with 2 × 10^4 TC-1/luc cells intravaginally then vaccinated intramuscularly with or without 40μg CRT/E7 DNA starting 4 days after tumor challenge for a total of 3 times with 3-day interval. 10 days after tumor challenge, mice were injected with or without 100ng GMCSF intravaginally for 3 times with 2-day interval. 21 days after tumor challenge, cervicovaginal tumors were harvested and stained with PE-conjugated HPV16 H-2 Db-RAHYNIVTF tetramer or FITC-conjugated IFN-y, together with APC-conjugated CD8 monoclonal antibody followed by flow cytometry analysis. (A) Representative flow cytometry showing the number of E7-specific CD8+ T cells in cervicovaginal tumor of various groups. (B) Bar graph depicting the number of E7-specific CD8+ T cells in all CD8+ T cells in pooled cervicovaginal tumor of various groups (mean ± s.d.). (C) Representative flow cytometry showing the number of IFN-y positive CD8+ T cells per 5 × 10^5 tumor cells of the various groups. (D) Bar graph depicting the number of IFN-y positive CD8+ T cells per 5 × 10^5 tumor cells of various groups (mean ± s.d.).* indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001.
Local administration of GMCSF promotes accumulation and maturation of dendritic cell in tumor
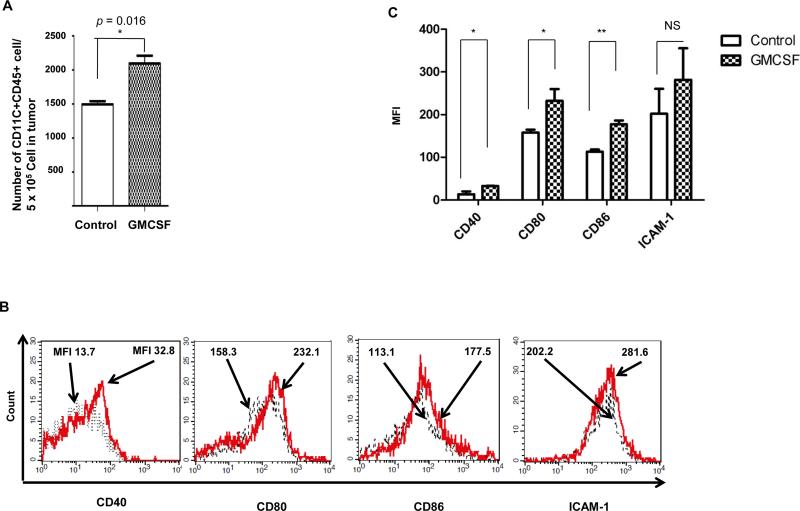
Next, we sought to determine the effect of GMCSF on dendritic cell accumulation and maturation in the tumor. C57BL/6 mice (5 per group) were challenged with TC-1 tumor cells and treated with or without GMCSF as described above. 21 days after tumor challenge, vaginal tumors were harvested and analyzed using flow cytometry. As shown in Figure 4A, tumors isolated from mice treated with GMCSF contain a greater amount of CD11c+ CD45+ dendritic cells compare to tumors collected from mice without GMCSF treatment, indicating that local injection of GMCSF lead to an accumulation of dendritic cells in the lesion site. Furthermore, DCs isolated from the vaginal tumor from mice treated with GMCSF showed significantly higher expressions of DC maturation markers CD40, CD80, and CD86 compared to DCs from untreated mice (Figure 4B-C). These data indicate that local GMCSF injection can promote the accumulation and maturation of dendritic cell in the local area.
Figure 4. Characterization of the effect of GMCSF on dendritic cell accumulation and maturation in vaginal tumor.
C57BL/6 mice (5 per group) were injected with 2 × 10^4 TC-1/luc cells intravaginally then vaccinated intramuscularly with or without 40μg CRT/E7 DNA starting 4 days after tumor challenge for a total of 3 times with 3-day interval. 10 days after tumor challenge, mice were injected with or without 100ng GMCSF intravaginally for 3 times with 2-day interval. 21 days after tumor challenge, vaginal tumors were harvested and stained with APC-CD11c+ and FITC-CD45, together with CD40, CD80, CD86, or ICAM-1 antibody followed by flow cytometry analysis. (A) Bar graph depicting the number of CD11c+ CD45+ cells per 5 × 10^5 tumor cells in cervicaovaginal tumors harvested from mice treated with or without GMCSF. (B) Representative flow cytometry histograms showing the number of cells with different DC maturation marker per 5 × 10^5 tumor cells in cervicaovaginal tumor harvested from mice treated with or without GMCSF. (C) Bar graph depicting the mean fluorescent intensity of various DC maturation marker in 5 × 10^5 cells in cervicaovaginal tumor harvested from mice treated with or without GMCSF. * indicates p < 0.05, ** indicates p < 0.01, NS indicates not significant.
GMCSF promotes cross presentation by dendritic cells
Finally, we determine the effect of GMCSF on dendritic cell mediated cross presentation. C57BL/6 mice (5 per group) were challenged with TC-1 tumor cell and treated with or without GMCSF as described above. 21 days after tumor challenge, pelvic lymph nodes were harvested and CD11c+ dendritic cells were isolated and cultured together with E7-specific CD8+ T cells at a 1:1 ratio for 12 hours. The activation of E7specific CD8+ T cells was analyzed by flow cytometry. As shown Figure 5A and B, a much greater percentage of E7-specific CD8+ T cells cultured with the dendritic cell isolated from the tumor-bearing mice treated with GMCSF secreted IFNy compared to the T cells cultured with dendritic cell isolated from tumor-bearing mice not treated with GMCSF. These results suggest that GMCSF can enhance antigen cross presentation by dendritic cell, which results in greater antigen specific CD8+ T cell activation, contributing to anti-tumor effect and leading to tumor control.
Figure 5. Characterization of the effect of GMCSF on antigen cross presentation by dendritic cell.
C57BL/6 mice (5 per group) were injected with 2 × 10^4 TC-1/luc cells intravaginally then vaccinated intramuscularly with or without 40μg CRT/E7 DNA starting 4 days after tumor challenge for a total of 3 times with 3-day interval. 10 days after tumor challenge, mice were injected with or without 100ng GMCSF intravaginally for 3 times with 2-day interval. 21 days after tumor challenge, pelvic lymph nodes were harvested and CD11c+ dendritic cells were purified through positive selection (CD11c kit; Miltenyi Biotec). 2 × 10^5 E7-Specific CD8 T cell were cultured together with isolated dendritic cells in 1:1 ratio for 12 hours and stained with FITC-conjugated IFN-y and APC-conjugated CD8 followed by flow cytometry analysis. (A) Representative flow cytometry showing the precentage of IFN-y positive CD8+ T cells in all CD8+ cells of the various groups. (B) Bar graph depicting the percentage of IFN-y positive CD8+ T cells in all CD8+ cells of various groups (mean ± s.d.). ** indicates p < 0.01
Discussion
In the current study, we examined the effects of GMCSF application following CRT/E7 DNA vaccine administration on antigen-specific CD8+ T cell-mediated immune responses and antitumor effects. We observed that following GMCSF treatment, the tissue in the cervical vaginal tract of mice exhibited significant increase in systemic and local E7-specific CD8+ T cells. In addition, we found that GMCSF treatment increased the accumulation and maturation of local dendritic cells. We also found that the dendritic cells obtained from mice treated with GMCSF have increase cross presentation effect compared to untreated mice. Finally, we show that mice treated with CRT/E7 DNA vaccine followed by intravaginal GMCSF injection, compared to either treatment alone, generated synergistic antitumor effects and drastically improved survival.
Here, we observed that intravaginal application of GMCSF following CRT/E7 DNA vaccination led to the accumulation of antigen-specific CD8+ T cells in the cervicovaginal tract. Several mechanisms may account for these observations. In our study we observed that local injection of GMCSF promotes the accumulation of dendritic cell in the cervicovaginal tract, and the maturation marker of local dendritic cells are upregulated following GMCSF application (Figure 4). In addition, we observed that the dendritic cells in the lymph node of tumor bearing mice treated with GMCSF have enhanced cross presentation function compared to untreated ones (Figure 5). Thus, it is possible that local application of GMCSF enhanced the accumulation and maturation of dendritic cell for antigen uptake for cross presentation, and the dendritic cells subsequently travel to the lymph node for the priming of CD8+ T cells, which then travel back to the local area and activated by the antigen presenting dendritic cells accumulated in the cervicovaginal tract. Of note, GMCSF may have an effect on various types of dendritic cells. Furthermore, dendritic cells in the lymph node consist of a heterogeneous population. It will be important to further investigate the effect of GMCSF on different types of dendritic cells, particularly myeloid dendritic cells, as well as migratory dendritic cells and lymph node resident dendritic cells, which should be done in future studies.
GMCSF is not the only substance that demonstrated the potential of attracting immune response to the local area. In the past, we have conducted a similar experiment through local deposition of imiquimod cream following intramuscular CRT/E7 DNA vaccination [18]. We observed that CXCL9/10, which has been shown to have the function of inducing IFNy and participate in attracting CXCR3+ T cells to the tissue [19], are upregulated following imiquimod application, and that IFNy is essential for the accumulation of antigen-specific CD8+ T cells in the cervicovaginal tract. GMCSF may function in a similar manner, inducing certain cytokine or chemokine secretion, which mediate CD8+ T cells migration to the cervicovaginal tract. Further study analyzing cytokine expression following GMCSF application can be performed to verify this possibility. The observation that more than one substance can enhance local antigen specific immune response after vaccination also suggests a direction for future study examining other cytokines and their ability to attract local immune response.
Before this treatment strategy is ready for translation to the clinic, some additional studies will be needed. One encouraging aspect about this treatment strategy is that clinical studies involving the use of GMCSF has been conducted in the past. One previous study reported an adjuvant treatment with continuous low-dose GMCSF can prolong survival of stage III/IV melanoma patients, and has substantial activity in heavily pretreated patients with metastatic breast cancer or female genital tract cancer [20]. Furthermore, another clinical trial had been performed in the past demonstrating that GMCSF applications are well tolerated by the human body, and that a significant increase of APC and cytotoxic T-lymphocyte infiltration was observed in women with cervical low-grade squamous intraepithelial lesions caused by HPV who are treated with GMCSF [21]. We have created numerous therapeutic HPV vaccines and are testing the efficacy of these vaccines using various regimens and administration methods in clinical trials. It will be important to determine the optimal combination for the regimen of local GMCSF application in relation to the therapeutic HPV vaccination regimen. Once we have identified the most potent vaccines and regimens for the generation of systemic HPV antigen-specific immune responses, GMCSF can potentially be used to enhance local recruitment of the therapeutic anti-lesion effects.
In summary, we found that therapeutic CRT/E7 DNA vaccination followed by local GMCSF application resulted in both systemic and local increase of antigen-specific CD8+ T cells in mice bearing cervicovaginal tumors, and that local application of GMCSF promotes local accumulation, maturation and enhances over all cross presentation of dendritic cell. This study strengthens the possibility for clinical translation of local administration of cytokines in combination with therapeutic HPV vaccines to generate potent cell-mediated immune responses and antitumor effects against HPV-associated lesions.
Acknowledgements
This work was funded by the United States National Institutes of Health (NIH) Cervical Cancer Specialized Program of Research Excellence (SPORE) (P50 CA098252), R01 grant (CA114425-01), and 1P20CA192988-01 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no conflicts of interest exist.
References
- 1.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2010;46:S20–6. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Armarnik S, Sheiner E, Piura B, Meirovitz M, Zlotnik A, Levy A. Obstetric outcome following cervical conization. Archives of gynecology and obstetrics. 2011;283:765–9. doi: 10.1007/s00404-011-1848-3. [DOI] [PubMed] [Google Scholar]
- 3.Cardosi RJ, Bomalaski JJ, Hoffman MS. Diagnosis and management of vulvar and vaginal intraepithelial neoplasia. Obstetrics and gynecology clinics of North America. 2001;28:685–702. doi: 10.1016/s0889-8545(05)70229-1. [DOI] [PubMed] [Google Scholar]
- 4.Abbasakoor F, Boulos PB. Anal intraepithelial neoplasia. The British journal of surgery. 2005;92:277–90. doi: 10.1002/bjs.4967. [DOI] [PubMed] [Google Scholar]
- 5.Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, et al. Immunotherapy for cervical cancer: Research status and clinical potential. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2010;24:109–29. doi: 10.2165/11532810-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. The Journal of clinical investigation. 2001;108:669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng S, Lyford-Pike S, Akpeng B, Wu A, Hung CF, Hannaman D, et al. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer immunology, immunotherapy : CII. 2013;62:171–82. doi: 10.1007/s00262-012-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang CM, Monie A, Hung CF, Wu TC. Treatment with imiquimod enhances antitumor immunity induced by therapeutic HPV DNA vaccination. Journal of biomedical science. 2010;17:32. doi: 10.1186/1423-0127-17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:361–7. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16- associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107–14. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. British journal of cancer. 2010;102:1129–36. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S, Song Y, Lee YH, Lee YR, Lee CK, Cho K, et al. Macrophage-colony stimulating factor enhances MHC-restricted presentation of exogenous antigen in dendritic cells. Cytokine. 2005;32:187–93. doi: 10.1016/j.cyto.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer research. 2000;60:3239–46. [PubMed] [Google Scholar]
- 15.Spitler LE, Grossbard ML, Ernstoff MS, Silver G, Jacobs M, Hayes FA, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:1614–21. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 16.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer research. 1996;56:21–6. [PubMed] [Google Scholar]
- 17.Wang TL, Ling M, Shih IM, Pham T, Pai SI, Lu Z, et al. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gene therapy. 2000;7:726–33. doi: 10.1038/sj.gt.3301160. [DOI] [PubMed] [Google Scholar]
- 18.Soong RS, Song L, Trieu J, Knoff J, He L, Tsai YC, et al. Toll like receptor agonist imiquimod facilitates antigen-specific CD8+ T cell accumulation in the genital tract leading to tumor control through interferon-gamma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-14-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology. 2011;89:207–15. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurbacher CM, Kurbacher JA, Cramer EM, Rhiem K, Mallman PK, Reichelt R, et al. Continuous low- dose GM-CSF as salvage therapy in refractory recurrent breast or female genital tract carcinoma. Oncology (Williston Park) 2005;19:23–6. [PubMed] [Google Scholar]
- 21.Hubert P, Doyen J, Capelle X, Arafa M, Renoux V, Bisig B, et al. Local applications of GM-CSF induce the recruitment of immune cells in cervical low-grade squamous intraepithelial lesions. Am J Reprod Immunol. 2010;64:126–36. doi: 10.1111/j.1600-0897.2010.00834.x. [DOI] [PubMed] [Google Scholar]