Abstract
Background
The effect of protein-based meningococcal vaccines on prevention of nasopharyngeal colonization has been difficult to investigate experimentally because a reliable animal colonization model did not exist.
Methods
Human CEACAM1 transgenic mice, which can be colonized by meningococci, were immunized IP with one of two meningococcal native outer membrane vesicle (NOMV) vaccines prepared from mutants with attenuated endotoxin (lpxL1 knockout) and over-expressed sub-family B Factor H-binding proteins (FHbp). Animals were challenged intranasally two weeks after the third dose with wild-type strain H44/76, or were treated IP with anti-NOMV serum before and during the bacterial challenge.
Results
The NOMV-1 vaccine, prepared from the serogroup B H44/76 mutant, elicited ~40-fold higher serum bactericidal antibody titers against the wild-type H44/76 challenge strain than the NOMV-2 vaccine prepared from a heterologous serogroup W mutant strain with different PorA and FHbp amino acid sequence variants. Compared to aluminum hydroxide-immunized control mice, the efficacy for prevention of any H44/76 colonization was 93% (95% confidence interval, 52-99, P<0.0001) for the NOMV-1 vaccine, and 19% (−3-36, P=0.23) for NOMV-2. NOMV-2-vaccinated mice had a 5.6-fold decrease in geometric mean CFU of bacteria per animal in tracheal washes compared to control mice (P=0.007). The efficacy of passive administration of serum from NOMV-1-vaccinated mice to immunologically naïve mice against colonization was 44% (17-61; P=0.002).
Conclusions
Both NOMV vaccines protected against meningococcal colonization but there was greater protection by the NOMV-1 vaccine with antigens matched with the challenge strain. Meningococcal vaccines that target protein antigens have potential to decrease colonization.
Keywords: vaccine, Factor H binding protein, FHbp, OMV, PorA, colonization model, Neisseria meningitidis
I. Introduction
Neisseria meningitidis is a common inhabitant of the human nasopharyngeal microflora. The organism can be sub-divided into encapsulated and non-encapsulated strains. Non-encapsulated strains are nearly always non-pathogenic with infection limited to the nasopharynx, while encapsulated strains can rarely spread to the bloodstream and cause disease.
Meningococcal polysaccharide-protein conjugate vaccines against capsular serogroups A, C, W and Y confer protection against both invasive meningococcal disease and meningococcal colonization [1]. Following introduction of meningococcal group C polysaccharide conjugate vaccines in the UK, approximately one-third of the overall decrease in serogroup C disease was attributed to herd immunity [1]. In contrast, plain (un-conjugated) meningococcal polysaccharide vaccines appeared to have minimal effect on colonization [2]. The reasons why conjugate vaccines, but not plain polysaccharide vaccines, confer protection against carriage are not known.
Serogroup B capsular polysaccharide is structurally similar to polysaccharides in human tissues [3]. Thus serogroup B vaccine development focused on use of noncapsular antigens such as detergent-treated outer membrane vesicles (dOMV) [4], recombinant proteins [5-7], or a combination of both [8, 9]. Native OMV (NOMV) vaccines with genetically attenuated endotoxin that do not require treatment with detergents to deplete endotoxin are also under investigation [10, 11]. Recently, a serogroup B vaccine containing recombinant Factor H binding protein (FHbp) was licensed in the United States, and a four-component serogroup B vaccine (called 4CMenB) that contains recombinant FHbp, two other recombinant proteins, and dOMV was licensed in Europe, Canada and Australia [12]. Both vaccines elicit broad serum bactericidal responses [8, 9, 13], and are expected to confer protection against invasive disease by the majority of serogroup B strains [14]. However, in a recent study in university students, the 4CMenB vaccine had only a modest effect on decreasing serogroup C and Y carriage [15] (the protein antigens in 4CMenB are also present in strains with other capsular groups), and did not decrease acquisition of serogroup B carriage [15].
The effect of vaccination on nasopharyngeal colonization of N. meningitidis has been difficult to investigate experimentally because the receptors important for meningococcal colonization, such as carcinoembryonic antigen-related cell adhesion molecules (CEACAMs), are human-specific [16]. Recently, Johswich et al [16] reported that transgenic mice expressing human CEACAM1 permitted establishment of meningococcal intranasal colonization. Further, human CEACAM1 transgenic mice immunized with a serogroup C polysaccharide-conjugate vaccine were protected against colonization caused by a N. meningitidis serogroup C strain. These results demonstrated the utility of this model for investigation of the effects of vaccination on carriage.
We are investigating the vaccine-potential of meningococcal NOMV vaccines prepared from mutants with genetically attenuated endotoxin and over-expressed FHbp. In mice and infant primates these vaccine elicited broad serum bactericidal antibody responses [11, 17-19]. The purpose of the present study was to investigate the ability of meningococcal NOMV vaccines to confer protection against nasopharyngeal colonization caused by a serogroup B strain.
2. Methods
2.1. Vaccine
The two NOMV vaccines, designated NOMV-1 (prepared from a mutant of serogroup B strain H44/76) and NOMV-2 (prepared from a mutant of serogroup W strain Su 1/06), have been previously described [19, 20]. In brief, endotoxin activity was attenuated by inactivation of the lpxL1 gene. The group W capsule was deleted by knocking out cssA-cssEw as described [20]. Factor H binding protein (FHbp) was over-expressed by chromosomal insertion of two copies of FHbp either ID 1 (H44/76 mutant) or ID 9 (Su 1/06 mutant) with an upstream modified PorA/NadA gene promoter [20]. The FHbp genes contained a single base pair substitution that introduced a serine at amino acid residue 41 instead of arginine (i.e. R41S). This mutation decreased binding of human Factor H (FH) to FHbp [21] and enhanced serum bactericidal antibody responses in human FH transgenic mice [19, 21]. The NOMV vaccines were prepared from membrane blebs released into bacterial culture supernatants and characterized as previously described [20]. By Western blot, the FHbp content of the NOMV vaccines was ~5-fold that of control NOMV vaccines prepared from the respective parental WT strains.
2.2. Ethical statement
All animal experiment procedures were approved by the Animal Ethics Review Committee of the University of Toronto (Permit Numbers: 20008007 and 20008657), which is subject to the ethical and legal requirements under the province of Ontario's Animals for Research Act and the Canadian Council on Animal Care (CCAC). All efforts were made to minimize suffering.
2.3. Transgenic mouse line
The human CEACAM1 mouse line in an FvB background has been described [16, 22]. The transgene is expressed under the control of the human ceacam1 promoter region, and its overall expression pattern on the mucosa of the olfactory and respiratory epithelium lining and the palate and the nasopharyngeal ducts matches well with humans [16].
2.4. Immunization
The 200 μl NOMV dose contained 2.5 μg of protein suspended in a solution of 3 mg/ml of aluminum hydroxide (Alhydrogel, Invivogen), 10 mM histidine (Sigma), and 150 mM NaCl (Sigma). A control vaccine containing aluminum hydroxide (Alhydrogel, Invivogen), 10 mM histidine and 150 mM NaCl (Sigma) was prepared without the addition of the NOMV.
On day 0, groups of mice (N=14 to 16), aged 6 to 8 weeks, were administered the NOMV vaccines or aluminum hydroxide by IP injection. The injections were repeated on days 14 and 28. On day 42, the animals were challenged intranasally with the wildtype serogroup B strain H44/76 (Figure 1). Three days later, the animals were sacrificed and nasotracheal washes were obtained for measurement of mucosal antibody and quantification of N. meningitidis CFU (See below).
Figure 1. Schema illustrating active and passive immunization protocols.
IN, intranasal; IP, intraperitoneal; CFU, colony forming units. In the active immunization, each NOMV dose contained 2.5 μg of protein. In the passive protection, the animals were given 100 μl of 1:5 dilutions of serum pools from NOMV- or aluminum hydroxideimmunized mice.
2.5. Measurement of antibodies to NOMV
The ELISA for measurement of mucosal antibodies to meningococcal NOMV was performed as described previously [16]. Diluted samples of nasal lavage fluid were added to the wells, incubated for two hours, and washed. Bound Ig was detected with 1:10,000 dilutions of AP-goat-anti-mouse IgG Fc(γ) or AP-goat-anti-mouse IgA (Abcam).
For measurement of serum antibodies to the NOMV, the ELISA was performed as previously described [19] with the only difference that 5 μg/ml of the NOMV vaccine diluted in PBS were added to the wells and incubated for 15 hrs at 4°C.
2.6. Serum bactericidal antibody activity
The assay was performed as previously described [23] except that the bacteria were grown to mid-exponential phase in Frantz media supplemented with 4mM D,L-Lactate (Sigma), and 2 mM cytidine 5’-monophospho-n-acetyl-neuramic acid (CMP-NANA; Carbosynth) as described by Costa et al [24]. The two test strains were the H44/76 wild type parent of the mutant used to prepare the NOMV-1 vaccine, and a serogroup W strain from Mali (Mali 29/07) that shares the same porA, and fhbp genes as that of Sudan 1/06 (the wild type parent strain of the mutant used to prepare the NOMV-2 vaccine). The complement source was IgG-depleted human serum [21]. The bactericidal titer was the serum dilution that resulted in a 50% decrease in CFU/ml after 60 minutes of incubation compared with CFU/ml in negative controls wells.
2.7. Mouse colonization
Mouse intranasal infection was conducted as previously described [16]. Mice were anesthetized with Isofluran (Baxter) inhalation. The bacteria were grown to mid-log phase in brain heart infusion broth (Becton Dickinson) supplemented with glucose (0.3 %, Sigma, St. Louis, MO, USA) and deferoxamine mesylate (60 μg/ml) as described [16]. A total of 10 μl inoculum of strain H44/76 and 16 μg/μl of human transferrin (Sigma) were applied to both nares (5 μl per nares for a total of 1x107-1x108 CFU). 72 hours after the challenge, the animals were sacrificed and CFU were determined by retrograde lavage of the upper airways through the trachea with 0.25 ml of PBS/Mg2++, followed by swabbing of the exposed nasal cavities using aluminum shaft applicators (Puritan Medical Products, Guilford, USA). Swabs were re-suspended into 500 μl of PBS/Mg2++. These samples were plated onto GC agar plates supplemented with IsoVitalex and VCNT inhibitor (Becton Dickinson) to suppress growth of nasal flora. The data were expressed as the sum of recovered CFU from each mouse.
2.8. Passive protection by antibody against colonization
Sera obtained three days after the bacterial challenge from mice immunized with the NOMV-1 vaccine or Al(OH) 3 alone were pooled and tested for passive protection against colonization in immunologically naïve CEACAM1 transgenic mice. Three hrs before the intranasal challenge, groups of human CEACAM1-transgenic mice were given 100 μl IP of the serum pools diluted 1:5 in sterile PBS. The mice received a second injection of the pooled serum diluted 1:5 at 24 h after challenge (Figure 1).
2.9. Statistical analyses
For calculation of geometric means, CFU in nasal washes, and IgG and bactericidal titers were transformed (Log10). The respective geometric means of two independent groups of mice were compared by a T test. The Fisher exact test was used to compare the proportions of treated mice and control mice with positive bacterial cultures in nasotracheal washes. Efficacy was calculated from the formula [colonization rate in the control group - the colonization rate in the vaccinated group]/ [colonization rate in the control group]. A two-tailed p value ≤0.05 was considered statistically significant.
3. Results
3.1. NOMV vaccination elicits serum and mucosal antibody responses and protects against acquisition of carriage
In the first experiment, animals were immunized with the NOMV-1 vaccine prepared from the serogroup B H44/76 mutant and challenged with the H44/76 WT parental strain. After three doses of NOMV-1, the human CEACAM1 transgenic mice developed serum and mucosal IgG anti-NOMV antibody responses (Figure 2, Panels A and B). There were no significant differences in the mucosal IgA anti-NOMV antibody responses between NOMV-1-vaccinated and control mice (data not shown). The animals also developed high serum bactericidal titers measured against the WT H44/76 strain (Figure 2, Panel C). A total of 13 of 14 NOMV-vaccinated animals challenged intranasally with the H44/76 WT strain had sterile cultures of the nasotracheal washes at 72 hrs, compared with 0 of 14 Al(OH) 3-vaccinated control mice (Figure 2, Panel D, P<0.0001). The efficacy of NOMV-1 vaccination against colonization was 93% (95% confidence interval, 53-99, Table 1).
Figure 2. Immunization with a mutant NOMV vaccine elicits mucosal and serum antibody responses and protects against colonization.
CEACAM1 transgenic mice immunized with NOMV vaccine (open circles), or Al(OH)3 adjuvant alone (grey squares). Panels A-C, each symbol represents the serum titer of an individual mouse. Panel D, each symbol represents the colony forming units (CFU) of an individual mouse. A. Serum IgG antibody titers to NOMV (ELISA). B. IgG antibody to NOMV in tracheal washes (ELISA). C. Serum bactericidal activity (human complement). D. Colony forming units (CFU) per animal recovered from tracheal washes obtained 72 hrs after intranasal inoculation with N. meningitidis serogroup B strain H44/76. In each panel, the respective differences between the geometric means of the two groups were significant (P<0.001).
Table 1.
Efficacy at preventing any meningococcal colonization by intervention
| % Any Colonization | Efficacy (95% CI) | P Value* | ||
|---|---|---|---|---|
| Control | Vaccinated | |||
| Active NOMV-FHbp immunization, homologous NOMV-1 vaccine | 100 | 7 | 93 (53 to 99) | <0.0001 |
| Active NOMV-FHbp immunization, heterologous NOMV-2 vaccine | 100 | 81 | 19** (−3 to 36) | 0.23 |
| Passive anti-NOMV-1-FHbp serum treatment | 86 | 48 | 44 (17 to 61) | 0.002 |
Fisher exact test
There was a 5.6-fold decrease in tracheal wash CFU per mouse in the vaccinated group, compared to control mice (P=0.007, see Figure 3).
In a second experiment, animals were immunized with the NOMV-2 vaccine prepared from the serogroup W mutant strain and challenged with the serogroup B H44/76 WT parental strain. As expected, the serum bactericidal titers against the heterologous serogroup B H44/76 challenge strain were ~10-fold lower than against a serogroup W test strain from Mali (29/07) that had the same PorA (P1.5,2) and sub-family B FHbp amino acid sequence variant (ID 9) as the mutant NOMV-2 vaccine (Figure 3, Panel A). Compared to aluminum hydroxide-treated control mice, the efficacy of the NOMV-2 vaccine for prevention of any H44/76 colonization was 19% (95% CI, −3-36, P=0.23, Table 1). However, the geometric mean CFU per animal in tracheal washes from the NOMV-2 vaccinated mice was 5.6-fold lower than in the negative control mice immunized with the adjuvant only (geometric mean of 23 vs 130, P=0.007) (Figure 3, Panel B).
Figure 3. Immunization of CEACAM1 transgenic mice with the NOMV-2 vaccine, prepared from a serogroup W mutant, elicits serum bactericidal antibody responses and protects against colonization by serogroup B strain H44/76 Panel.
A. Serum bactericidal activity. Left, a serogroup W test strain (Mali 29/07) with PorA P1.5,2 and FHbp ID9 that matched the NOMV-2 vaccine; Right, serogroup B H44/76 wildtype strain with a heterologous PorA P1. 7,16 and FHbp ID9 to the NOMV2 vaccine. Each symbol represents the titer of pooled serum from four animals. Panel B, Bacterial CFU in tracheal washes obtained 72 hrs after challenge with wild-type serogroup B strain H44/76; each symbol represents CFU of an individual mouse. The respective difference between the geometric means of the CFU in the two groups is significant (P<0.01)
3.2. Serum antibodies passively protect against meningococcal serogroup B intranasal colonization
To investigate whether passively administered antibodies confer protection against colonization, immunologically naïve, human CEACAM1 transgenic mice were injected IP with 1:5 dilutions of serum pools from mice previously immunized with either the NOMV-1 vaccine or Al(OH)3 control. (See Figure 1). In each of the three experiments (Figure 4), animals given the anti-NOMV serum and challenged with strain H44/76 had lower CFU in nasotracheal washes than animals given the negative control serum (P<0.05 in experiments A and C, and P=0.10 in experiment B (two-tailed probability values). When the data from all three experiments were combined, the efficacy of passively administered antibody against colonization was 44% (95% CI, 17-61, P=0.002, Table 1). Thus, serum antibodies contribute to protection against meningococcal colonization.
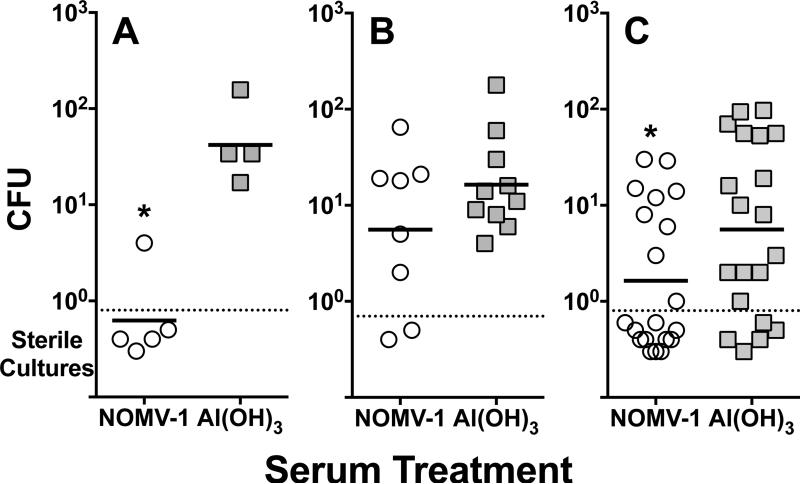
Figure 4. Sera from human CEACAM transgenic mice immunized with a mutant NOMV vaccine confer passive protection against nasotracheal colonization.
Mice were challenged intranasally at time 0 with N. meningitidis serogroup B strain H44/76 and CFU were determined in nasotracheal washes at 72 hrs. At time -3 hrs and + 24 hrs, the mice were treated IP with serum pools diluted 1:5 from mice immunized with the NOMV vaccine or Al(OH)3. Each panel represents an independent experiment. Each symbol represents the CFU isolated from an individual mouse. Open circles, sera from NOMV-vaccinated animals; grey squares, sera from negative control mice immunized with Al(OH)3. Horizontal bars denote the geometric mean CFU recovered per group. Asterisks denote statistically significant differences. Mice treated with sera from NOMV-vaccinated mice had significantly lower geometric means of the CFU (P<0.05 in experiment 1, Panel A, and experiment 3, Panel C; P=0.1 in experiment 2, Panel B).
4. Discussion
The extent to which meningococcal vaccines that target protein antigens can protect against nasopharygenal carriage remains uncertain. In Norway and New Zealand, dOMV vaccines appeared to have had minimal effects on decreasing nasopharygeal carriage [25-27]. In Normandy, France, a dOMV vaccine was reported to decrease carriage but confounding biases may have contributed to the results [28]. The ability of two recently licensed serogroup B vaccines (4CMenB [12] or a bivalent FHbp [29]) to prevent colonization in the population is not known [30].
Several groups investigated the vaccine-potential of meningococcal NOMV vaccines with attenuated endotoxin activity for prevention of invasive meningococcal disease [10, 31-33]. In one experimental study, an NOMV-FHbp vaccine elicited broader serum bactericidal activity in mice against genetically diverse serogroup B strains than a dOMV vaccine used to control a meningococcal outbreak in Norway [18]. The NOMVFHbp vaccine also elicited broader bactericidal activity than the three recombinant proteins used in the 4CMenB vaccine [18]. NOMV-FHbp vaccines such as the one used in experiment 2 of the present study also are being developed for prevention of epidemic meningococcal serogroup A, C and X disease in Sub-Sahara Africa [20, 34, 35]. While promising for prevention of invasive disease, an important gap in knowledge is whether NOMV vaccines will decrease transmission of meningococci in the population.
In the present study we investigated whether meningococcal NOMV vaccines elicited protection against colonization. When the challenge strain had PorA, FHbp and other antigens that exactly matched those of the NOMV-1 vaccine, there was near-complete protection against colonization. Further, the observed protection can be attributed at least in part to serum antibodies in that passively administered post-vaccination serum pools from NOMV-1 vaccinated mice also protected immunologically naïve CEACAM1 transgenic mice against H44/76 colonization. In these experiments, we did not correlate the specific antibody titers with protection against colonization or identity the specific antibodies responsible for protection. However, in previous studies, antibodies to FHbp and/or PorA were responsible for nearly all of the complement-mediated serum bactericidal activity in NOMV-FHbp-vaccinated mice [17, 18, 36].
In experiment 2, we immunized transgenic mice with a NOMV-2 vaccine prepared from a serogroup W mutant with a heterologous PorA sequence type, and a sub-family B FHbp amino acid sequence variant that was 94% percent identical with the sub-family B FHbp ID 1 in the serogroup B H44/76 challenge strain. While the NOMV-2 vaccine elicited high serum bactericidal antibody responses against strain H44/76, the magnitude of the titers was ~40-fold lower than in experiment 1 where the mice had been immunized with the NOMV-1 vaccine prepared from a mutant of strain H44/76. Perhaps related to these lower serum titers, the NOMV-2 vaccine in experiment 2 had much lower efficacy against any H44/76 colonization (19%) than the NOMV-1 vaccine in experiment 1 (93%). However, in experiment 2, there was a 5.6-fold decrease in the ”density” of colonization in the vaccinated mice compared to control mice immunized with aluminum hydroxide alone.
There is ample evidence that pneumoocccal, Haemophilus influenzae type b (Hib) and meningococcal serogroups A and C polysaccharide-protein conjugate vaccines decrease transmission of these organisms in the population [37-41]. For example, in the U.S. Hib conjugate vaccination at 18 to 72 months of age decreased disease incidence in children <18 months of age who at the time were not being vaccinated [42]. However, the effect of Hib conjugate vaccination on colonization was not absolute. For example, Murphy et al reported 65% efficacy against any Hib colonization in children immunized with a Hib conjugate vaccine and exposed to Hib carriers in a day care center [43]. Barbour et al found that of major of effect of Hib conjugate vaccination on colonization may be by decreasing the “density” of bacteria in the nasopharynx, and not by eliminating colonization [44]. Therefore, the 5.6-fold decrease in meningococcal colonization in experiment 2 in mice immunized with the NOMV-2 vaccine provides experimental support for the potential of this vaccine to decrease transmission of meningococci caused by strains with heterologous PorA and FHbp antigens.
In the present study we did not investigate the mechanism by which vaccination conferred protection against colonization. For other pathogens such as Streptococcus pneumoniae [45], nontypeable Haemophilus influenzae [46], N. gonorrhoeae [47, 48], and Bordetella pertussis [49] whole cell vaccines, CD4+ TH17 T-cells appeared to be important for protection against mucosal infections. When these are considered alongside our results, NOMV-mediated protection against meningococcal colonization most likely resulted from eliciting both meningococcal specific antibody-dependent mechanisms to the homologous PorA and FHbp antigens, and antibody-independent mechanisms, which may include specific T-cell subsets and inflammation-recruited neutrophils.
Finally, several possible limitations of our study should be considered. First, we investigated the effect of vaccination on meningococcal colonization in a mouse model. In previous work, mouse models may have falsely predicted the ability of vaccination to protect against colonization in humans or animal models more relevant than mice. For example acellular pertussis vaccination, which protected mice from Bordertella pertussis colonization, failed to protect against colonization in a baboon model [49]. We also investigated only a single time point in the mice, 14 days after a third dose of the NOMV vaccines, to test whether NOMV-induced immunity against colonization was possible. Future studies should consider vaccine dosage and number, the kinetics of response and duration of protection after immunization, but these endpoints were beyond the scope of our study. Another possible limitation of our study was the use of sera from NOMV-1 and aluminum hydroxide-immunized mice in the passive protection experiments that had been obtained three days after the intranasal bacterial challenge, which may have stimulated antibodies or inflammatory mediators. In a separate experiment, mice immunized with the NOMV-2 vaccine and given an intranasal challenge with the bacteria had indistinguishable serum bactericidal titers 3 days after the challenge than immunized mice that had not been challenged (geometric mean titers of 550 and 650, respectively, P=0.93). Since the passive serum protection experiments primarily explored the role of serum antibodies, it seems unlikely that meningococcal nasopharyngeal challenge three days prior to serum collection significantly affected the results.
In summary, development of meningococcal serogroup B vaccines remains a high public health priority. At a population level, vaccines that both prevent disease in individuals and that elicit herd immunity are the most effective for controlling disease and therefore prove cost-effective. Our results suggest that mutant meningococcal NOMV vaccines, which in previous studies elicited broad serum bactericidal antibody responses, also have the potential to prevent colonization.
Highlights.
Human CEACAM1 transgenic mice were immunized with native meningococcal OMV vaccines with attenuated endotoxin and over-expressed FHbp.
The mice developed serum bactericidal antibodies and mucosal antibodies to the OMV.
Mice were protected against nasotracheal serogroup B meningococcal infection.
Passively administered serum antibody also conferred protection against colonization.
Meningococcal vaccines that target protein antigens may decrease carriage.
Acknowledgements
This work was supported by the Alberta Heritage Foundation for Medical Research (AHFMR) through the Interdisciplinary Team in Vaccine Design and Implementation (SDG-O), the Canadian Institutes of Health Research operating grant MOP-130273 (SDG-O) and fellowship MFE-135542 (CMB), and grants AI046464 and AI082263 (DMG) from the National Institute of Allergy and Infectious Diseases, NIH. The work at Children's Hospital Oakland Research Institute (CHORI) was performed in a facility funded by Research Facilities Improvement Program grant number C06 RR016226 from the National Center for Research Resources, NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RP and DMG are inventors on patent applications in the field of meningococcal vaccines.
Non-standard abbreviations
- NOMV
native outer membrane vesicle
- dOMV
detergent-treated OMV
- CEACAM
carcinoembryonic antigen-related cell adhesion molecule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CMB, KOJ and SDG-O report no conflicts.
References
- 1.Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellicour S, Greenwood B. Systematic review: Impact of meningococcal vaccination on pharyngeal carriage of meningococci. Trop Med Int Health. 2007;12:1409–21. doi: 10.1111/j.1365-3156.2007.01929.x. [DOI] [PubMed] [Google Scholar]
- 3.Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–7. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 4.Taha MK, Zarantonelli ML, Alonso JM, Naess LM, Holst J, Feiring B, et al. Use of available outer membrane vesicle vaccines to control serogroup B meningococcal outbreaks. Vaccine. 2007;25:2537–8. doi: 10.1016/j.vaccine.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infection and immunity. 2004;72:2088–100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–9. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–99. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010;29:e71–9. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 9.Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51:1127–37. doi: 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- 10.Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine. 2011;29:1413–20. doi: 10.1016/j.vaccine.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, et al. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine. 2011;29:4728–34. doi: 10.1016/j.vaccine.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews SM, Pollard AJ. A vaccine against serogroup B Neisseria meningitidis: dealing with uncertainty. Lancet Infect Dis. 2014;14:426–34. doi: 10.1016/S1473-3099(13)70341-4. [DOI] [PubMed] [Google Scholar]
- 13.Marshall HS, Richmond PC, Nissen MD, Wouters A, Baber J, Jiang Q, et al. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine. 2013;31:1569–75. doi: 10.1016/j.vaccine.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Frosi G, Biolchi A, Lo Sapio M, Rigat F, Gilchrist S, Lucidarme J, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31:4968–74. doi: 10.1016/j.vaccine.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31. doi: 10.1016/S0140-6736(14)60842-4. [DOI] [PubMed] [Google Scholar]
- 16.Johswich KO, McCaw SE, Islam E, Sintsova A, Gu A, Shively JE, et al. In vivo adaptation and persistence of Neisseria meningitidis within the nasopharyngeal mucosa. PLoS Pathog. 2013;9:e1003509. doi: 10.1371/journal.ppat.1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeberling O, Giuntini S, Seubert A, Granoff DM. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin Vaccine Immunol. 2009;16:156–62. doi: 10.1128/CVI.00403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis. 2008;198:262–70. doi: 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog. 2012;8:e1002688. doi: 10.1371/journal.ppat.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajon R, Fergus AM, Granoff DM. Mutant native outer membrane vesicles combined with a serogroup A polysaccharide conjugate vaccine for prevention of meningococcal epidemics in Africa. PLoS One. 2013;8:e66536. doi: 10.1371/journal.pone.0066536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol. 2011;186:3606–14. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu A, Zhang Z, Zhang N, Tsark W, Shively JE. Generation of human CEACAM1 transgenic mice and binding of Neisseria Opa protein to their neutrophils. PLoS One. 2010;5:e10067. doi: 10.1371/journal.pone.0010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infection and immunity. 2011;79:3751–9. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa I, Pajon R, Granoff DM. Human Factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enchance FH binding. mBio. 2014;5 doi: 10.1128/mBio.01625-14. doi:10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold R, Galloway Y, McNicholas A, O'Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29:7100–6. doi: 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 26.Kelly C, Arnold R, Galloway Y, O'Hallahan J. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am J Epidemiol. 2007;166:817–23. doi: 10.1093/aje/kwm147. [DOI] [PubMed] [Google Scholar]
- 27.Bjune G. “Herd immunity” and the meningococcal vaccine trial in Norway. Lancet. 1992;340:315. doi: 10.1016/0140-6736(92)92411-8. [DOI] [PubMed] [Google Scholar]
- 28.Delbos V, Lemee L, Benichou J, Berthelot G, Deghmane AE, Leroy JP, et al. Impact of MenBvac, an outer membrane vesicle (OMV) vaccine, on the meningococcal carriage. Vaccine. 2013;31:4416–20. doi: 10.1016/j.vaccine.2013.06.080. [DOI] [PubMed] [Google Scholar]
- 29.Richmond PC, Nissen MD, Marshall HS, Lambert SB, Roberton D, Gruber WC, et al. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine. 2012;30:6163–74. doi: 10.1016/j.vaccine.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 30.Taha MK, Deghmane AE. Meningococcal carriage: the dilemma of 4CMenB vaccine. Lancet. 2014 doi: 10.1016/S0140-6736(14)60935-1. [DOI] [PubMed] [Google Scholar]
- 31.Pinto VB, Moran EE, Cruz F, Wang XM, Fridman A, Zollinger WD, et al. An experimental outer membrane vesicle vaccine from N. meningitidis serogroup B strains that induces serum bactericidal activity to multiple serogroups. Vaccine. 2011;29:7752–8. doi: 10.1016/j.vaccine.2011.07.124. [DOI] [PubMed] [Google Scholar]
- 32.Keiser PB, Gibbs BT, Coster TS, Moran EE, Stoddard MB, Labrie JE, 3rd, et al. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine. 2010;28:6970–6. doi: 10.1016/j.vaccine.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 33.Weynants V, Denoel P, Devos N, Janssens D, Feron C, Goraj K, et al. Genetically modified L3,7 and L2 lipooligosaccharides from Neisseria meningitidis serogroup B confer a broad cross-bactericidal response. Infection and immunity. 2009;77:2084–93. doi: 10.1128/IAI.01108-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koeberling O, Ispasanie E, Hauser J, Rossi O, Pluschke G, Caugant DA, et al. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine. 2014;32:2688–95. doi: 10.1016/j.vaccine.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 35.Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. Meningococcal factor H binding proteins in epidemic strains from Africa: Implications for vaccine development. PLoS Negl Trop Dis. 2011;5:e1302. doi: 10.1371/journal.pntd.0001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koeberling O, Delany I, Granoff DM. A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin Vaccine Immunol. 2011;18:736–42. doi: 10.1128/CVI.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibarz-Pavon AB, Maclennan J, Andrews NJ, Gray SJ, Urwin R, Clarke SC, et al. Changes in serogroup and genotype prevalence among carried meningococci in the United Kingdom during vaccine implementation. J Infect Dis. 2011;204:1046–53. doi: 10.1093/infdis/jir466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daugla DM, Gami JP, Gamougam K, Naibei N, Mbainadji L, Narbe M, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study. Lancet. 2014;383:40–7. doi: 10.1016/S0140-6736(13)61612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristiansen PA, Diomande F, Ba AK, Sanou I, Ouedraogo AS, Ouedraogo R, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56:354–63. doi: 10.1093/cid/cis892. [DOI] [PubMed] [Google Scholar]
- 40.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien KL, Millar EV, Zell ER, Bronsdon M, Weatherholtz R, Reid R, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–20. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 42.Murphy TV, White KE, Pastor P, Gabriel L, Medley F, Granoff DM, et al. Declining incidence of Haemophilus influenzae type b disease since introduction of vaccination. JAMA. 1993;269:246–8. [PubMed] [Google Scholar]
- 43.Murphy TV, Pastor P, Medley F, Osterholm MT, Granoff DM. Decreased Haemophilus colonization in children vaccinated with Haemophilus influenzae type b conjugate vaccine. J Pediatr. 1993;122:517–23. doi: 10.1016/s0022-3476(05)83529-2. [DOI] [PubMed] [Google Scholar]
- 44.Barbour ML, Booy R, Crook DW, Griffiths H, Chapel HM, Moxon ER, et al. Haemophilus influenzae type b carriage and immunity four years after receiving the Haemophilus influenzae oligosaccharide-CRM197 (HbOC) conjugate vaccine. Pediatr Infect Dis J. 1993;12:478–84. doi: 10.1097/00006454-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Trzcinski K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infection and immunity. 2008;76:2678–84. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noda K, Kodama S, Umemoto S, Nomi N, Hirano T, Suzuki M. Th17 cells contribute to nontypeable Haemophilus influenzae-specific protective immunity induced by nasal vaccination with P6 outer membrane protein and alpha-galactosylceramide. Microbiol Immunol. 2011;55:574–81. doi: 10.1111/j.1348-0421.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- 47.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–21. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Islam EA, Jarvis GA, Gray-Owen SD, Russell MW. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-beta-dependent mechanisms. Mucosal Immunol. 2012;5:320–31. doi: 10.1038/mi.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111:787–92. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]