Abstract
Background: The question of whether the metabolic syndrome truly reflects a single disease entity with a common underlying pathology remains unclear. In this study, we assess whether metabolic syndrome represents an underlying disease construct in a large population-based sample of Andean Hispanic adults and examine its relationship to subclinical atherosclerosis.
Methods: The study sample was comprised of 2513 participants. Confirmatory factor analysis (CFA) was used to identify a metabolic syndrome latent factor using waist circumference, systolic and diastolic blood pressure, high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), and glucose levels as indicators. The relationship with subclinical atherosclerosis, measured by carotid intima media thickness (cIMT), was assessed using structural equation modeling.
Results: Results supported the proposed structure of the metabolic syndrome latent factor evidenced by adequate fit indexes. HDL-C did not significantly load on the metabolic syndrome latent factor (standardized factor loading=0.01, P=0.88). The metabolic syndrome latent factor was significantly associated with cIMT in women (B=0.007, P<0.001) and men (B=0.008, P<0.001) after controlling for age, low-density lipoprotein cholesterol and smoking.
Conclusions: Our findings suggest that metabolic syndrome components, such as waist circumference, blood pressure, TGs, and glucose levels, but not HDL-C, share a common underlying pathophysiology that may contribute to the progression of atherosclerosis in Andean Hispanics. Its longitudinal association with cardiovascular disease should be the focus of future research.
Introduction
The metabolic syndrome, a cluster of anthropometric, hemodynamic, and metabolic disturbances, is associated with increased risk of cardiovascular disease (CVD)1 and type 2 diabetes mellitus (T2DM).2 A recently published joint interim statement issued by several organizations has established an updated metabolic syndrome definition, recognizing central adiposity, abnormal glucose regulation, elevated triglycerides (TGs), lowered high-density lipoprotein cholesterol (HDL-C), and elevated blood pressure as components.3 The debate surrounding the definition of the metabolic syndrome, however, is still ongoing, and criticisms have been raised regarding several issues, most importantly the somewhat arbitrary selection of components and their cutoff values. Thus, the question of whether the metabolic syndrome truly reflects a single disease entity with a common underlying pathology remains unclear.
In this study, we aimed to: (1) Test whether the metabolic syndrome represents a single underlying factor using confirmatory factor analysis (CFA) in a population-based sample of Andean Hispanic adults; (2) examine differences in the metabolic syndrome factor between men and women; and (3) assess the relationship between the metabolic syndrome factor and carotid artery media thickness (cIMT), a well validated marker of subclinical atherosclerosis.4–6
CFA is used to understand whether the shared variance of measured variables (indicators) can be attributed to an underlying construct (latent factor). Additionally, CFA allows for the examination of models in which both a latent factor and its correspondence with relevant indicators are explicitly specified on the basis of previous literature. Previous studies have attempted to model the metabolic syndrome factor structure using CFA.7,8 These studies have identified a metabolic syndrome factor model with glucose dysregulation [measured by both fasting blood glucose (FBG) and insulin level], obesity [measured by body mass index (BMI) and waist circumference (WC)], dyslipidemia (measured by TGs and HDL-C), and systolic blood pressure (SBP) and diastolic blood pressure (DBP) as central components.7,8 More recently, Stevenson and colleagues9 developed a more parsimonious metabolic syndrome model with five components commonly used in clinical practice—adiposity, SBP, HDL-C, TGs, and glucose. Possible gender differences and the examination of a metabolic syndrome latent factor model in Hispanics, however, have yet to be investigated and are of high importance.
Gender and ethnicity are important determinants for the risk of having metabolic syndrome.10–18 In the third National Health and Nutrition Examination Survey (NHANES III), differences by gender were observed among ethnic minorities.10 African American and Mexican American women had higher prevalence rates of metabolic syndrome than African American and Mexican American men, respectively, whereas the risk in Caucasians was similar between genders.10 Moreover, Mexican Americans were shown to have the highest prevalence rates of the metabolic syndrome.10
Although Hispanics are a highly heterogeneous group, until recently, most studies had been conducted in Mexican Americans. Results from other Hispanic subgroups are still scarce; however, recently published (and ongoing) studies are providing important data regarding the prevalence and patterns of metabolic syndrome in different Hispanic subgroups, including those from Andean countries. Results from our group,18–23 and other large epidemiological studies conducted in Venezuela,10 Chile,24 and Colombia,25 have provided evidence of a different phenotypic pattern of the metabolic syndrome among Andean Hispanics. This pattern is characterized by a high prevalence of abdominal obesity and dyslipidemia, with low HDL-C levels being particularly prevalent in women and hypertriglyceridemia being particularly prevalent in men. Given the distinct presentation of the syndrome in this subgroup, a more detailed characterization of the metabolic syndrome and its relationship with subclinical cardiovascular disease is warranted.
Methods
Study population and sampling design
Study participants were enrolled in the Peruvian Study of Cardiovascular Disease Prevalence (PREVENCION). This population-based study was undertaken in Arequipa, the second largest city in Peru. Its population is comparable to other urban populations in Peru and resembles urban populations in Andean countries, such as Bolivia and Ecuador. This population consists largely of Mestizos (“mixed”), with the degree of admixture being predominantly Andean Amerindian (i.e., autochthonous Quechua and Aymara), small contributions from Spanish whites, and minimal contributions from West African populations. The first phase of PREVENCION was designed to determine the prevalence of CVD and cardiovascular risk factors in the adult study population. Details regarding the sampling strategy, general objectives, and design of the PREVENCION study have been published previously.18
Anthropometric, blood pressure, and biochemical measurements
Height and weight were measured with a stadiometer and a calibrated scale, respectively, with the participant barefoot and wearing light clothing. BMI was calculated as weight divided by height squared (kg/m2). WC was measured at the umbilical level while subjects were standing.
Blood pressure was measured between 7 a.m. and 10 a.m. using a mercury sphygmomanometer after a resting period of at least 5 min, with the auscultatory method, according to recommendations from the seventh report of the Joint National Committee for the diagnosis, evaluation, and treatment of high blood pressure.26 At least two measurements were performed in each of two separate days, and all measurements were used to calculate a final mean value for SBP and DBP.
Samples of venous blood were obtained after at least 8 hr of fast and serum was used for biochemical measurements. Low-density lipoprotein cholesterol (LDL-C), glucose, and TGs were measured enzymatically by automated methods (Cobas Mira Assay; Roche, Basel, Switzerland). HDL-C was measured after precipitation of apolipoprotein B (ApoB)-containing lipoproteins.27 All coefficient of variations for these measurements were <10%.
Measurements of cIMT
High-resolution B-mode carotid ultrasonography was performed with a linear-array, 10-MHz transducer in B mode (Sonosite Titan; Sonosite, Bothell, WA). With the subject in the supine position, images were obtained bilaterally from the anterior, posterior, and lateral views. Both carotid arteries were examined with the head tilted slightly upward in the mid-line position. The transducer was manipulated so that the near and far walls of the common carotid artery were parallel to the transducer footprint and the lumen diameter was maximized in the longitudinal plane. A region 1.0 cm proximal to the carotid bulb was identified, and cIMT of the near and far walls was evaluated as the distance between the lumen–intima interface and the media–adventitia interface. If plaques were present, these were included in the measurements. Measurements of cIMT were performed on a frozen frame of a suitable longitudinal image using the Sonocalc software (Sonosite Titan; Sonosite, Bothell, WA), which performs multiple automated measurements along 1 cm and averages them, thereby increasing the accuracy of measurements. All measurements were performed offline in a blinded fashion.
Statistical analysis
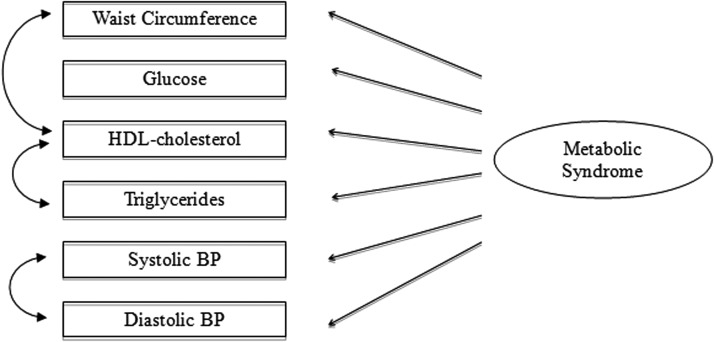
Preliminary statistical analyses included descriptive statistics and assessment of normality of distributions. Nonnormal variables (glucose and cIMT) were log-transformed. CFA was used to examine the metabolic syndrome construct (latent factor) and its correspondence to measured variables (indicators): WC, SBP, DBP, HDL-C, TGs, and glucose (model depicted in Fig. 1). Correlated residuals were specified between HDL-C and TGs, and between SBP and DBP, given that these factors are known to share common physiological characteristics beyond their contribution to the metabolic syndrome construct. The comparative fit index (CFI), root mean squared error of approximation (RMSEA), and standardized root mean square residual (SRMR) were used to evaluate model fit. Once a final model was established, it was reassessed using multiple group comparisons to examine gender differences. Within this approach, a model was estimated separately for men and women. The correspondence between the indicators and the metabolic syndrome factor were compared by gender using chi-squared difference tests of nested models. Finally, cIMT was regressed on the metabolic syndrome factor using structural equation modeling. Age, LDL-C, and smoking were included as control variables.28 The model was again tested by gender using the multiple groups approach. Mplus version 6 (Muthén and Muthén, http://www.statmodel.com/) was used for all analyses.
FIG. 1.
Metabolic syndrome measurement model.
Missing data
Complete data were available for all subjects for the estimation of the metabolic syndrome factor. However, given that the assessment of cIMT was implemented late in the study, missing data were present in 40% of the cIMT variable (1009 subjects with missing data). Analyses were conducted using both full information maximum likelihood, a state-of-the-art method for the estimation of model parameters in the presence of missing data,29 and listwise deletion. Both methods yielded comparable parameter estimates (results not shown), suggesting that no bias was introduced by missingness in the variables included in the model. Therefore, data from all subjects was retained for analyses.
Results
Sample characteristics
The study population consisted of 2513 participants (1141 men and 1372 women). Median age was 51 years. Characteristics of the study sample are shown in Table 1. Men demonstrated a higher prevalence of smoking and alcohol consumption and were significantly taller, heavier, and had higher WC measurements, but BMI was comparable between genders. Men demonstrated higher DBP, total cholesterol, LDL-C, and TG levels and significantly lower HDL-C. There was no difference in glucose, insulin, prevalence of T2DM, or prevalence of CVD. Men had significantly greater cIMT than women.
Table 1.
Demographic, Clinical, and Laboratory Characteristics of the Study Sample
| Men (n=1141) | Women (n=1372) | P value | |
|---|---|---|---|
| Age, years | 51 (37–64) | 51 (36–73) | 0.777 |
| Current smoking (%) | 26.2 | 11.3 | <0.001* |
| Body mass index (kg/m2) | 26.4 (24.0–29.0) | 26.1 (23.0–30.0) | 0.116 |
| Waist circumference (cm) | 94 (87–101) | 87 (79–96) | <0.001* |
| Systolic blood pressure (mmHg) | 120 (110–130) | 116 (102–130) | 0.183 |
| Diastolic blood pressure (mmHg) | 80 (72–83) | 75 (70–82) | <0.001* |
| HDL-C (mg/dL) | 44.0 (38.7–51.4) | 48.0 (41.0–55.2) | <0.001* |
| LDL-C (mg/dL) | 113.0 (96.3–132.3) | 117.0 (100.0–140.0) | <0.001* |
| Triglycerides (mg/dL) | 166 (118–240) | 141 (100–196) | <0.001* |
| Glucose (grams/dL) | 78.0 (73.0–86.0) | 78.6 (73.0–86.0) | 0.727 |
| Cardiovascular disease (%) | 2.3 | 2.6 | 0.526 |
| Carotid intima media thickness (mm) | 0.67 (0.56–0.78) | 0.64 (0.54–0.75) | 0.024* |
P<0.05.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Measurement model
The initial model was assessed and showed adequate fit to the data as evidenced by the following fit indexes: CFI=0.979, RMSEA=0.059, SRMR=0.029. However, after analysis of the residual matrix, modification indices, and theoretical considerations, a correlation was specified between the residuals of HDL-C and WC. This modified model showed significantly better fit χ2(1)=53.232, P<0.001, with CFI=0.997, RMSEA=0.023, SRMR=0.009, and supported a common factor structure of the metabolic syndrome measured by six indicators—WC, SBP, DBP, HDL-C, TGs, and glucose levels.
Structure of the metabolic syndrome
The individual contribution of each indicator was assessed by examining the standardized factor loadings. WC showed the highest correlation with the metabolic syndrome factor (standardized factor loading=0.68, P<0.001), followed by SBP (0.53, P<0.001), DPB (0.52, P<0.001), TGs (0.45, P<0.001), and glucose (0.33, P<0.001). HDL-C was not significantly correlated with the metabolic syndrome factor (standardized factor loading=0.01, P=0.88), beyond its association with TGs (r=−0.10, P<0.001) and WC (r=−0.23, P<0.001), suggesting that HDL-C is not an indicator of a common underlying metabolic syndrome construct in this population.
The metabolic syndrome explained 46% of the variance in WC, 28% of the variance in SBP, 27% of the variance in DBP, 20% of the variance in TGs, and 11% of the variance in glucose levels.
Gender comparison
Significant differences were found for all indicators: SBP (χ2(1)=35.09, P<0.001), DBP (χ2(1)=5.024, P=0.025], HDL-C [χ2(1)=5.97, P=0.015], TGs (χ2(1)=5.26, P=0.022), and glucose (χ2(1)=8.37, P=0.003), suggesting significant gender differences in all loadings. The model was therefore estimated separately by gender. Table 2 presents loadings estimated independently for men and women.
Table 2.
Loadings of Metabolic Syndrome Latent Factor
| Loadings for men | Loadings for women | |||
|---|---|---|---|---|
| Unstandardized | Standardized | Unstandardized | Standardized | |
| Waist circumference | 1.00 | 0.74 | 1.00 | 0.61 |
| Systolic blood pressure | 1.02 | 0.45 | 1.77 | 0.61 |
| Diastolic blood pressure | 0.51 | 0.45 | 0.72 | 0.56 |
| HDL-C (mg/dL) | 0.12 | 0.10a | -0.01 | 0.01a |
| Triglycerides | 4.77 | 0.39 | 5.27 | 0.49 |
| Glucose (log) | 0.01 | 0.28 | 0.01 | 0.39 |
Indicator did not significantly load to the metabolic syndrome latent factor.
HDL-C, high-density lipoprotein cholesterol.
The metabolic syndrome factor and cIMT
Given the difference in the loadings across men and women, the association between the metabolic syndrome latent factor and cIMT was estimated separately by gender. The metabolic syndrome factor was significantly associated with cIMT in men (B=0.014, β=531, P<0.001) and women (B=0.015, β=615, P<0.001). In a model that adjusted for age, LDL-C and smoking, the metabolic syndrome factor remained significantly associated with cIMT in both genders (B for men=0.003, β=0.145, P=0.004; B for women=0.004, β=0.179, P<0.001). Other significant correlates of cIMT in men were age (B=0.006, β=0.578, P<0.001) and LDL-C (B=0.001, β=0.102, P<0.001). Age was also a significant correlate of cIMT in women (B=0.006, β=179, P<0.001). The model explained 44% and 47% of the variance of cIMT in men and women, respectively.
The metabolic syndrome factor vs. the current diagnostic criteria
A regression model was fitted using the current National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for metabolic syndrome as a correlate of cIMT to compare it to the metabolic syndrome latent factor. The current NCEP ATP III criteria were significantly associated with cIMT in both men (β=0.22, P<0.001) and women (β=0.26, P<0.001). The diagnostic metabolic syndrome criteria, however, explained only 7% and 5% of the variance in cIMT in men and women, respectively, whereas the metabolic syndrome factor alone explained 28% of the cIMT variance in men and 38% in women (Table 3). Even after controlling for the metabolic syndrome criteria, age, LDL-C, and smoking, the metabolic syndrome factor explained significant additional variance in cIMT (R2 change in men=0.09, P<0.001; R2 change in women=0.03, P<0.001).
Table 3.
The Metabolic Syndrome Latent Factor vs. the Current Metabolic Syndrome NCEP ATP III Criteria
| B | β | P | cIMT R2 | |
|---|---|---|---|---|
| Men | ||||
| Metabolic syndrome latent factor | 0.014 | 0.531 | <0.001* | 0.282 |
| Metabolic syndrome NCEP ATP III criteria | 0.100 | 0.266 | <0.001* | 0.071 |
| Women | ||||
| Metabolic syndrome latent factor | 0.015 | 0.615 | <0.001* | 0.379 |
| Metabolic syndrome NCEP ATP III criteria | 0.082 | 0.220 | <0.001* | 0.048 |
P<0.001.
NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III.
Discussion
Our study reports on the use of a continuous variable approach to examine the metabolic syndrome as an underlying disease construct in Andean Hispanics. Our results support a common metabolic syndrome underlying factor measured by five indicators—WC, SBP, DBP, TGs, and glucose levels. HDL-C was not found to significantly load to the metabolic syndrome factor, suggesting that it is not a component of the metabolic syndrome in Andean Hispanics. Differences in the contribution of each indicator to the metabolic syndrome factor were found between men and women. Furthermore, we provide novel data assessing the relationship between a metabolic syndrome factor and cIMT in a community-based sample. The metabolic syndrome factor was significantly associated with subclinical atherosclerosis in this sample in both men and women after controlling for the effects of age, LDL-C, and smoking.
Previous studies assessed the factor structure of the metabolic syndrome in primarily Caucasian populations. Shen et al.7 examined a hierarchical structure of the syndrome with metabolic syndrome as a second-order factor and insulin resistance (measured by glucose and insulin levels), obesity (WC and BMI), lipid levels (HDL-C and TGs), and blood pressure (SBP and DBP) as first-order factors. This model differs from ours in that it uses a hierarchical approach and incorporates additional indicators. Specifically, the insulin resistance factor is comprised of both glucose and insulin levels, and the obesity factor is measured by both BMI and WC. Stevenson and colleagues recently tested a more parsimonious model with five components—BMI, SBP, HDL-C, TGs, and glucose.9 It differs from our model in that Stevenson et al. used BMI and not WC as a proxy for abdominal obesity. A commonality among studies in Caucasian samples and the results of our examination of Andean Hispanics, however, is that all studies support the presence of a single underlying metabolic syndrome construct.7,9 In line with previous results and theoretical considerations,7,9 our study reports evidence of the significant contribution of WC, SBP and DBP, TGs, and glucose levels on the metabolic syndrome latent factor.
In contrast to the aforementioned studies on the metabolic syndrome factor structure,7,9 our study found that HDL-C was not significantly correlated to the metabolic syndrome latent factor. This suggests that, as opposed to its significance among Caucasians, HDL-C may not be an important contributor to the metabolic syndrome in Andean Hispanics. This discrepancy might reflect ethnic differences on the predictive value of HDL-C as a component of the metabolic syndrome. Previous reports have consistently reported a strikingly high prevalence of low HDL-C among Andean Hispanics, despite a low prevalence of metabolic syndrome.10,19,30 Similarly, the levels of lipid profile components were found to vary across ethnicities in a US sample of older adults, with Hispanics having lower HDL-C levels than non-Hispanic whites.31 In line with these findings are results from two prospective designs that showed HDL-C was not associated with risk of myocardial infarction in Hispanics.32,33 Another population that has been identified as having particularly low levels of HDL-C is Turkish adults.34 A recent review showed levels of HDL-C are lower among Turkish adults when compared to estimates in other European countries and the United States.34 Interestingly, HDL-C estimates among the Turkish population were comparable to those reported in Andean Hispanics.10,19 Research examining the contribution of HDL-C on the metabolic syndrome, however, is not available in this population.
Important differences between men and women in the metabolic syndrome components have been previously reported in the literature.1,2,19 Shen et al. reported differences between men and women in the contribution of lipids such as HDL-C and TGs as well as WC.7 In our study, we found that for men, central adiposity appeared to be the most important component of the metabolic syndrome, whereas for women, both SBP and WC had the important contribution to the metabolic syndrome construct.
Our study is the first to report on a positive association between a metabolic syndrome factor and cIMT. These results are consistent with previous reports showing increased cIMT in subjects with the metabolic syndrome.35–38 The relationship between the metabolic syndrome factor and cIMT was significant in both genders after controlling for age, LDL-C, and smoking status. Although some reports have indicated gender differences in this relationship,38,39 most studies suggest a robust association in both genders.35–38 Furthermore, we found that the metabolic syndrome factor explained significantly greater variability in cIMT compared to the current NCEP ATP III criteria. This is not surprising given that a latent variable approach confers the important advantage of measuring constructs as continuous rather than dichotomous variables using potentially arbitrary cutoff scores, as is the case with all current metabolic syndrome diagnostic definitions. Because risk associated with pathophysiological constructs is progressive as opposed to simply absent or present, the present methodology provides a novel means to capture the metabolic syndrome as a continuous and uninterrupted process in line with biological assumptions. This approach, therefore, presents a more detailed characterization of this debated syndrome.
Important strengths of our study include our large sample size and our population-based sampling strategy. Our study is limited by its cross-sectional nature. Additionally, our sample includes a single ethnic group and did not allow for comparison across ethnic groups. A longitudinal study is needed to better assess the relationship between a latent metabolic syndrome construct and the risk of cardiovascular events; however, pending prospective data, our findings provide important insights into the association between metabolic syndrome and subclinical atherosclerosis in this population.
Conclusions
Our findings suggest that in Andean Hispanics the metabolic syndrome represents a common underlying disease entity measured by five indicators—WC, SBP, DBP, TGs, and glucose. This underlying metabolic syndrome factor is strongly associated with subclinical carotid atherosclerosis, measured by cIMT, in both genders after adjustment for age, LDL-C, and smoking. Additionally, we found the factor explained a greater amount of variability in subclinical disease compared to currently used metabolic syndrome diagnostic criteria. Gender differences were found in the contribution of indicators to the metabolic syndrome factor; however, HDL-C was not found to be a component of the metabolic syndrome in either men or women. Further studies regarding the metabolic syndrome and its association with cardiovascular risk are warranted in other Hispanic populations.
Author Disclosure Statement
The authors of this manuscript have no conflict of interest to disclose.
References
- 1.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683–689 [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo C, Okoloise M, Williams K, et al. The metabolic syndrome as predictor of type 2 diabetes: The San Antonio heart study. Diabetes Care 2003;26:3153–3159 [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 4.Kitamura A, Iso H, Imano H, et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke 2004;35:2788–2794 [DOI] [PubMed] [Google Scholar]
- 5.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: The Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000;151:478–487 [DOI] [PubMed] [Google Scholar]
- 6.Hollander M, Hak AE, Koudstaal PJ, et al. Comparison between measures of atherosclerosis and risk of stroke: The Rotterdam Study. Stroke 2003;34:2367–2372 [DOI] [PubMed] [Google Scholar]
- 7.Shen BJ, Goldberg RB, Llabre MM, et al. Is the factor structure of the metabolic syndrome comparable between men and women and across three ethnic groups: The Miami Community Health Study. Ann Epidemiol 2006;16:131–137 [DOI] [PubMed] [Google Scholar]
- 8.Klaus JR, Hurwitz BE, Llabre MM, et al. Central obesity and insulin resistance in the cardiometabolic syndrome: Pathways to preclinical cardiovascular structure and function. J Cardiometab Syndr 2009;4:63–71 [DOI] [PubMed] [Google Scholar]
- 9.Stevenson JE, Wright BR, Boydstun AS. The metabolic syndrome and coronary artery disease: A structural equation modeling approach suggestive of a common underlying pathophysiology. Metabolism 2012;61:1582–1588 [DOI] [PubMed] [Google Scholar]
- 10.Florez H, Silva E, Fernandez V, et al. Prevalence and risk factors associated with the metabolic syndrome and dyslipidemia in White, Black, Amerindian and Mixed Hispanics in Zulia State, Venezuela. Diabetes Res Clin Pract 2005;69:63–77 [DOI] [PubMed] [Google Scholar]
- 11.Hwang LC, Bai CH, Chen CJ. Prevalence of obesity and metabolic syndrome in Taiwan. J Formosan Med Assoc=Taiwan yi zhi 2006;105:626–635 [DOI] [PubMed] [Google Scholar]
- 12.Bouguerra R, Ben Salem L, Alberti H, et al. Prevalence of metabolic abnormalities in the Tunisian adults: A population based study. Diabetes Metab 2006;32:215–221 [DOI] [PubMed] [Google Scholar]
- 13.He Y, Jiang B, Wang J, et al. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol 2006;47:1588–1594 [DOI] [PubMed] [Google Scholar]
- 14.Lee WY, Park JS, Noh SY, et al. Prevalence of the metabolic syndrome among 40,698 Korean metropolitan subjects. Diabetes Res Clin Pract 2004;65:143–149 [DOI] [PubMed] [Google Scholar]
- 15.Azizi F, Salehi P, Etemadi A, et al. Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract 2003;61:29–37 [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Deedwania PC, Gupta A, et al. Prevalence of metabolic syndrome in an Indian urban population. Int J Cardiol 2004;97:257–261 [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo C, Serrano-Rios M, Martinez-Larrad MT, et al. Geographic variations of the International Diabetes Federation and the National Cholesterol Education Program-Adult Treatment Panel III definitions of the metabolic syndrome in nondiabetic subjects. Diabetes Care 2006;29:685–691 [DOI] [PubMed] [Google Scholar]
- 18.Medina-Lezama J, Chirinos JA, Zea Diaz H, et al. Design of PREVENCION: A population-based study of cardiovascular disease in Peru. Int J Cardiol 2005;105:198–202 [DOI] [PubMed] [Google Scholar]
- 19.Medina-Lezama J, Zea-Diaz H, Morey-Vargas OL, et al. Prevalence of the metabolic syndrome in Peruvian Andean hispanics: The PREVENCION study. Diabetes Res Clin Pract 2007;78:270–281 [DOI] [PubMed] [Google Scholar]
- 20.Medina-Lezama J, Pastorius CA, Zea-Diaz H, et al. Optimal definitions for abdominal obesity and the metabolic syndrome in Andean Hispanics: The PREVENCION study. Diabetes Care 2010;33:1385–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina-Lezama J, Zea-Diaz H, Morey-Vargas OL, et al. Prevalence and patterns of hypertension in Peruvian Andean Hispanics: The PREVENCION study. J Am Soc Hypertens 2007;1:216–225 [DOI] [PubMed] [Google Scholar]
- 22.Medina-Lezama J, Morey-Vargas OL, Zea-Diaz H, et al. Prevalencia de Sobrepeso y Obesidad en la Poblacion Adulta de Arequipa Metropolitana: Resultados del Estudio Prevencion. Revista Peruana de Cardiología. 2006;XXXII:194–209 [Google Scholar]
- 23.Medina-Lezama J, Morey-Vargas OL, Zea-Diaz H, et al. Estimaciones del Riesgo Cardiovascular Global en la Población Adulta de Arequipa Metropolitana: Resultados del Estudio Prevencion. Revista Peruana de Cardiología 2006;XXXII:129–144 [Google Scholar]
- 24.Chile MdSd. Resumen Ejecutivo Encuesta Nacional de Salud, 2003. El Vigia 2004;6:1–20 [Google Scholar]
- 25.Bautista LE, Orostegui M, Vera LM, et al. Prevalence and impact of cardiovascular risk factors in Bucaramanga, Colombia: rResults from the Countrywide Integrated Noncommunicable Disease Intervention Programme (CINDI/CARMEN) baseline survey. Eur J Cardiovasc Prev Rehabil 2006;13:769–775 [DOI] [PubMed] [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 27.Grauholt AM, Grande P, Horby-Petersen J, et al. High-density lipoprotein cholesterol assay by magnesium dextransulphate precipitation. Scand J Clin Lab Invest 1986;46:715–721 [DOI] [PubMed] [Google Scholar]
- 28.Pastorius CA, Medina-Lezama J, Corrales-Medina F, et al. Normative values and correlates of carotid artery intima-media thickness and carotid atherosclerosis in Andean-Hispanics: The Prevencion Study. Atherosclerosis 2010;211:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psycholog Methods 2001;6:330–351 [PubMed] [Google Scholar]
- 30.Medina-Lezama J, Morey-Vargas OL, Zea-Diaz H, et al., Prevalence of lifestyle-related cardiovascular risk factors in Peru: The PREVENCION study. Revista panamericana de salud publica=Pan Am J Public Health 2008;24:169–179 [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez C, Pablos-Mendez A, Palmas W, et al. Comparison of modifiable determinants of lipids and lipoprotein levels among African-Americans, Hispanics, and Non-Hispanic Caucasians≥65 years of age living in New York City. Am J Cardiol 2002;89:178–183 [DOI] [PubMed] [Google Scholar]
- 32.Hanley AJ, Williams K, Stern MP, et al. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio Heart Study. Diabetes Care 2002;25:1177–1184 [DOI] [PubMed] [Google Scholar]
- 33.Willey JZ, Rodriguez CJ, Carlino RF, et al. Race-ethnic differences in the association between lipid profile components and risk of myocardial infarction: The Northern Manhattan Study. Am Heart J 2011;161:886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onat A. Risk factors and cardiovascular disease in Turkey. Atherosclerosis 2001;156:1–10 [DOI] [PubMed] [Google Scholar]
- 35.Bertoni AG, Wong ND, Shea S, et al., Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2007;30:2951–2956 [DOI] [PubMed] [Google Scholar]
- 36.Kullo IJ, Cassidy AE, Peyser PA, et al. Association between metabolic syndrome and subclinical coronary atherosclerosis in asymptomatic adults. Am J Cardiol 2004;94:1554–1558 [DOI] [PubMed] [Google Scholar]
- 37.Tzou WS, Douglas PS, Srinivasan SR, et al. Increased subclinical atherosclerosis in young adults with metabolic syndrome: The Bogalusa Heart Study. J Am Coll Cardiol 2005;46:457–463 [DOI] [PubMed] [Google Scholar]
- 38.Skilton MR, Moulin P, Serusclat A, et al. A comparison of the NCEP-ATPIII, IDF and AHA/NHLBI metabolic syndrome definitions with relation to early carotid atherosclerosis in subjects with hypercholesterolemia or at risk of CVD: Evidence for sex-specific differences. Atherosclerosis 2007;190:416–422 [DOI] [PubMed] [Google Scholar]
- 39.Kawamoto R, Tomita H, Ohtsuka N, et al. Metabolic syndrome, diabetes and subclinical atherosclerosis as assessed by carotid intima-media thickness. J Atheroscler Thromb 2007;14:78–85 [DOI] [PubMed] [Google Scholar]