Abstract
Background. Incomplete control of troublesome regurgitation and extraesophageal manifestations of chronic gastroesophageal reflux disease (GERD) is a known limitation of proton pump inhibitor (PPI) therapy. This multicenter randomized study compared the efficacy of transoral incisionless fundoplication (TIF) against PPIs in controlling these symptoms in patients with small hiatal hernias. Methods. Between June and August 2012, 63 patients were randomized at 7 US community hospitals. Patients in the PPI group were placed on maximum standard dose (MSD). Patients in the TIF group underwent esophagogastric fundoplication using the EsophyX2 device. Primary outcome was elimination of daily troublesome regurgitation or extraesophageal symptoms. Secondary outcomes were normalization of esophageal acid exposure (EAE), PPI usage and healing of esophagitis. Results. Of 63 randomized patients (40 TIF and 23 PPI), 3 were lost to follow-up leaving 39 TIF and 21 PPI patients for analysis. At 6-month follow-up, troublesome regurgitation was eliminated in 97% of TIF patients versus 50% of PPI patients, relative risk (RR) = 1.9, 95% confidence interval (CI) = 1.2-3.11 (P = .006). Globally, 62% of TIF patients experienced elimination of regurgitation and extraesophageal symptoms versus 5% of PPI patients, RR = 12.9, 95% CI = 1.9-88.9 (P = .009). EAE was normalized in 54% of TIF patients (off PPIs) versus 52% of PPI patients (on MSD), RR = 1.0, 95% CI = 0.6-1.7 (P = .914). Ninety percent of TIF patients were off PPIs. Conclusion. At 6-month follow-up, TIF was more effective than MSD PPI therapy in eliminating troublesome regurgitation and extraesophageal symptoms of GERD.
Keywords: transoral incisionless fundoplication (TIF), EsophyX, extraesophageal GERD symptoms, regurgitation, proton pump inhibitor (PPI), heartburn
Introduction
Adequate control of troublesome regurgitation and extraesophageal manifestation such as laryngitis, asthma, chronic cough, and dental erosions1 in chronic gastroesophageal reflux disease (GERD) patients remains a therapeutic concern.2,3 Medical therapy with proton pump inhibitors (PPIs) causes a modest and considerably less symptomatic relief of regurgitation4 and extraesophageal symptoms5-10 compared with heartburn. On the other hand, despite its proven long-term effectiveness, laparoscopic Nissen fundoplication (LNF) is associated with potential side effects such as gas bloat, dysphagia, and uncontrolled flatulence.11 Additionally, failure of medical therapy has been considered predictive of nonsatisfactory outcomes of LNF.3 Recently, many attempts have been made to develop an alternative and less invasive treatment that would bridge the gap between medical therapy and LNF.
Several retrospective and prospective studies have reported that transoral incisionless fundoplication (TIF) performed with EsophyX device (EndoGastric Solutions, Redmond, WA) is capable of improving GERD symptoms and patient satisfaction of those suffering from chronic GERD when the associated hiatal hernia defect is small (≤2 cm).12 A notable absence of randomized studies evaluating the procedure has prevented a better definition of its role in the management of chronic GERD.13
The TEMPO trial (TIF EsophyX vs Medical PPI Open Label Trial) compared the efficacy of the TIF procedure against maximal dose PPI therapy in controlling regurgitation and extraesophageal symptoms of GERD in patients who partially responded to PPIs. The primary hypothesis was that TIF would be more effective than PPIs in eliminating daily troublesome regurgitation or extraesophageal GERD symptoms at 6-month follow-up. The secondary hypotheses were that the majority of TIF patients would normalize their esophageal acid exposure (EAE) compared with baseline and that the majority of TIF patients would be completely off PPIs.
Methods
Study Design
This multicenter, open label, randomized, comparative study was conducted at 7 study sites in the United States. The institutional review board of the participating institutions approved the study protocol. Written consent was obtained from all patients before randomization after all critical information about the study had been explained in detail. Patients who had met the eligibility criteria were randomly assigned to receive either TIF or maximum dose PPI therapy with a target allocation ratio of 2:1. There were no important changes to methods after study initiation and no interim analyses for efficacy were performed.
Patients
Patients experiencing persistent daily troublesome regurgitation or extraesophageal GERD symptoms (with or without heartburn) on daily PPIs were deemed eligible for the study if they had documented abnormal EAE as determined by ambulatory 48-hour pH monitoring while off PPI therapy for at least 7 days (% total time pH < 4 occurred for >5.3% of the recording time14) and hiatal hernia measurements not exceeding 2 cm in both axial length and in greatest transverse dimension. A complete list of inclusion and exclusion criteria is presented in Table 1. Eligible patients were randomly assigned to receive either TIF or maximum standard dose (MSD) PPI therapy. Patients in the PPI group were required to take the MSD of currently used PPI in an attempt to optimize control of their GERD symptoms. The same brand of PPI used by individual patients at screening was prescribed by investigators at the maximal allowed dose per manufacturer’s recommendations and provided free of charge to each patient randomized to the PPI group. A complete listing of PPI brands used in this study is provided in Table 2. Patients in the TIF group underwent endoscopic fundoplication using the latest iteration of the EsophyX2 device to perform the standardized TIF-2.0 protocol previously described elsewere.15,16 In brief, under general anesthetic, the EsophyX device was gently introduced over the flexible endoscope into the stomach under constant endoscopic visualization. The helical retractor was engaged into the tissue slightly distal to Z line. Then, in combination with the tissue manipulating elements, the fundus of the stomach was folded up and around the distal esophagus. After tissue handling elements were appropriately positioned and locked into place, the invaginator was activated to allow the separation of the gastroesophageal junction from the diaphragm. The polypropylene “H” fasteners were delivered through the tissue. The same maneuvers were repeated at 3 additional positions to create full thickness, partial, gastroesophageal fundoplication. TIF patients were generally discharged 24 hours postprocedure and were asked to follow the standard dietary and physical restrictions previously described.15 Patients were evaluated and followed in community-based practices by clinical teams led by 4 surgeons and 3 gastroenterologists. TIF procedures were performed in the associated community hospitals.
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age: 18-80 years | Body mass index (BMI) >35 kg/m2 |
| Gastroesophageal reflux disease duration: >1 year | Hiatal hernia >2 cm in axial length and/or >2 cm in greatest transverse dimensions |
| History of daily proton pump inhibitors (PPIs) use >6 months | Esophagitis grade C or D; Barrett’s esophagus >2 cm; esophageal ulcer; fixed esophageal stricture or narrowing |
| Troublesome atypical symptoms and/or regurgitation (with or without heartburn) while on daily PPI therapy | Portal hypertension and/or varices |
| Abnormal 48-hour pH off PPIs (total % time pH < 4 > 5.3%) | Active gastroduodenal ulcer disease |
| Hill grade I or II | Gastroparesis, gastric outlet obstruction, or stenosis |
| Willingness to undergo pH testing | Coagulation disorder |
| Willingness to adhere to postoperative diet for 6 weeks | History of any of the following: resective gastric or esophageal surgery, antireflux surgery with anatomy unsuitable for transoral incisionless fundoplication (TIF) procedure per physician judgment, cervical spine fusion, Zenker’s diverticulum, esophageal epiphrenic diverticulum, achalasia, scleroderma ordermatomyositis, eosinophilic esophagitis, or cirrhosis |
| Availability for follow-up visits | Pregnancy or plans of pregnancy in the 12 months following treatment |
| Willingly and cognitively signed informed consent | Enrollment in another device or drug study that may confound the results |
Table 2.
Baseline Characteristics of Study Patients.a
| Characteristics | TIF Group (n = 39) | PPI Group (n = 21) | P Valuesb |
|---|---|---|---|
| Female, n (%) | 20 (51) | 13 (62) | .587 |
| Age in years, median (range)c | 54.8 (35.7-73.3) | 50.1 (32.5-63.3) | .206 |
| < 50, n (%) | 14 (36) | 10 (48) | .418 |
| 50-65, n (%) | 20 (51) | 11 (52) | >.999 |
| > 65, n (%) | 5 (13) | 0 (0) | .152 |
| Body mass index, kg/m2, median (range)c | 28.9 (20.5-34.9) | 28.3 (24.5-34.9) | .871 |
| GERD symptom duration in years, median (range)c | 10 (2-50) | 10 (1-20) | .586 |
| PPI therapy duration in years, median (range)c | 7 (1-25) | 8 (1-22) | .861 |
| Barrett’s esophagus, <2 cm, n (%) | 1 (3) | 0 (0) | >.999 |
| Esophagitis (Los Angeles grade), n (%) | 20 (51) | 13 (62) | .587 |
| A, n (%) | 1 (5) | 4 (31) | .066 |
| B, n (%) | 19 (95) | 9 (69) | .066 |
| Hill grade, n (%) | 37 (95) | 18 (86) | .332 |
| I, n (%) | 5 (14) | 2 (11) | >.999 |
| II, n (%) | 32 (86) | 16 (89) | >.999 |
| Hiatal hernia, n (%) | 36 (92) | 16 (76) | .114 |
| Axial length ≤1cm, n (%) | 14 (39) | 3 (19) | .208 |
| Axial length >1cm and ≤2 cm, n (%) | 22 (61) | 13 (81) | .208 |
| GERD Health-Related Quality of Life score, median (range) | |||
| On PPIsc | 27 (4-48) | 26 (16-39) | .896 |
| Off PPIsc | 34 (7-50) | 34 (21-49) | .536 |
| Heartburn score, median (range) | |||
| On PPIsc | 19 (4-30) | 17 (7-27) | .560 |
| Off PPIsc | 23.5 (4-30) | 24 (16-30) | .733 |
| Reflux Symptom Index score, median (range) | |||
| On PPIsc | 23 (3-43) | 23 (4-35) | .774 |
| Off PPIsc | 25 (2-42) | 27 (17-42) | .211 |
| Reflux Disease Questionnaire score, median (range) | |||
| On PPIsc | 3.2 (0-5) | 3.4 (0.3-4.0) | .721 |
| Off PPIsc | 3.9 (0.6-5.0) | 4.0 (2.3-5.0) | .191 |
| Total % time pH < 4, median (range)1 | 9.6 (5.4-19.5) | 9.3 (5.4-17.2) | .636 |
| Patients on single dose of PPI at entry, n (%) | 27 (69) | 16 (76) | .765 |
| Patients on Omeprazole at entry, n (%) | 16 (41) | 10 (48) | .785 |
| Patients on Esomeprazole at entry, n (%) | 9 (23) | 8 (38) | .243 |
| Patients on Lansoprazole at entry, n (%) | 5 (13) | 1 (5) | .412 |
| Patients on Pantoprazole at entry, n (%) | 6 (15) | 1 (5) | .404 |
| Patients on Dexlansoprazole at entry, n (%) | 3 (8) | 1 (5) | >.999 |
Abbreviations: TIF, transoral incisionless fundoplication; PPI, proton pump inhibitor; GERD, gastroesophageal reflux disease.
Hill grade and esophagitis were evaluated with screening endoscopy. Of 12 patients in the TIF group who were taking double-dose PPIs at entry, 6 (50%) patients were on Omeprazole, 3 (25%) on Pantoprazole, 2 (17%) on Lansoprazole, and 1 (8%) on Esomeprazole. In the PPI group, of 5 patients who were taking double-dose PPIs, 3 (60%) patients were on Esomeprazole and 2 (40%) were on Omeprazole.
P values were calculated using 2-tailed Fisher exact test unless indicated otherwise.
Mann–Whitney U test.
Preprocedure Evaluation
All patients enrolled in the study underwent thorough preprocedure evaluation, including complete history, physical examination, symptom assessment, esophagogastroduodenoscopy (EGD), and 48-hour pH monitoring. Gastroesophageal manometry was performed in selected patients to rule out diagnoses of achalasia or severe esophageal dysmotility disorders, whenever warranted by clinical suspicion. In some cases, a barium swallow was obtained to further evaluate esophageal anatomy and esophageal clearance and to rule out any suspected esophageal structural problems.
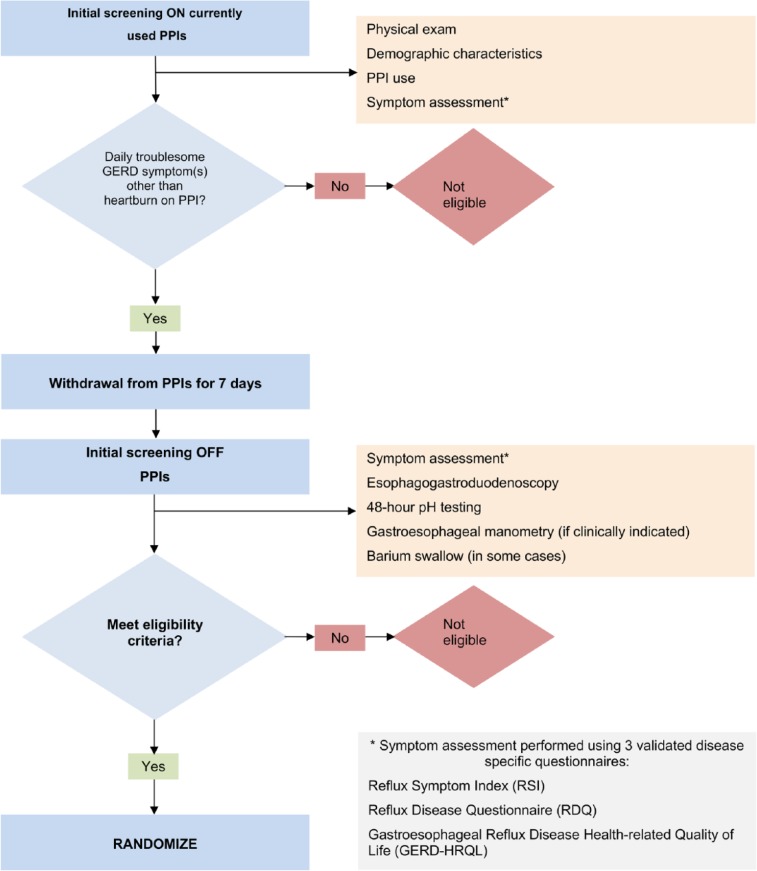
All patients underwent two screening visits (Figure 1). During the first screening visit, demographic characteristic of study patients, frequency, dose, and duration of GERD medication usage were collected while patients were on currently used PPIs. Symptom assessment was carried out using three validated GERD-specific instruments: (a) Gastroesophageal Health-Related Quality of Life (GERD-HRQL), (b) Reflux Symptom Index (RSI), and (c) Reflux Disease Questionnaire (RDQ). Patients who suffered from daily troublesome GERD symptoms other than heartburn, such as atypical GERD symptoms or regurgitation while taking daily PPIs were eligible to undergo further testing. These patients were asked to discontinue PPI therapy for 7 days and to return for the second screening visit.
Figure 1.
Screening assessments of study patients.
Symptom assessment was repeated off PPI therapy for 7 days during the second screening visit using the same questionnaires. All patients underwent EGD and 48-hour pH test off PPIs to objectively confirm the diagnosis of GERD. EGD was used to confirm the presence of esophagitis, assess the size of hiatal hernia, and evaluate the appearance of gastroesophageal junction using Hill grade classification. Biopsies were performed whenever indicated by the findings at endoscopy. A 48-hour pH test was performed after discontinuation of PPIs for 7 days. Per protocol, EAE was considered abnormal if % total time pH <4 occurred for >5.3% of the recording time.14
Follow-up Evaluation
Three follow-up visits were scheduled (2 weeks, 3 months, and 6 months) for all patients. Postprocedure symptoms, adverse events, and medication usage for all patients were recorded at all follow-up visits. Patients in the TIF group were required to completely discontinue PPI usage 14 days after the procedure and to follow the postprocedure diet for a total of 6 weeks.
At the 6-month follow-up visit, patients in the PPI (control) group completed the GERD-HRQL, RSI, and RDQ questionnaires and underwent EGD and 48-hour pH monitoring while on MSD PPIs. Patients in the TIF group completed the same 3 questionnaires and tests 6 months postprocedure while off PPIs. In addition, the minority of patients in the TIF group who had resumed taking PPIs 6 months postprocedure were asked to complete the same questionnaires while on PPI therapy.
Study End Points and Efficacy Assessments
The primary endpoint was elimination of daily troublesome GERD symptoms other than heartburn as evaluated by GERD-HRQL, RSI, and RDQ instruments at 6-month follow-up. GERD-HRQL is designed and validated to evaluate typical GERD symptoms by measuring 10 items (6 related to heartburn, 2 to dysphagia, 1 to bloating, and 1 to the impact of medications on daily life) on the visual analog scale ranging from 0 (no symptoms) to 5 (worst symptoms).17 A higher total GERD-HRQL score (range from 0 to 50) indicates more severe GERD.15 RSI is a 9-item validated questionnaire used to measure atypical GERD symptoms such as hoarseness, throat clearing, excess throat mucus, dysphagia, and cough.18 The scale for each individual item ranges from 0 (no problem) to 5 (severe problem), with a maximum total score of 45 and a normality threshold of ≤13.18 RDQ is a 12-item questionnaire that was designed to assess the frequency and severity of heartburn (4 items measuring the frequency and severity of pain and burning behind the breastbone), regurgitation (4 items measuring the frequency and severity of acid taste in the mouth and movement of the material upward from the stomach), and dyspeptic complaints (4 items measuring the frequency and severity of pain or burning in the upper stomach).19 Response options range from 0 (not present) to 5 (daily) for frequency and 0 (not present) to 5 (severe) for severity. Each patient’s score is calculated as the mean of item responses with higher scores indicating more severe or frequent symptoms.19 In the current study, elimination of daily troublesome symptoms was defined as a score ≤2 on the GERD-HRQL and RSI questionnaires; in the case of RDQ, elimination of moderate to severe regurgitation with the frequency reduced to 1 day a week or less (this corresponds to scores of ≤2 for frequency and severity of regurgitation). The RSI questionnaire was used to assess atypical GERD symptoms while RDQ was used to assess regurgitation. GERD-HRQL was used to assess heartburn, bloating, and satisfaction with current health condition.
Secondary efficacy endpoints were normalization of EAE, healing of esophagitis, and PPI use. Complete discontinuation of PPI therapy was considered clinically significant for TIF patients.
In addition to primary and secondary endpoints, patient satisfaction, incidence of de novo postfundoplication symptoms (bloating, excess flatulence, and dysphagia) and serious adverse events were documented.
Sample Size and Randomization
An unequal randomization allocation of 2:1 was chosen primarily to reduce the costs associated with this trial. A sample size of n = 42 (28 TIF, 14 PPI) was needed for an 80% power to detect a significant difference (α of .05) between the 2 groups. The power calculation was based on the assumption that >70% of the patients randomized to the TIF group would have their daily troublesome symptoms eliminated compared with ≤20% in the PPI group. To account for eventual attrition due to loss of follow-up and individual patient refusal to undergo EGD or 48-hour pH monitoring at follow-up, 12 more patients were allocated to TIF group and 9 more patients were allocated to PPI group (n = 63; 40 TIF, 23 PPI). Using a random number generator software, an independent statistician established the randomization sequence in blocks of 9 with stratification according to participating centers. Patient assignment was provided to participating centers via sealed opaque envelopes, and was concealed from clinical staff and patients during the 2 screening visits. The envelopes with randomization were opened after the second screening visit, in the presence of patients who had met all the eligibility criteria.
Statistical Analysis
Data were collected on a 37-page case report form and then transferred to a study specific and secured electronic database (FileMaker Pro 10, Santa Clara, CA). The Shapiro–Wilk test was used for normality testing. Continuous, normally distributed data were presented as the mean ± standard deviation (SD). Data with skewed distribution were reported as the median (range). Fisher exact test was used to determine the significance of 2 × 2 contingency tables. Unadjusted relative risk (RRs) and 95% confidence intervals (CIs) were calculated to estimate the relative effect of TIF as compared with that of PPI therapy for all outcomes. Two-tailed Mann–Whitney U test was used to assess the difference between nonparametric data. The P values for changes at follow-up compared with those at baseline within the same treatment group were calculated using the 2-tailed paired t test or the Wilcoxon signed rank test; in case of proportions, McNemar’s test was used. A P value of less than .05 was considered significant. Statistical analyses were performed using JMP 10.0 software.
Results
Baseline Characteristics of the Patients
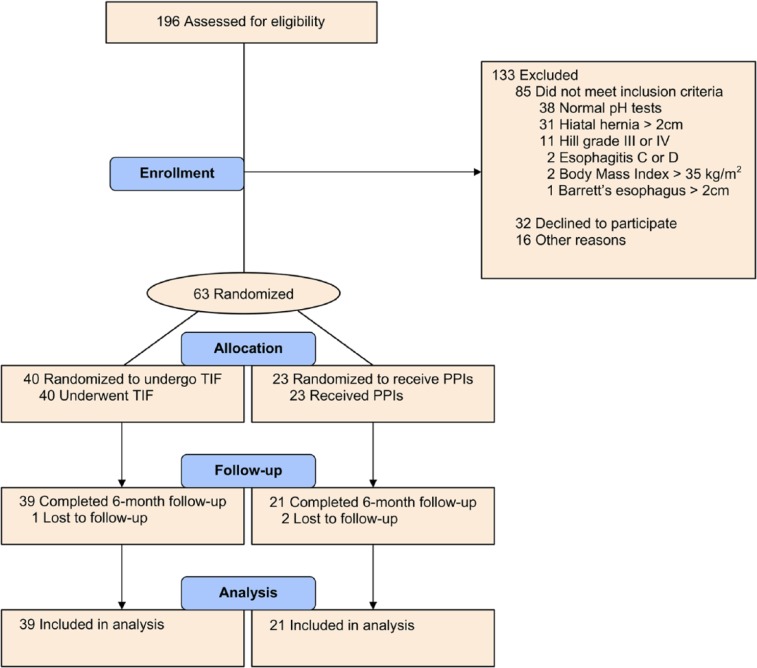
Between June and August 2012, 63 patients were randomized into the study; 40 patients into the TIF group and 23 patients into the PPI group. All randomized patients had abnormal distal esophageal acid exposure. The flowchart of screened, enrolled, and analyzed patients is shown in Figure 2. The treatment groups were well matched at entry (Table 2). There were no differences between treatment groups in the clinical features of the disease, such as duration of symptoms, duration of PPI use before procedure and/or severity of the disease based on symptom score and abnormal acid exposure as expressed in % time pH < 4. Overall, a slight majority of patients were female (33 of 63, 52%) and only 5 of 63 patients (8%) were older than 65 years. Medication dosage and PPIs used before randomization are shown in Table 2.
Figure 2.
CONSORT flowchart of study patients.
Abbreviations: PPIs, proton pump inhibitors; TIF, transoral incisionless fundoplication.
Safety and Procedure Outcomes
All TIF procedures were performed under general anesthesia and were completed successfully without conversion to open or laparoscopic approaches. In 37 of 39 (95%) cases no related issues with the EsophyX2 device were reported. In one case, the anterior wire used to transfer fasteners from the cartridge to a stylet was damaged, leading the physician to complete procedure with the posterior wire only. In another case, the gastric distention was suboptimal, making the procedure technically more challenging. The average time required to complete the procedure, measured from device introduction to removal, was 38 minutes (range = 20-68 minutes, SD = 14 minutes). On average, 21 (range = 16-30, SD = 4) contributing fasteners were used to create an esophagogastric fundoplication with a mean length of 2.8 cm (range = 2.5-4 cm, SD = 0.5 cm) and a circumference of 290° (range 240° to 340°, SD = 18°) as evaluated by immediate postprocedure endoscopy. All 31 patients who were assigned a preprocedure Hill grade II were converted to Hill grade I. Postoperative valve adherence to the endoscope was tight in 79% (31/39) and moderate in 21% (8/39) of patients. All 36 hiatal hernias present at screening were reduced. Ninety-eight percent of patients were discharged within 24 hours. Two patients (5%) stayed in the hospital for 2 days; one for the management of postoperative dizziness and nausea and one because of allergic reaction to pain medication. There were no reports of any serious adverse event or hospital readmission associated with the TIF procedure.
Primary Outcome
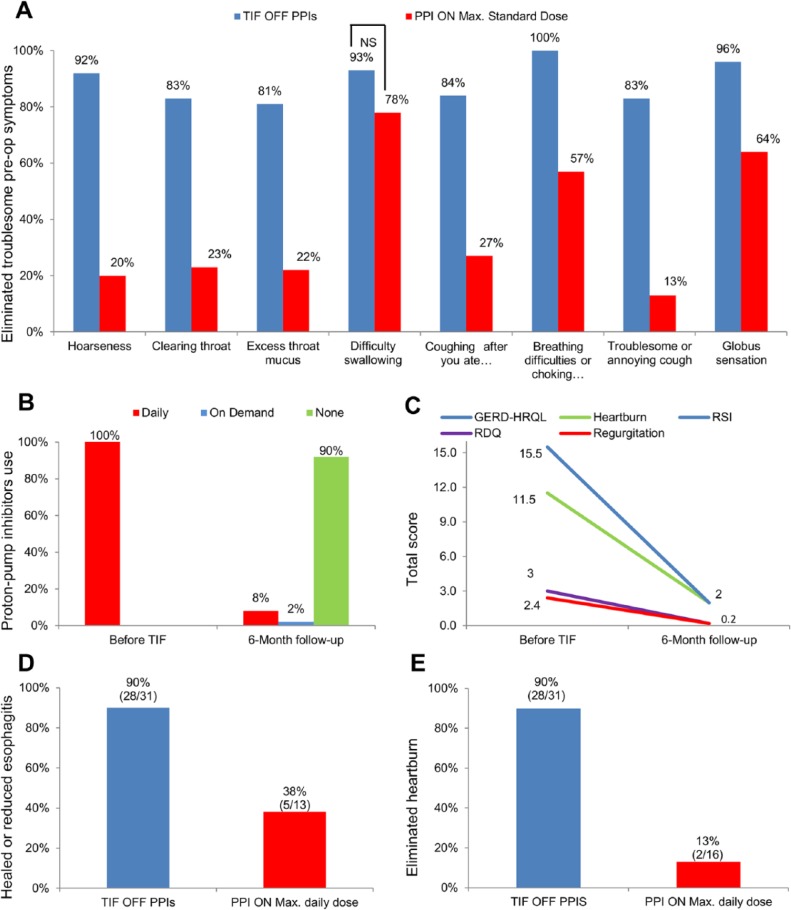
Troublesome regurgitation, as evaluated by RDQ questionnaire, was eliminated in 97% (29/30) of patients in the TIF group (off PPIs) versus 50% (9/18) of patients in the PPI group (on MSD), RR = 1.9, 95% CI = 1.2-3.1 (P < .001). Elimination of atypical GERD symptoms such as throat clearing, troublesome or annoying cough, and hoarseness is charted in Figure 3. Globally, at 6-month follow-up, complete elimination of all daily troublesome GERD symptoms other than heartburn was observed in 62% (24/39) of patients in the TIF group compared with 5% (1/21) in the PPI group, RR = 12.9, 95% CI = 1.9-88.9 (P < .001).
Figure 3.
(A) Elimination of daily troublesome atypical symptoms as evaluated by RSI questionnaires at 6-month follow-up. (B) PPI use before TIF and at 6-month follow-up. (C) Quality-of-life scores in patients back on some form of PPI regimen. (D) Rate of healing or reduction of reflux esophagitis in both treatment arms. (E) Elimination of daily troublesome heartburn as evaluated by GERD-HRQL questionnaires.
Abbreviations: GERD-HRQL, gastroesophageal reflux disease health-related quality of life; PPIs, proton pump inhibitors; RDQ, Reflux Disease Questionnaire; RSI, Reflux Symptom Index; TIF, transoral incisionless fundoplication.
Secondary Outcomes
Proton Pump Inhibitor Use
All patients in the TIF group were on daily PPI therapy before enrollment and randomization. At 6-month follow-up, 90% (35/39, 95% CI = 0.76-0.97) of patients in the TIF group had completely stopped taking PPIs; 3% (1/39, 95% CI= <.0001 to 0.14) of patients were taking PPIs on demand and 8 % (3/39, 95% CI = 0.02-0.21) were back on daily PPIs (Figure 3). Out of the 3 patients who were back on daily PPIs, 1 had reduced the original dose and 2 patients were on a higher dose. Of the 4 patients who were back on some form of PPI therapy, 1 remained dissatisfied with current health condition compared with 4 dissatisfied before TIF. Before enrollment in this study, of these 4 patients, 2 used PPIs for >20 years, one patient was on PPIs for 18 years and 1 was taking PPIs for 7 years. Two of these 4 patients reported PPI use at 3-month follow-up; another 2 patients started taking the PPIs after 3-month follow-up visit. Of these 4 patients, 1 had normalized distal esophageal acid exposure (% total time pH < 4 was reduced from 9.9 to 3.1), 1 had improved (from 8.3 to 6.7), and 2 experienced increase in % total time pH < 4 (from 16.8 to 23.2 and from 15.7 to 22.2). Quality of life scores in patients back on PPIs are shown in Figure 3.
Objective Outcomes
In the TIF group, 54% (21/39) of patients had normalized esophageal acid exposure (off PPIs) compared to 52% (11/21) of patients on maximum dose PPI in the control group, RR = 1.0, 95% CI = 0.6-1.7 (P = .914). A significant reduction of all 48-hour pH Bravo parameters was achieved in the TIF group; PPI therapy did not reach significant reduction in the duration of the longest reflux episode (Table 3). Of patients with abnormal acid exposure at 6 months postprocedure, 11% (2/18) in the TIF group were dissatisfied with their current health condition versus 100% dissatisfied (10/10) in the PPI group, RR = 0.13, 95% CI = 0.05-0.42 (P < .001). The reduction in the total GERD-HRQL, RSI, and RDQ scores in patients with abnormal esophageal acid exposure at 6-month follow-up is shown in Table 4.
Table 3.
Changes in Mean 48-Hour pH Parameters From Before Treatments to 6-Month Follow-Up in Both Treatment Groups.
| pH Parameters | TIF group (n = 39); Difference in Means (95% CI) | P Valuesa | PPI group (n = 21); Difference in Means (95% CI) | P Valuesa |
|---|---|---|---|---|
| Number of refluxes | −59.9 (−80.7 to −39.2 | <.001 | −99.1 (−133.7 to −64.5) | <.001 |
| Number of long refluxes (>5 minutes) | −4.1 (−6.1 to −2.0) | <.001 | −7.9 (−10.9 to −4.9 | <.001 |
| Duration of longest reflux, minutes | −7.9 (−15.6 to −0.3) | .042 | −2.7 (−13.3 to 7.9) | .598 |
| Fraction time pH < 4, % | −3.4 (−4.9 to −1.9) | <.001 | −5.5 (−7.4 to −3.6) | <.001 |
| DeMeester score | −11.6 (−17.4 to −5.9) | <.001 | −16.5 (−23.0 to −10.0) | <.001 |
Abbreviations: PPI, proton pump inhibitor; TIF, transoral incisionless fundoplication; CI, confidence interval.
P values were calculated with paired t test.
Table 4.
Changes in Mean Symptom Scores in Patients With Abnormal Distal Esophageal Acid Exposure From Before Treatments to 6-Month Follow-up in Both Treatment Groups.
| Questionnaires/Symptoms | TIF Group (n = 18); Difference in Means (95% CI) | P Valuesa | PPI Group (n = 10); Difference in Means (95% CI) | P Values |
|---|---|---|---|---|
| GERD-HRQL score | −17.9 (−25.8 to −10.1) | <.001 | −3.6 (−9.6 to 2.4) | .206 |
| Heartburn score | −12.2 (−18.1 to −6.3) | <.001 | −2.6 (−5.7 to 0.5) | .090 |
| Regurgitation score | −2.9 (−3.5 to −2.2) | <.001 | −0.7 (−1.4 to 0) | .042 |
| RDQ score | −2.7 (−3.3 to −2.0) | <.001 | −0.7 (−1.5 to 0.1) | .071 |
| RSI score | −15.3 (−20.3 to −10.3) | <.001 | −1.3 (−6.3 to 3.7) | .574 |
Abbreviations: GERD-HRQL, gastroesophageal reflux disease health-related quality of life; RDQ, Reflux Disease Questionnaire; RSI, Reflux Symptom Index; PPI, proton pump inhibitor; TIF, transoral incisionless fundoplication.
P values were calculated using paired t test.
Endoscopic Assessment
All patients underwent endoscopic evaluation at 6-month follow-up. Complete healing or reduction in reflux esophagitis at 6 months was achieved in 90% (18/20) of patients in the TIF group (off PPIs) compared with 38% (5/13) in PPI group (on MSD), RR = 2.3, 95% CI = 1.2-4.7 (P = .018; Figure 3). Hiatal hernias remained reduced in all patients (36/36) who had a hiatal hernia before the TIF procedure. The valve appearance was judged as a Hill grade I in all cases. One patient who had short segment Barrett’s (<2 cm) before the procedure was found to have healed esophageal erosions. This patient was off PPIs at 6-month follow-up and % total time pH < 4 was reduced from 9 before procedure to 1.5 at 6-month follow-up.
Ancillary Analyses
Heartburn, Patient Satisfaction, and Quality of Life
Ninety percent (28/31) of patients in the TIF group (off PPIs) reported elimination of daily troublesome heartburn versus 13% (2/16) of patients in the PPI group (maximum daily dose); RR = 7.2, 95% CI = 2.0-26.6 (P = .003; Figure 3).
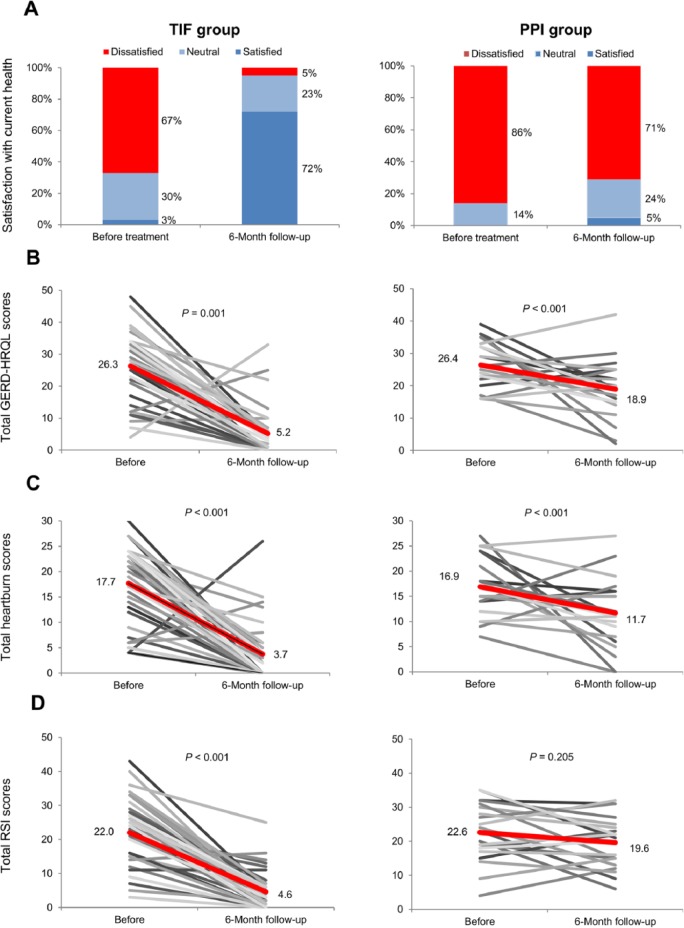
The median heartburn score in the TIF group, as evaluated by the GERD-HRQL questionnaire, improved significantly falling from 19 (range = 4-30) on PPIs before TIF to 2 (range = 0-26) off PPIs at 6-month follow-up (P < .001); in the PPI group the median heartburn score also improved, decreasing from 17 (range = 7-27) on screening to 11 (range = 0-27) on maximum PPI dose (P = .012). Figure 4 charts the progression of individual heartburn scores for all study patients from pretreatment values to 6-month follow-up. The median total GERD-HRQL score in the TIF group improved significantly from 27 (range = 4-48) on PPIs before TIF to 4 (range = 0-33) off PPIs at 6-month follow-up (P < .001); in the PPI group, the median total GERD-HRQL score also significantly improved from 26 (range = 16-39) to 19 (range = 2-42) on maximum PPI dose (P = .009).
Figure 4.
(A) Patient satisfaction with current health condition as evaluated by GERD-HRQL questionnaire. (B) Individual total GERD-HRQL scores in all patients before treatments and at 6-month follow-up. (C) Individual total heartburn scores in all patients before treatments and at 6-month follow-up. (D) Individual total RSI scores in all patients before treatments and at 6-month follow-up.
Abbreviations: GERD-HRQL, gastroesophageal reflux disease health-related quality of life; PPIs, proton pump inhibitors; RSI, reflux symptom index; TIF, transoral incisionless fundoplication. Red lines represent improvement in the mean scores.
Patient satisfaction with current health condition, as evaluated by GERD-HRQL, improved significantly in the TIF group compared with baseline on PPIs (72% [28/39] satisfied while off PPIs vs 3% [1/39] satisfied before TIF while on PPIs [P < .001]). In the PPI group, patient satisfaction did not improve significantly (5% [1/21] satisfied while on maximum PPI dose vs 0% [0/21] satisfied at screening [P > .999]). Between-group analysis revealed significantly more patients in the TIF group (72%, 28/39) were satisfied with their current health condition after treatment compared with PPI group (5%, 1/21), RR = 15.1, 95% CI = 2.2-103.1 (P = .006). Satisfaction with current health condition is charted in Figure 4.
The median total RSI score in the TIF group decreased significantly from 23 (range = 0-43) on PPIs before procedure to 3 (range = 0-25) off PPIs at 6-month follow-up (P < .001). A minor improvement in the median total RSI score in the control group on MSD PPI therapy did not reach statistical significance (from 23 [range = 4-35] at screening to 21 [range = 6-32] [P = .205]). Individual RSI scores are shown in Figure 4.
The median total RDQ score in the TIF group was significantly decreased from 3.2 (range = 0-5) before TIF on PPIs to 0.2 (range = 0-2.4) off PPIs at 6-month follow-up (P < .001); the median total score for the regurgitation component of the RDQ questionnaire also significantly improved from 3.2 (range = 0-5) to 0 (range = 0-1.3; P < .001). In the PPI group, the median total RDQ score significantly improved declining from 3.4 (range = 0.3-4.0) to 2.0 (range = 0.3-4.1; P < .001); however, the median total regurgitation score was insignificantly improved from 3.0 (range = 0.3-5.0) to 2.5 (range = 0.5-4.3) at 6-month follow-up on maximum daily PPI dose (P = .111).
Dysphagia, Bloating, and Flatulence
Twelve of 39 (31%) patients in the TIF group and 8 of 21 (38%) patients in the PPI group suffered from daily troublesome dysphagia at screening (score >2). The elimination of daily troublesome dysphagia was experienced in 92% (11/12) of patients in the TIF group compared to 75% (6/8) in the PPI group, RR = 1.2, 95% CI = 0.8-1.9 (P = .366). In the TIF group, 1 patient reported worsening dysphagia (score from 1 to 4); in the PPI group, 2 patients reported worsening (score from 1 to 3 and score from 2 to 3). There were no reports of de novo dysphagia in either group. Bloating was improved in 79% (19/24) of patients in the TIF group, compared with 25% (4/16) in the PPI group, RR = 3.2, 95% CI = 1.3-7.6 (P = .009). There were no reports of de novo bloating in either group. In the TIF group, of 5 patients who reported daily troublesome bloating at 6 month follow-up, 3 improved slightly (scores from 4 to 3) and 2 patients reported unchanged severity of bloating (scores 3 to 3 and scores 5 to 5); in the PPI group, of 12 patients who reported daily troublesome bloating at 6-month follow-up, 3 patients reported unchanged symptoms and 9 patients reported worsening of bloating. Seventeen of 21 patients (81%) in the TIF group and 2 of 12 (17%) in the PPI group reported elimination of daily troublesome flatulence at 6-month follow-up, RR = 4.9, 95% CI = 1.3-17.5; P = .016). There were no reports of de novo troublesome flatulence in either group.
Discussion
The 3 major findings of this multicenter randomized study comparing TIF to maximum dose PPIs in a select group of patients with chronic GERD and hiatal hernias measuring less than 2 cm were as follows: (a) TIF was more effective than PPIs in elimination of troublesome regurgitation, (b) a majority of TIF patients (90%) were completely off PPIs at 6-month follow-up, and (c) TIF was equivalent to PPIs in normalizing distal EAE. To the best of our knowledge, this is the first time that an endoscopic anti-reflux procedure involving reconstruction of the gastroesophageal junction, was found to be more effective than PPIs in controlling troublesome typical and atypical GERD symptoms.20-22
The study population in both group consisted of patients who were partial responders to PPI therapy, as indicated by lower QOL scores on PPIs compared with the QOL scores off PPIs at screening (Table 2). We chose to use MSD PPI as the control group because patients suffering ongoing symptoms despite medical therapy are routinely stepped up to maximum dosage in an attempt to control troublesome symptoms. Methodologically this design is justified by the fact that more than two thirds of patients were not on MSD at time of enrollment (Table 2). Patients with persistent symptoms despite MSD PPI therapy choosing to undergo traditional surgical therapy11 may face an additional risk to develop persistent postfundoplication symptoms (gas bloat, dysphagia, and inability to belch) suggesting that this population is left with limited treatment options. In contrast, as confirmed with present study, current published literature on TIF suggests that incidence of persistent postfundoplication side effects is low after TIF.12
The study was conducted by general surgeons (4 centers) and gastroenterologists (3 centers) acting as investigators and device operators. This mix was intentional to eliminate a specialty bias. The similarity of outcomes achieved by investigators across specialties is encouraging and suggests that the TIF procedure can be performed equally well by both foregut surgeons and gastroenterologists with advanced endoscopic skills.
The Montreal consensus defined regurgitation as the perception of flow of refluxed gastric content into the mouth or hypopharynx.1 Troublesome symptoms are defined as mild symptoms occurring 2 or more days a week, or moderate to severe symptoms occurring more than 1 day a week.1 Control of troublesome regurgitation is viewed in the current literature as the principal shortcoming of PPI therapy.2 Kahrilas et al4 concluded that PPIs caused a modest and considerably less symptomatic relief of regurgitation compared with heartburn. In our study, troublesome regurgitation was eliminated in 97% of patients in the TIF arm when compared with 50% in the PPI group when MSD were administered. This difference suggests that rebuilding the mechanical and geometrical characteristics of the gastroesophageal valve is important when attempting to control symptoms potentially arising from proximal extend of the refluxate. This study confirms the previously reported outcomes with regards to elimination of regurgitation15,23,24 and suggests that TIF could be an alternative treatment option for this subgroup of patients.
Review of the literature yields success rates for LNF in eliminating troublesome extraesophageal symptoms ranging between 48% and 65%.25-27 In this study, TIF was significantly more effective than MSD PPIs (Figure 3) in eliminating throat clearing (83% vs 23%), troublesome or annoying cough (83% vs 13%), and hoarseness (92% vs 20%). We believe that patients with extraesophageal symptoms achieved positive results because of the fact that they had objective evidence of GERD, and had relatively small anatomic defects. TIF could be considered a viable alternative for the patient population with a significant extraesophageal component provided that the same inclusion criteria are used in clinical practice.
Beyond the clinical relevance of the major findings of this study related to TIF, we found that MSD of PPIs were not better than TIF in eliminating heartburn, healing of reflux esophagitis, and normalizing distal EAE. We speculate that the unexpectedly low rate of healing of esophagitis in the PPI group (38%) may indicate that a higher proportion of patients in this study suffered from non-acid reflux or weakly acid reflux. The published data on the normalization of EAE after PPI treatment are limited.14 Milkes et al28 reported that 50% of patients had persistent abnormal EAE despite taking PPIs twice daily. Our study reproduces these results in the PPI group. Although we performed a medication count and it appears that patients complied with their prescribed medical regimens, we recognize that full control of patients’ compliance is difficult to achieve. Therefore, we cannot discount the possibility that some patients did not comply with prescribed medications influencing the rate of EAE normalization in the control group.
In the TIF group, significant symptoms control (up to 97%) was not followed by the similar rate of pH normalization (54%). Improvement in patient quality of life and healing of esophagitis are often cited as the goals of treatments for GERD, and accordingly are often the endpoints of many studies related to GERD therapies.15 The level of esophageal acid exposure in patients who were asymptomatic on PPI therapy is poorly addressed in the literature. Among 4 studies we found, 17% to 80% of patients demonstrated abnormal esophageal acid exposure while being asymptomatic on PPI therapy.29-32 As this matter relates to newer technologies for GERD, Bell et al15 did not find association between symptomatic outcomes and normalization of esophageal acid exposure after TIF, mirroring findings from another study.33 Future studies involving pH impedance may better define the relationship between symptomatic outcomes and normalization of esophageal acid exposure post TIF and further elucidate the effects of TIF and PPIs in patients with nonacid reflux.
Patients in the TIF group had a significant improvement in every 48-hour pH parameter, but did not reach the levels of pH normalization reported for laparoscopic fundoplication.34 It has been argued that the higher rates of pH normalization reported post–laparoscopic fundoplication result from the creation of a “supercompetent” gastroesophageal valve, which may also cause postfundoplication side effects such as dysphagia (11%), bloating (40%), diarrhea (16%), and flatulence (57%) in a significant number of patients at 5-year follow-up.11 Such occurrences result in patient dissatisfaction, additional health care and societal costs and the need for revisional procedures or endoscopic esophageal dilatations in cases of severe persistent dysphagia. In this study, none of the patients in the TIF group reported de novo bloating, dysphagia, or flatulence at 6-month follow-up. Although, the comparison of postfundoplication symptoms after the TIF procedure and after LNF is based on different time intervals, we believe that the very limited incidence of side effects combined with a solid safety record (no reported mortality in more than 13 000 cases performed worldwide) represents one of the most attractive aspects of TIF procedure.
The only patient with short segment Barrett’s in this trial was randomized to the TIF group and was completely off PPIs with a normalized distal esophageal exposure at 6-month follow-up. The role of TIF in the management of Barrett’s metaplasia remains to be defined and will no doubt require additional studies. This patient will be kept under strict endoscopic surveillance.
Our findings must be interpreted while considering the limitations of this study, most notably the short-term follow-up and the potential placebo effect in the TIF group. We felt that reporting these encouraging short-term results is reasonable given that the recommended treatment for patients with predominant extraesophageal symptomatology is aggressive acid reduction using PPIs twice daily short-term over a duration of 3 to 4 months.35 To address the potential placebo effect, another randomized trial which includes a sham arm is currently underway with early results expected in the near future. One may argue that the small sample size (39 in the TIF group vs 21 in the PPI group) and unequal randomization allocation could affect our findings. In this context, it is worth pointing that the use of unequal randomization ratios will only significantly reduce the power of the study if a ratio of 3:1 or more is used.36 Furthermore, the study has been appropriately powered (80%) to detect significant differences between the 2 treatment arms. A further potential limitation of this study was that the study population was heterogeneous with regards to predominant symptomatology; we feel that this is reflective of the fact that specialized GERD practices are increasingly evaluating and treating patients with mixed symptomatology. And finally, we used randomization and objective pH testing to minimize potential bias from an open-label study design. To further minimize this bias, all PPI patients were offered crossover to TIF after completion of 6-month follow-up. We will report these outcomes as results become available.
With regard to predictors of success or failures following TIF, a critical analysis of the 4 patients who continued acid suppressive medication after TIF revealed important findings. As previously suggested,15 our study also demonstrates that patients with more severe disease appear to have a higher likelihood of requiring PPIs after TIF. All 4 patients suffered from severe heartburn (GERD-HRQL scores >30 on PPIs). Although 10% of patients were back on PPI therapy, the significant improvement in the QOL scores postprocedure (Figure 3) suggests that TIF may be a useful therapeutic adjunct to PPIs in patients with incomplete symptom control. We attempted to define factors predictive of post-treatment normalization of esophageal acid exposure. The fact that all patients in the study underwent pH testing at screening and at 6-month follow-up presented a unique opportunity to define these factors. We observed that preprocedure % total time pH < 4 inferior to 10 was associated with a high rate of pH normalization after TIF (74% [17/23] normalized vs 25% [4/12] normalized if % time pH < 4 was ≥10; [P = .004]). Interestingly, the same observation could not be made in the PPI group (66% normalized [8/12] if % time pH < 4 was inferior to 10 before vs 33% [3/9] if % time was ≥10 after taking maximal PPI dose for 6 months [P = .198]). Beyond the previously defined factors associated with successful outcomes such as hiatal hernia ≤2 cm and GERD-HRQL <30, this study suggests that preprocedure total % time pH < 4 inferior to 10 could be an important factor in selecting the most appropriate patients and setting appropriate expectations for the TIF procedure in individual cases. We plan to report a comprehensive evaluation of factors associated with successful outcomes after TIF at a longer term follow-up. Also, in this study, we reported our results in terms of complete elimination of troublesome symptoms and pH normalization over 48 hours rather than ≥50% reduction in total scores and % total time pH < 4 based on 24 hours. We believe that these stricter and more comprehensive evaluation criteria may provide a useful reference in assessing patients for less invasive GERD therapies.
Based on the results of this study, it would appear that the TIF procedure is ideally suited as a treatment alternative for GERD patients who fall in the so-called “therapy gap,” a term often used to describe the 30% to 40% of patients who take daily PPIs and who remain unsatisfied because of incomplete symptom control.3 These patients are often unwilling to undergo a laparoscopic fundoplication for fear of its side effects. TIF may offer these patients the opportunity to safely eliminate their troublesome typical and atypical symptoms without the risk of developing postfundoplication syndromes.
Conclusion
In conclusion, among select patients presenting with persistent troublesome GERD symptoms and small hiatal hernias (≤2 cm), TIF, compared to maximal standard dose PPI therapy, resulted in better control of regurgitation and a wide range of chronic GERD symptoms while avoiding the undesirable postfundoplication syndromes associated with laparoscopic antireflux procedure in some patients. Patients in the TIF and PPI arms were found to have similar rates of distal esophageal pH normalization. Despite encouraging results from this study, longer term follow-ups are warranted.
Acknowledgments
The authors thank Emir Deljkich, MD, for his assistance with this research.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Trad and Dr Simoni have reported that they have a consulting agreement with EndoGastric Solutions. Dr Trad, Dr Barnes, Dr Simoni, Dr Shughoury, Dr Mavrelis, Dr Raza, Dr Heise, Dr Turgeon, and Dr Fox reported that their institutions have received grant for research from EndoGastric Solutions for pre- and postoperative testing, TIF procedure, medications, and support for study coordinator.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded in total by EndoGastric Solutions, Inc, Redmond, Washington.
References
- 1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. [DOI] [PubMed] [Google Scholar]
- 2. El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720-737. [DOI] [PubMed] [Google Scholar]
- 3. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. [DOI] [PubMed] [Google Scholar]
- 4. Kahrilas PJ, Howden CW, Hughes N. Response of regurgitaion to proton pump inhibitir therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1419-1425. [DOI] [PubMed] [Google Scholar]
- 5. Chan WW, Chiou E, Obstein KL, Tignor AS, Whitlock TL. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med. 2011;171:620-629. [DOI] [PubMed] [Google Scholar]
- 6. Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2011;19:CD004823. [DOI] [PubMed] [Google Scholar]
- 7. Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:2646-2654. [DOI] [PubMed] [Google Scholar]
- 8. Noordzij JP, Khidr A, Evans BA, et al. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope. 2001;111:2147-2151. [DOI] [PubMed] [Google Scholar]
- 9. Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131:342-350. [DOI] [PubMed] [Google Scholar]
- 10. Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116:254-260. [DOI] [PubMed] [Google Scholar]
- 11. Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969-1977. [DOI] [PubMed] [Google Scholar]
- 12. Wendling MR, Melvin WS, Perry KA. Impact of transoral incisionless fundoplication (TIF) on subjective and objective GERD indices: a systematic review of the published literature. Surg Endosc. 2013;27:3754-3761. [DOI] [PubMed] [Google Scholar]
- 13. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Endoluminal treatments for gastroesophageal reflux disease (GERD). http://www.sages.org/publication/id/CSR1/ Accessed December 8, 2013.
- 14. Hirano I, Richter JE, Practice Committee of the American College of Gastroenterology. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol. 2007;102:668-685. [DOI] [PubMed] [Google Scholar]
- 15. Bell RC, Mavrelis PG, Barnes WE, et al. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215:794-809. [DOI] [PubMed] [Google Scholar]
- 16. Bell RC, Cadiere GB. Transoral rotational esophago-gastric fundoplication: technical, anatomical, and safety consideration. Surg Endosc. 2011;25:2387-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Velanovich V, Vallance SR, Gusz JR, Tapia FV, Harkabus MA. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg. 1996;183:217-224. [PubMed] [Google Scholar]
- 18. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274-279. [DOI] [PubMed] [Google Scholar]
- 19. Shaw M, Talley NJ, Beebe T, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:52-57. [DOI] [PubMed] [Google Scholar]
- 20. Coron E, Sebille V, Cadiout G, et al. Clinical trial: radiofrequency energy delivery in proton pump inhibitor-dependent gastro-oesophageal reflux disease patients. Aliment Pharmacol Ther. 2008;28:1147-1158. [DOI] [PubMed] [Google Scholar]
- 21. Comay D, Adam V, da Silveira EB, Kennedy W, Mayrand S, Barkun AN. The Stretta procedure versus proton pump inhibitors and laparoscopic Nissen fundoplication in the management of gastroesophageal reflux disease: a cost-effectiveness analysis. Can J Gastroenterol. 2008;22:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parker M, Smith CD. Comparing the effectiveness of endoscopic full-thickness plication and endoscopic radiofrequency treatments for patients with GERD. Expert Rev Gastroenterol Hepatol. 2010;4:387-390. [DOI] [PubMed] [Google Scholar]
- 23. Trad KS, Turgeon DG, Deljkich E. Long-term outcomes after transoral incisionless fundoplication in patients with GERD and LPR symptoms. Surg Endosc. 2012;26:650-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bell RC, Freeman KD. Clinical and pH-metric outcomes of transoral esophago-gastric fundoplication for the treatment of gastroesophageal reflux disease. Surg Endosc. 2011;25:1975-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oelschlager BK, Eubanks TR, Oleynikov D, Pope C, Pellegrini CA. Symptomatic and physiologic outcomes after operative treatment for extraesophageal reflux. Surg Endosc. 2002;16:1032-1036. [DOI] [PubMed] [Google Scholar]
- 26. Duffy JP, Maggard M, Hiyama DT, et al. Laparoscopic Nissen fundoplication improves quality of life in patients with atypical symptoms of gastroesophageal reflux. Am Surg. 2003;69:833-838. [PubMed] [Google Scholar]
- 27. Wright RC, Rhodes KP. Improvement of laryngopharyngeal reflux symptoms after laparoscopic Hill repair. Am J Surg. 2003;185:455-461. [DOI] [PubMed] [Google Scholar]
- 28. Milkes D, Gerson LB, Triadafilopoulus G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal and intragastric pH in patients with gastroesophageal reflux disease (GERD). Am J Gastroenterol. 2004;99:991-996. [DOI] [PubMed] [Google Scholar]
- 29. Sarela AI, Hick DG, Verbeke CS, Casey JF, Guillou PJ, Clark GW. Persistent acid and bile reflux in asymptomatic patients with Barrett esophagus receiving proton pump inhibitor therapy. Arch Surg. 2004;139:547-551. [DOI] [PubMed] [Google Scholar]
- 30. Oritz A, Martínez de Haro LF, Parrilla P, Molina J, Bermejo J, Munitiz V. 24-hour pH monitoring is necessary to assess acid reflux suppression in patients with Barrett’s oesophagus undergoing treatment with proton pump inhibitors. Br J Surg. 1999;86:1472-1474. [DOI] [PubMed] [Google Scholar]
- 31. Ouatu-Lascar R, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol. 1998;93:711-716. [DOI] [PubMed] [Google Scholar]
- 32. Katzka DA, Castell DO. Successful elimination of reflux symptoms does not insure adequate control of acid reflux in patients with Barrett’s oesophagus. Am J Gastroenterol. 1994;89:989-991. [PubMed] [Google Scholar]
- 33. Velanovich V, Karmy-Jones R. Measuring gastroesophageal reflux disease: relationship between the health-related quality of life and physiologic parameters. Am Surg. 1998;64:649-653. [PubMed] [Google Scholar]
- 34. Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647-2669. [DOI] [PubMed] [Google Scholar]
- 35. Richter JE. Review article: extraesophageal manifestations of gastroesophageal reflux disease. Aliment Pharmacol Ther. 2005;22(suppl 1):70-80. [DOI] [PubMed] [Google Scholar]
- 36. Dumville JC, Hahn S, Miles JN, Torgerson DJ. The use of unequal randomization ratios in clinical trials: a review. Contemp Clin Trials. 2006;27:1-12. [DOI] [PubMed] [Google Scholar]