Abstract
In POINT X, a study designed to reflect clinical practice and patient treatment choices, 254 European patients received open-label collagenase for Dupuytren’s contracture. The most severely affected joint was treated first in 74% of patients. In total, 52%, 41%, 7%, and 1% of patients selected the little, ring, middle, and index finger, respectively; 79% had one or two joints treated. Only 9% of patients (n = 24) received 4 or 5 injections. The mean improvement in total passive extension deficit (TPED) was 34° on day 1, improving further by day 7 to 42°. This secondary improvement was maintained by day 90 and month 6. The mean number of injections/joint was 1.2 for the metacarpophalangeal joint and 1.25 for the proximal interphalangeal joint. Median time to recovery was 4 days; the mean improvement in hand function was clinically relevant as measured by the Unité Rhumatologique des Affections de la Main (URAM) score. In total, 87% and 86% of patients and physicians, respectively, were very satisfied or satisfied with treatment at month 6, although correlation between TPED and patient satisfaction was weak (Spearman −0.18, 95% CI −0.32 to −0.06). Collagenase was well tolerated, with 10 (3.9%) patients experiencing severe adverse events. As a real-world study, the POINT X findings can be generalized to the at-large population.
Keywords: Collagenase, Dupuytren’s disease, efficacy, tolerability, open label, patient-reported outcomes
Introduction
Surgery has been the mainstay of treatment for Dupuytren’s contracture (DC). In recent years, there has been an increased interest in less invasive approaches, including splinting, percutaneous needle fasciotomy (PNF), and collagenase injection (Larocerie-Salgado and Davidson, 2012; Rahr et al., 2011; van Rijssen et al., 2012). There may even be a role for splinting. Collagenase Clostridium histolyticum (CCH) is the first nonsurgical, pharmacological treatment for DC with a palpable cord approved for use in the United States (2010) and Europe (2011). Clinical trials (Badalamente and Hurst, 2007; Gilpin et al., 2010; Hurst et al., 2009; Witthaut et al., 2013) and post-marketing studies (Peimer et al., 2012; Pess et al., 2012) have demonstrated the efficacy and favourable safety profiles of CCH for treating DC.
By design, clinical trials are highly controlled with structured treatment regimens and rigorous, prespecified endpoints. In earlier trials (CORD I [Hurst et al., 2009] and CORD II [Gilpin et al., 2010]) and open-label JOINT I and II studies (Witthaut et al., 2013), joint selection was based on and ranked by contracture severity. The stringent primary endpoint was “clinical success,” defined as a reduction in fixed-flexion contracture (FFC) to ≤ 5° of normal extension, and assessments were done in the clinic on days 1, 7, and 30 after each injection. The POINT X (Prospective, Open-label Investigation of the Nonsurgical Treatment with collagenase Clostridium histolyticum [Xiapex®]) study was uniquely designed to be more reflective of the choices in clinical practice and to emphasize patient-reported outcome measures.
Methods
Study design and patient population
POINT X was an open-label phase IIIb study conducted at 28 European sites in eight countries. Patients (aged 18–70) were eligible for enrolment if they had a palpable cord with contracture of an MP joint and/or PIP joint ≥ 20° (other than the thumb). Key exclusion criteria included treatment for DC on the selected joint within 90 days of enrolment, taking an anticoagulant within 7 days of the first injection, and having another chronic disorder affecting the hands. The study was approved by the institutional review board or independent ethics committee at each participating site. All patients continued taking their normal medications, and all provided written informed consent.
At screening, patients ranked the joints they wanted to be treated and were asked to provide a reason for this choice. Each treatment cycle consisted of a CCH injection, finger extension procedure, and 30 day follow-up period. At each decision point patients, in consultation with the investigator, had three options: receive another injection for the same joint (≤ 3 injections/joint) if the correction was not satisfactory after the previous injection, start another treatment cycle (≤ 5 cycles) by switching to the next joint in rank order, or receive no further injections.
Investigators were permitted to use local anaesthesia during the finger extension procedure the day after injection. Follow-up visits were scheduled for 1, 7, and 30 days after injection for each cycle; final follow-ups occurred 90 days and 6 months after the last treatment cycle.
Assessments
Efficacy
Finger goniometry was used to measure passive extension deficit (PED), total passive extension deficit (TPED), and range of motion (ROM). To improve inter-rater reliability the same examiner, when practical, measured the same patients for the study duration. The examiner was either the treating surgeon or appropriately trained personnel.
Patients and physicians rated disease severity on a 4-point scale (normal [no contracture], mild, moderate, severe) and satisfaction with treatment on a 5-point scale (very satisfied, satisfied, neither satisfied nor dissatisfied, dissatisfied, very dissatisfied). Disease severity was assessed at baseline, on day 30 of each treatment cycle, and day 90 and month 6 after the last injection. Satisfaction with treatment was assessed at all of these time points except baseline.
Recovery of hand function and resumption of normal activities
Recovery of hand function was assessed using the validated, 9-item Unité Rhumatologique des Affections de la Main (URAM) scale (Beaudreuil et al., 2011) on injection day (before injection) and on day 30 of each treatment cycle, day 90, and month 6.
To assess the time to recovery and resumption of normal daily activities, patients completed a daily diary for the first 2 weeks of each treatment cycle and a weekly diary from day 14 after each injection through day 90 after the last injection. Patients rated how treatment affected their ability to do regular activities on a visual analogue scale (VAS), ranging from 0 (no effect on activity) to 10 (completely prevented activity). Work- and hobby-related questions were binary (yes/no responses). In the weekly diary, patients who answered affirmatively to the binary questions were asked to provide the number of days/hours affected. Time to recovery was defined as the number of days between the initial injection date and date on which the patient provided a VAS response ≤ 3 in the diary.
Healthcare resources
The Healthcare Resource Utilization (HRU) questionnaire, developed specifically for the POINT X study, contains seven items related to outpatient visits and/or procedures, physical and/or occupational therapy, emergency department visits, acute hospital admission and length of stay, diagnostic tests, rehabilitative care, and use of assistive devices.
Safety and tolerability
All observed and reported adverse events (AEs) were recorded and adjudicated. Blood samples were drawn at screening and month 6 for immunogenicity. Pain management was evaluated by recording the type and amount of analgesic medications used after each injection and follow-up visit.
Statistical analysis
Efficacy was analysed for all patients who received one or more CCH injections and had one or more post-injection efficacy assessments; safety data were analysed for all patients who received one or more CCH injections. Continuous variables were summarized as means with standard deviations (SDs) or medians with inter-quartile ranges (IQRs), depending on the data distribution. Categorical variables were summarized as frequency counts and percentages.
Potential relationships between objective (i.e., TPED, ROM) and patient- and physician-rated satisfaction with treatment were assessed by calculating Spearman’s correlations and 95% confidence intervals (CIs). Time to recovery was evaluated using a Kaplan–Meier analysis, which included the number of patients who recovered, number of patients who did not recover at the end of the daily diary collection (i.e., were censored), and median time to recovery with 95% CI.
Inferential statistics were conducted post-hoc on TPED and URAM results using a one-sample t-test. Likewise, URAM total score at baseline was compared with that at month 6. All analyses were conducted using SAS® software version 9.2 or higher (SAS Institute, Cary, North Carolina, USA).
Results
Patient disposition
The first subject’s first visit was December 22, 2010, and the last subject’s last visit was October 31, 2012. A total of 249 patients (98%) completed the study (Figure 1, Table 1). No patient discontinued because of a safety or tolerability concern. In total, 52% and 41% of patients selected the little and ring finger, respectively, as their first choice for treatment. Far fewer patients selected the middle finger (7%) or index finger (0.8%) for the first injection. Eighty-three percent of patients cited impaired function as the primary reason for their choice; having it as the only contracted joint was the second most common reason (35%). Other reasons included appearance (29%), following their doctor’s advice (28%), and recurrence (11%). Virtually all (98%) of the physicians’ rankings paralleled the patients’ responses.
Figure 1.
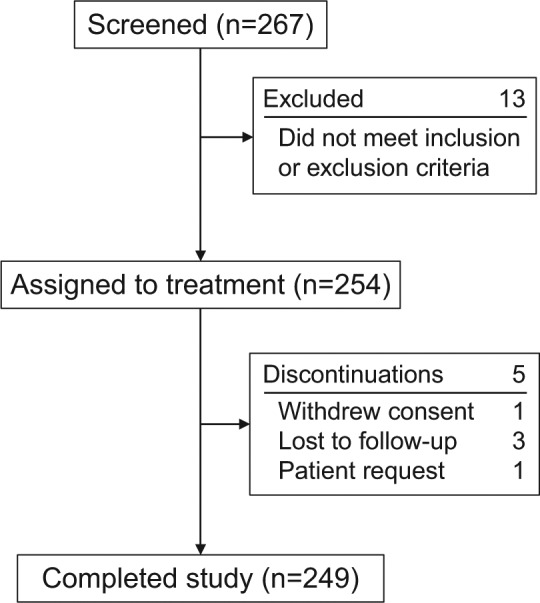
Patient flow.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristic | Patients (N = 254) |
|---|---|
| Mean (SD) age, y | 60 (7) |
| Male gender, n (%) | 223 (88) |
| Affected hands, n (%) | |
| Both | 115 (45) |
| Left | 62 (24) |
| Right | 77 (30) |
| Affected fingers, n (%) | |
| 1 | 90 (35) |
| 2 | 73 (29) |
| 3 | 38 (15) |
| 4 | 27 (11) |
| 5 | 14 (6) |
| ≥ 6 | 12 (5) |
| Patients by finger affected, n (%) | |
| Little | 191 (75) |
| Ring | 175 (69) |
| Middle | 75 (30) |
| Index | 23 (9) |
| Thumb | 6 (2) |
| Affected joint, n (%) | |
| MP | 392 (58) |
| PIP | 271 (40) |
| DIP | 16 (2) |
DIP: distal interphalangeal; MP: metacarpophalangeal; PIP: proximal interphalangeal.
In 74% of patients, the first joint treated was the most severely contracted. In the little and ring fingers, more patients received CCH for MP (39% and 34%, respectively) than for PIP joints (14% and 6%, respectively). Irrespective of the finger treated, 79% of patients had an MP joint treated first; 21% had a PIP joint treated first. In the subgroup of patients that received only 1 injection (n = 142), 44% had the MP joint of the little finger treated, and 32% had the MP joint of the ring finger treated. In the subgroup of patients that received multiple injections (n = 112), 79% had one or two joints treated even though they could receive up to 3 injections per joint. Only 9% of patients (n = 24) received 4 or 5 injections. No distinct patterns emerged with regard to the treatment of joints relative to the number of joints affected by DC.
Overall, 375 joints received one CCH injection; 66 and 15 joints received 2 and 3 injections, respectively. For cycle 1, the finger extension procedure was performed on all but one patient (whose cord ruptured spontaneously), and local anaesthesia was used during the manipulation on all but 11 (4%) patients. For cycles 2 through 5, the procedure was performed on all patients treated during those cycles, and local anaesthesia was used during the procedure on all but six patients (3%). Across all treatment cycles, 45% of patients took pain medication for a median duration of 2.0 (IQR 1.0−6.5) days.
Efficacy
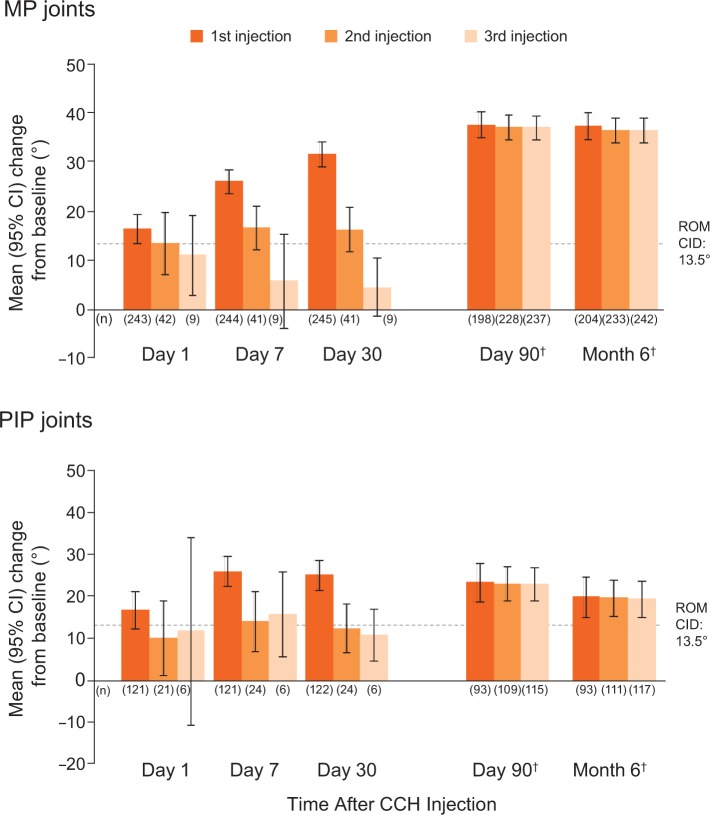
MP joints showed the largest reduction in PED 30 days after the first injection (Figure 2, top). The contracture improved even further at day 90, and this improvement was maintained through month 6. Across treatment cycles, changes in ROM increased from day 1 to day 30 for MP joints (Figure 2, top). The contracture was even more improved at day 90, and this improvement was maintained through month 6. For PIP joints, changes in ROM were largest after the first injection, and these improvements were maintained through day 90 and month 6 (Figure 2, bottom).
Figure 2.
Change in ROM by joint type and time after CCH injection.*
*Across all five treatment cycles.
†Legend definitions change for these time points: 1st injection = joints that received only 1 injection; 2nd injection = joints that received 1 or 2 injections; 3rd injection = joints that received 1, 2, or 3 injections.
*Across all five treatment groups.
CI: confidence interval; CCH: collagenase Clostridium histolyticum; CID: clinically important difference; MP: metacarpophalangeal; PIP: proximal interphalangeal; ROM: range of motion.
At baseline, 83% of patients and 80% of physicians rated the severity of DC as moderate or severe. These values were reduced to 21% and 25% at day 90, and 30% and 27% at month 6, respectively. Thus, by study end more than 70% of patients and physicians rated disease severity as normal/mild. Likewise, 89% of patients and physicians were very satisfied or satisfied with treatment at day 90; these values were 87% and 86%, respectively, at month 6. For changes in TPED, the Spearman correlation with patient satisfaction was weak: −0.184 (95% CI −0.302, −0.061); with physician satisfaction, the correlation was also weak: −0.206 (95% CI −0.322, −0.358). For changes in ROM, the correlation with patient satisfaction was moderate: 0.335 (95% CI 0.218, 0.442); with physician satisfaction, it was also moderate: 0.371 (95% CI 0.257, 0.474).
Recovery of hand function and resumption of normal activities
Baseline URAM total scores ranged from 9.3–15.2 for cycles 1–5 (Figure 3). After cycle 1, the mean reduction in score (i.e., improvement in hand function) was −9.5 (SD 7.0; min, max: −25, −5); smaller changes were observed for the other treatment cycles; however, the largest changes were observed at day 90 and month 6 (Figure 3). Among all evaluable patients (n = 83), the mean change from baseline to month 6 was statistically significant (−11.0, 95% CI −12.9, −9.1; p < 0.0001). In general, mean changes in total URAM score exceeded the clinically important change of 2.9 points (Beaudreuil et al., 2011) at all time points.
Figure 3.
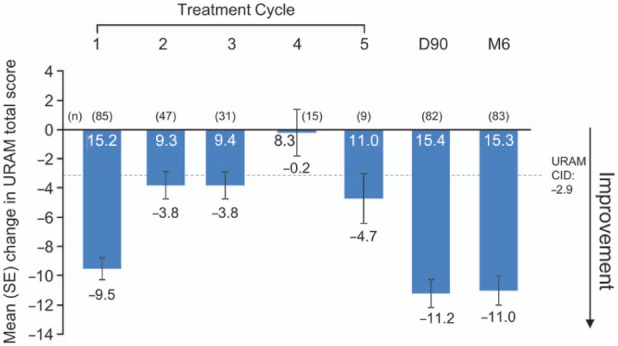
Changes in URAM total score by treatment cycle.*
*30 days after the first injection in each treatment cycle (with ≤ 4 responses, total score was recorded as missing; with ≥ 5 responses, total score was calculated using mean score for answered items as an imputed score for missing items).
Note: values inside the bars are baseline scores (pre-injection) for that cycle.
CID: clinically important difference; SE: standard error; URAM: Unité Rhumatologique des Affections de la Main.
The median time to recovery after cycle 1 was 3 days (95% CI 3, 4); 14% of patients did not achieve full recovery during the first 14 days (Figure 4). No patient had to miss work, < 1% had to reduce their work hours, and only 3% had to modify their usual job duties.
Figure 4.
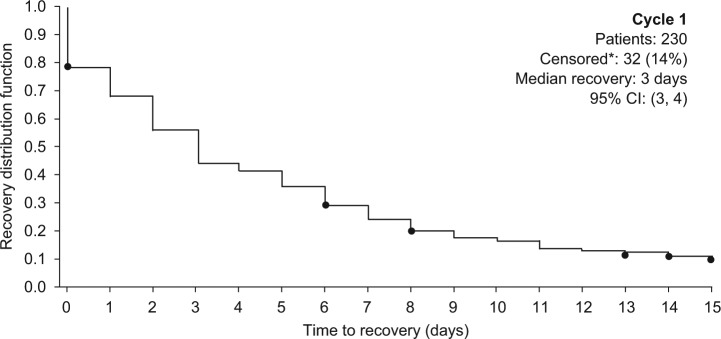
Kaplan–Meier analysis of time to recovery after the first injection in cycle 1.
*For patients who did not provide a response ≤ 3, the censored time to recovery was defined as the number of days between the first injection and last day within the cycle on which the patient responded to the question.
CI: confidence interval.
Healthcare resources
By day 30 of cycle 1, < 5.6% of patients had used any of the healthcare resource services as assessed using the HRU questionnaire. Few patients used physical or hand or occupational therapy after CCH for the duration of the study. By month 6, < 5% of patients had undergone an outpatient or day-case procedure related to their DC.
Safety and tolerability
Eighty-eight percent of patients experienced one or more treatment-emergent AEs; in 93% of these patients, the events were considered to be treatment related (Table 2). Nearly all treatment-emergent AEs were mild to moderate in severity. Ten patients experienced serious AEs; in two cases, the event was considered to be treatment related (increased transaminases, pain in extremity). At screening, only small percentages of patients tested positive for anti-Clostridial type I (2%) or type II (1%) collagenase. After 6 months, 92% and 90% of patients were positive for type I and II collagenases. None tested positive for cross-reactivity to endogenous MMPs.
Table 2.
Treatment-emergent adverse events* by severity occurring in ≥5% of patients† (N=254) Severity.
| Adverse event, n (%) | All | Mild | Moderate | Severe |
|---|---|---|---|---|
| Oedema peripheral | 111 (44) | 75 (30) | 34 (13) | 2 (0.8) |
| Pain in extremity | 65 (26) | 38 (15) | 25 (10) | 2 (0.8) |
| Injection site pain | 53 (21) | 43 (17) | 9 (4) | 1 (0.4) |
| Haematoma | 49 (19) | 45 (18) | 4 (2) | 0 |
| Injection site haematoma | 39 (15) | 34 (13) | 5 (2) | 0 |
| Skin laceration | 37 (15) | 24 (9) | 11 (4) | 2 (0.8) |
| Contusion | 34 (13) | 27 (11) | 7 (3) | 0 |
| Procedural pain | 31 (12) | 27 (11) | 4 (2) | 0 |
| Injection site swelling | 23 (9) | 15 (6) | 8 (3) | 0 |
| Injection site oedema | 20 (8) | 17 (7) | 3 (1) | 0 |
| Ecchymosis | 17 (7) | 14 (6) | 2 (0.8) | 1 (0.4) |
| Lymphadenopathy | 17 (7) | 16 (6) | 1 (0.4) | 0 |
| Surgical skin tear | 18 (7) | 16 (6) | 2 (0.8) | 0 |
| Arthralgia | 16 (6) | 11 (4) | 4 (2) | 1 (0.4) |
| Tenderness | 15 (6) | 14 (6) | 1 (0.4) | 0 |
Discussion
The POINT X study can be distinguished from the phase III clinical trials of CCH in several important ways. First, only five patients (2%) withdrew from POINT X (four during cycle 1, one during cycle 5). None discontinued because of the safety or tolerability of CCH. Ninety-eight percent of patients provided one or more post-baseline assessments, which is a remarkably high retention rate for a clinical study. Unlike the CORD and JOINT studies, which had strict objective criteria for the selection of joints to be treated, POINT X patients played a crucial role in this process. That is, in consultation with the investigator, patients helped decide the order of joints to be treated, and these decisions were made based on personal preference rather than externally imposed criteria. In POINT X, one of the key, prespecified efficacy measures was the patient diary, completed on a daily basis for 14 days after each injection. Thus, for the first time in the clinical trial program for CCH, treatment-related changes were assessed for post-injection days 1 through 7. In the phase III studies, the earliest evaluation of efficacy was day 7 after CCH injection. Indeed, in POINT X early treatment effects were observed; the median time to recovery of normal activities was ~3 days.
Further, although not permitted in previous studies, local anaesthesia was used by nearly all POINT X investigators to mitigate discomfort during the finger extension procedure. In contrast to the phase III studies, in which the primary endpoint was a reduction in PED to ≤ 5° of normal extension, POINT X included a broader spectrum of objective and subjective patient-reported outcome measures.
After treatment with CCH, the magnitude of the changes in PED and ROM in POINT X were largely consistent with those previously reported for the phase III clinical trials, and for ROM, changes beyond the predetermined clinically important difference of 13.5° (Witthaut et al., 2011) were observed as early as day 1 after injection (mean 16.4°). The median (IQR) number of CCH injections required per patient was 1 (1, ≤4), and most POINT X patients required only 1 CCH injection to achieve an acceptable outcome in a prioritized joint. These data are not available from the phase III studies, as clinical success was measured after up to 3 injections. Interestingly, the use of local anaesthesia during the finger extension procedure did not reduce the mean number of injections given per joint compared with the phase III studies. Thus, the use of anaesthesia alleviated patient discomfort and probably reduced the number of physician attempts to disrupt the cord after injection. In this study, by protocol the manipulation was performed the day after injection, although for logistical reasons such as clinic scheduling there is unlikely to be any detriment in delaying manipulation (Manning et al., 2013). Whilst we did not study those who recurred or had an unsatisfactory result, the use of CCH does not seem to preclude surgery if eventually needed (Hay et al., 2013).
Spearman’s correlations between objective (i.e., TPED and ROM) and subjective endpoints (patient- and physician-rated satisfaction with treatment) were low to moderate. The highest correlation was 0.37 between changes in ROM and physician satisfaction. This weak correlation suggests that traditional objective measurement of angular deformity in DC does not properly reflect the benefit or otherwise of treatment of DC.
The safety profile for POINT X is largely consistent with data from the phase III studies, showing that CCH was well tolerated and most AEs were transient injection site reactions. However, there were no reports of tendon rupture or ligament injury in POINT X as were reported in the CORD I and CORD II trials, respectively. This could be due—at least in part—to the comprehensive training provided to POINT X investigators before the study start.
The POINT X study is not without limitations. The open-label design confers some advantages but limits comparability because of the lack of a control group. Despite different levels of experience of the POINT X investigators, it was not possible to evaluate a potential learning curve. However, all investigators received standardized training and strict guidance for performing the injection procedure. Finally, the complexity of the study design provided for the collection of an enormous amount of data, the analyses for which are on-going and will be published separately.
Unique among CCH clinical trials, the POINT X study was designed to reflect clinical practice, with an emphasis on patient choice for prioritizing treatment and the use of patient-reported outcome measures to assess treatment efficacy. CCH was efficacious for reducing contracture consistent with the phase III studies. Patient- and physician-rated satisfaction was high, and recovery was prompt and uneventful, with few joints needing more than 1 injection to achieve a favourable outcome. CCH was well tolerated, and there was low use of additional healthcare resources. As a real-world study, the POINT X findings can be generalized to the at-large population of patients with clinical features of DC similar to those of the study population.
POINT X investigators
DENMARK: Arne Borgwardt, Christianshavns Kirurgiske Klinik, Copenhagen; Per Sondergaard, Regions Hospital Silkeborg, Silkeborg.
FRANCE: Michel Chammas, Service de chirurgie de la main et du Membre Supérieur, Chirurgie des Nerfs Périphériques, Hôpital Lapeyronie, CHU Montpellier, Montpellier; Berengere Chignon-Sicard, CHU Nice, Hôpital de Cimiez, Nice; Caroline Leclercq, Clinique Jouvenet, Service de chirurgie, Institut de la Main, Paris; Emmanuel H. Masmejean, Georges Pompidou European Hospital (HEGP), Paris Descartes University, Paris.
GERMANY: Michael Horst Lehnert,* Aerztezentrum Meviva, Berlin; Andreas Nusche, Klinik für Hand, Plastische, Rekonstruktive und Verbrennungschirurgie, Berufsgenossenschaftliche Unfallklinik Tuebingen, Tuebingen; Karl-Josef Prommersberger, Rhoen-Klinikum AG/Klinik für Handchirurgie, Bad Neustadt; Michael J. Raschke, Klinik und Poliklinik für Unfall-, Hand- und Wiederherstellungschirurgie, Universitaetsklinikum Muenster, Muenster; Bert Reichert, Klinik fur Plastische, Wiederherstellende und Handchirurgie, Zentrum für Schwerbrandverletzte, Klinikum Nürnberg Süd, Nürnberg; Martin Richter, Malteser-Krankenhaus Abteilung Plastische, Hand-und Wiederherstellungschirurgie, Bonn.
*Centre did not enrol patients.
HUNGARY: Zsolt Szabo, Borsod-Abauj-Zemplen Megyei Korhaz es Egyetemi, Miskolc.
ITALY: Antonio Landi, Azienda Ospedaliero-Universitaria di Modena, Dipartimento IX Patologie dell’Apparato Locomotore, Struttura Complessa di Chirurgia della Mano e Microchirurgia, Modena; Giorgio Eugenio Pajardi, San Giuseppe MultiMedica University Hospital and Università degli Studi di, Milan; Mario Igor Rossello, Centro regionale di chirurgia della mano, ospedale S. Paolo, Savona.
SPAIN: Joaquim Casanas Sintes, Hospital De Bellvitge/Servicio de Traumatologia, Hospitalet De Llobregat, Barcelona; Angel Ferreres Claramunt, Institut Kaplan, Barcelona; Miguel Cuadros Romero, Hospital Clinico Universitario Virgen de la Victoria, Servicio de Cirugia Ortopédica y Traumatologia, Málaga; Joaquin Fores, Hospital Clinic de Barcelona, Servicio de Cirugía Ortopédica y Traumatologia, Barcelona; Enrique MacKenney Carrasco, Hospital General de Elche, Servicio de Cirugia de Mano, Alicante.
SWEDEN: Marianne Arner, Department of Hand Surgery Södersjukhuset and Department of Clinical Science & Education, Karolinska Institutet, Stockholm, Sweden; Lars B. Dahlin, Lund University, Skåne University Hospital, Malmö; Stephan L. Wilbrand, Department of Hand Surgery, Uppsala University Hospital, Uppsala.
UNITED KINGDOM: Chris Bainbridge, Royal Derby Hospital, Derby; Adrian Chojnowski, Norwich & Norfolk Hospital, Norwich; Grzegorz Sianos, Glasgow Royal Infirmary, Glasgow; David Warwick, Wellcome Trust Clinical Research Facility, University Hospital Southampton.
Acknowledgments
Medical writing assistance was provided by Linda Goldstein of Engage Scientific Solutions and was funded by Pfizer.
Footnotes
Conflict of interests: D. Warwick, M. Arner, G. Pajardi, E. H. Masmejean, Z. Szabo, and J. Fores were study investigators for the CCH clinical trial program; they also received honoraria and travel expenses for advisory board work with Pfizer. B. Reichert was a consultant and speaker for Pfizer; he also received honoraria and travel expenses for presentations at the Dupuytren Summit sponsored by Pfizer. A. Seghouani was a consultant for Pfizer Ltd. D. Chapman and R. A. Gerber are employees of and own stock in Pfizer Inc. F. Huard is an employee of and owns stock in Pfizer Global Research and Development. P. P. Szczypa is an employee of and owns stock in Pfizer Ltd.
Ethical approval: The study was approved by the institutional review board or independent ethics committee at each of the 28 European sites that participated. The study was conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organizations of Medical Sciences and World Health Organization), Guideline for Good Clinical Practice (International Conference on Harmonisation), and the Declaration of Helsinki (World Medical Association). The POINT X study is registered at ClinicalTrials.gov (NCT01229436).
Funding: The POINT X study was sponsored by Pfizer.
References
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007, 32: 767–74. [DOI] [PubMed] [Google Scholar]
- Beaudreuil J, Allard A, Zerkak D, et al. ; URAM Study Group. Unité Rhumatologique des Affections de la Main (URAM) scale: development and validation of a tool to assess Dupuytren’s disease-specific disability. Arthritis Care Res (Hoboken). 2011, 63: 1448–55. [DOI] [PubMed] [Google Scholar]
- Council for International Organizations of Medical Sciences, World Health Organization. International ethical guidelines for biomedical research involving human subjects. http://www.cioms.ch/images/stories/CIOMS/guidelines/guidelines_nov_2002_blurb.htm (26 November 2013). [PubMed]
- Gilpin D, Coleman S, Hall S, et al. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s contracture. J Hand Surg Am. 2010, 35: 2027–38.e1. [DOI] [PubMed] [Google Scholar]
- Hay DC, Louie DL, Earp BE, Kaplan FTD, Akelman E, Blazar PE. Surgical findings in the treatment of Dupuytren’s disease after initial treatment with clostridial collagenase (Xiaflex). J Hand Surg Eur. Epub ahead of print 6 May, 2013. DOI 1753193413488305. [DOI] [PubMed] [Google Scholar]
- Hurst LC, Badalamente MA, Hentz VR, et al. ; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009, 361: 968–79. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonisation. Guidelines for good clinical practice: efficacy guidelines. http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (25 November 2013).
- Larocerie-Salgado J, Davidson J. Nonoperative treatment of PIPJ flexion contractures associated with Dupuytren’s disease J Hand Surg Eur. 2012, 37, 722–7. [DOI] [PubMed] [Google Scholar]
- Manning CJ, Delaney R, Hayton M. Efficacy and tolerability of Day 2 manipulation and local anaesthesia after collagenase injection in patients with Dupuytren’s contracture. J Hand Surg Eur. Epub ahead of print May 29, 2013. DOI: 1753193413490899. [DOI] [PubMed] [Google Scholar]
- Peimer C, McGoldrick C, Fiore G. Nonsurgical treatment of Dupuytren’s contracture: 1-year US post-marketing safety data for collagenase clostridium histolyticum. Hand (N Y). 2012, 7: 143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pess G, Peimer C, Skodny P, Tursi J, Szczypa P, Gerber R. Efficacy and effectiveness of collagenase Clostridium histolyticum for Dupuytren’s contracture: comparison of real-world data with clinical trial results. Presented at: XVII Congress of the Federation of European Societies for Surgery of the Hand; 29 May–1 June 2012; Antalya, Turkey. [Google Scholar]
- Rahr L, Sondergaard P, Bisgaard T, Baad-Hansen T. Percutaneous needle fasciotomy for primary Dupuytren’s contracture. J Hand Surg Eur. 2011, 36: 548–52. [DOI] [PubMed] [Google Scholar]
- van Rijssen AL, Ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012, 129: 469–77. [DOI] [PubMed] [Google Scholar]
- Witthaut J, Bushmakin A, Gerber R, Cappelleri J, Le Graverand-Gastineau M-P. Determining clinically important changes in range of motion in patients with Dupuytren’s contracture: secondary analysis of the randomized, double-blind, placebo-controlled CORD I study. Clin Drug Invest. 2011, 31: 791–8. [DOI] [PubMed] [Google Scholar]
- Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013, 38: 2–11. [DOI] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki — Ethical principles for medical research involving human subjects. http://www.wma.net/en/30publications/10policies/b3/ (26 November 2013). [DOI] [PubMed]