Abstract
Objective:
Mucopolysaccharidosis I (MPS I) is a progressive, debilitating, and life-threatening genetic disease, which, owing to the nonspecific nature of the early symptoms, is often unrecognized and associated with significant diagnostic delays. To improve early recognition leading to early diagnosis and initiation of treatment, we characterized the extent of airway-related symptoms and surgeries among patients with MPS I.
Methods:
Analysis of the frequency of airway-related symptoms and surgeries from 1041 patients enrolled in the MPS I Registry and correlation with other systemic manifestations of MPS I.
Results:
Airway-related symptoms (macroglossia, enlarged tonsils, reactive airway disease/asthma, or sleep disturbances) were reported for as many as 85% of Hurler, 83% of Hurler-Scheie, and 65% of Scheie patients—very often before the diagnosis of MPS I was established. Surgeries for an airway indication were reported in 39% of patients and many had at least 1 airway-related surgery before the diagnosis of MPS I was confirmed. The mean percentage of patients with airway-related symptoms for whom hernias and/or dysostosis multiplex were also reported was 84% and 54%, respectively.
Conclusion:
Airway-related symptoms and surgeries are common and often the earliest presenting feature in MPS I. Improved recognition of early MPS I disease manifestations may lead to earlier diagnosis and treatment.
Keywords: airway, mucopolysaccharidosis I, registries, surgeries, symptoms
Introduction
Mucopolysaccharidosis type I (MPS I) is a rare genetic disorder caused by deficient activity of the lysosomal enzyme, α-L-iduronidase. This enzyme degrades glycosaminoglycans (GAGs, specifically dermatan and heparan sulfate), and its deficiency leads to the pathologic accumulation of GAGs in multiple tissue types, particularly those of the respiratory, skeletal, nervous, cardiovascular, and gastrointestinal systems. Mucopolysaccharidosis type I is 1 of a family of mucopolysaccharide disorders, each having a distinct genetic cause but overlapping symptomatology. Studies have noted a combined incidence of 2 to 5 per 100 000 live births1 for MPS disorders, which may be an underestimate because of undiagnosed patients with these conditions.
The clinical presentation in patients with MPS I is extremely variable. Patients are broadly categorized as having Hurler syndrome (most severe, onset in infancy with marked neurocognitive manifestations), Hurler-Scheie syndrome (intermediate severity, onset in early childhood with mild to moderate cognitive impairment), or Scheie syndrome (least severe, onset in childhood with no cognitive impairment). Hurler-Scheie and Scheie syndromes, which have the most clinical variability, are often grouped together and referred to as attenuated MPS I.2
Treatment options for MPS I include hematopoietic stem cell transplantation (HSCT) for children with the most severe, Hurler phenotype, and enzyme replacement therapy with laronidase (Aldurazyme; Genzyme, a Sanofi company, Cambridge, Massachusetts, USA, and BioMarin Pharmaceutical, San Rafael, California, USA) for children with Hurler syndrome for whom HSCT is not an option, and for children and adults with attenuated MPS I.3 Early and sustained treatment is critical for optimal outcome in this otherwise debilitating and progressive disease. Unfortunately, because the early signs and symptoms of MPS I can be nonspecific and the disease is not well recognized, diagnostic delays are common. This is especially true for attenuated patients, in whom diagnosis may be delayed for many years.4
Because airway manifestations occur early in all forms of MPS I, undiagnosed patients are frequently referred to otolaryngologists, who thus have an early opportunity to recognize MPS I. Symptoms such as “noisy breathing,” sleep apnea, frequent respiratory and ear infections, chronic nasal discharge, and enlargement of the tongue, tonsils, and adenoids will often pre-date a definitive MPS I diagnosis by several years, particularly in patients with an attenuated phenotype.5 In addition, children and adults with MPS I typically undergo multiple surgical procedures to address the multisystemic manifestations of this disease.6 These patients are at increased risk for anesthetic complications due to enlarged tonsils, macroglossia, restricted mouth opening, tracheobronchomalacia, and mucosal swelling, at least in part due to inflammation and GAG accumulation in the laryngopharynx and trachea (Figure 1).7,8 Other factors that contribute to the high rates of perioperative morbidity and mortality include short and stiff neck, unstable joints, cardiac disease, and increased susceptibility to respiratory infections. A recent MPS I Registry analysis found that about 20% of deaths associated with surgery in patients with MPS I are directly related to airway obstruction or difficult intubations.9 Therefore, a clear understanding of the airway manifestations of MPS I and their potential effect on endotracheal intubation and airway management during anesthesia is vital if the “can’t intubate, can’t ventilate” scenario is to be avoided. Increasing awareness and knowledge of the disease among clinicians, such as otolaryngologists and anesthesiologists, with prompt recognition of the early general and airway-related signs and symptoms, has the potential to significantly reduce the morbidity and mortality in patients with MPS I.
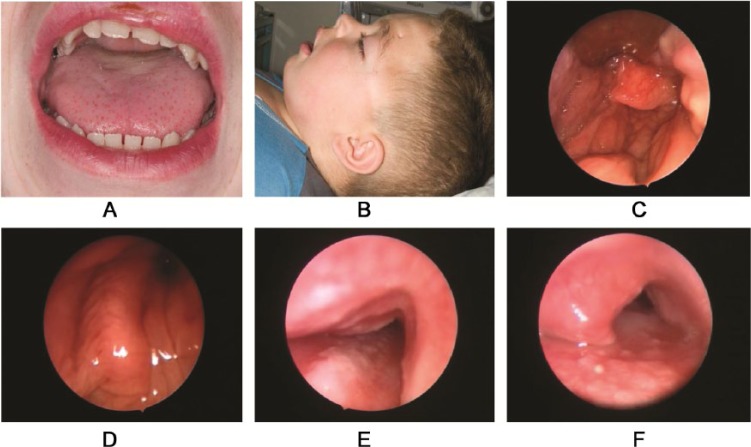
Figure 1.
(A) Macroglossia in a child with mucopolysaccharidosis type I (MPS I). (B) Anatomical features complicating anesthesia in MPS I: large head and short stiff neck10 (written permission obtained and held by institution’s medical illustration department). (C) Laryngopharyngeal deposits in MPS I. (D) Significant laryngeal obstruction in MPS I. (E) Tracheomalacia in MPS. (F) Tracheal narrowing due to MPS deposit within the tracheal lumen.
Reflecting the fact that airway-related symptoms are often among the first to emerge in patients with MPS I, and because these can contribute to substantial morbidity and mortality, we sought to better characterize these disease manifestations by carefully evaluating data from patients enrolled in the MPS I Registry. To further characterize the early clinical presentation in patients with MPS I, we determined the percentage of patients with an airway-related symptom who also had hernias or dysostosis multiplex, 2 of the key early manifestations of MPS I.2
Methods
The global MPS I Registry (clinicaltrials.gov NCT00144794) was initiated in 2003 as part of an effort to help health care professionals involved in the treatment or diagnosis of MPS I better understand the disease and its management and to help create MPS I treatment monitoring guidelines.11 All data provided for this analysis were obtained as of July 2013. Patient participation in the MPS I Registry is voluntary. Each independent site is responsible for obtaining a patient’s informed consent to submit his or her health information to the Registry and to use and disclose this information in subsequent aggregate analyses, such as journal articles, annual reports, education materials, and public health reports. All patient data are de-identified and entered by participating sites in accordance with applicable privacy regulations. At enrollment, detailed medical histories and baseline data are collected. Physicians are encouraged to follow a recommended schedule of clinical assessments and to report the results of those assessments. The time intervals between data entries for each patient vary and can encompass any periods from birth until the most recent clinic visit.
Data involving frequency and age of onset of the following airway-related symptoms, which are indicated by “yes/no” responses in the MPS I Registry, were extracted from the database: “macroglossia,” “enlarged tonsils,” “reactive airway disease/asthma,” and “sleep disturbances.” In addition, data on the frequency of “hernia” and “dysostosis multiplex,” also indicated by yes/no responses, were extracted for those patients who were noted to have the above airway-related symptoms. Surgical data, which are collected in the Registry as free text entries in fields for “surgical indication” and “surgeries performed,” were extracted by searching for terms consistent with airway obstruction and airway-related surgeries. Irrelevant entries (eg, “obstruction” relating to a vetriculoperitoneal [VP] shunt) were omitted. Data were analyzed by symptom, procedure type, and patient phenotype, both overall and prior to diagnosis of MPS I. In addition, median ages at MPS I diagnosis, at first reported airway-related symptoms, and at first airway surgery were calculated. For some patients in the Registry, the MPS I phenotype was reported as unknown or was missing; data for these patients were analyzed separately and the cohort was labeled as “unknown.”
Results
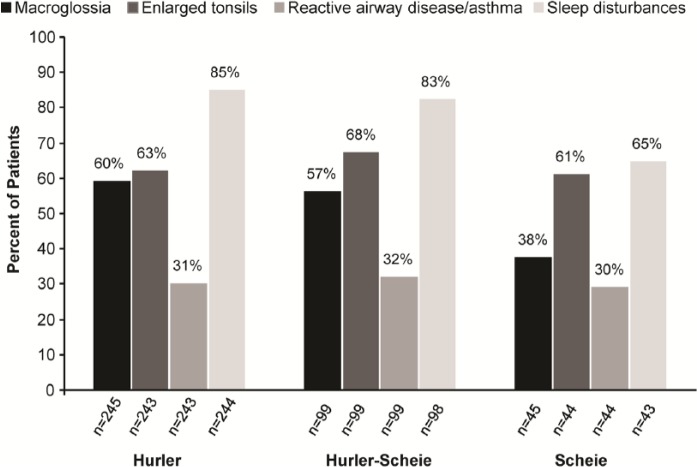
As of July 2013, 1041 patients were enrolled in the MPS I Registry and categorized by clinical phenotype as follows: 615 Hurler, 229 Hurler-Scheie, 127 Scheie, and 70 unknown. The percentage of patients, by phenotype, for whom macroglossia, enlarged tonsils, reactive disease/asthma, and sleep disturbances were reported, is shown in Figure 2. Sleep disturbances were the most common airway-related symptoms among all 3 phenotypes, as shown in Figure 2. In many cases, airway-related symptoms were reported before the diagnosis of MPS I was made. The percentage of patients, by phenotype, for whom macroglossia, enlarged tonsils, reactive airway disease/asthma, and sleep disturbances were reported prior to diagnosis, is shown in Figure 3. Airway-related symptoms were reported at a very early age (Table 1); macroglossia and sleep disturbances were first reported at an overall median age of 1.7 years, enlarged tonsils at 2.5 years, and reactive airway disease/asthma at 2.8 years. All airway-related symptoms were reported at a much earlier age (1.3-1.8 years) in patients with the most severe, Hurler phenotype, compared to patients with attenuated forms of MPS I.
Figure 2.
Proportion of patients with airway-related symptoms reported. The percentages of patients with macroglossia, enlarged tonsils, reactive airway disease/asthma, and sleep disturbances were determined among all patients for whom a “yes/no” response was recorded in the registry. Symptom frequency is shown by phenotype for Hurler, Hurler-Scheie, and Scheie patients.
Figure 3.
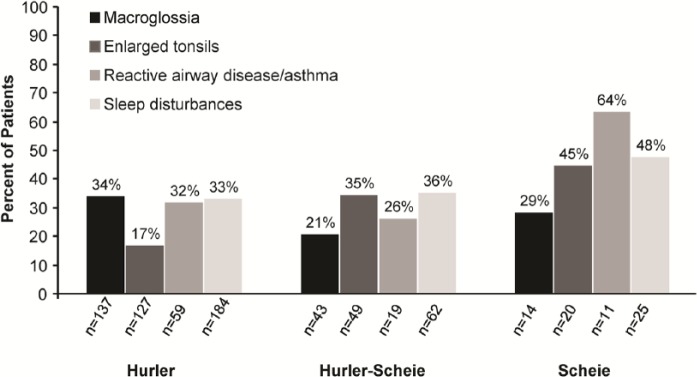
Proportion of patients with airway-related symptoms reported prior to diagnosis of mucopolysaccharidosis type I. Percentages were assessed by phenotype among patients with known symptom-onset and diagnosis dates.
Table 1.
Age (Years) at First Reported Airway-Related Symptoms (All Registry Patients).
| Hurler | Hurler-Scheie | Scheie | Alla | |
|---|---|---|---|---|
| Macroglossia, No. | 137 | 43 | 14 | 203 |
| Median age first reported | 1.4 | 5.0 | 9.5 | 1.7 |
| Range | 0.2-12.2 | 0.2-22.7 | 4.0-38.8 | 0.2-38.8 |
| Enlarged tonsils, No. | 127 | 49 | 20 | 205 |
| Median age first reported | 1.7 | 4.0 | 5.2 | 2.5 |
| Range | 0.1-14.0 | −0.2-15.2 | 1.5-16.5 | −0.2-16.5 |
| Reactive airway disease/asthma, No. | 59 | 19 | 11 | 89 |
| Median age first reported | 1.8 | 6.1 | 5.6 | 2.8 |
| Range | −0.1-13.0 | 0.1-14.8 | 0.2-37.8 | −0.1-37.8 |
| Sleep disturbances, No. | 184 | 62 | 25 | 278 |
| Median age first reported | 1.3 | 4.0 | 6.8 | 1.7 |
| Range | −0.1-12.9 | −0.3-28.9 | 0.0-38.6 | −0.3-38.6 |
Includes patients with unknown phenotype.
The prevalence of hernias and dysostosis multiplex, 2 of the key early manifestations of MPS I, was determined among patients with an airway-related symptom. Dysostosis multiplex presents on chest radiograph as broad “oar shaped” ribs on anteroposterior view and vertebral abnormalities on lateral view. The vast majority (86% Hurler, 85% Hurler-Scheie, and 87% Scheie) of patients with a reported airway-related symptom also had hernias. The prevalence of dysostosis multiplex, which was noted in 66% of Hurler, 54% of Hurler-Scheie, and 50% of Scheie patients with any airway-related symptom, correlated with disease severity. We also determined the percentage of patients with an airway-related symptom prior to diagnosis of MPS I, for whom hernias and dysostosis multiplex were also reported. Hernias were reported prior to diagnosis in 84% of Hurler, 74% of Hurler-Scheie, and 87% of Scheie patients with an airway-related symptom; dysostosis multiplex was reported in 73% of Hurler, 51% of Hurler-Scheie, and 39% of Scheie patients.
Demographic data for patients for whom an airway-related surgery was reported are presented in Table 2. Overall, 39% of patients (403/1041) in the MPS I Registry had at least 1 surgical procedure for an airway indication. A total of 698 airway-related surgeries were reported for these patients, ranging from 1 to 14 surgeries per patient. Adenoidectomy/tonsillectomy were the most commonly reported airway-related procedures and accounted for 84%, 88%, and 96% of airway surgeries among Hurler, Hurler-Scheie, and Scheie patients, respectively (Figure 4). Tracheostomy and bronchoscopy were less common, accounting for 9% (59/698) and 13% (87/698) of all airway-related surgeries, respectively. The percentage of patients for whom a tracheostomy or bronchoscopy was reported is shown in Figure 4.
Table 2.
Demographics of Patients With Airway-Related Surgery.
| Hurler | Hurler-Scheie | Scheie | Alla | |
|---|---|---|---|---|
| Patients reporting at least 1 airway-related surgery | 246 | 99 | 45 | 403 |
| Median age at diagnosis, y | 1.1 | 4.0 | 8.3 | 1.4 |
| Range | −0.6-11.3 | −0.4-11.6 | 0-40.3 | −0.6-43.9 |
| Median number of airway-related surgeries per patient | 1.0 | 2.0 | 1.0 | 1.0 |
| Range | 1.0-14.0 | 1.0-7.0 | 1.0-3.0 | 1.0-14.0 |
| Number of patients with a known surgery date and diagnosis date | 242 | 93 | 43 | 390 |
| Age at first airway surgery, y | 2.0 | 4.7 | 5.5 | 3.0 |
| Range | 0-14.4 | 0.1-21.1 | 0.8-18.8 | 0-21.1 |
| Percentage of patients with airway surgery before diagnosis | 11.2 | 34.4 | 65.1 | 22.3 |
| Total number of airway-related surgeries | 440 | 178 | 64 | 698 |
Includes patients with unknown phenotype.
Figure 4.
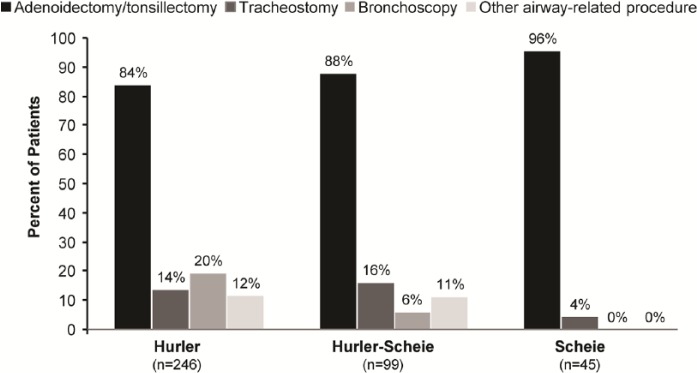
Airway-related surgeries among mucopolysaccharidosis type I patients. The percentages of patients with adenoidectomy/tonsillectomy, tracheostomy, bronchoscopy, or “other airway-related procedure” reported were assessed among 246 Hurler, 99 Hurler-Scheie, and 45 Scheie patients.
The mean overall age at first airway-related surgery was 3 years. In addition, 11.2% of Hurler, 34.4% of Hurler-Scheie, and 65.1% of Scheie patients underwent at least 1 airway-related surgery before the diagnosis of MPS I was established (Table 2). The percentage of patients, by phenotype, for whom an adenoidectomy/tonsillectomy, tracheostomy, and/or bronchoscopy was reported prior to diagnosis with MPS I is shown in Figure 5.
Figure 5.
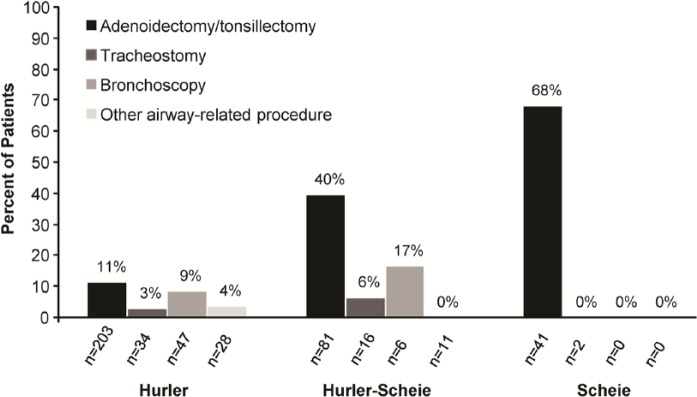
Frequency of airway-related surgeries prior to diagnosis of mucopolysaccharidosis type I (MPS I). Percentages were assessed by phenotype among patients with airway-related procedures reported along with MPS I diagnosis and surgery dates.
Discussion
The upper and lower airways in patients with MPS I are often affected early in the disease process. Therefore, otolaryngologists may be among the first physicians to evaluate these patients. Although symptoms such as sleep disturbances and enlarged tonsils are common in pediatric patients, other multisystemic features associated with MPS I may be present and should raise suspicion of a possible underlying MPS I diagnosis (Table 3). Hepatomegaly, abnormal spine curvature, coarse facial features, corneal clouding, joint contractures, hernia, and cardiac valve abnormalities may be present, in varying combinations and to varying degrees. Indeed, our analysis showed that hernias and dysostosis multiplex, 2 of the earliest presenting symptoms of MPS I, were found in the vast majority of patients who had an airway-related symptom. The prevalence rates of dysostosis multiplex observed in this study are consistent with rates noted previously in patients with Hurler, Hurler-Scheie, and Scheie syndrome, prior to the initiation of treatment.12 In addition, hernias and dysostosis multiplex were present prior to diagnosis of MPS I in the majority of patients who also had an airway-related symptom. Thus, the presence of hernias or dysostosis multiplex in combination with macroglossia, enlarged tonsils, reactive airway disease/asthma, sleep disturbances, or other airway-related symptoms should prompt a consideration of MPS I in the differential diagnosis.
Table 3.
Physical Manifestations Suggestive of Mucopolysaccharidosis Type I (MPS I).a
| General manifestations |
| Macrocephaly |
| Dysmorphic facial featuresb |
| Short stature |
| Thick, coarse hair |
| Cardiac valve abnormalities |
| Splenomegaly |
| Hepatomegaly |
| Umbilical or inguinal hernias and prominent abdomen |
| Corneal clouding |
| Kyphosis of lumbar spine (gibbus) |
| Contractures and joint stiffness |
| Dysostosis multiplex |
| Spinal cord compression |
| Ear, nose, and throat manifestations |
| Macroglossia |
| Recurrent otitis media |
| Chronic rhinitis |
| Upper airway obstruction with snoring and/or sleep apnea |
All manifestations may be seen in both severe (Hurler) and attenuated (Hurler-Scheie and Scheie) forms of MPS I and are also characteristic of 1 or more other types of MPS. Clinical presentation is heterogeneous and not all manifestations are seen in any given patient.
Facial dysmorphisms tend to be more subtle in more attenuated forms of MPS I and patients are more likely to present with other symptoms.
The ability to recognize and diagnose MPS I is critical to initiate early disease-specific treatment and to address the multiple and complex medical, surgical, and psychosocial issues facing individuals with MPS I. In particular, airway-related symptoms and airway obstruction may require surgical intervention. Life-threatening sequelae may arise among all phenotypes, as indicated by the number of patients requiring tracheostomy, for example (Figure 4), particularly if symptoms progress without recognition. As shown in this analysis, many patients with MPS I, particularly those with the more attenuated phenotypes (Hurler-Scheie and Scheie), will undergo airway-related surgeries prior to MPS I diagnosis (Figure 5 and Table 2). The higher number of surgeries among patients with attenuated MPS I may represent a beneficial treatment effect of HSCT, which is most commonly performed in MPS I patients with the Hurler phenotype.
A recent study describing an algorithm for the early determination of phenotypic severity in patients with MPS I found that signs and symptoms of upper airway obstruction (including excessive snoring during sleep, chronic rhinorrhea, obstructive sleep apnea, and feeding difficulties due to obstructed nasal breathing) are clinical characteristics present early in life (within the first month) in patients with the Hurler phenotype.13 Our findings indicate that these airway-related symptoms are also present in the attenuated phenotypes. Awareness of the underlying disease and its associated symptoms can enable otolaryngologists, general surgeons, and anesthesiologists to facilitate early diagnosis and employ appropriate management strategies, including special precautions during surgery.
Because this study involved analysis of data from the MPS I Registry, there may be limitations based on the types of information collected in the database as well as the terms used in the clinical report form. For example, the term sleep disturbances is a broad category and encompasses insomnias; disorders of sleep-wake schedule; disorders of excessive somnolence; dysfunctions related to sleep, sleep stages, or parasomnias; obstructive sleep apnea; and central apnea.14 In addition, analysis of data collected in the Registry may not represent the total incidences of airway-related symptoms and surgeries in patients with MPS I. The MPS I Registry is a voluntary, observational disease database, which is inherently subject to some degree of selection bias, reporting bias, incomplete or inaccurate data entries, and patient loss to follow-up.11 Although the overall source-to-database error rate in the MPS I Registry is relatively low (< 4%),15 other sources of error are not excluded. Our method of analysis may have underestimated the actual prevalence of airway-related symptoms and surgeries, as this was calculated based on the patients for whom a yes/no response was provided, and it was assumed that patients for whom no response was provided did not have an airway-related symptom or surgery. Despite these limitations, the data demonstrate the high frequency of early airway-related symptoms and surgeries in patients with MPS I and highlight the importance of recognizing these manifestations to prevent the complications associated with the underlying disease.
Conclusion
An awareness of early MPS I disease characteristics and the potential complications associated with this disorder may not be widespread among otolaryngologists and other pediatric specialists. Greater understanding and recognition of the early signs and symptoms of this complex disorder have the potential to prompt earlier diagnosis and initiation of appropriate treatment. In addition, identification of the underlying disease process can lead to special management strategies, including anesthetic precautions, to avoid surgical complications often seen in patients with MPS I who present with upper airway obstruction. Mucopolysaccharidosis type I should be considered in the differential diagnosis of patients who present with airway-related symptoms, such as macroglossia, recurrent otitis media, chronic rhinitis, and upper airway obstruction, particularly when accompanied by hernia or dysostosis multiplex. If a diagnosis of MPS I is suspected, patients should be referred to an appropriate clinician, such as a geneticist or metabolic disease specialist, for further testing and management.
Acknowledgments
The authors are grateful to Lisa Underhill, MS, and Iva Ivanovska Holder, PhD, Genzyme Global Medical Affairs, for assistance with medical writing, helpful discussions, and critical review of the manuscript, and to Marta Cizmarik, MS, Genzyme Global Medical Operations, for help with biostatistical analyses of the data.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.A. was the chair of the North American Board of Advisors, and a member of the International Board of Advisors, for the MPS I Registry. I.A.B. has spoken as an unpaid invited speaker at medical meetings on MPS that were sponsored by Genzyme, a Sanofi company. H.T. and S.F. are employees of Genzyme.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Genzyme, a Sanofi company.
References
- 1. Lin HY, Lin SP, Chuang CK, et al. Incidence of the mucopolysaccharidoses in Taiwan, 1984-2004. Am J Med Genet A. 2009;149A:960-964. [DOI] [PubMed] [Google Scholar]
- 2. Muenzer J, Wraith JE, Clarke LA. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics. 2009;123:19-29. [DOI] [PubMed] [Google Scholar]
- 3. de Ru MH, Boelens JJ, Das AM, et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J Rare Dis. 2011;6:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D’Aco K, Underhill L, Rangachari L, et al. Diagnosis and treatment trends in mucopolysaccharidosis I: findings from the MPS I Registry. Eur J Pediatr. 2012;171:911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas JA, Beck M, Clarke JTR, Cox GF. Childhood onset of Scheie syndrome, the attenuated form of mucopolysaccharidosis I. J Inherit Metab Dis. 2010;33:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arn P, Wraith J, Underhill L. Characterization of surgical procedures in patients with mucopolysaccharidosis type I: findings from the MPS I Registry. J Pediatr. 2009;154:859-864. [DOI] [PubMed] [Google Scholar]
- 7. Muhlenbach MS, Shaffer CB, Georges L, Abode K, Muenzer J. Bronchoscopy and airway management in patients with mucopolysaccharidoses (MPS). Pediatr Pulmonol. 2013;48:601-607. [DOI] [PubMed] [Google Scholar]
- 8. Chan YL, Lin SP, Man TT, Cheng CR. Clinical experience in anesthetic management for children with mucopolysaccharidoses: report of ten cases. Acta Paediatr Taiwan. 2001;42:306-308. [PubMed] [Google Scholar]
- 9. Arn P, Whitley C, Wraith JE, et al. High rate of postoperative mortality in patients with mucopolysaccharidosis I: findings from the MPS I Registry. J Pediatr Surg. 2012;47:477-484. [DOI] [PubMed] [Google Scholar]
- 10. Shinhar SY, Zablocki H, Madgy DN. Airway management in mucopolysaccharide storage disorders. Arch Otolaryngol Head Neck Surg. 2004;130:233-237. [DOI] [PubMed] [Google Scholar]
- 11. Pastores GM, Arn P, Beck M, et al. The MPS I Registry: design, methodology, and early findings of a global disease registry for monitoring patients with mucopolysaccharidosis type I. Mol Genet Metab. 2007;91:37-47. [DOI] [PubMed] [Google Scholar]
- 12. Beck M, Arn P, Giugliani R, et al. The natural history of MPS I: global perspectives from the MPS I Registry [published online March 27, 2014]. Genet Med. 10.1038/gim.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kingma SDK, Langereis EJ, de Klerk CM, et al. An algorithm to predict phenotypic severity in mucopolysaccharidosis type I in the first month of life. Orphanet J Rare Dis. 2013;8:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cormier RE. Sleep disturbances. In: Walker HK, Hall WD, Hurst JW. eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:398-403. [PubMed] [Google Scholar]
- 15. Verhulst K, Artiles-Carloni L, Beck M, et al. Source document verification in the Mucopolysaccharidosis Type I Registry. Pharmacoepidemiol Drug Saf. 2012;21:749-752. [DOI] [PubMed] [Google Scholar]