Abstract
Background:
Patients with multiple sclerosis (MS) have a deficiency of circulating CD8+ T cells, which might impair control of Epstein–Barr virus (EBV) and predispose to MS by allowing EBV-infected autoreactive B cells to accumulate in the central nervous system. Based on the expression of CD45RA and CD62L, CD4+ T cells and CD8+ T cells can be subdivided into four subsets with distinct homing and functional properties, namely: naïve, central memory, effector memory (EM) and effector memory re-expressing CD45RA (EMRA) cells.
Objective:
Our aim was to determine which memory subsets are involved in the CD8+ T cell deficiency and how these relate to clinical course.
Methods:
We used flow cytometry to analyze the memory phenotypes of T cells in the blood of 118 MS patients and 112 healthy subjects.
Results:
MS patients had a decreased frequency of EM (CD45RA–CD62L–) and EMRA (CD45RA+CD62L–) CD8+ T cells, which was present at the onset of disease and persisted throughout the clinical course. The frequencies of CD4+ EM and EMRA T cells were normal.
Conclusion:
Deficiency of effector memory CD8+ T cells is an early and persistent feature of MS and might underlie the impaired CD8+ T cell control of EBV.
Keywords: Multiple sclerosis, CD8+ T cell, Epstein–Barr virus, immunological memory, CD45RA, CD62L
Introduction
A large body of evidence indicates that infection with the Epstein–Barr virus (EBV) plays a major role in the pathogenesis of multiple sclerosis (MS), although its exact role is incompletely understood.1,2 EBV is a ubiquitous human herpesvirus that has the unique ability to infect, activate and latently persist in B lymphocytes for the lifetime of the infected individual.3 Normally EBV infection is kept under tight control by EBV-specific immune responses, especially by cytotoxic CD8+ T cells which eliminate proliferating and lytically infected B cells.4 Since 1980 it has been recognized that MS patients have a decreased proportion and number of CD8+ T cells and increased CD4/CD8 T cell ratio in peripheral blood.5–13 This was initially interpreted as a decrease in suppressor T cells leading to disinhibition of autoimmune responses5,6 but later attributed to sequestration of CD8+ T cells in the central nervous system (CNS).11 An alternative explanation is that the low number of circulating CD8+ T cells is genetically determined and results in a decreased CD8+ T cell response to EBV,2 which in turn allows EBV-infected autoreactive B cells to accumulate in the CNS and lead to the development of MS.14
Based on the expression of CD45RA, CCR7 and CD62L, human CD4+ T cells and CD8+ T cells can be subdivided into four major subsets with distinct homing and functional properties, namely: naïve (CD45RA+CCR7+CD62L+), central memory (CM) (CD45RA–CCR7+CD62L+), effector memory (EM) (CD45RA–CCR7–CD62L–) and effector memory re-expressing CD45RA (EMRA) (CD45RA+CCR7–CD62L–) cells.15,16 Naïve and CM CD8+ T cells home to secondary lymphoid organs whereas EM and EMRA CD8+ T cells traffic to inflamed non-lymphoid tissues and exhibit effector functions such as interferon-γ production and cytotoxicity. Recent studies suggest it may be possible to subdivide memory T cells into additional types but this remains controversial.17 In the current study we analyzed the memory phenotypes of CD4+ T cells and CD8+ T cells in MS patients and healthy subjects to determine which memory subsets are involved in the CD8+ T cell deficiency and how these subsets relate to clinical course.
Methods
Patients and controls
This study was approved by the Royal Brisbane and Women’s Hospital Human Research Ethics Committee and The University of Queensland Medical Research Ethics Committee. Blood was collected from 118 MS patients and 112 age- and sex-matched healthy subjects following informed consent. The MS patient group included 14 patients with the first attack of the type seen in MS (clinically isolated syndrome (CIS)), 34 with relapsing–remitting MS (RRMS), 35 with secondary progressive MS (SPMS) and 35 with primary progressive MS (PPMS). Of the 48 patients with CIS or RRMS, 17 had had a clinical attack within 30 days of venesection, and 31 were in remission. All patients with RRMS, SPMS and PPMS met the 2005 and/or the 2010 Revised McDonald Criteria for a diagnosis of MS, and five of the CIS patients also met these criteria at later follow-up. The patients had not received corticosteroids or immunomodulatory therapy for at least three months prior to venesection. The demographic and clinical details of the healthy subjects and patients with MS are presented in Table 1.
Table 1.
General characteristics of healthy subjects and patients with MS.
| HC(n = 112) | All MS(n = 118) | CIS(n = 14) | RRMS(n = 34) | SPMS(n = 35) | PPMS(n = 35) | |
|---|---|---|---|---|---|---|
| Age, years | 41.0 (28.0–52.0) | 45.5 (38.0–54.0) | 38.5 (27.3–42.3) | 39.0 (32.3–47.3) | 48.0 (40.0–55.0) | 54.0 (46.0–56.0) |
| Sex, % female | 78.6 | 71.2 | 71.4 | 82.4 | 71.4 | 60.0 |
| Age of onset of MS, years | 34.5 (26.9–42.2) | 37.3 (25.0–41.9) | 32.0 (26.8–37.5) | 29.0 (24.0–39.0) | 40.0 (34.0–49.0) | |
| Duration of MS, years | 7.5 (2.8–17.0) | 0.6 (0.1–1.3) | 4.0 (1.6–11.5) | 16.0 (10.0–24.0) | 9.0 (5.0–17.0) | |
| EDSS score | 5.0 (2.5–6.5) | 1.0 (0.0–1.5) | 2.3 (1.0–3.5) | 6.5 (5.5–8.0) | 6.5 (5.5–7.0) | |
| MSSS | 6.6 (3.8–8.8) | 2.4 (0.7–4.2) | 4.4 (2.2–6.2) | 7.8 (5.8–9.1) | 8.5 (6.6–9.5) |
All values except for sex presented as medians (interquartile ranges).
HC: healthy control subjects; All MS: total group of MS patients; CIS: clinically isolated syndrome; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS; PPMS: primary progressive MS; EDSS: Expanded Disability Status Scale; MSSS: MS Severity Score.
Flow cytometry
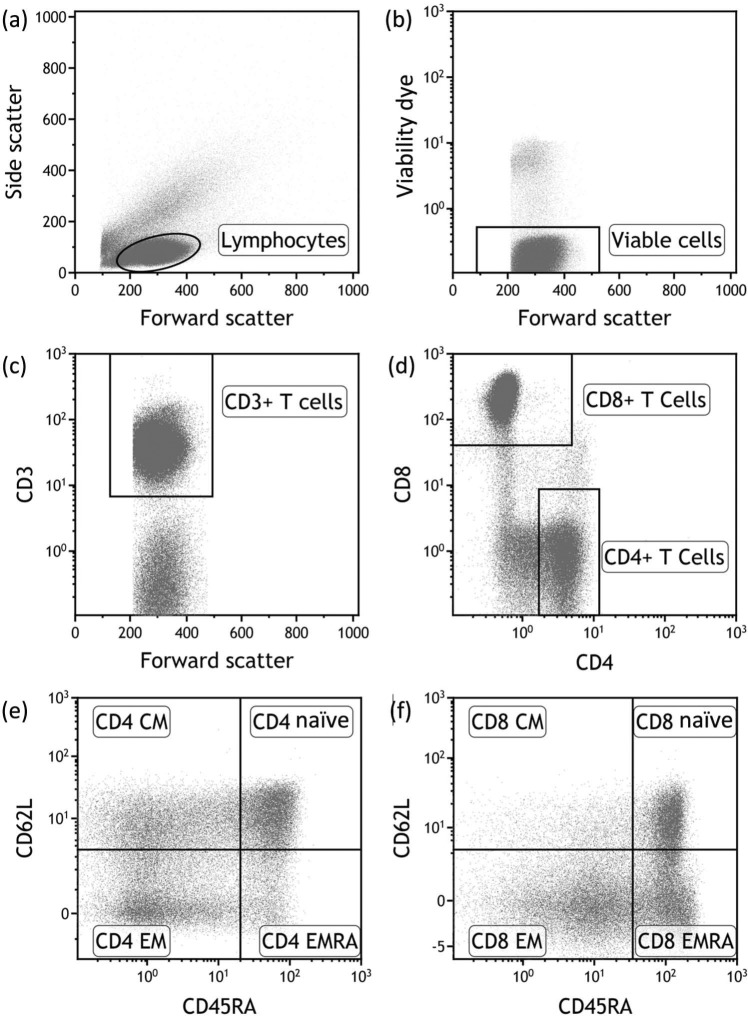
Peripheral blood mononuclear cells (PBMCs) were separated by density centrifugation and cryopreserved. Prior to analysis on a Beckman Coulter Gallios flow cytometer with seven color acquisition, cryopreserved PBMC samples were thawed and cultured for 24 h before use to allow cells to rest and re-express cell surface molecules. Cells were stained with Aqua Live/Dead cell exclusion dye (Invitrogen) and the following fluorochrome-conjugated antibodies: anti-CD3-allophycocyanin (APC) (Becton Dickinson), anti-CD4-V450 (Becton Dickinson), anti-CD8-APC-A700 (Beckman Coulter), anti-CD45RA-phycoerythrin-Cy7 (Becton Dickinson), anti-CD62L-APC-Cy7 (BioLegend) and anti-CCR7-peridinin chlorophyll protein-Cy5.5 (Becton Dickinson). Single labeled tubes for each antibody, isotype-matched control antibodies, fluorescence-minus-one controls, dead cell exclusion and doublet discrimination were used to ensure accurate positive cut-off values and compensation matrices and to validate cell phenotype detection sensitivity and resolution. Figure 1 shows the gating strategy used to identify the memory T cell subsets. Flow cytometry data was analyzed using Kaluza 1.2 software (Beckman Coulter).
Figure 1.
Flow cytometric gating strategy. Within the PBMCs of a healthy subject, lymphocytes (a) were identified on the basis of forward scatter and side scatter. We then identified the viable population of lymphocytes (b). The CD3+ T cell component of the viable cells (c) was then divided into CD4+ T cells and CD8+ T cells (d). Finally the CD4+ T cells and CD8+ T cells were subdivided into naïve, central memory (CM), effector memory (EM) and effector memory re-expressing CD45RA (EMRA) subsets according to the expression of CD45RA and CD62L ((e) and (f), respectively).
Data analysis and statistics
Statistical analyses were performed using GraphPad Prism version 6.00 (Graphpad Software Inc., San Diego, CA, USA) and Sigmaplot 12.5 (Systat Software Inc., San Jose, CA, USA). Because most of the data was not normally distributed, the results have been presented as medians with interquartile ranges. For single comparisons between the whole group of MS patients and healthy subjects, Student’s t test or the Mann–Whitney rank sum test was used, according to the distribution of the data. For comparisons between each of the subtypes of MS (CIS, CIS + RRMS, SPMS, PPMS, and All MS) and healthy subjects, we used Kruskal–Wallis non-parametric analysis of variance corrected for multiple comparisons (Dunn’s Test). To assess the relationships between T cell frequencies and age, disease duration, Expanded Disability Status Scale (EDSS) score and MS Severity Score (MSSS) we used Spearman rank correlation. Differences were considered significant for p <0.05.
Results
The proportion of CD8+ T cells in the PBMC was decreased and the CD4:CD8 ratio was increased in MS patients compared with healthy subjects (Table 2). Analysis of T cell memory phenotype based on CD45RA and CD62L expression revealed that the decreased frequency of total CD8+ T cells was due to a decrease in EM and EMRA T cells, which was present in all subtypes of MS (CIS + RRMS; SPMS; and PPMS) and at the onset of disease (CIS) (Table 3). This was particularly evident when the frequencies of CD8+ EM and EMRA T cells were combined. In CIS/RRMS patients the T cell subset frequencies during attacks were not significantly different from those during remission. The absolute number of CD8+ EM/EMRA T cells was also decreased in the MS patients for whom this data was available (not shown). In contrast the frequencies of CD4+ EM and EMRA T cells were normal. The proportion of CD8+ CM T cells in the blood was higher in patients with MS than in healthy subjects, although the difference was not statistically significant after correction for multiple comparisons. In PPMS there was also a decrease in naïve CD8+ T cells (Table 3). Reduced frequencies of CD8+ EM and EMRA T cells were also evident in MS patients when CCR7 was used instead of CD62L to analyze memory phenotype although the differences were not as significant as with CD62L (not shown). The frequencies of CD8+ EM, EMRA and EM/EMRA T cells within PBMCs were significantly lower in MS patients at all ages compared with healthy subjects (Figure 2) but were not significantly correlated with disease duration, disability (EDSS) or severity (MSSS) (not shown).
Table 2.
Peripheral blood T cell frequencies.
| % CD3+ | % CD4+CD3+ | % CD8+CD3+ | CD4:CD8 ratio | |
|---|---|---|---|---|
| HC (n = 112) | 69.11 (63.32–73.66) | 44.48 (38.48–50.29) | 18.61 (15.65–23.00) | 2.27 (1.76–3.07) |
| All MS (n = 118) | 66.68 (59.18–72.88) | 46.07 (39.47–52.07) | 15.74 (12.62–20.43) | 2.97 (2.16–3.77) |
| p value | 0.045a | 0.189b | <0.001b | <0.001a |
Values are presented as median (interquartile range) percentages of T cells within PBMCs.
Mann–Whitney rank sum test.
Student’s t test.
HC: healthy control subjects; All MS: total group of MS patients.
Table 3.
Frequencies of memory T cell subsets.
| CD4+
|
CD8+
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | CM | EM | EMRA | N | CM | EM | EMRA | EM + EMRA | |
| HC (n = 112) | 8.21 | 6.26 | 15.54 | 8.09 | 3.01 | 1.12 | 6.08 | 7.17 | 13.89 |
| (5.63–14.12) | (3.95–11.09) | (12.72–19.76) | (4.90–12.06) | (1.90–4.79) | (0.64–1.81) | (4.29–7.84) | (5.18–9.99) | (10.91–17.09) | |
| All MS (n = 118) | 8.40 | 6.80 | 18.02 | 8.79 | 2.71 | 1.42 | 4.87 | 5.25 | 10.57 |
| (5.12–12.69) | (4.21–10.06) | (13.70–22.96) | (5.81–13.78) | (1.51–4.56) | (0.81–2.30) | (3.31–6.54) | (3.61–7.68) | (8.01–14.05) | |
| p value | >0.999 | >0.999 | 0.119 | >0.999 | >0.999 | 0.110 | 0.001 | <0.0001 | <0.0001 |
| CIS + RRMS (n = 48) | 8.04 | 6.94 | 17.29 | 8.79 | 3.97 | 1.34 | 4.61 | 5.95 | 11.12 |
| (4.40–15.41) | (4.05–9.48) | (10.44–20.84) | (5.70–12.17) | (2.16–6.19) | (0.94–2.18) | (2.98–6.43) | (3.90–8.11) | (8.34–13.95) | |
| p value | >0.999 | >0.999 | >0.999 | >0.999 | 0.880 | 0.364 | 0.006 | 0.082 | 0.010 |
| SPMS (n = 35) |
8.67 | 4.79 | 18.10 | 8.92 | 2.73 | 1.43 | 4.66 | 5.80 | 9.86 |
| (5.44–11.99) | (3.89–10.03) | (15.18–22.94) | (6.08–14.57) | (1.83–4.04) | (0.80–2.41) | (3.39–6.09) | (4.15–8.38) | (8.73–14.39) | |
| p value | >0.999 | >0.999 | 0.241 | >0.999 | >0.999 | 0.385 | 0.022 | 0.087 | 0.005 |
| PPMS (n = 35) | 8.42 | 8.10 | 19.25 | 8.00 | 1.84 | 1.43 | 5.35 | 3.98 | 10.26 |
| (5.09–12.33) | (5.62–10.82) | (13.73–24.70) | (5.62–14.39) | (0.99–3.05) | (0.60–2.49) | (3.27–6.97) | (2.73–6.72) | (7.28–13.40) | |
| p value | >0.999 | >0.999 | 0.112 | >0.999 | 0.009 | 0.971 | 0.488 | <0.0001 | 0.001 |
| CIS (n = 14) | 8.04 | 7.97 | 18.47 | 8.47 | 5.59 | 1.75 | 3.48 | 5.86 | 10.24 |
| (6.01–16.05) | (5.15–10.38) | (10.19–21.29) | (6.34–15.60) | (2.53–9.59) | (1.06–2.62) | (2.81–5.16) | (4.75–8.57) | (8.26–12.23) | |
| p value | >0.999 | >0.999 | >0.999 | >0.999 | 0.194 | 0.202 | 0.004 | >0.999 | 0.044 |
Values are presented as median (interquartile range) percentages of memory T cell subsets within PBMCs. All p values are for comparisons with healthy control subjects (HC) using Kruskal–Wallis non-parametric analysis of variance corrected for multiple comparisons (Dunn’s Test). Significant p values are highlighted in bold.
N: naïve T cells; CM: central memory; EM: effector memory; EMRA: effector memory re-expressing CD45RA; HC: healthy control subjects; All MS: total group of MS patients; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS; PPMS: primary progressive MS; CIS: clinically isolated syndrome
Figure 2.
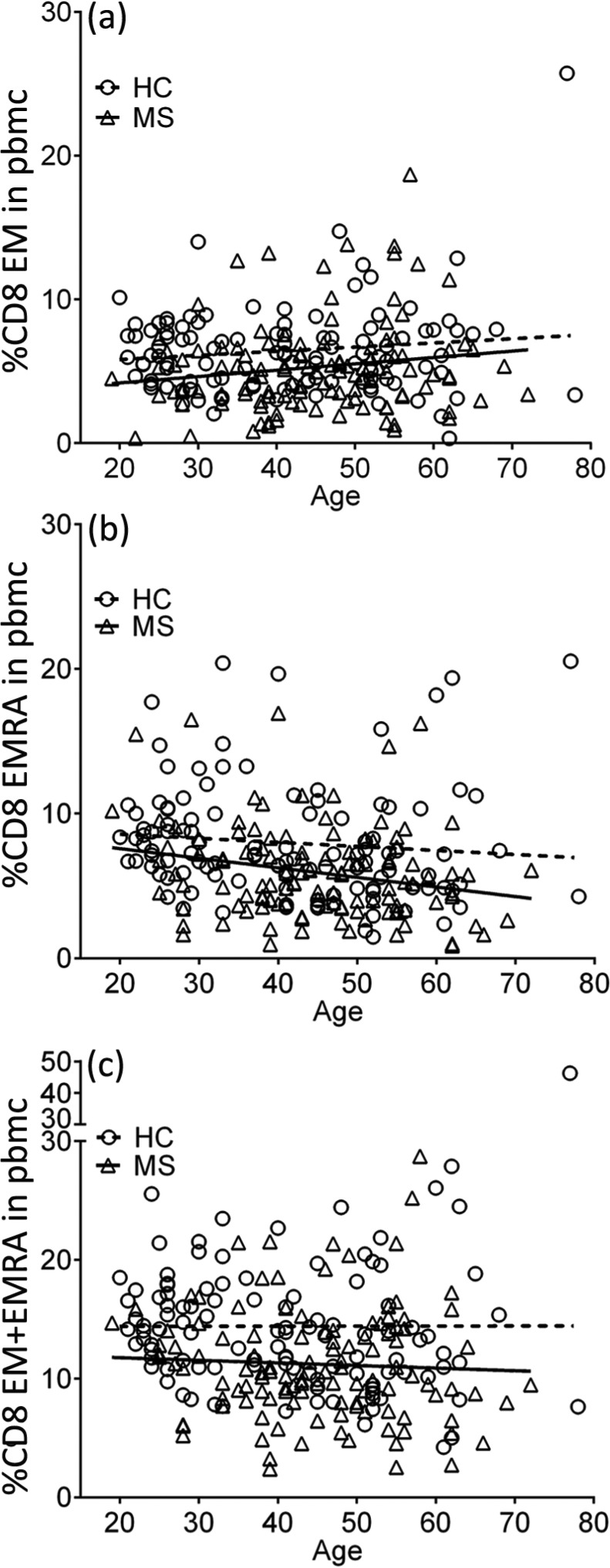
Percentages of CD8+ EM (a), EMRA (b) and EM/EMRA (c) T cells in PBMCs in healthy subjects (HC) and the total group of MS patients (MS) plotted against age in years. On multiple linear regression analysis, the slopes of the regression lines are not significantly different but the y intercepts (elevations) for the MS patients are significantly different from those for the healthy subjects (p = 0.0027; p = 0.0001; p <0.0001, respectively).
Discussion
In this study we have shown that patients with MS have a deficiency of CD8+ EM and EMRA T cells in peripheral blood. This deficiency is present at the onset of MS and persists throughout the clinical course. The decrease in CD8+ EM and EMRA T cells in the blood accounts for the well described decrease in total CD8+ T cells and increase in CD4:CD8 ratio in MS.5–13 Previous studies on T cell memory subsets in MS have measured the frequencies of the subsets only within the total CD4+ and CD8+ T cell populations and not within the peripheral blood.18–20 Therefore the conflicting findings of increased EM18,20 and increased CM, normal EM and decreased EMRA19 within the CD8+ population would not necessarily have applied to the actual frequencies of these subsets within the PBMC in the setting of a decreased total number of CD8+ T cells. This is illustrated in our study where the proportion of EM T cells within the CD8+ population in the total group of MS patients (32.5%) was the same as in the healthy control group (33.3%) despite the presence of a highly significant reduction in the frequency of CD8+ EM T cells in the PBMCs in the MS group (p = 0.001; Table 3). In contrast to the reduction in CD8+ EM and EMRA T cells, the frequencies of CD4+ EM and EMRA T cells in the PBMC were not decreased in MS patients.
A major question is whether the reduction in CD8+ EM and EMRA T cells in MS is due to a primary deficiency of these cells or is secondary to the disease process. The increased CD4:CD8 ratio and reduced total CD8+ T cells in the blood have been attributed to sequestration of CD8+ T cells in the CNS11 because CD8+ cells predominate over CD4+ cells in the T cell infiltrate within the CNS parenchyma.21 However, the predominance of CD8+ over CD4+ T cells does not apply to other compartments of the CNS in MS, with equal numbers of CD4+ and CD8+ cells occurring in the perivascular infiltrates21,22 and with CD4+ predominating over CD8+ T cells in the cerebrospinal fluid.23 Therefore if lymphocyte sequestration in the inflamed CNS were to be incriminated as the cause of the reduction in circulating CD8+ T cells it would also be expected to cause a reduction in circulating CD4+ T cells, which is not the case. Indeed the frequency of CD4+ EM T cells in the blood was actually higher in MS patients than in healthy controls, although this difference was not statistically significant after allowing for multiple comparisons. Furthermore, even if CD8+ T cells were to predominate over CD4+ T cells within the sum total of CNS lymphocytes this would not necessarily increase the CD4:CD8 T cell ratio in the blood because a predominance of CD8+ over CD4+ T cells within the liver in chronic hepatitis C is not accompanied by an increased CD4:CD8 T cell ratio in the blood.24 For the following reasons we propose that the deficiency of CD8+ EM and EMRA T cells is the result of a primary defect rather than being secondary to the MS disease process. First, the deficiency is present at the onset of MS and at all stages of the clinical course, including the relapsing–remitting and progressive phases. Second, it persists unchanged throughout the disease course and is not related to disability or disease severity; in particular, contrary to what would be expected if it were secondary to the disease process, it does not worsen with increasing severity or duration of MS. Third, CD8+ T cell deficiency and an increased CD4/CD8 ratio are features of many human chronic autoimmune diseases and are also present in the healthy blood relatives of patients with these diseases,25 indicating that the abnormalities are genetically determined and not secondary to the disease process. In view of the cross-sectional nature of our study we do not know whether the T cell subset frequencies fluctuate over time in individual patients, particularly during concurrent infections or clinical attacks of MS. Longitudinal studies will be needed to answer this. Possible mechanisms causing deficiency of CD8+ EM and EMRA T cells include: (i) decreased proliferation and differentiation in response to antigen or homeostatic cytokines; and (ii) reduced cellular lifespan due to increased apoptosis. Our finding of an increased number of CD8+CD45RA–CD62L+ CM T cells in MS suggests impaired generation of EM and EMRA T cells from CD8+ CM, which normally acquire an EM phenotype after activation by antigen and an EM or EMRA phenotype in response to homeostatic cytokines.26
In our study, patients with a primary progressive course of MS had a reduced frequency of naïve CD8+ T cells as well as a reduction in EM and EMRA cells. Moreover, the rate of decline in naïve CD8+ T cells with age in these patients was greater than the normal age-related decline in healthy controls (not shown). In healthy subjects this decline is due to thymic involution.27 Our findings are consistent with a previous report of reduced thymic output of CD8+ T cells in PPMS.28 In contrast, naïve CD4+ T cells were not decreased in PPMS in either our study or the previous report despite a reduction in the thymic output of naïve CD4+ T cells.28 By reducing the number of naïve CD8+ T cells available to generate effector T cells in response to stimulation by antigen, the accelerated age-related decline in naïve CD8+ T cells is likely to contribute to the reduction of CD8+ EM and EMRA T cells in PPMS, especially in older patients. Accelerated age-related decline of naïve CD8+ T cells in PPMS accounts for our previous finding that the total CD8+ T cell population in the blood declines more rapidly in PPMS than in healthy subjects29 and might explain why the onset of PPMS occurs on average 10 years later than that of RRMS.
In conclusion, we have shown that patients with MS have a deficiency of CD8+ EM and EMRA T cells in peripheral blood. This deficiency could underlie the defective CD8+ T cell control of EBV-infected B cells in MS.8,30,31
Acknowledgments
We are grateful to Dr Stefan Blum, Kaye Hooper and Bernie Gazzard for assistance in the collection of blood samples.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by Multiple Sclerosis Research Australia [grant number 12-002] and the Trish Multiple Sclerosis Research Foundation.
Contributor Information
Michael P Pender, The University of Queensland, School of Medicine, Brisbane, Australia/Royal Brisbane and Women’s Hospital, Australia/QIMR Berghofer Medical Research Institute, Brisbane, Australia.
Peter A Csurhes, The University of Queensland, School of Medicine, Brisbane, Australia/The University of Queensland Centre for Clinical Research, Brisbane, Australia.
Casey MM Pfluger, The University of Queensland, School of Medicine, Brisbane, Australia/The University of Queensland Centre for Clinical Research, Brisbane, Australia.
Scott R Burrows, The University of Queensland, School of Medicine, Brisbane, Australia/QIMR Berghofer Medical Research Institute, Brisbane, Australia.
References
- 1. Ascherio A, Munger KL. Epstein–Barr virus infection and multiple sclerosis: A review. J Neuroimmune Pharmacol 2010; 5: 271–277. [DOI] [PubMed] [Google Scholar]
- 2. Pender MP. The essential role of Epstein–Barr virus in the pathogenesis of multiple sclerosis. Neuroscientist 2011; 17: 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004; 350: 1328–1337. [DOI] [PubMed] [Google Scholar]
- 4. Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu Rev Microbiol 2000; 54: 19–48. [DOI] [PubMed] [Google Scholar]
- 5. Reinherz EL, Weiner HL, Hauser SL, et al. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med 1980; 303: 125–129. [DOI] [PubMed] [Google Scholar]
- 6. Bach M-A, Phan-Dinh-Tuy F, Tournier E, et al. Deficit of suppressor T cells in active multiple sclerosis. Lancet 1980; 316: 1221–1223. [DOI] [PubMed] [Google Scholar]
- 7. Compston A. Lymphocyte subpopulations in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 1983; 46: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Craig JC, Hawkins SA, Swallow MW, et al. Subsets of T lymphocytes in relation to T lymphocyte function in multiple sclerosis. Clin Exp Immunol 1985; 61: 548–555. [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson AJ, Brazil J, Whelan CA, et al. Peripheral blood T lymphocyte changes in multiple sclerosis: A marker of disease progression rather than of relapse? J Neurol Neurosurg Psychiatry 1986; 49: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trotter JL, Clifford DB, McInnis JE, et al. Correlation of immunological studies and disease progression in chronic progressive multiple sclerosis. Ann Neurol 1989; 25: 172–178. [DOI] [PubMed] [Google Scholar]
- 11. Kreuzfelder E, Shen G, Bittorf M, et al. Enumeration of T, B and natural killer peripheral blood cells of patients with multiple sclerosis and controls. Eur Neurol 1992; 32: 190–194. [DOI] [PubMed] [Google Scholar]
- 12. Michałowska-Wender G, Wender M. Mononuclear subsets in the peripheral blood of multiple sclerosis patients in relation to results of brain gadolinium-enhancing imaging. Folia Neuropathol 2006; 44: 67–71. [PubMed] [Google Scholar]
- 13. Pender MP, Csurhes PA, Pfluger CMM, et al. Decreased CD8+ T cell response to Epstein-Barr virus infected B cells in multiple sclerosis is not due to decreased HLA class I expression on B cells or monocytes. BMC Neurology 2011; 11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol 2003; 24: 584–588. [DOI] [PubMed] [Google Scholar]
- 15. Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401: 708–712. [DOI] [PubMed] [Google Scholar]
- 16. Amyes E, McMichael AJ, Callan MFC. Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets. J Immunol 2005; 175: 5765–5773. [DOI] [PubMed] [Google Scholar]
- 17. Farber DL, Yudanin NA, Restifo NP. Human memory T cells: Generation, compartmentalization and homeostasis. Nat Rev Immunol 2014; 14: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haegele KF, Stueckle CA, Malin J-P, et al. Increase of CD8+ T-effector memory cells in peripheral blood of patients with relapsing–remitting multiple sclerosis compared to healthy controls. J Neuroimmunol 2007; 183: 168–174. [DOI] [PubMed] [Google Scholar]
- 19. Liu G-Z, Fang L-B, Hjelmström P, et al. Increased CD8+ central memory T cells in patients with multiple sclerosis. Mult Scler 2007; 13: 149–155. [DOI] [PubMed] [Google Scholar]
- 20. Mikulkova Z, Praksova P, Stourac P, et al. Imbalance in T-cell and cytokine profiles in patients with relapsing–remitting multiple sclerosis. J Neurol Sci 2011; 300: 135–141. [DOI] [PubMed] [Google Scholar]
- 21. Booss J, Esiri MM, Tourtellotte WW, et al. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci 1983; 62: 219–232. [DOI] [PubMed] [Google Scholar]
- 22. Lassmann H, Wekerle H. The pathology of multiple sclerosis. In: Compston A. (ed.) McAlpine’s Multiple Sclerosis. 4th ed. London: Churchill Livingstone, 2005, pp.557–599. [Google Scholar]
- 23. Cepok S, Jacobsen M, Schock S, et al. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain 2001; 124: 2169–2176. [DOI] [PubMed] [Google Scholar]
- 24. Tran A, Yang G, Doglio A, et al. Phenotyping of intrahepatic and peripheral blood lymphocytes in patients with chronic hepatitis C. Dig Dis Sci 1997; 42: 2495–2500. [DOI] [PubMed] [Google Scholar]
- 25. Pender MP. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency and steps to autoimmunity: A unifying hypothesis. Autoimmune Dis 2012; 2012: 189096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 2003; 101: 4260–4266. [DOI] [PubMed] [Google Scholar]
- 27. McFarland RD, Douek DC, Koup RA, et al. Identification of a human recent thymic emigrant phenotype. Proc Natl Acad Sci U S A 2000; 97: 4215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haegert DG, Hackenbroch JD, Duszczyszyn D, et al. Reduced thymic output and peripheral naïve CD4 T-cell alterations in primary progressive multiple sclerosis (PPMS). J Neuroimmunol 2011; 233: 233–239. [DOI] [PubMed] [Google Scholar]
- 29. Pender MP, Csurhes PA, Pfluger CMM, et al. CD8 T cell deficiency impairs control of Epstein–Barr virus and worsens with age in multiple sclerosis. J Neurol Neurosurg Psychiatry 2012; 83: 353–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pender MP, Csurhes PA, Lenarczyk A, et al. Decreased T cell reactivity to Epstein-Barr virus infected lymphoblastoid cell lines in multiple sclerosis. J Neurol Neurosurg Psychiatry 2009; 80: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lindsey JW, Hatfield LM. Epstein-Barr virus and multiple sclerosis: Cellular immune response and cross-reactivity. J Neuroimmunol 2010; 229: 238–242. [DOI] [PubMed] [Google Scholar]