ABSTRACT
Autophagy is an important cell survival pathway which is up-regulated under stress conditions.1) It is a well regulated catabolic process and enables the cell to recycle its constituents and organelles for re-use.1) Autophagy has been implicated to play an important role in a variety of disorders such as cancer and protein aggregatory neurodegenerative diseases e.g., Alzheimer's disease, Parkinson's disease and Huntington's disease.2) Iron is a critical metal required for normal cellular functioning.3) A very tightly regulated balance of iron levels is required for the normal physiological functioning of the cell.3) Both an excess and deficiency of iron can lead to cellular stress, and thereby, alters the autophagic status within the cell. Thus, it is important to completely understand how iron can affect the autophagic pathway and its potential implications under physiological as well as pathological conditions.
Key Words: Autophagy, Iron metabolism, Chelators
AUTOPHAGY
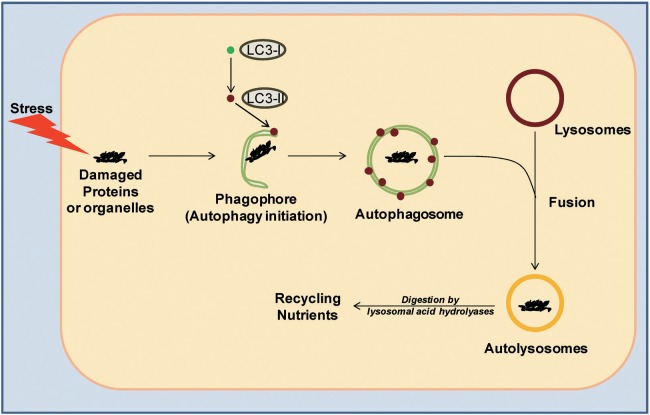
Autophagy is essentially a pro-survival, catabolic process in response to stress stimuli.1) It is a recycling mechanism by which cells digest damaged or effete constituents and/or organelles ("cargo") and recycle the nutrients (e.g., metals, lipids, amino acids etc.) back to the cell for essential processes.1) It is a well orchestrated system that starts with the formation of a crescent-shaped structure, known as a phagophore, around the damaged cargo1) (Fig. 1). This is followed by the recruitment of lipidated LC3-II protein on to the membrane, which then leads to formation of a double membrane-bound organelle, known as the autophagosome, that encapsulates the cargo.1) These autophagosomes then fuse with lysosomes to form autolysosomes1) (Fig. 1). The acidic hydrolases in the lysosomes digest the cargo and facilitate recycling of nutrients back to the cytoplasm for re-utilization.1)
Fig. 1.
The autophagic pathway: When cells experience stress it leads to an increase in damaged organelles and cellular constituents ("cargo"). This event triggers autophagic initiation and results in the formation of a crescent-shaped structure, the phagophore, around the damaged cargo. This is followed by lipidation of LC3-I to form LC3-II, which is then recruited onto the phagophore membrane. It is followed by membrane elongation and formation of double membranous organelle, known as autophagosome, around the damaged cargo. Notably, the autophagosomes are a hallmark of autophagy. The autophagosomes then fuse with lysosomes to form autolysosomes, where lysosomal acid hydrolases digest the cargo and recycle nutrients back into the cytoplasm.
Autophagy plays a role in cellular detoxification and helps in clearing damaged cellular constituents.1) De-regulation of autophagy can lead to accumulation of damaged and toxic molecules, which can result in pathological conditions such as the neurodegenerative disorders: Alzheimer's, Parkinson's and Huntington's disease.2) Moreover, autophagy is also known to play significant roles in tumor progression and metastasis.4, 5) Autophagy has been shown to have both pro- as well as anti-oncogenic effects, depending on tumor stage and its microenvironment.4) Some studies have demonstrated the involvement of autophagy in metastasis via its protective role in anoikis (i.e., loss-of-anchorage-dependent cell death) and cell dormancy, which are characteristics of metastatic cells.6-8)
IRON METABOLISM AND AUTOPHAGY
Iron is a requisite metal in almost all biological systems.3) It is required for numerous critical processes such as DNA synthesis, heme and iron-sulfur cluster synthesis etc.3) It also plays an important role in the active sites of various enzymes such as cytochrome c, aconitase, and ribonucleotide reductase.3) Hence, iron is an essential bio-metal required for normal physiological functioning of the cell. However, the levels of iron in the cell need to be tightly balanced, as an excess of iron can have damaging effects due to the generation of iron-catalyzed reactive oxygen species (ROS).9) In fact, iron can participate in the Fenton and Haber-Weiss reactions,9) leading to ROS generation, and thus, oxidative stress. Therefore, either deprivation of iron or excessive iron levels in the cell can lead to stress and this can result in up-regulation of stress-induced autophagy. In fact, Khan et al. have shown that the exogenous application of iron oxide nanoparticles to cells in culture can lead to induction of autophagy due to ROS generation.10)
As lysosomes are involved in autophagic turnover of organelles and long-lived proteins (many of which contain iron), they become enriched in low molecular weight iron.11) This increases the susceptibility of lysosomes towards oxidative stress via the Fenton and Haber-Weiss reactions.11) Moreover, the lysosomal vacuoles engaged in autophagic degradation of iron-rich proteins or organelles become more prone to oxidative stress than those in the resting state.11) The cytosol contains a number of iron-binding proteins (such as HSP70 and ferritin) and autophagocytosis of these proteins can potentially sequester redox-active iron in lysosomes and decrease lysosomal vulnerability to oxidative stress.12, 13) Cells rich in these proteins have been demonstrated to be much more resistant to oxidative stress.14) Thus, autophagy plays a critical role in balancing the redox-status of the cell via its ability to increase lysosomal iron levels as well as by autophagocytosis of iron-binding proteins.
Autophagy is also known to play an important role in maintaining physiological iron balance in the cell, through its role in the degradation of the iron-storage protein, ferritin.13, 15-23) Ferritin is known to be involved in removal of excess iron from the cytoplasm and its storage in a non-redox active form.24) Indeed, ferritin has been shown to be degraded in cells by lysosomal acid hydrolases16) and it is known that ferritin enters lysosomes through the autophagic pathway.15) Thus, autophagy plays a critical role in the mechanism via which iron homeostasis is maintained in the cell for normal physiological functioning.
EFFECT OF IRON CHELATORS ON AUTOPHAGY
Currently, very little is known regarding the effect of the iron chelators on autophagy. This is important to understand considering the use of these agents for the treatment of iron overload disease, but also potentially other conditions, including cancer.25) Autophagy is usually characterized experimentally by measurement of autophagosomes.26) This can be done either directly by microscopic visualization, or indirectly by measuring the levels of lipidated LC3-II protein, which is a classical marker for autophagosomes.26) As autophagy is a dynamic process, the levels of autophagosomes could increase either due to elevated autophagic initiation or due to decreased lysosome-mediated degradation of autophagosomes.26) To determine which part of autophagy pathway (i.e., initiation or degradation) is affected, it is important to utilize late-stage autophagic inhibitors, such as bafilomycin A1 or chloroquine, in the experimental protocol.26)
A recent investigation from our laboratory has demonstrated that the chelator, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT), which forms redox-active iron and copper complexes, increases autophagosomes and LC3-II levels in pancreatic cancer cells.27) The effect of Dp44mT on autophagy was compared with the classical iron chelator, desferrioxamine (DFO). Although, DFO also led to a significant increase in LC3-II levels, it was markedly less effective relative to Dp44mT.27) Furthermore, the role of redox activity of Dp44mT was assessed by its ability to induce LC3-II. The metal complexes of Dp44mT were able to induce LC3-II levels, while the DFO metal complexes did not, as they are redox-inactive.27) Moreover, the anti-oxidant, N-acetylcysteine, was able to suppress the Dp44mT-mediated increase in LC3-II levels.27) These results indicated that ROS generation by Dp44mT-metal complexes plays a more significant role in autophagic induction than iron chelation alone.27)
Using the late-stage autophagy inhibitor, bafilomycin A1,26) we demonstrated that Dp44mT increases initiation of the autophagic pathway.27) However, over-expression of the metastasis suppressor, N-myc downstream regulated gene-1 (NDRG1),28, 29) which is also known to be up-regulated by iron chelators including, Dp44mT,30) resulted in suppression of Dp44mT-mediated autophagic initiation.27) It was concluded that Dp44mT initially increases activation of the autophagic initiation machinery, which is then subsequently suppressed by NDRG1 up-regulation when cells were exposed to this chelator for prolonged periods of time.27)
Interestingly, a very recent study from our laboratory has compared the autophagic-induction efficacy of Dp44mT relative to DFO in breast cancer cells.31) It was shown that both Dp44mT and DFO led to an increase in autophagosome number and LC3-II levels.31) While Dp44mT was able to induce the autophagic-initiation pathway, it also led to permeabilization of the lysosomal membrane, and thus, rendered tumor cells unable to complete autophagy.31) In contrast, DFO was unable to initiate autophagy and mainly affected the lysosomal-mediated degradation phase of autophagy.31)
In another study, Wu et al. demonstrated that DFO induced autophagy and exerted its neuro-protective effects in the rotenone-treated SH-SY5Y cell model of Parkinson's disease.32) The authors further showed that DFO leads to the induction of autophagy via a hypoxia inducible factor-1α (HIF-1α)-dependent pathway.32) This latter investigation indicates an important role of autophagy in protein aggregatory neurodegenerative disorders such as Parkinson's disease. The difference in the ability of DFO to induce autophagy in these neuron-like cell models32) relative to breast cancer cells (Gutierrez et al., unpublished data), is potentially due to the different cell-types examined.
As discussed above, ferritin is primarily known to be degraded in the cell via autophagy.15, 16) Interestingly, Domenico et al. have shown that different iron chelators can specifically determine the pathway via which ferritin is degraded in cells.33) These authors demonstrated that poorly permeant DFO led to lysosome-mediated degradation of ferritin via autophagy.33) On the other hand, the membrane-permeant iron chelators, Desferasirox (DFX) and Deferiprone (DP), diverted ferritin degradation towards the proteasomal pathway.33) In contrast, another recent study has shown that both DFO and DFX lead to increased autophagy by suppression of the mammalian target of rapamycin (mTOR) signaling pathway.34) Again, the observed differences in the effect of DFX on the autophagic pathway may be cell-type dependent.
Critically, examining the studies above,33, 34) it is notable that both investigations only visualized LC3-II-containing autophagosomes as a marker of autophagy. Considering that autophagy is a dynamic pathway,26) the observed increase in autophagosomes could be either due to increased autophagic initiation or because of suppression of the lysosome-mediated autophagic degradation step.26) Hence, in order to ascertain the reason for the observed increase in autophagosome number,33, 34) it is imperative to use late-stage autophagy inhibitors,26) such as bafilomycin A1 or choloroquine, in combination with iron chelators. Thus, more detailed and better controlled studies will be required to determine the effect of different iron chelators on autophagy.
FUTURE PROSPECTIVES
Although, iron and autophagy play a crucial role under both physiological as well as pathological conditions, little work has been done to understand the intricate relationship between this important biological metal and how it influences the autophagic pathway and vice versa. More detailed investigations are required to understand the mechanisms via which de-regulation of iron metabolism can affect autophagy.
ACKNOWLEDGEMENTS
D.R.R. is the recipient of a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship and Project Grants. D.J.R.L thanks the NHMRC for an Early Career Postdoctoral Fellowship [1013810] and Cancer Institute of New South Wales (CINSW) for an Early Career Research Fellowship. P.J.J. acknowledges the CINSW for an Early Career Research Fellowship.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- 1).Hamacher-Brady A. Autophagy regulation and integration with cell signaling. Antioxid Redox Signal, 2012; 17: 756–765. [DOI] [PubMed]
- 2).Sahni S, Merlot AM, Krishan S, Jansson PJ, Richardson DR. Gene of the month: BECN1. J Clin Pathol, 2014; 67: 656–660. [DOI] [PubMed]
- 3).Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta, 1997; 1331: 1–40. [DOI] [PubMed]
- 4).Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol, 2010; 22: 241–245. [DOI] [PMC free article] [PubMed]
- 5).Tsuchihara K, Fujii S, Esumi H. Autophagy and cancer: dynamism of the metabolism of tumor cells and tissues. Cancer Lett, 2009; 278: 130–138. [DOI] [PubMed]
- 6).Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell, 2008; 19: 797–806. [DOI] [PMC free article] [PubMed]
- 7).Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer, 2009; 9: 274–284. [DOI] [PubMed]
- 8).White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell, 2004; 6: 159–170. [DOI] [PubMed]
- 9).Stohs S, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med, 1995; 18: 321–336. [DOI] [PubMed]
- 10).Khan MI, Mohammad A, Patil G, Naqvi S, Chauhan L, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials, 2012; 33: 1477–1488. [DOI] [PubMed]
- 11).Kurz T, Eaton JW, Brunk UT. The role of lysosomes in iron metabolism and recycling. Int J Biochem Cell Biol, 2011; 43: 1686–1697. [DOI] [PubMed]
- 12).Kurz T, Brunk UT. Autophagy of HSP70 and chelation of lysosomal iron in a non-redox-active form. Autophagy, 2009; 5: 93–95. [DOI] [PubMed]
- 13).Kurz T, Gustafsson B, Brunk UT. Cell sensitivity to oxidative stress is influenced by ferritin autophagy. Free Radic Biol Med, 2011; 50: 1647–1658. [DOI] [PubMed]
- 14).Karlsson M, Frennesson C, Gustafsson T, Brunk UT, Nilsson SEG, Kurz T. Autophagy of iron-binding proteins may contribute to the oxidative stress resistance of ARPE-19 cells. Exp Eye Res, 2013; 116: 359–365. [PubMed]
- 15).Hultcrantz R, Glaumann H. Intracellular fate of ferritin in HeLa cells following microinjection. Exp Cell Res, 1987; 171: 203–212. [DOI] [PubMed]
- 16).Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol, 2006; 291: C445–C455. [DOI] [PubMed]
- 17).Zhang Y, Mikhael M, Xu D, Li Y, Soe-Lin S, Ning B, Li W, Nie G, Zhao Y, Ponka P. Lysosomal proteolysis is the primary degradation pathway for cytosolic ferritin and cytosolic ferritin degradation is necessary for iron exit. Antioxid Redox Signal, 2010; 13: 999–1009. [DOI] [PubMed]
- 18).Bridges KR, Hoffman KE. The effects of ascorbic acid on the intracellular metabolism of iron and ferritin. J Biol Chem, 1986; 261: 14273–14277. [PubMed]
- 19).Garner B, Roberg K, Brunk UT. Endogenous ferritin protects cells with iron-laden lysosomes against oxidative stress. Free Radic Res, 1998; 29: 103–114. [DOI] [PubMed]
- 20).Konijn AM, Glickstein H, Vaisman B, Meyron-Holtz EG, Slotki IN, Cabantchik ZI. The cellular labile iron pool and intracellular ferritin in K562 cells. Blood, 1999; 94: 2128–2134. [PubMed]
- 21).Kwok J, Richardson D. Examination of the mechanism (s) involved in doxorubicin-mediated iron accumulation in ferritin: studies using metabolic inhibitors, protein synthesis inhibitors, and lysosomotropic agents. Mol Pharmacol, 2004; 65: 181–195. [DOI] [PubMed]
- 22).Radisky D, Kaplan J. Iron in cytosolic ferritin can be recycled through lysosomal degradation in human fibroblasts. Biochem J, 1998; 336: 201–205. [DOI] [PMC free article] [PubMed]
- 23).Roberts S, Bomford A. Ferritin iron kinetics and protein turnover in K562 cells. J Biol Chem, 1988; 263: 19181–19187. [PubMed]
- 24).Theil EC. Iron, ferritin, and nutrition. Annu Rev Nutr, 2004; 24: 327–343. [DOI] [PubMed]
- 25).Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev, 2005; 57: 547–583. [DOI] [PubMed]
- 26).Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell, 2010; 140: 313–326. [DOI] [PMC free article] [PubMed]
- 27).Sahni S, Bae D-H, Lane DJ, Kovacevic Z, Kalinowski DS, Jansson PJ, Richardson DR. The Metastasis Suppressor, N-myc Downstream-regulated Gene 1 (NDRG1), Inhibits Stress-induced Autophagy in Cancer Cells. J Biol Chem, 2014; 289: 9692–9709. [DOI] [PMC free article] [PubMed]
- 28).Bae D-H, Jansson PJ, Huang ML, Kovacevic Z, Kalinowski D, Lee CS, Sahni S, Richardson DR. The role of NDRG1 in the pathology and potential treatment of human cancers. J Clin Pathol, 2013; 66: 911–917. [DOI] [PubMed]
- 29).Fang BA, Kovacevic Z, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta, 2014; 1845: 1–19. [DOI] [PubMed]
- 30).Le NT, Richardson DR. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood, 2004; 104: 2967–2975. [DOI] [PubMed]
- 31).Gutierrez E, Jansson PJ, Richardson DR. The anticancer agent di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes prosurvival autophagy by two mechanisms: persistent induction of autophagosome synthesis and impairment of lysosomal integrity. J Biol Chem, 2014; 289: 33568–33589. [DOI] [PMC free article] [PubMed]
- 32).Wu Y, Li X, Xie W, Jankovic J, Le W, Pan T. Neuroprotection of deferoxamine on rotenone-induced injury via accumulation of HIF-1 alpha and induction of autophagy in SH-SY5Y cells. Neurochem Int, 2010; 57: 198–205. [DOI] [PubMed]
- 33).De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood, 2009; 114: 4546–4551. [DOI] [PMC free article] [PubMed]
- 34).Pullarkat V, Meng Z, Donohue C, Yamamoto VN, Tomassetti S, Bhatia R, Krishnan A, Forman SJ, Synold TW. Iron chelators induce autophagic cell death in multiple myeloma cells. Leuk Res, 2014; 38: 988–996. [DOI] [PubMed]