ABSTRACT
Groundwater contamination of arsenic is the major cause of a serious health hazard in Bangladesh. No specific treatment is yet available to manage the large number of individuals exposed to arsenic. In this study, we evaluated the protective effects of Phyllanthus emblica (Indian gooseberry or Amla) leaf extract (PLE) on arsenic-mediated toxicity in experimental mice. Male Swiss albino mice were divided into three different groups (n=6/group). ‘Control’ mice received arsenic free water together with normal feed. Mice in the remaining two groups designated ‘SA’ and ‘SA+PLE’ were exposed to sodium arsenite (SA, 10 µg/g body weight/day) through drinking water in addition to receiving normal feed and PLE-supplemented feed, respectively. The weight gain of SA-exposed mice was decreased compared with the controls; however, this decrease in body weight gain was prevented when the feed was supplemented with PLE. A secondary effect of arsenic was enlargement of the liver, kidney and spleen of SA-group mice. Deposition of arsenic in those organs was demonstrated by ICP-MS. When PLE was supplemented in the feed the enlargement of the organs was minimized; however, the deposition of arsenic was not significantly reduced. These results indicated that PLE may not block arsenic deposition in tissue directly but rather may play a protective role to reduce arsenic-induced toxicity. Therefore, co-administration of PLE in arsenic-exposed animals might have a future therapeutic application for protecting against arsenic-mediated toxicity.
Key Words: sodium arsenite, Phyllanthus emblica, growth retardation, mice, antioxidant
INTRODUCTION
Among several heavy metals that are present in the environment naturally, arsenic is recognized as one of the most dangerous poison. Millions of people around the globe are chronically exposed to arsenic through drinking contaminated ground water. Arsenic contamination of ground water in Bangladesh has so far been reported to be a major health hazard because of the large number of people affected.1-4) Arsenic is known to be a potent sulfhydryl-reactive chemical capable of binding and cross-linking cellular proteins5, 6) thereby altering multiple cellular pathways including expression of growth factors, suppression of cell cycle checkpoint proteins, promotion of apoptosis, inhibition of DNA repair, decreasing immunosurveillance, and increasing oxidative stress. These alterations in cellular pathways play key roles in various diseases in humans such as carcinogenicity, genotoxicity, diabetes, hypertension, weight loss, and cardiovascular and neurologic disorders. In addition, there has been increasing evidence of the correlation between arsenic exposure and the generation of reactive oxygen species (ROS) leading to tumor promotion.7, 8) Moreover, overproduction or an ineffective elimination of ROS may induce oxidative stress and cause damage or malfunctioning of various organs including liver, lungs, kidney and spleen.9-12) Scientists have started to use the antioxidant properties of medicinal plant sources to ameliorate the effect of arsenic toxicity. Turmeric and curcumin, a yellow pigment isolated from turmeric, have been shown to reduce adverse effects of arsenic in mice by virtue of their antioxidant potentials.13, 14) Recently aqueous extract of Psidium guajava leaves and Trichosanthes dioica fruit have been reported to partially protect against the degenerative changes in kidney and liver of arsenic-intoxicated rats by restoring various oxidative stress markers.12, 15) Some earlier studies also showed the protective efficacies of Spirulina fusiformis,16) Oscimum sanctum17) and Lycopersicon esculentum18) against oxidative stresses generated by various heavy metals such as mercury, arsenic, lead and cadmium. We have recently reported that arsenic-induced growth retardation in mice and elevation of serum enzymes such as LDH, ALP and SGPT were prevented by dietary supplementation of water hyacinth root powder.19)
Phyllanthus emblica (syn. Emblica officinalis), commonly known as Indian gooseberry or ‘amla’, is a major rejuvenating component of herbal drugs used in ayurvedic systems of medicine. It is extensively found all over India, as well as Sri Lanka, Malaysia, China, Pakistan and Bangladesh. This plant is an important dietary source of vitamin C and various minerals, and it also contains phenolic compounds such as tannins, phyllembelic acid, phyllembelin, rutin, curcum-inoids emblicol, emblicanin A and B, gallic acid, and ellagic acid.20, 21) Various parts of the plant show antidiabetic, antibacterial, antiulcerogenic, hepatoprotective, gastroprotective, and chemopreventive properties.21) Strong antioxidant activities of different components in this plant may be a reason for these various effects.22)
The present study investigated whether P. emblica leaf extract (PLE) could reduce or prevent arsenic-mediated adverse effects in experimental mice. We found that PLE had such activity and might be a good candidate to reduce the adverse effects of arsenic.
MATERIALS AND METHODS
Plant materials
The leaves of Phyllanthus emblica were collected from orchards at the University of Dhaka, Bangladesh. The plant was identified and authenticated and a voucher specimen (Accession no. DABC-35000) of the plant was deposited in Bangladesh National Herbarium.
Preparation of Phyllanthus emblica leaf extract (PLE)
The leaves of P. emblica were cleaned and washed repeatedly, air-dried at room temperature (~30°C) in a cool dry place away from direct sunlight for 7 to 10 days and finally ground to a coarse powder. Dried leaf powder (250 g) was suspended in 1 L ethanol (95%) in a flask. The flask was covered and kept for extraction at room temperature for 10 days. The extract was then filtered using Whatman filter paper (no. 11) to collect filtrate and to remove residual particulates. The residue was re-suspended in 700 ml ethanol (95%) to obtain additional extract. The filtrates were then concentrated using vacuum rotary evaporator. A gummy substance obtained thereby was subjected to drying at room temperature to prepare the powdered form. The powdered extract was weighed and stored at 4ºC for further work. From 250.0 g of dried leaf powder, 34.0 g (13.6%) of extract was finally obtained. The extract was dissolved in 40% ethanol before using. This powder (50 µg/g body weight/day) was mixed with mice feed purchased from International Centre for Diarrheal Disease Research, Bangladesh (ICDDR, B).
Animal maintenance
Male Swiss albino mice (6 weeks of age) of average body weight approximately 15 g were purchased from the Animal Division of ICDDR, B. Mice were randomly selected and housed in plastic cages with wood-cob bedding (6 mice per cage). After seven days of acclimation, mice were divided into three groups namely control, sodium arsenite (SA) and SA+PLE. Control mice were supplied with miliQ water using feeding bottles and normal mice feed. The SA-group mice were given normal feed and sodium arsenite (NaAsO2) containing water (prepared in miliQ water, 10 µg/g body weight/day) while the SA+PLE-group mice were provided with PLE (50 µg/g body weight/day) containing feed and SA containing water. These different groups of mice were maintained for 10 weeks. All these procedures and experiments using mice were undertaken following the ethical issues set by the Faculty of Biological Sciences, University of Dhaka, Bangladesh.
Determination of the body and organ weight of mice
Each mouse was weighed every two weeks and recorded accordingly. After 10 weeks mice were sacrificed by cervical dislocation and the abdomen was exposed surgically by ventral incision. The organs kidney, liver and spleen were collected and weighed.
Measurement of arsenic deposition in different organs
Levels of arsenic in the samples were measured by the method described previously.23) Briefly, liver, spleen and kidney samples were placed in a 15 ml polypropylene tube in the presence of 3 ml of nitric acid (61%). The tubes were capped and incubated at 80°C for 48 hrs, followed by cooling for 1 hr to room temperature. After cooling, 3 ml of hydrogen peroxide (30%) was added to each tube, followed by incubation at 80°C for 3 hrs. After suitable dilution of the digested materials with ultrapure water, levels of elements in the samples were determined by an inductively coupled plasma-mass spectrometer (ICP-MS; 7500cx, Agilent Technologies, Inc.) with a reaction cell for absence of ArCl ion interference.
Statistical analysis
Data were statistically analyzed using Two way ANOVA with GraphPad Prism 6.
RESULTS
PLE supplementation prevented arsenic-mediated decrease in body weight gain
In this study, we first examined whether PLE could play a protective role against arsenic-mediated retardation of growth in mice. The initial average body weights of control, SA-group, SA+PLE group of mice were 14.85±0.24, 15.22±0.26, and 14.82±0.25 g, respectively (Fig. 1). After 10 weeks, the average body weight of the control, SA, and SA+PLE was 40.13±0.54, 20.22±0.12, and 32.72±0.29 g, respectively. A continual increase in body weight was observed in the control mice. However, this increase in body weight was disrupted in the SA-group mice during the 10 weeks study period indicating arsenic-mediated growth retardation (Fig.1 and 2). The growth pattern of mice receiving feed supplemented with PLE was found to be similar to that of the controls indicating no apparent effects of PLE alone on mice growth (data not shown). When PLE was combined with SA, the reduced body weight gain observed in the SA-only mice was partially prevented (Fig. 1). Body weights of SA+PLE mice were significantly higher at each 2 weeks interval (p<0.05) compared with the SA-only mice. This result indicated that PLE might play some role in mitigating the growth retardation associated with arsenic exposure.
Fig. 1.
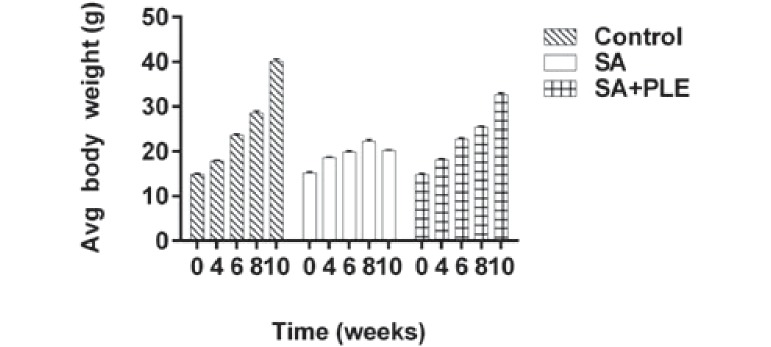
Protective effects of PLE on arsenic-mediated loss of body weight in mice. Body weight of each group of mice were taken at every two weeks and continued up to 10 weeks. Data of average (Avg) body weight of 4, 6, 8 and 10 weeks were plotted. Data shown as mean±SD (n=6 per group). p<0.01 control vs. SA or SA+PLE.
Fig. 2.
A representative photograph of one from each group (as indicated) after 10 weeks of arsenic exposure.
PLE prevented enlargement of spleen, liver and kidney in arsenic-exposed mice
Compared with the controls, the collected organs (spleen, liver and kidney) from mice in the SA group were enlarged. Based on the organ/body weight (bw) ratios, mice exposed to arsenic for 10 weeks had approximately 4-, 2.5-, and 4-fold larger spleens, livers and kidneys than control mice (Fig. 3). This increase in organ/bw ratios was mostly prevented when PLE was supplemented in feed as demonstrated by the comparable organ/bw ratios in both the control and SA+PLE groups (Fig.3). This result suggested that PLE might prevent arsenic-mediated toxic effects on those organs.
Fig. 3.
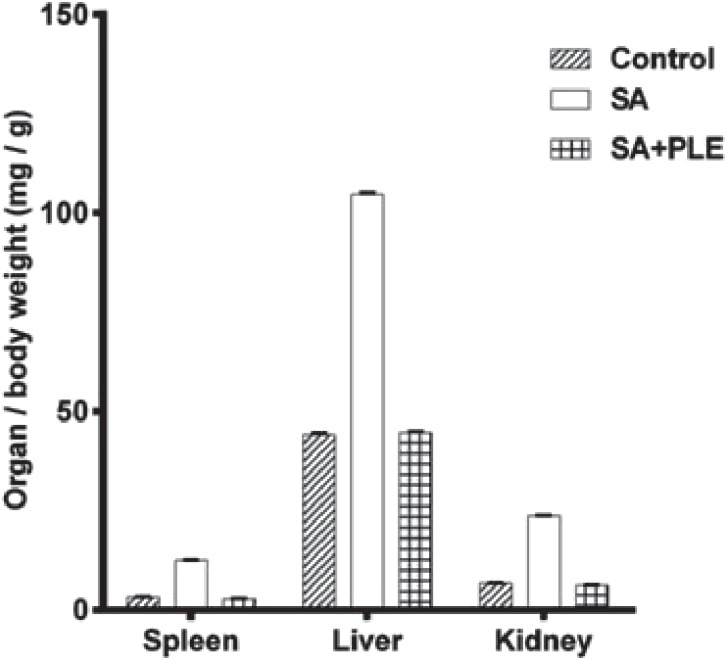
Arsenic-induced increase in organ/body weight ratio was blocked by PLE. The average organ/body weight (mg/g) ratio was plotted for each organ. Data shown as mean±SD (n=6 per group). p<0.01 control vs. SA or SA+PLE.
Tissue analysis demonstrated that arsenic was deposited at increased concentrations in the liver (2.74±0.1 µg/g body weight), kidney (1.50±0.075 µg/g body weight) and spleen (1.1±.0.06 µg/g body weight) of the SA-group mice (Fig. 4). PLE supplementation partially, but not significantly, reduced the level of arsenic deposition in those organs (liver 2.24±0.01, kidney 0.77±0.011 and spleen 0.83±0.012 µg/g body weight) (Fig. 4). These results indicated that the PLE could likely to reduce the arsenic-mediated toxic effects rather than its direct involvement to block the deposition of arsenic in those organs.
Fig. 4.
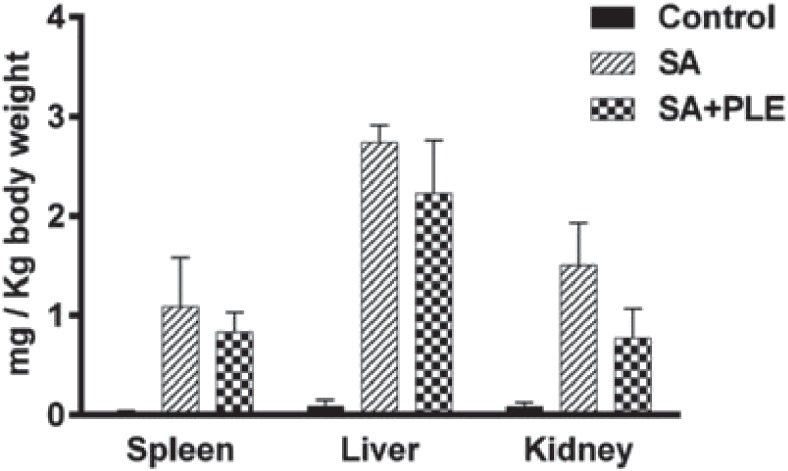
Deposition of arsenic (mg/Kg body weight) in different organs. Data shown as mean±SD (n=6 per group). p<0.01 control vs. SA or SA+PLE.
DISCUSSION
Epidemiological studies have shown that chronic exposure of arsenic can lead to a range of adverse health effects in human.1, 2, 24, 25) Arsenic produces free radicals that affect different organs including liver, kidney and brain resulting in development of various diseases.12, 26, 27) In this study, we explored using a well-known indigenous plant, Phyllanthus emblica, to mitigate some of the toxic effects of arsenic exposure in a mouse model. The overall toxicity of ingested arsenic caused growth retardation in mice (Fig. 1 and 2) probably by interfering with metabolic processes.28, 29) The decreased mice body weights observed in this study was comparable to earlier results from our group19) and others.13, 30) What our results have shown for the first time is that PLE supplemented in feed prevented the effects on body weight gain in mice co-exposed to arsenic. The exact mechanism behind the effects is still unclear; however, the high antioxidant contents of PLE might play roles in scavenging free radicals generated by arsenic.22, 31) This view was supported by antioxidant properties of the polyphenols of tea extracts that showed efficacy against arsenic- and lead-induced toxicity in experimental animals.32, 33) In addition, some earlier studies reported reduction of arsenic-mediated adverse effects in mice when they were orally administered turmeric and curcumin.13, 14) This action of turmeric and curcumin might also be explained by scavenging the free radicals generated by arsenic.34, 35)
An association between environmental arsenic exposure and hepatomegaly, hepatoportal sclerosis and liver fibrosis has been previously reported.36-39) The incidence of hepatomegaly was greatly increased in hospitalized arsenicosis patients of West Bengal, India, and a positive correlation between level of arsenic in drinking water and hepatic arsenic content was shown.9) In our study, increased liver, spleen and kidney weights were clearly observed in SA-exposed mice demonstrated by increased organ/body weight ratio (Fig. 3). Splenic enlargement as increases organ/body weight ratios was shown previously in cGMP-dependent protein kinase type I-deficient mice.40) Swine exposed to heavy metals were also shown to have enlarged kidneys.41) In our study, PLE supplementation in the feed blocked this increase in organ weights, and we found that the weights of the organs in these mice were almost similar to those of the control mice than to the arsenic only mice.
In cases of chronic ingestion, arsenic is known to accumulate in the liver, kidneys, heart, lungs, muscles and spleen.42, 43) ICP-MS analysis of the organ samples in our study also showed deposition of arsenic in the liver, kidney and spleen. Electrothermal atomic absorption spectrometry studies performed earlier showed that the highest concentration of arsenic was in the liver and kidneys,42) that are consistent with our present observations. Although the ability of PLE to prevent arsenic-mediated decrease in body weight gain and enlargement of organ weights was firmly evident (Figs. 1 and 3), its ability to reduce arsenic deposition in organs, however, was not significant (Fig. 4).
P. emblica is very common and native tree in Bangladesh and West Bengal in India, where groundwater arsenic contamination has become a menacing problem. Further investigation is needed to explain the mechanism of PLE for the reduction of arsenic toxicity and to explore its use as a potential candidate to remediate arsenic effect.
ACKNOWLEDGEMENT
This research received no specific grant. We thank Shoko Ohnuma for ICP-MS analysis of the samples.
DISCLOSURE STATEMENT
All authors declare to have no actual or potential conflicts of interest.
REFERENCES
- 1).Khan MMH, Aklimunnessa K, Ahsan N, Kabir M, Mori M. Case-control study of arsenicosis in some arsenic contaminated villages of Bangladesh. Sapporo Med J, 2006; 75: 51–61.
- 2).Chowdhury UK, Rahman MM, Mondal BK, Paul K, Lodh D, Biswas BK, Basu GK, Chanda CR, Saha KC, Mukharjee SC, Roy S, Das R, Kaies I, Barua AK, Palit SK, Quamruzzaman Q, Chakraborti D. Groundwater arsenic contamination and human suffering in West Bengal, India and Bangladesh. Environ Sci, 2010; 8: 393–415.
- 3).Mazumder DNG, Ghoshal UC, Saha J, Santra A, De BK, Chatterjee A, Dutta S, Angle CR, Centeno JA. Randomized placebo-controlled trial of 2, 3-dimercaptosuccinic acid in therapy of chronic arsenicosis due to drinking arsenic-contaminated subsoil water. J Toxicol Clin Toxicol, 1998; 36: 683–690. [DOI] [PubMed]
- 4).McLellan F. Arsenic contamination affects millions in Bangladesh. Lancet, 2002; 359: 1127. [DOI] [PubMed]
- 5).Hossain K, Akhand AA, Kato M, Du J, Takeda K, Wu J, Takeuchi K, Liu W, Suzuki H, Nakashima I. Arsenite induces apoptosis of murine T lymphocytes through membrane raft-linked signaling for activation of c-Jun amino-terminal kinase. J Immunol, 2000; 165: 4290–4297. [DOI] [PubMed]
- 6).Akhand AA, Du J, Liu W, Hossain K, Miyata T, Nagase F, Kato M, Suzuki H, Nakashima I. Redox-linked cell surface-oriented signaling for T-cell death. Antioxid Redox Signal, 2004; 4: 445–454. [DOI] [PubMed]
- 7).Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H. Evidence for induction of oxidative stress caused by chronic exposures of Chinese residents to arsenic contained in drinking water. Environ Health Perspect, 2002; 110: 331–336. [DOI] [PMC free article] [PubMed]
- 8).Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell Biochem, 2004; 25567–25578. [DOI] [PubMed]
- 9).Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Mazumder DNG. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J Toxicol Clin Toxicol, 2000; 38: 395–405. [DOI] [PubMed]
- 10).Ali N, Hoque MA, Haque A, Salam KA, Karim MR, Rahman A, Islam K, Saud ZA, Khalek MA, Akhand AA, Hossain M, Mandal A, Karim MR, Miyata H, Himeno S, Hossain K. Association between arsenic exposure and plasma cholinesterase activity: a population based study in Bangladesh. Environ Health, 2010; 10: 9:36. [DOI] [PMC free article] [PubMed]
- 11).Alarifi S, Ali D, Alkahtani S, Siddiqui MA, Ali BA. Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco Targets Ther, 2013; 6: 75–84. [DOI] [PMC free article] [PubMed]
- 12).Tandon N, Roy M, Roy S, Gupta N. Protective Effect of Psidium guajava in Arsenic-induced Oxidative Stress and Cytological Damage in Rats. Toxicol Int, 2012; 19: 245–249. [DOI] [PMC free article] [PubMed]
- 13).Karim MR, Haque A, Islam K, Ali N, Salam KA, Saud ZA, Hossain E, Fazol A, Akhand AA, Himeno S, Hossain K. Protective effects of the dietary supplementation of turmeric (Curcuma longa L.) on sodium arsenite-induced biochemical perturbation in mice. Bangladesh Med Res Counc Bull, 2010; 36: 82–88. [DOI] [PubMed]
- 14).Biswas J, Roy S, Mukherjee S, Sinha D, Roy M. Indian spice curcumin may be an effective strategy to combat the genotoxicity of arsenic in Swiss albino mice. Asian Pac J Cancer Prev, 2010; 11: 239–247. [PubMed]
- 15).Bhattacharya S, Haldar PK. Trichosanthes dioica fruit ameliorates experimentally induced arsenic toxicity in male albino rats through the alleviation of oxidative stress. Biol Trace Elem Res, 2012; 148: 232–41. [DOI] [PubMed]
- 16).Kumar M, Sharma MK, Kumar A. Spirulina fusiformis: a food supplement against mercury induced hepatic toxicity. J Health Sci, 2005; 51: 424–430.
- 17).Sharma MK, Kumar M, Kumar A. Ocimum sanctum aqueous leaf extract provides protection against mercury induced toxicity in Swiss albino mice. Indian J Exp Biol, 2002; 40: 1079–1082. [PubMed]
- 18).Nwokocha CR, Nwokocha MI, Aneto I, Obi J, Udekweleze DC, Olatunde B, Owu DU, Iwuala MO. Comparative analysis on the effect of Lycopersicon esculentum (tomato) in reducing cadmium, mercury and lead accumulation in liver. Food Chem Toxichol, 2012; 50: 2070–2073. [DOI] [PubMed]
- 19).Sarker RSJ, Ahsan N, Hossain K, Goush PK, Akhand AA. Protective effects of water hyacinth (Eichornia crassipes) on sodium arsenite-mediated adverse effects in mice. Avicenna J Med Biotech, 2012; 4: 148–154. [PMC free article] [PubMed]
- 20).Zang Y, Nagao T, Tanaka T, Yang CR, Okabe H, Kouno I. Antiproliferative activity of the main constituents from Phyllanthus emblica Biol Pharm Bull, 2004; 27: 251–255. [DOI] [PubMed]
- 21).Krishnaveni M, Mirunalini S. Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol, 2010; 21: 93–105. [DOI] [PubMed]
- 22).Jain PK, Ravichandran V, Sharma S, Agarwal RK. The antioxidant activity of some medicinal plants. Turk J Biol, 2008; 32: 197–202.
- 23).Kato M, Kumasaka MY, Ohnuma S, Furuta A, Kato Y, Shekhar HU, Kojima M, Koike Y, Dinh Thang N, Ohgami N, Ly TB, Jia X, Yitte H, Naito H, Ichihara G, Iajima I. Comparison of Barium and Arsenic Concentrations in Well Drinking Water and in Human Body Samples and a Novel Remediation System for These Elements in Well Drinking Water. PLoS ONE, 2013; 8: e66681. [DOI] [PMC free article] [PubMed]
- 24).Abernathy AS, Grissom RE, Charles O. Estimating Total Arsenic Exposure in the United States. In: Arsenic Exposure and Health Effects III, edited by Chappell WR, Abernathy CO, Calderon RL. pp. 51–60, 1999, 51–60. Elsevier.
- 25).Chowdhury U, Chakraborti D, Rahman MM, Paul K, Sengupta M. Arsenic calamity in the Indian subcontinents: What lessons have been learned? Talanta, 2002; 58: 3–22. [DOI] [PubMed]
- 26).Piao F, Ma N, Hiraku Y, Murata M, Oikawa S, Cheng F, Zhong L, Yamauchi T, Kawanishi S, Yokoyama K. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J Occupational Health, 2005; 47: 445–449. [DOI] [PubMed]
- 27).Mahfuzar R, Martin T, Ahmad SA, Olav A. Diabetes Mellitus Associated with Arsenic Exposure in Bangladesh. Am J Epidemiol, 1998; 148: 198–203. [DOI] [PubMed]
- 28).Tu C, Ma LQ. Effects of arsenic concentrations and forms on arsenic uptake by the hyperaccumulator ladder brake. J Environ Qual, 2002; 31: 641–647. [PubMed]
- 29).Florea AM, Ebenezer N, Yamoah, Dopp E. Intracellular Calcium Disturbances Induced by Arsenic and Its Methylated Derivatives in Relation to Genomic Damage and Apoptosis Induction. Environ Health Perspect, 2005; 113: 659–664. [DOI] [PMC free article] [PubMed]
- 30).Verma RJ, Vasu A, Saiyed AA. Arsenic toxicity in mice and its possible amelioration. J Environ Sci (China), 2004; 16(3): 447–53. [PubMed]
- 31).Poltanov EA, Shikov AN, Dorman HJ, Pozharitskaya ON, Makarov VG, Tikhonov VP, Hiltunen R. Chemical and antioxidant evaluation of Indian gooseberry (Emblica officinalis Gaertn., syn. Phyllanthus emblica L.) supplements. Phytother Res, 2009; 23: 1309–1315. [DOI] [PubMed]
- 32).Sinha D, Roy S, Roy M. Antioxidant potential of tea reduces arsenite induced oxidative stress in Swiss albino mice. Food Chem Toxicol, 2010; 48: 1032–1039. [DOI] [PubMed]
- 33).Mehana EE, Meki AR, Fazili KM. Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp Toxicol Pathol, 2012; 64: 291–295. [DOI] [PubMed]
- 34).Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res, 2002; 62: 3868–3875. [PubMed]
- 35).Tirkey N, Kaur G, Vij G, Chopra K. Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacology, 2005; 5: 189–196. [DOI] [PMC free article] [PubMed]
- 36).Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect, 2000; 108: 655–666. [DOI] [PMC free article] [PubMed]
- 37).Centeno JA, Mullick FG, Martinez L, Page NP, Gibb H, Longfellow D, Thompson C, Ladich ER. Pathology related to chronic arsenic exposure. Environ Health Perspect, 2002; 110: 883–886. [DOI] [PMC free article] [PubMed]
- 38).Chiu HF, Ho SC, Wang LY, Wu TN, Yang CY. Does arsenic exposure increases the risk for liver cancer? J Toxicol Environ Health, 2004; 67: 1491–1500. [DOI] [PubMed]
- 39).Mazumder DNG. Effect of chronic intake of arsenic contaminated water on liver. Toxicol Appl Pharmacol, 2005; 206: 160–175. [DOI] [PubMed]
- 40).Föller M, Feil S, Ghoreschi K, Koka S, Gerling A, Thunemann M, Hofmann F, Schuler B, Vogel J, Pichler B, Kasinathan RS, Nicolay JP, Huber SM, Lang F, Feil R. Anemia and splenomegaly in cGKI-deficient mice. Proc Natl Acad Sci (USA), 2008; 105: 6771–6776. [DOI] [PMC free article] [PubMed]
- 41).Milićević DR, Milijan J, Verica JB, Zoran PI, Dubravka VŽ. Assessment of toxic elements’ content in swine kidneys: Pathomorphological analysis. Archive Oncol, 2010; 18: 17–22.
- 42).Benramdane L, Accominotti M, Fanton L, Malicier D, Vallon JJ. Arsenic speciation in human organs following fatal arsenic trioxide poisoning—a case report. Clin Cheb, 1999; 45: 301–306. [PubMed]
- 43).Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J, 2003; 79: 391– 396. [DOI] [PMC free article] [PubMed]


