ABSTRACT
Carbon nanotubes (CNTs) are a novel synthetic material comprising only carbon atoms. Based on its rigidity, its electrical and heat conductivity and its applicability to surface manufacturing, this material is expected to have numerous applications in industry. However, due to the material’s dimensional similarity to asbestos fibers, its carcinogenicity was hypothesized during the last decade, and indeed, we have shown that multi-wall CNTs (MWCNTs) of 50 nm in diameter are potently carcinogenic to mesothelial cells after intraperitoneal injection. Additionally, we suggested that inflammogenicity after intraperitoneal injection can predict mesothelial carcinogenesis. However, few data have been published on the intraperitoneal inflammogenicity of single-wall CNTs (SWCNTs). Here, we conducted a series of studies on SWCNTs using both intraperitoneal injection into rats and MeT5A mesothelial cells. Intraperitoneal injection of 10 mg SWCNTs caused no remarkable inflammation in the abdominal cavity, and the exposure of MeT5A cells to up to 25 μg/cm2 SWCNTs did not alter proliferation. MWCNTs of 50 nm in diameter were used as a positive control, and tangled MWCNTs of 15 nm in diameter were used as a negative control. The results suggest that SWCNTs are a low-risk material with respect to mesothelial carcinogenesis.
Key Words: carbon nanotube, inflammation, mesothelioma, human risk assessment
INTRODUCTION
Novel synthetic materials in industry often change our daily life dramatically. Without the invention of blue light-emitting diodes for energy-saving white light sources,1) the development of numerous consumer electronics, including liquid-crystal television, smart phones and Blu-ray discs, would not have been accomplished.2) Carbon nanotubes (CNTs) are one such synthetic nanomaterial, consisting only of carbon.3) These are classified into either single-wall CNTs (SWCNTs) or multi-wall CNTs (MWCNTs), and the diameter of these nanotubes ranges from 1–200 nm, which is comparable to the size of a single biomolecule of a bacterium. CNTs are already on the consumer market as a material for battery cells4) and liquid-crystal film.5)
MWCNTs are distinct from SWCNTs due to their difference in diameter, which ultimately results in a difference in rigidity.6, 7) In the early 2000s, toxicologists recognized that MWCNTs are similar to asbestos in dimensions, rigidity and biological persistence and warned about this material’s possible carcinogenicity to mesothelial cells.8-10) Indeed, MWCNTs induced malignant mesothelioma (MM) in p53+/– knockout mice11) and in the tunica vaginalis of Fischer-344 rats.12) We have reported that the diameter of MWCNTs is a critical factor for inflammogenicity and the subsequent mesothelial carcinogenesis.13) Inflammogenicity and mesothelial carcinogenesis were proportionally associated. MWCNTs with a diameter of 50 nm were most carcinogenic to mesothelial cells,13) whereas tangled MWCNTs with a diameter of 15 nm proved non-carcinogenic.14) Therefore, MWCNTs with a diameter of 50 nm should be handled with extreme care.7)
In contrast, SWCNTs have been a good candidate for drug delivery systems,15, 16) in addition to other industrial uses.17) However, toxicological data are still lacking.7) Here, we evaluated two different types of SWCNTs via an electron microscopy analysis, a cell culture analysis and intraperitoneal injection into rats. These data showed much lower toxicity and inflammogenicity for SWCNTs than for MWCNTs.
MATERIALS AND METHODS
Characterization of fibers
Two different types of pristine (non-functional) SWCNT fibers (Lot No. HT0209-DA000 and HT0209-SA001, called SWCNT1 and SWCNT2, respectively) were obtained from Zeon Corporation (Tokyo, Japan). The surface areas were 1,100 and 650 m3/g, respectively. For transmission electron microscopy analyses, SWCNTs suspended in ethanol via vigorous sonication were spread directly over a copper grid with a plastic mesh and a carbon coat (Cat. No. 653; Nisshin EM). After drying, the fibers were analyzed. High-resolution transmission electron microscopy (HRTEM) images were obtained with a JEM-2100F high-resolution transmission electron microscope with a field emission gun (JEOL, Japan) operated at 80 keV under a pressure of <10−6 Pa at room temperature.
MWCNTs were also used as positive (MWCNT-50) and negative (MWCNT-tngl) controls. These fibers were previously fully characterized.13) Briefly, MWCNT-50 has a diameter of 50 nm and is highly inflammogenic and highly carcinogenic, whereas MWCNT-tngl has a diameter of 15 nm, is scarcely inflammogenic and induces no MM.14)
Animal experiments
SWCNT1 was used for animal experiments. SWCNTs were dispersed at a concentration of 5 mg/ml in physiological saline solution containing 0.5% crystalline bovine serum albumin (GIBCO Life Technologies, Carlsbad, CA) via sonication for 10 min immediately before use. The animal experiment committee of Nagoya University Graduate School of Medicine approved this experiment. In total, 24 6-week-old male Wistar rats (~120 g body weight) were obtained from SLC Japan (Shizuoka, Japan) and maintained under controlled temperature (24±1°C), humidity (55±5%), and light (light on from 7:00–19:00 h) conditions, with free access to standard laboratory chow (CE-2, CLEA Co. Ltd., Osaka, Japan) and water under specific pathogen-free conditions, as previously described.13) The rats were divided into 8 groups of 3 animals each, receiving 0.1 mg SWCNTs, 0.3 mg SWCNTs, 1.0 mg SWCNTs, 3.0 mg SWCNTs, 10 mg SWCNTs, 10 mg MWCNT-50, 10 mg MWCNT-tngl or vehicle control (3 ml of 0.5% albumin in physiological saline). Each fiber or vehicle was intraperitoneally injected into the rats at a single dose, and the animals were then closely observed until euthanasia 4 weeks later. We fully examined the abdominal cavity. The liver, spleen and kidneys were excised; fixed in 10% neutral buffered formalin; and subjected to routine paraffin-embedded sectioning, followed by hematoxylin and eosin staining.
Cell experiments
MeT5A, an immortalized human mesothelial cell line, was obtained from ATCC (Manassas, VA). The MeT5A cells were maintained in Medium 199 containing 0.685 mM L-glutamine, 2.2 g/l sodium bicarbonate, 10 ng/ml epidermal growth factor, 400 nM hydrocortisone, 870 nM recombinant human insulin, 25 mM HEPES, 2.8 ml/l Trace Elements B Solution from Mediatech (Manassas, VA) and 10% fetal bovine serum in 5% CO2 in air at 37°C. Then, 5 × 103 cells were seeded on a 96-well plate, which was followed by the addition of fibers or vehicle at the indicated concentration (0.5–25 μg/cm2). Thereafter, cell viability was quantified at 24, 48 and 72 h with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay.
Statistical analysis
Unpaired Student’s t-test was used for analysis. P<0.05 was considered statistically significant.
RESULTS
SWCNTs exhibited single- or double-wall tubes with flattened portions
The HRTEM images were analyzed. Both types of SWCNTs contained few impurities but showed a minimal amount of particulate matter that could serve as a possible catalyst for synthesis (Fig. 1A). Both types of SWCNTs were tangled like a spider web (Fig. 1A and 1B), so it was not possible to determine the length of the fibers. The SWCNTs showed either a single- or a double-wall structure (Fig. 1C). Both types of SWCNTs showed flattened and even twisted portions (Fig. 1D), and those portions were observed more often in SWCNT2 than in SWCNT1. Importantly, the SWCNTs with a diameter larger than approximately 4 nm showed so-called flattened CNT (FNT) structures, as previously reported by our research group.18) The distribution of the widest diameter for SWCNT1 was measured for the single- and double-wall structures. The outer layer of the double-wall CNTs (mean ± SEM, 4.30±0.59 nm) was not significantly thicker than that of the single-wall CNTs (3.83±0.165 nm; P=0.3545). However, more variability in diameter was observed among the double-wall CNTs (Fig. 2).
Fig. 1.
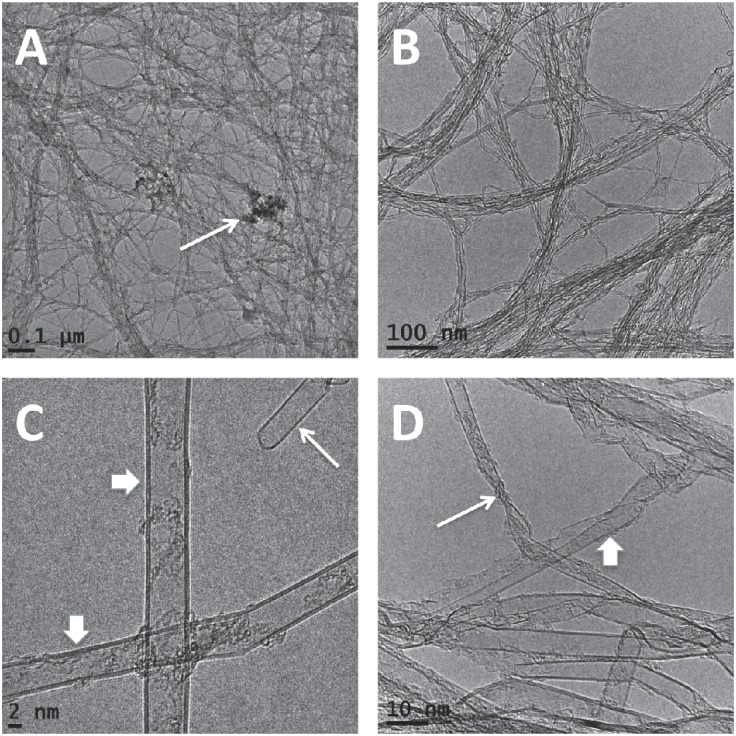
Electron microscopy observation of the SWCNTs used. A) Low-magnification image of SWCNT1. Note the diffuse meshwork structure, even after sonication in ethanol. The arrow shows a minimal amount of particulate matter. B) Low-magnification image of SWCNT2. Note the diffuse meshwork structure. C) HRTEM image of SWCNT1. The long arrow shows a single-wall CNT, and the short arrows indicate double-wall CNTs. D) HRTEM image of SWCNT2. The long arrow shows kinking, and the short arrow indicates a flattened portion.
Fig. 2.
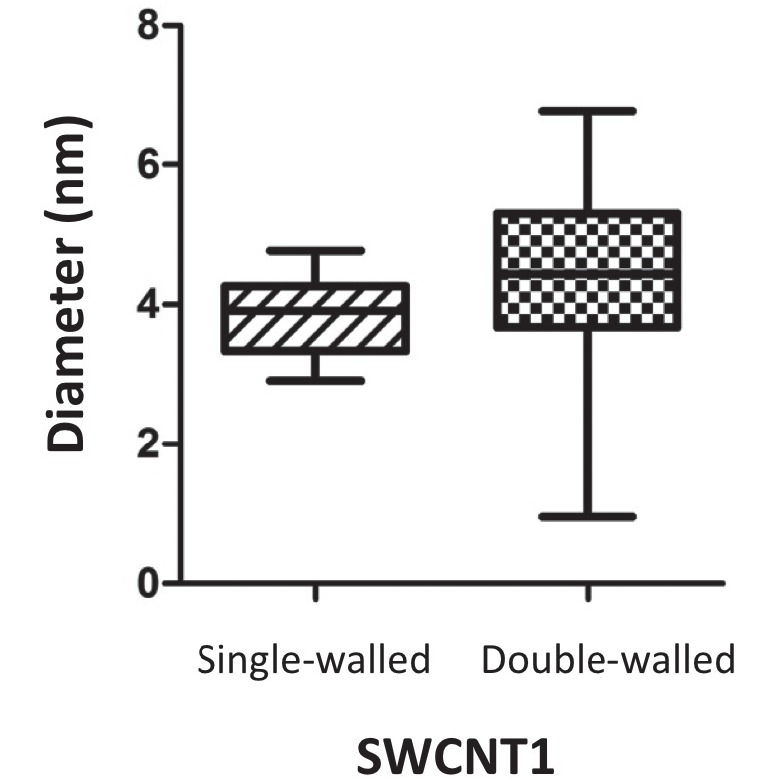
Distribution of the diameter of SWCNT1. The widest diameter of the double-wall portion was not significantly larger than that of the single-wall portion but showed much variability. The range corresponds to 5–95% of each group (N=13 for single-wall tubes; N=8 for double-wall tubes).
SWCNTs did not induce recognizable inflammation in the peritoneal cavity
Intraperitoneal injection of 10 mg MWCNT-50 fibers induced severe peritonitis, as we previously reported.13) Peritoneal fibrosis was evident, as confirmed by the dull edge of the liver (Fig. 3). However, SWCNT1 caused no recognizable alteration macroscopically, except for the presence of aggregated SWCNTs, observed as black foreign bodies (Fig. 3). Collectively, the observations were similar to those for MWCNT-tngl.
Fig. 3.
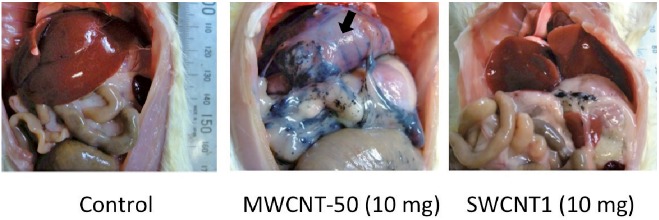
Macroscopic appearance of the rat peritoneal cavity 4 weeks after a single injection of each type of CNT. MWCNT-50 showed massive fibrosis with chronic inflammation. SWCNT1 showed nearly no change, except for the presence of residual CNTs. MWCNT-50, multi-wall CNTs of 50 nm in diameter.
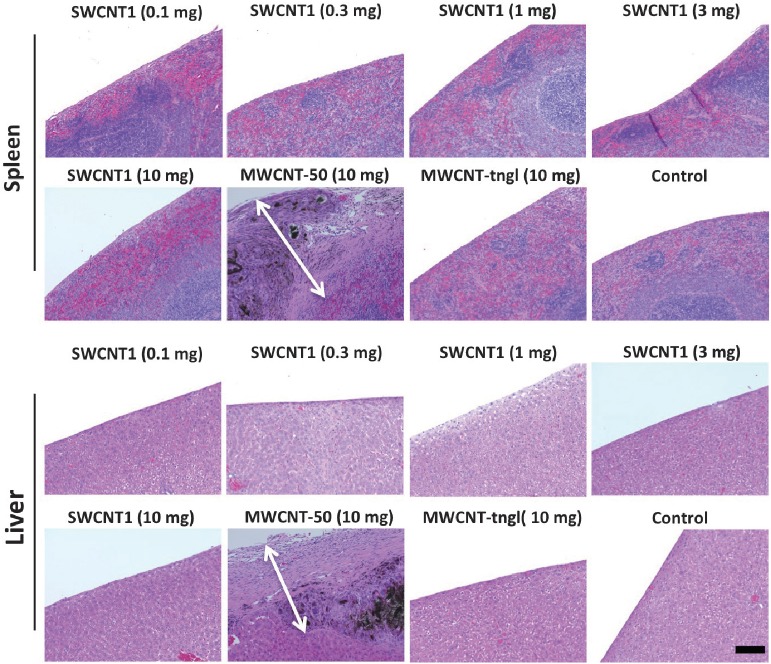
The pathologic alteration was confirmed with histological analysis. MWCNT-50 caused massive fibrosis on the surface of the intraperitoneal organs and peritoneum. The thickness of the fibrosis was over 500 μm (Fig. 4), corresponding to a white membranous appearance macroscopically (Fig. 3). The fibrotic lesion was extremely diffuse, with a scattered presence of foreign-body reactions. However, these fibrotic lesions were absent for SWCNT1 and MWCNT-tngl (Fig. 4).
Fig. 4.
Fig. 4 Histological analysis of the peritoneal surface of the spleen and liver after a single injection of each type of CNT. Only MWCNT-50 showed massive fibrosis on the surface of the spleen and liver. The arrows show the thickness of the fibrosis. The black material is the injected MWCNT-50. MWCNT-tngl, tangled (diameter = 15 nm) multi-wall CNTs (hematoxylin and eosin staining; bar = 100 μm).
SWCNTs did not induce cytotoxicity to the human mesothelial cell line MeT5A
The two different types of SWCNTs were not toxic to MeT5A cells up to a concentration of 25 μg/cm2, whereas at a concentration as low as 5 μg/cm2, MWCNT-50 was significantly toxic (Fig. 5).
Fig. 5.
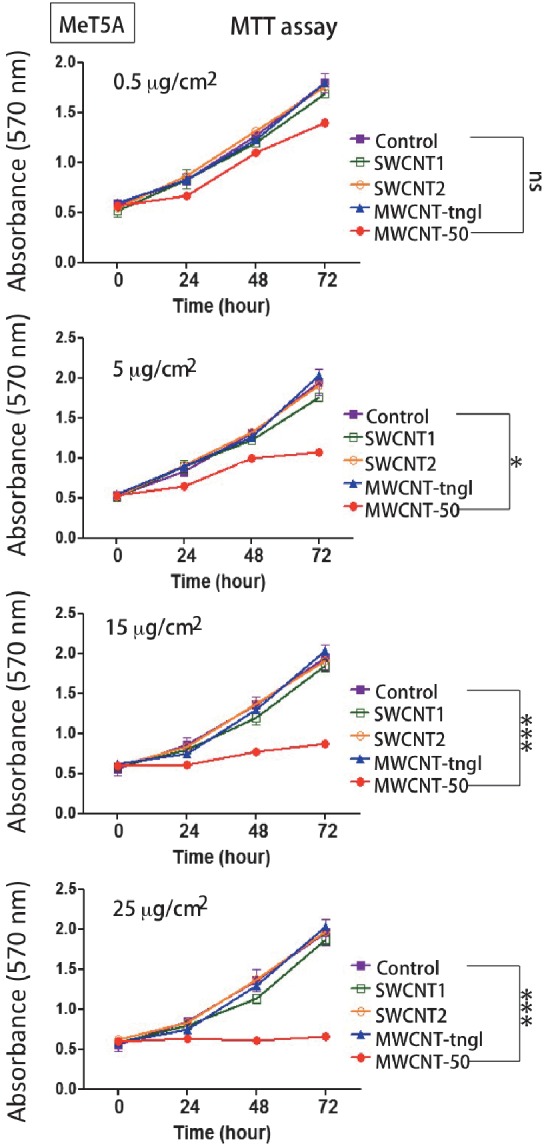
Cytotoxicity of CNTs to human mesothelial cells. Only MWCNT-50 showed a dose-dependent toxicity to MeT5A cells. ns, not significant; *, P<0.05; ***, P<0.001.
DISCUSSION
In the present study, we analyzed the dimensions of manufactured pristine SWCNTs, confirmed the distribution of their size and found that they contain FNT portions. Importantly, pristine SWCNTs showed minimal inflammogenicity following intraperitoneal administration. This was in clear contrast to the results for MWCNTs, and especially those of 50 nm in diameter, which are potently carcinogenic to mesothelial cells following intraperitoneal administration.13)
In our previous experiments, we demonstrated that the results of a 4-week study of intraperitoneal injection predicted eventual mesothelial carcinogenicity.13) The MWCNTs that showed nearly no inflammogenicity in the 4-week study (MWCNT-tngl) caused no MM at all.14) Therefore, our present results suggest that SWCNTs are a low-risk material with respect to mesothelial carcinogenesis, even without considering the respiratory system, through which humans are exposed to these nanomaterials. This conclusion depends on our hypothesis that toxicity to and the following damage to mesothelial cells are critical factors for mesothelial carcinogenesis.13) Confirmation via carcinogenesis is preferable. However, considering the high cost, large amount of time and ethical issues involved in animal carcinogenesis studies, we believe that a 4-week intraperitoneal injection test for mass screening can be used as a substitute.
SWCNTs move inside the body much more flexibly than MWCNTs do. It was reported that certain MWCNTs are transported by the blood flow and finally excreted in the urine.19) These results of other investigators suggest that we need to further study the effect of SWCNTs on the cardiovascular and nervous systems to complete the assessment of major human health risks. Unfortunately, there are no effective labeling or detection techniques for SWCNTs thus far, requiring further development.
In conclusion, this intraperitoneal injection study suggests that SWCNTs have low carcinogenicity to mesothelial cells.
ACKNOWLEDGMENTS
This work was supported in part by the National Cancer Center Research and Development Fund (25-A-5); a grant-in-aid for research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (24390094; 221S0001-04; 24108001); and a grant from Zeon Corporation. We thank Nobuaki Misawa (Department of Pathology and Biological Responses, Nagoya University Graduate School of Medicine) for excellent technical assistance.
CONFLICT OF INTEREST
Single-wall carbon nanotubes were donated by Zeon Corporation (Tokyo, Japan). This study was supported in part by Zeon Corporation.
Abbreviations
- CNT
carbon nanotube
- FNT
flattened CNT
- HRTEM
high-resolution transmission electron microscope
- MM
malignant mesothelioma
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MWCNT
multi-wall carbon nanotube
- SWCNT
single-wall carbon nanotube
REFERENCES
- 1).Akasaki I, Amano H, Kito M, Hiramatsu K. Photoluminescence of Mg-doped p-type GaN and electroluminescence of GaN p-n junction LED. J Lumin, 1991; 48–49: 666–670.
- 2).Sheu J-K, Chang S-J, Kuo CH, Su Y-K, Wu LW, Lin YC, Lai WC, Tsai JM, Chi G-C, Wu RK. White-light emission from near UV InGaN-GaN LED chip precoated with blue/green/red phosphors. IEEE Photonics Technol Lett, 2003; 15: 18–20.
- 3).Iijima S. Helical Microtubules of Graphitic Carbon. Nature, 1991; 354: 56–58.
- 4).Reddy ALM, Shaijumon MM, Gowda SR, Ajayan PM. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Lett, 2009; 9: 1002–1006. [DOI] [PubMed]
- 5).Lynch MD, Patrick DL. Organizing carbon nanotubes with liquid crystals. Nano Lett, 2002; 2: 1197–1201.
- 6).Nagai H, Toyokuni S. Biopersistent fiber-induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys, 2010; 502: 1–7. [DOI] [PubMed]
- 7).Toyokuni S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv Drug Deliv Rev, 2013; 65: 2098–2110. [DOI] [PubMed]
- 8).Huczko A, Lange H, Całko E, Grubek-Jaworska H, Droszcz P. Physiological testing of carbon nanotubes: are they asbestos-like? Fullerene Sci Technol, 2001; 9: 251–254.
- 9).Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett, 2007; 168: 121–131. [DOI] [PubMed]
- 10).Osmond-McLeod MJ, Poland CA, Murphy F, Waddington L, Morris H, Hawkins SC, Clark S, Aitken R, McCall MJ, Donaldson K. Durability and inflammogenic impact of carbon nanotubes compared with asbestos fibres. Part Fibre Toxicol, 2011; 8. [DOI] [PMC free article] [PubMed]
- 11).Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J. Induction of mesothelioma in p53+/– mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci, 2008; 33: 105–116. [DOI] [PubMed]
- 12).Sakamoto Y, Nakae D, Fukumori N, Tayama K, Maekawa A, Imai K, Hirose A, Nishimura T, Ohashi N, Ogata A. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci, 2009; 34: 65–76. [DOI] [PubMed]
- 13).Nagai H, Okazaki Y, Chew S, Misawa N, Yamashita Y, Akatsuka S, Yamashita K, Ishihara T, Yoshikawa Y, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y, Shinohara H, Toyokuni S. Diameter of multi-walled carbon nanotubes is a critical factor in mesothelial injury and subsequent carcinogenesis. Proc Natl Acad Sci U S A, 2011; 108: E1330–1338. [DOI] [PMC free article] [PubMed]
- 14).Nagai H, Okazaki Y, Chew SH, Misawa N, Miyata Y, Shinohara H, Toyokuni S. Intraperitoneal administration of tangled multiwalled carbon nanotubes of 15 nm in diameter does not induce mesothelial carcinogenesis in rats. Pathol Int, 2013; 63: 457–462. [DOI] [PubMed]
- 15).Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol, 2005; 9: 674–679. [DOI] [PubMed]
- 16).Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev, 2006; 58: 1460–1470. [DOI] [PubMed]
- 17).Tisch U, Haick H. Nanomaterials for cross-reactive sensor arrays. MRS Bulletin, 2010; 35: 797–803.
- 18).Choi DH, Wang Q, Azuma Y, Majima Y, Warner JH, Miyata Y, Shinohara H, Kitaura R. Fabrication and characterization of fully Flattened carbon nanotubes: a new graphene nanoribbon analogue. Sci Rep, 2013; 3: 1617. [DOI] [PMC free article] [PubMed]
- 19).Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci U S A, 2006; 103: 3357–3362. [DOI] [PMC free article] [PubMed]