ABSTRACT
The purpose of this study is to investigate the morphological characteristics of renal tumors which affect the surgeons’ decision-making for the selection of open or laparoscopic partial nephrectomy. We included 147 patients who underwent partial nephrectomy for renal masses with elective indications in this study. Laparoscopic partial nephrectomy (LPN) and open partial nephrectomy (OPN) were performed in 72 and 75 patients, respectively. Preoperative trans-sectional images were used to assess tumor characteristics such as tumor size, endophyticity, distance from the sinus, distance from the kidney equator, hilar designation, inside designation, and R.E.N.A.L. nephrometry score. Univariate logistic regression analyses demonstrated that tumor size, endophyticity, distance from the sinus, hilar designation, inside designation, and R.E.N.A.L. nephrometry score were associated with decision of laparoscopic partial nephrectomy. Among these factors, multiple regression analyses showed that endophyticity (odds ratio = 0.92, p = 0.007) and distance from the sinus (odds ratio = 1.201, p < 0.001) had statistically significant associations with the type of operation performed. ROC analyses demonstrated cut-off values of 16 mm for endophyticity (sensitivity 69%, specificity 77%) and of 4 mm for distance from the sinus (sensitivity 79%, specificity 65%) for predicting the selection of laparoscopic surgery. In conclusion, this study revealed that endophyticity and distance from the sinus were important for the surgical planning of partial nephrectomy.
Key Words: Kidney neoplasms, Laparoscopy, Nephrectomy, ROC Analyses, Urologic Surgical Procedures
INTRODUCTION
In recent years, partial nephrectomy has been shown to achieve equivalent oncological outcomes and better overall survival than radical nephrectomy, as well as to reduce the risk of end-stage renal disease.1-5) The latest guidelines for the treatment of renal tumors recommended partial nephrectomy as the first choice surgical option for cT1a or cT1b renal tumors if feasible technically.6, 7) Laparoscopic partial nephrectomy (LPN) has emerged as a viable alternative to open partial nephrectomy (OPN) due to its minimal invasiveness,8) although it is challenging technically. Robotic partial nephrectomy was developed to reduce the invasiveness and overcome problems associated with LPN; however, it is not yet used widely and currently can only be performed at the specific institutes. Therefore, because LPN or OPN remain the two major surgical options to treat localized small renal tumors, surgical planning to select LPN versus OPN is a clinically important issue.
It is necessary to resect tumors at oncologically appropriate lines9) with minimal margins,10) within safe ischemic time,11, 12) and without postoperative complications.13) Therefore, renal tumors with low complexity would be good indications for LPN. The following factors are associated with tumor complexity: tumor size, endophyticity, nearness to the sinus, polar location, hilar designation, and inside designation. Many studies reported the tumor characteristics resected using LPN and OPN.14-18) However, few studies have reported on the factors that influence the decision of the surgeon during surgical planning for LPN or OPN, or on the cut-off points that determine the indication for the two procedures.
In the current study, we evaluated the relationship between tumor morphology and surgery type and determined the cut-off values that predict the choice made by surgeons regarding the laparoscopic approach.
METHODS
The Ethics Committee at Nagoya University Graduate School of Medicine approved the study protocol.
Subjects
Between January 2009 and December 2013, a total of 176 patients underwent partial nephrectomy for clinically diagnosed renal cell carcinoma at our institution. Because the purpose of the study was to evaluate the relationship between tumor characteristics and the decision making process regarding the surgical approach for early-stage renal cell carcinoma, we excluded the following patients: 10 patients with a solitary kidney, 4 patients who underwent OPN during gastrointestinal surgery, 2 patients with cT3a and poor renal function, 3 patients with multiple tumors, 7 patients who underwent robotic partial nephrectomy, which has just been implemented at our hospital, and 3 patients for other reasons. Consequently, a total of 147 patients who underwent surgery for ≤ cT2 renal cell carcinoma were included in this study. LPN and OPN were performed in 72 and 75 patients, respectively. Postoperative histopathology showed renal cell carcinoma in 130 patients, angiomyolipoma in 9, hemorrhagic cysts in 3, and oncocytoma in 5. In our institution, LPN was considered first, OPN second, and finally laparoscopic radial nephrectomy as a third option for localized small renal masses. The surgical plan is determined based on preoperative trans-sectional images and patient clinical information at institutional conferences.
Surgical procedures
The key surgical procedures were performed similarly in the LPN and OPN groups. The surface of the renal cortex bordering the lesion was stripped of fatty tissue to enable visualization of the tumor’s lateral margins. The renal artery and/or vein were clamped prior to tumor resection. The tumor margins were then excised by cold cutting, starting at approximately 5 mm from the tumor edge. Clamping of the renal blood flow was released after the closure of the renal defect using knot-tying sutures, if necessary, over Surgicel® bolsters.
Trans-sectional images
Contrast-enhanced CT scans were obtained using a slice thickness of 1 mm by intravenous injection of iodinated-contrast agent. For patients who were allergic to iodine, contrast-enhanced MRI was performed using a slice thickness of 0.5 mm by intravenous injection of gadolinium-contrast agent. Tumor characteristics such as tumor size, clinical T stage, endophyticity (length from the renal surface to the tumor bottom), distance from the sinus to the deepest portion of the tumor, distance from the kidney equatorial plane to the tumor’s nearest edge, polar position, hilar designation (for lesions in contact with renal vessels), inside designation (for lesions located inside the kidney), and R.E.N.A.L. nephrometry score on preoperative trans-sectional images.
Statistics
All values are presented as means ± standard deviations. Student’s t-tests were used to compare parametric values. Chi-square tests were used to compare the ratios between groups. Univariate and multiple logistic regression analyses were used to examine the relationship between tumor morphological features and type of surgery. Receiver operating characteristics – area under the curve (ROC-AUC) analyses were performed to calculate the cut-off values for predicting the selection of LPN. All tests were 2-sided, and p values less than 0.05 were considered to be statistically significant. All statistical analyses were performed using SPSS® software.
RESULTS
Renal lesions were successfully excised in all patients. No major perioperative complications occurred in any patient. Postoperative hemorrhage occurred in 2 patients, who were managed conservatively. Postoperative urine leakage was not noted in any patients.
Table 1 shows the patient characteristics (Table 1). Statistically significant differences in tumor size, clinical T stage, endophyticity, distance from the sinus, hilar designation, inside designation, and R.E.N.A.L. nephrometry score were noted between the LPN and OPN groups.
Table 1.
Patient characteristics
| LPN group | OPN group | p value | |
|---|---|---|---|
| No. patients | 72 | 75 | |
| Patient age at surgery Mean ± SD (range) | 60.7 ± 11.2 (29 – 82) | 58.3 ± 13.7 (23 – 86) | 0.256 |
| Gender (male / female) | 50 / 22 | 57 / 18 | 0.372 |
| Side (R / L) | 40 / 32 | 40 / 35 | 0.787 |
| Tumor size (mm) | 24.8 ± 7.3 (11 – 42) | 29.5 ± 9.3 (13 – 52) | 0.001 |
| Clinical T stage (1a / 1b) | 71 / 1 | 67 / 8 | 0.019 |
| Endophyticity (mm) | 14.4 ± 6.5 (4 – 31) | 21.0 ± 7.2 (7 – 44) | <0.001 |
| Distance from the sinus (mm) | 7.6 ± 5.3 (0 – 23) | 2.9 ± 3.4 (0 – 13) | <0.001 |
| Distance from the renal equator (mm) | 12.1 ± 12.1 (0 – 38) | 11.1 ± 10.4 (0 – 38) | 0.591 |
| Hilar designation (y / n) | 2 / 70 (2.8%) | 12 / 63 (16.0%) | 0.006 |
| Polar location; upper / middle / lower pole | 10 / 39 / 23 | 14 / 38 / 23 | 0.691 |
| Inside designation (y / n) | 12 / 60 (16.7%) | 29 / 46 (38.7%) | 0.003 |
| R.E.N.A.L. nephrometry score | 6.4 ± 1.4 (4 – 9) | 7.7 ± 1.4 (5 – 10) | <0.001 |
Next, we performed multivariate logistic regression analysis to determine tumor morphological features for selecting LPN. Univariate analysis demonstrated that tumor size, endophyticity, distance from the sinus, hilar designation, inside designation, and R.E.N.A.L. nephrometry score were associated with the selection of LPN (Table 2). Among these, multivariate analysis showed that tumors with less endophyticity and those located further from the sinus were more often chosen LPN than OPN. Tumor size, distance from the kidney equatorial plane, hilar designation, inside designation, and R.E.N.A.L. nephrometry score were not associated with the choice of surgical approach.
Table 2.
Tumor morphological features for selecting laparoscopic partial nephrectomy
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) |
p-value | Odds ratio (95% CI) |
p-value | ||
| Tumor size (mm) | 0.934 (0.895–0.974) |
0.0016 | |||
| Endophyticity (mm) | 0.865 (0.815–0.917) |
<0.0001 | 0.920 (0.861–0.983) |
0.0131 | |
| Distance from the sinus (mm) | 1.291 (1.171–1.423) |
<0.0001 | 1.201 (1.078–1.338) |
0.0009 | |
| Distance from the kidney equator (mm) | 1.008 (0.979–1.038) |
0.5885 | |||
| Hilar designation (y / n) | 0.150 (0.032–0.696) |
0.0154 | |||
| Inside designation (y / n) | 0.317 (0.146–0.688) |
0.0037 | |||
| R.E.N.A.L. nephrometry score | 0.522 (0.398–0.687) |
<0.0001 | |||
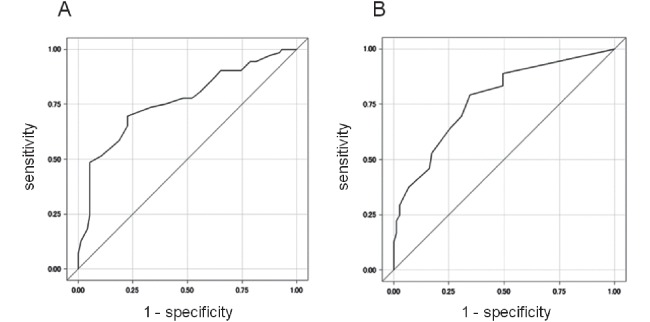
ROC-AUC analyses revealed the sensitivity and specificity of endophyticity and the distance from the sinus for the selection of LPN (Fig. 1). Using the optimal cut-off point for endophyticity 16 mm, the sensitivity and specificity were 69.4% and 77.3%, respectively, and the AUC was 0.7623. Using the optimal cut-off point for distance from the sinus 4 mm, the sensitivity and specificity were 79.2% and 65.3%, respectively, and the AUC was 0.7735. When the tumor had an endophyticity of <16 mm and a distance from the sinus ≥4mm, 80.4% of cases were treated with LPN (positive predictive value = 80.4%; Table 3). When the tumor had an endophyticity of ≥16 mm and a distance from the sinus of <4 mm, 17.6% of cases were treated with LPN (negative predictive value = 82.4%).
Fig. 1.
Sensitivity and specificity of endophyticity and distance from the sinus in ROC curves
ROC-AUC analyses demonstrated cut-off values of 16 mm for endophyticity (sensitivity 69.4%, specificity 77.3%) (A) and of 4 mm for distance from the sinus (sensitivity 79.2%, specificity 65.3%) (B) for predicting laparoscopic partial nephrectomy.
Table 3.
Number of patients who underwent laparoscopic partial nephrectomy
| Endophyticity | |||
|---|---|---|---|
| <16 mm (n = 64) | ≥16 mm (n = 83) | ||
| Distance from the sinus | ≥4 mm (n = 83) | 41 / 51 (80.4%) | 16 / 32 (50.0%) |
| <4 mm (n = 64) | 6 / 13 (46.2%) | 9 / 51 (17.6%) | |
DISCUSSION
The results of this study demonstrated that most tumor morphological characteristics (tumor size, endophyticity, distance from the sinus, hilar designation, inside designation, and R.E.N.A.L. nephrometry score) were associated with decision-making for the optimal surgical procedures to use, according to univariate analyses. Among these, endophyticity and distance from the sinus were the most important morphological characteristics for selecting LPN versus OPN, according to multivariate analyses. These results suggest that endophyticity and distance from the sinus represent tumor complexities that affect a surgeon’s decision.19) When evaluating endophyticity, we assessed both the endophytic ratio and the actual length from the renal surface to the bottom of the tumor. Of these, the latter showed a greater association with the surgical approach (data not shown).
Several studies have reported that less complex tumors are resected more frequently with LPN, whereas high complexity lesions are more likely to undergo OPN or radical nephrectomy.14-18) Broughton et al. reported that tumor size and R.E.N.A.L. nephrometry score were independent factors for planning OPN over LPN or radical nephrectomy.15) Gill et al. compared the tumor characteristics of 771 LPN and 1029 OPN cases and reported that tumor size (2.6 cm in LPN vs 3.3 cm in OPN) and endophytic designation (34.4% in LPN vs 53.3% in OPN) were significantly different between the two procedures.16) In addition to these comparisons of two procedures, some authors prompted to identify cut-offs to determine which patients might be suitable for a particular approach. Naya et al. evaluated the morphological factors of tumors from 68 patients treated with laparoscopic radical nephrectomy and 74 patients treated with LPN.17) They reported that tumor size as well as DAP and R.E.N.A.L. nephrometry score were significant factors for the selection of LPN, and that DAP score of 6 and R.E.N.A.L. score of 8 were the optimal cut-off values for the selection of LPN. Esen et al. compared tumor factors in 32 and 23 patients who underwent robot-assisted and open partial nephrectomy, respectively, and high R.E.N.A.L. score (cut-off, 6.5) and high P.A.D.U.A. score (cut-off, 7.5) were significant predictors for selecting robotic over open surgery.18) Our study indicated that only endophyticity (cut-off, 16 mm) and distance from the sinus (cut-off, 4 mm) affected surgical planning. Meanwhile, contrary to our expectations, tumor size was not a significant factor that affected surgical decision-making in the multivariate analyses. Our results suggested that surgeons selected laparoscopic surgery if the tumor was exophytic and distant from the sinus, even if the tumor size was large.
In this study, we included patients who were treated by either LPN or OPN. Because the movability of surgical devices is restricted during laparoscopic surgery, LPN is not suitable for resecting complicated renal masses or closing large renal defects. Consequently, in order to achieve both low invasiveness and improved operationality, robotic partial nephrectomy has been developed and spreading gradually. This new technique can ease the difficulties associated with LPN, and some studies demonstrated that robotic partial nephrectomy could be performed for more complicated tumors;20) therefore, it might be an alternative to OPN and LPN for select patients. Future studies assessing the cut-off points for determining whether robotic partial nephrectomy or OPN is preferable will be important.
Our study has several limitations that must be noted. Due to its retrospective nature, it is not clear whether the criterion of judgment we proposed in this study is universal. Nine surgeons performed partial nephrectomy in the patients included in this study, which might have affected decision-making regarding surgical type. In addition, the sensitivities and specificities determined by ROC analyses were not adequately high. A larger sample size and a prospective study are needed.
In conclusion, we investigated the tumor characteristics that affected surgical decision-making regarding LPN or OPN. We revealed that endophyticity and distance from the sinus were the most important determinants for surgical planning for partial nephrectomy.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1).Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, Sinescu IC. Renal cell carcinoma guideline. Eur Urol, 2007; 51: 1502–1510. [DOI] [PubMed]
- 2).Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, Blute ML. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol, 2008; 179: 468–471; discussion 472–463. [DOI] [PubMed]
- 3).Peycelon M, Hupertan V, Comperat E, Renard-Penna R, Vaessen C, Conort P, Bitker MO, Chartier-Kastler E, Richard F, Roupret M. Long-term outcomes after nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol, 2009; 181: 35–41. [DOI] [PubMed]
- 4).Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. Jama, 2012; 307: 1629–1635. [DOI] [PMC free article] [PubMed]
- 5).Yap SA, Finelli A, Urbach DR, Tomlinson GA, Alibhai SM. Partial Nephrectomy for the Treatment of Renal Cell Carcinoma and the Risk of End Stage Renal Disease. BJU Int, 2014: in press. [DOI] [PubMed]
- 6).Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Derweesh IH, Gupta S, Hancock SL, Kim JJ, Kuzel TM, Lam ET, Lau C, Levine EG, Lin DW, Margolin KA, Michaelson MD, Olencki T, Pili R, Plimack ER, Rampersaud EN, Redman BG, Ryan CJ, Sheinfeld J, Sircar K, Somer B, Wang J, Wilder RB, Dwyer MA, Kumar R. Kidney cancer, version 2.2014. J Natl Compr Canc Netw, 2014; 12: 175–182. [DOI] [PubMed]
- 7).Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol, 2010; 58: 398–406. [DOI] [PubMed]
- 8).Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol, 2009; 55: 1171–1178. [DOI] [PubMed]
- 9).Azhar RA, Luis de Castro Abreu A, Broxham E, Sherrod A, Ma Y, Cai J, Gill TS, Desai M, Gill IS. Histological Analysis of the Kidney Tumor-Parenchyma Interface. J Urol, 2014: in press. [DOI] [PubMed]
- 10).Mir MC, Campbell RA, Sharma N, Remer EM, Simmons MN, Li J, Demirjian S, Kaouk J, Campbell SC. Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology, 2013; 82: 263–268. [DOI] [PubMed]
- 11).Funahashi Y, Hattori R, Yamamoto T, Kamihira O, Kato K, Gotoh M. Ischemic renal damage after nephron-sparing surgery in patients with normal contralateral kidney. Eur Urol, 2009; 55: 209–215. [DOI] [PubMed]
- 12).Funahashi Y, Hattori R, Yamamoto T, Sassa N, Fujita T, Gotoh M. Effect of warm ischemia on renal function during partial nephrectomy: assessment with new 99mTc-mercaptoacetyltriglycine scintigraphy parameter. Urology, 2012; 79: 160–164. [DOI] [PubMed]
- 13).Hung AJ, Cai J, Simmons MN, Gill IS. "Trifecta" in partial nephrectomy. J Urol, 2013; 189: 36–42. [DOI] [PubMed]
- 14).Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol, 2009; 182: 844–853. [DOI] [PubMed]
- 15).Broughton GJ, Clark PE, Barocas DA, Cookson MS, Smith JA, Jr., Herrell SD, Chang SS. Tumour size, tumour complexity, and surgical approach are associated with nephrectomy type in small renal cortical tumours treated electively. BJU Int, 2012; 109: 1607–1613. [DOI] [PubMed]
- 16).Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, Jr., Frank I, Permpongkosol S, Weight CJ, Kaouk JH, Kattan MW, Novick AC. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol, 2007; 178: 41–46. [DOI] [PubMed]
- 17).Naya Y, Kawauchi A, Oishi M, Ueda T, Fujihara A, Naito Y, Nakamura T, Hongo F, Kamoi K, Okihara K, Miki T. Comparison of diameter-axial-polar nephrometry and RENAL nephrometry score for treatment decision-making in patients with small renal mass. Int J Clin Oncol, 2014: in press. [DOI] [PubMed]
- 18).Esen T, Acar O, Musaoglu A, Vural M. Morphometric profile of the localised renal tumors managed either by open or robot-assisted nephron-sparing surgery: the impact of scoring systems on the decision making process. BMC Urol, 2013; 13: 63. [DOI] [PMC free article] [PubMed]
- 19).Leslie S, Gill IS, de Castro Abreu AL, Rahmanuddin S, Gill KS, Nguyen M, Berger AK, Goh AC, Cai J, Duddalwar VA, Aron M, Desai MM. Renal Tumor Contact Surface Area: A Novel Parameter for Predicting Complexity and Outcomes of Partial Nephrectomy. Eur Urol, 2014: in press. [DOI] [PubMed]
- 20).Volpe A, Garrou D, Amparore D, De Naeyer G, Porpiglia F, Ficarra V, Mottrie A. Perioperative and renal functional outcomes of elective robot-assisted partial nephrectomy (RAPN) for renal tumours with high surgical complexity. BJU Int, 2014: in press. [DOI] [PubMed]