ABSTRACT
Oxidative stress and the mineralocorticoid receptor (MR) are implicated in the pathogenesis of salt-induced left ventricular (LV) diastolic dysfunction associated with metabolic syndrome (MetS). We recently characterized DahlS.Z-Leprfa/Leprfa (DS/obese) rats, derived from a cross between Dahl salt-sensitive and Zucker rats, as a new animal model of MetS. We investigated the pathophysiological roles of increased oxidative stress and MR activation in cardiac injury with this model. DS/obese rats were treated with the antioxidant tempol (1 mmol/L in drinking water) or the selective MR antagonist eplerenone (15 mg/kg per day, per os) for 5 weeks beginning at 10 weeks of age. The increased systolic blood pressure and LV hypertrophy that develop in untreated DS/obese rats were substantially ameliorated by eplerenone but not by tempol. Eplerenone also attenuated LV fibrosis and diastolic dysfunction more effectively than did tempol in DS/obese rats, whereas cardiac oxidative stress and inflammation were reduced similarly by both drugs. Both the ratio of plasma aldosterone concentration to plasma renin activity and cardiac expression of the MR and serum/glucocorticoid–regulated kinase 1 genes were decreased to a greater extent by eplerenone than by tempol. Our results indicate that both increased oxidative stress and MR activation in the heart may contribute to the development of LV remodeling and diastolic dysfunction in DS/obese rats. The superior cardioprotective action of eplerenone is likely attributable to its greater antihypertensive effect, which is likely related to its greater inhibition of aldosterone-MR activity in the cardiovascular system.
Key Words: metabolic syndrome, cardiac remodeling, oxidative stress, mineralocorticoid receptor, renin-angiotensin-aldosterone system
INTRODUCTION
Metabolic syndrome (MetS), a complex of highly debilitating disorders including hypertension, diabetes mellitus, and dyslipidemia, is associated with the development of visceral obesity.1) Obesity is also separately associated with the development of hypertension2) and a consequent increase in cardiovascular disease risk.3) Salt loading induces left ventricular (LV) hypertrophy or diastolic dysfunction in several hypertension models.4) Furthermore, some patients with MetS exhibit LV diastolic dysfunction,5) which eventually leads to diastolic heart failure with a poor prognosis.6)
Reactive oxygen species (ROS), which are generated physiologically by cellular metabolism, have been implicated in cardiac functional damage.4, 7) Increased oxidative stress is also thought to contribute to MetS,8) possibly as a result in part of the ROS-induced production of adipocytokines.4) The fact that oxidative stress is also a common factor linked separately to each of the components of MetS further supports its importance in the etiopathogenesis of this condition.9)
The renin-angiotensin-aldosterone system (RAAS) is also implicated in the pathogenesis of MetS.10) The effects of aldosterone are mediated by the mineralocorticoid receptor (MR), which belongs to the nuclear receptor superfamily. Aldosterone and MR are thought to contribute both to the development of MetS and to the progression of target organ damage associated with this condition. Renal and cardiac injury in an experimental model of MetS have thus been found to be dependent on activation of the aldosterone-MR system.11-13) Furthermore, MR antagonists have proved effective in clinical trials for the treatment of patients with severe heart failure.14) MR blockade reduces LV mass in hypertensive individuals with LV hypertrophy and with or without type 2 diabetes mellitus,14) and it ameliorates markers of inflammation in those with diabetes.15) The relation between the aldosterone-MR system and oxidative stress in cardiac pathology associated with MetS has remained unclear, however.
We recently established a new animal model of MetS, the DahlS.Z-Leprfa/Leprfa (Dahl salt-sensitive (DS)/obese) rat, by crossing DS rats with Zucker rats, which harbor a missense mutation in the leptin receptor gene (Lepr). When fed a normal diet, DS/obese rats develop a phenotype similar to MetS in humans, including hypertension,16, 17) suggesting that salt sensitivity of blood pressure is enhanced in these animals. DS/obese rats also develop LV diastolic dysfunction as well as LV hypertrophy and fibrosis, and these changes are associated with the increases in cardiac oxidative stress and inflammation as well as in cardiac RAAS gene expression.17) We have now investigated the effects of an antioxidant and a selective MR antagonist on cardiac remodeling and diastolic dysfunction in DS/obese rats.
METHODS
Animals and experimental protocol
Animal experiments were approved by the Animal Experiment Committee of Nagoya University Graduate School of Medicine (Daiko district, approval Nos. 021-030, 022-008, 023-009, 024-008, and 025-007). Eight-week-old male inbred DS/obese rats were obtained from Japan SLC Inc. (Hamamatsu, Japan) and were handled in accordance with the guidelines of Nagoya University Graduate School of Medicine as well as with the Guide for the Care and Use of Laboratory Animals (U.S. NIH publication no. 85-23, revised 1996). Weaning rats were fed normal laboratory chow containing 0.36% NaCl. DS/obese rats fed a 0.36% NaCl diet after 5 weeks of age develop LV hypertrophy and diastolic dysfunction attributable to hypertension at 15 weeks.16, 17) The rats were randomly allocated to three groups: (1) the MetS group (n = 8); (2) the Temp group (n = 8), in which the animals were administered the superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethyl-piperidine-N-oxyl (tempol; Sigma, St. Louis, MO, USA) at 1 mmol/L in drinking water from 10 to 15 weeks of age; and (3) the EPL group (n = 8), in which the animals were administered the selective MR antagonist eplerenone (Pfizer Inc., New York, NY, USA) at 15 mg per kilogram of body weight per day orally via a gastric tube from 10 to 15 weeks of age. The dose of eplerenone was determined on the basis of results of a previous study.18) Age-matched male homozygous lean littermates of DS/obese rats (DahlS.Z-Lepr+/Lepr+, or DS/lean, rats) served as control animals (CONT group, n = 8). Both diet and tap water were provided ad libitum throughout the experimental period. Body weight was measured weekly. At 15 weeks of age, all animals were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg) and were subjected to hemodynamic and echocardiographic analyses. The heart was subsequently excised, and LV tissue was either immediately stored at –80°C for molecular analysis or fixed with paraformaldehyde for pathological analysis.
Hemodynamic and echocardiographic analyses
Systolic blood pressure (SBP) and heart rate were measured weekly in conscious animals by tail-cuff plethysmography (BP98A; Softron, Tokyo, Japan). At 15 weeks of age, rats were subjected to transthoracic echocardiography, as described previously.17, 19, 20) M-mode echocardiography was performed with a 12.5-MHz transducer (Xario SSA-660A; Toshiba Medical Systems, Tochigi, Japan). LV end-diastolic (LVDd) and end-systolic (LVDs) dimensions as well as the thickness of the interventricular septum (IVST) and LV posterior wall (LVPWT) were measured, and LV fractional shortening (LVFS), relative wall thickness (RWT), and LV mass were calculated as described previouly.21-23) LV ejection fraction (LVEF) was calculated with the formula of Teichholz.24) For assessment of LV diastolic function, we calculated the peak flow velocities at the mitral level during rapid filling (E) and during atrial contraction (A), the E/A ratio, and the deceleration time (DcT), from the pulsed Doppler echocardiographic data. After echocardiography, cardiac catheterization was performed as described previously.25) Tracings of LV pressure and the electrocardiogram were digitized to determine LV end-diastolic pressure (LVEDP). The time constant of isovolumic relaxation (τ) was calculated by the derivative method of Raff and Glantz as described previously.26)
Measurement of metabolic parameters
Blood was collected from the right carotid artery of rats that had been deprived of food overnight and was centrifuged at 1400 × g for 10 min at room temperature in the absence or presence of anticoagulant. The serum concentration of glucose was measured with a routine enzymatic assay. The plasma levels of insulin and leptin were determined with mouse/rat enzyme-linked immunosorbent assay kits (Morinaga Bioscience Institute, Yokohama, Japan). The homeostasis model assessment of insulin resistance (HOMA-IR) index, which predicts insulin sensitivity, was calculated from the glucose and insulin values according to the empirical formulae: HOMA-IR = fasting insulin (µU/mL) × fasting glucose (mmol/L)/22.5.27) Plasma renin activity and the plasma concentration of aldosterone were determined with radioimmunoassays (renin RIA beads from Abbott Japan, Tokyo, and a DPC aldosterone kit from Mitsubishi Chemical Medicine, Tokyo, Japan, respectively).
Histology and immunohistochemistry
LV tissue was fixed in ice-cold 4% paraformaldehyde for 48 to 72 h, embedded in paraffin, and processed for histology as described.28) Transverse sections (thickness, 3 μm) were stained either with hematoxylin-eosin for routine histological examination or with Azan-Mallory solution for evaluation of fibrosis. For evaluation of macrophage infiltration into the myocardium, frozen sections (thickness, 5 μm) that had been fixed with acetone were subjected to immunostaining for the monocyte-macrophage marker CD68 as described previously.17)
Superoxide production
Nicotinamide adenine dinucleotide phosphate (NADPH)-dependent superoxide production by homogenates prepared from freshly frozen LV tissue was measured with an assay based on lucigenin-enhanced chemiluminescence, as described previously.18) The chemiluminescence signal was sampled every minute for 10 min with a microplate reader (Wallac 1420 ARVO MX/Light; Perkin-Elmer, Waltham, MA), and the respective background counts were subtracted from experimental values. Superoxide production in tissue sections was examined by staining with dihydroethidium (Sigma, St. Louis, MO) as described previously.17, 29)
RT and real-time PCR analysis
Total RNA was extracted from LV tissue and treated with DNase with the use of a spin-vacuum isolation kit (Promega, Madison, WI). Complementary DNA was synthesized from 2 μg of total RNA by reverse transcription (RT) with random primers (Invitrogen, Carlsbad, CA) and MuLV reverse transcriptase (Applied Biosystems, Foster City, CA). After RT, real-time PCR analysis was performed with the use of a Prism 7000 Sequence Detector (Perkin-Elmer), as previously described,30) and with primers and TaqMan probes specific for atrial natriuretic peptide (ANP),20) brain natriuretic peptide (BNP),20) β-myosin heavy chain (β-MHC),20) collagen type I,31) or type III,17) transforming growth factor-β1 (TGF-β1),20) connective tissue growth factor (CTGF),18) monocyte chemoattractant protein-1 (MCP-1),18) osteopontin,18) cyclooxygenase-2 (COX-2),32) angiotensin-converting enzyme (ACE),20) the type 1A receptor for angiotensin II (AT1A receptor),20) MR, serum/glucocorticoid–regulated kinase 1 (Sgk1),17) and the p22phox,33) gp91phox,33) p67phox,17) and Rac117) subunits of NADPH oxidase. Reagents for the detection of human GAPDH mRNA (Applied Biosystems) were used to quantify rat GAPDH mRNA as an internal standard.
Statistical analysis
Data are presented as means ± SEM. Differences among groups of rats at 15 weeks of age were assessed by one-way factorial analysis of variance (ANOVA) followed by Fisher’s multiple-comparison test. The time course of body weight or SBP was compared among groups by two-way repeated-measures ANOVA. A P value of <0.05 was considered statistically significant.
RESULTS
LV geometry and function and metabolic characteristics
Body weight was significantly increased in DS/obese rats compared with DS/lean rats at 9 weeks of age and thereafter, and this increase was not affected by either tempol or eplerenone (Figure 1A, Table 1). SBP was also significantly higher in DS/obese rats than in DS/lean rats at 9 weeks of age and thereafter, and this increase in SBP was substantially attenuated by eplerenone but not by tempol (Figure 1B, Table 1). At 15 weeks of age, the ratio of LV weight to tibial length, an index of LV hypertrophy, was decreased in the EPL group but not in the Temp group compared with the MetS group (Table 1). The fasting serum glucose concentration at 15 weeks of age did not differ among the MetS, Temp, and EPL groups. The fasting plasma insulin level and homeostasis model assessment of insulin resistance (HOMA-IR) were not affected by either tempol or eplerenone. The plasma concentration of leptin at 15 weeks of age was also similar in the three experimental groups.
Fig. 1.
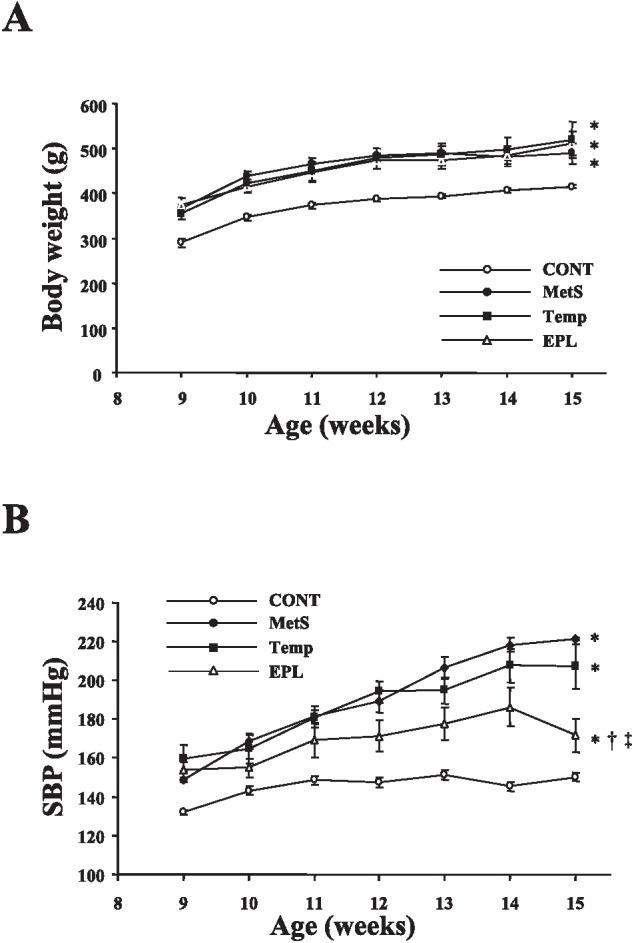
Time courses of body weight (A) and SBP (B) in rats of the four experimental groups. Data are means ± SEM (n = 8 rats per group). *P < 0.05 versus CONT group; †P < 0.05 versus MetS group; ‡P < 0.05 versus Temp group.
Table 1.
Anatomic, metabolic, and hormonal parameters for rats in the four experimental groups at 15 weeks of age
| Parameter | MetS | Temp | EPL |
|---|---|---|---|
| Body weight (g) | 481.8 ± 67.5 | 511.3 ± 62.0 | 519.8 ± 89.5 |
| Tibial length (mm) | 35.8 ± 1.1 | 36.0 ± 0.6 | 35.6 ± 1.3 |
| SBP (mmHg) | 221.0 ± 18.0 | 207.0 ± 12.0 | 171.7 ± 20.2*† |
| Heart rate (beats per min) | 355.8 ± 36.4 | 354.9 ± 39.6 | 356.3 ± 20.9 |
| Heart weight/tibial length (mg/mm) | 41.0 ± 0.9 | 40.6 ± 1.4 | 34.6 ± 0.9*† |
| LV weight/tibial length (mg/mm) | 31.6 ± 0.7 | 30.2 ± 0.5 | 24.9 ± 0.7*† |
| Serum glucose (mg/dL) | 147.0 ± 14.3 | 148.8 ± 14.6 | 156.3 ± 16.0 |
| Plasma insulin (ng/mL) | 3.42 ± 0.46 | 3.45 ± 2.14 | 3.98 ± 0.52 |
| HOMA-IR | 26.9 ± 6.3 | 24.1 ± 7.8 | 25.5 ± 4.9 |
| Plasma leptin (ng/mL) | 29.59 ± 1.40 | 28.70 ± 1.61 | 28.89 ± 0.68 |
Data are means ± SEM for eight rats per group. *P < 0.05 versus MetS group; †P < 0.05 versus Temp group.
Echocardiography revealed that the IVST, LVPWT, RWT, and LV mass were significantly greater, and that the LVDs was significantly smaller, in the MetS group than in the CONT group (Table 2). Treatment of DS/obese rats with eplerenone attenuated all of these changes except LVDs. LVFS was also increased in untreated DS/obese rats compared with DS/lean rats, and this change was not affected by either drug (Table 2). The DcT and time constant of τ were significantly prolonged and the E/A ratio was significantly decreased in the MetS group compared with the CONT group (Table 2). The ratio of LVEDP to the LVDd, an index of LV diastolic stiffness, was also increased in the MetS group (Table 2). These changes in DcT, the E/A ratio, and the LVEDP/LVDd ratio were attenuated to a greater extent in the EPL group than in the Temp group. These data thus indicated that treatment with eplerenone inhibited LV remodeling, preserved LV systolic function, and attenuated LV diastolic dysfunction to a greater extent than did tempol.
Table 2.
Cardiac functional parameters for rats in the four experimental groups at 15 weeks of age
| Parameter | CONT | MetS | Temp | EPL |
|---|---|---|---|---|
| IVST (mm) | 1.60 ± 0.21 | 2.10 ± 0.12* | 1.93 ± 0.03* | 1.78 ± 0.22† |
| LVDd (mm) | 8.20 ± 0.79 | 8.40 ± 0.89 | 8.22 ± 0.45 | 8.01 ± 1.10 |
| LVPWT (mm) | 1.50 ± 0.08 | 2.00 ± 0.18* | 1.87 ± 0.04* | 1.61 ± 0.32†‡ |
| LVDs (mm) | 5.10 ± 0.27 | 4.48 ± 0.82* | 3.95 ± 0.05* | 3.68 ± 0.10† |
| LVFS (%) | 38.3 ± 1.1 | 46.5 ± 2.1* | 52.1 ± 1.6* | 52.6 ± 1.9* |
| LVEF (%) | 74.7 ± 1.0 | 81.3 ± 2.7* | 83.8 ± 1.0* | 80.6 ± 1.3* |
| LV mass (mg) | 931 ± 17 | 1390 ± 28* | 1187 ± 52* | 1096 ± 61†‡ |
| RWT | 0.37 ± 0.01 | 0.50 ± 0.01* | 0.45 ± 0.01*† | 0.40 ± 0.02†‡ |
| E/A | 1.85 ± 0.16 | 1.27 ± 0.05* | 1.54 ± 0.02*† | 1.86 ± 0.04† |
| DcT (ms) | 39.6 ± 0.9 | 56.0 ± 1.2* | 48.1 ± 2.1*† | 41.4 ± 1.1†‡ |
| τ (ms) | 25.1 ± 1.9 | 36.0 ± 2.7* | 27.9 ± 1.4† | 24.9 ± 0.7† |
| LVEDP (mmHg) | 2.1 ± 0.6 | 9.6 ± 0.1* | 8.1 ± 0.1*† | 5.3 ± 0.7*†‡ |
| LVEDP/LVDd (mmHg/mm) | 0.24 ± 0.06 | 1.24 ± 0.02* | 1.02 ± 0.02*† | 0.79 ± 0.05*†‡ |
Data are means ± SEM for eight rats per group. *P < 0.05 versus CONT group; †P < 0.05 versus MetS group; ‡P < 0.05 versus Temp group.
Cardiomyocyte hypertrophy, cardiac fibrosis, and gene expression
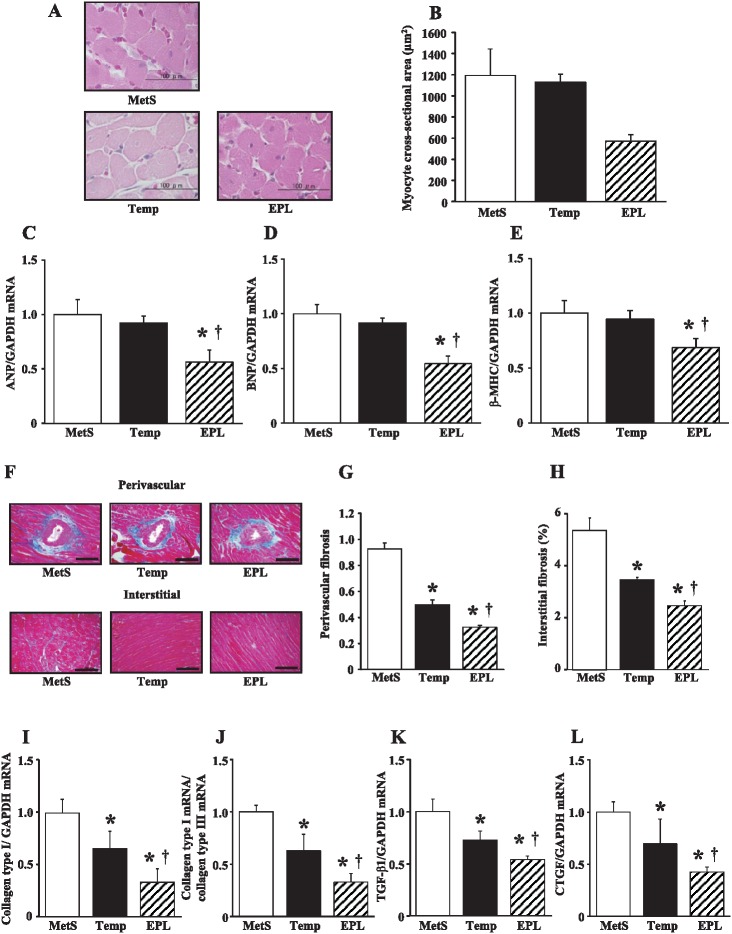
Microscopic analysis revealed that the cross-sectional area of cardiac myocytes was reduced in the EPL group but not in the Temp group compared with the MetS group at 15 weeks (Figure 2A, B). The expression of ANP, BNP, and β-MHC genes in the left ventricle of DS/obese rats was also down-regulated by eplerenone but not by tempol (Figure 2C–E). Azan-Mallory staining revealed that the extent of fibrosis in perivascular and interstitial regions of the LV myocardium was decreased in the Temp group and to a greater extent in the EPL group compared with the MetS group (Figure 2F–H). The abundance of collagen type I mRNA (Figure 2I); the ratio of collagen type I to type III mRNA abundance, which correlates with myocardial diastolic stiffness (Figure 2J); and the amounts of TGF-β1 and CTGF mRNAs (Figure 2K, L), which correlate with cardiac fibrosis and growth, were all decreased to a greater extent in the EPL group than in the Temp group compared with the MetS group.
Fig. 2.
Cardiomyocyte size, cardiac fibrosis, expression of fetal-type cardiac genes and fibrosis-related genes in the left ventricle of rats in the three experimental groups at 15 weeks of age. (A) Hematoxylin-eosin staining of transverse sections of the LV myocardium. Scale bars, 100 µm. (B) Cross-sectional area of cardiac myocytes determined from sections similar to those in (A). (C–E) Quantitative RT-PCR analysis of the relative abundance of ANP, BNP, and β-MHC mRNAs normalized by the amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, respectively. (F) Collagen deposition as revealed by Azan-Mallory staining in perivascular (upper panels) and interstitial (lower panels) regions of the LV myocardium. Scale bars, 200 µm. (G, H) Relative extents of perivascular and interstitial fibrosis, respectively, in the LV myocardium as determined from sections similar to those in (F). (I–L) Quantitative RT-PCR analysis of collagen type I mRNA, the ratio of collagen type I to collagen type III mRNAs, TGF-β1 mRNA, and CTGF mRNA, respectively. All quantitative data are means ± SEM (n = 8 rats per group). *P < 0.05 versus MetS group; †P < 0.05 versus Temp group.
Cardiac oxidative stress
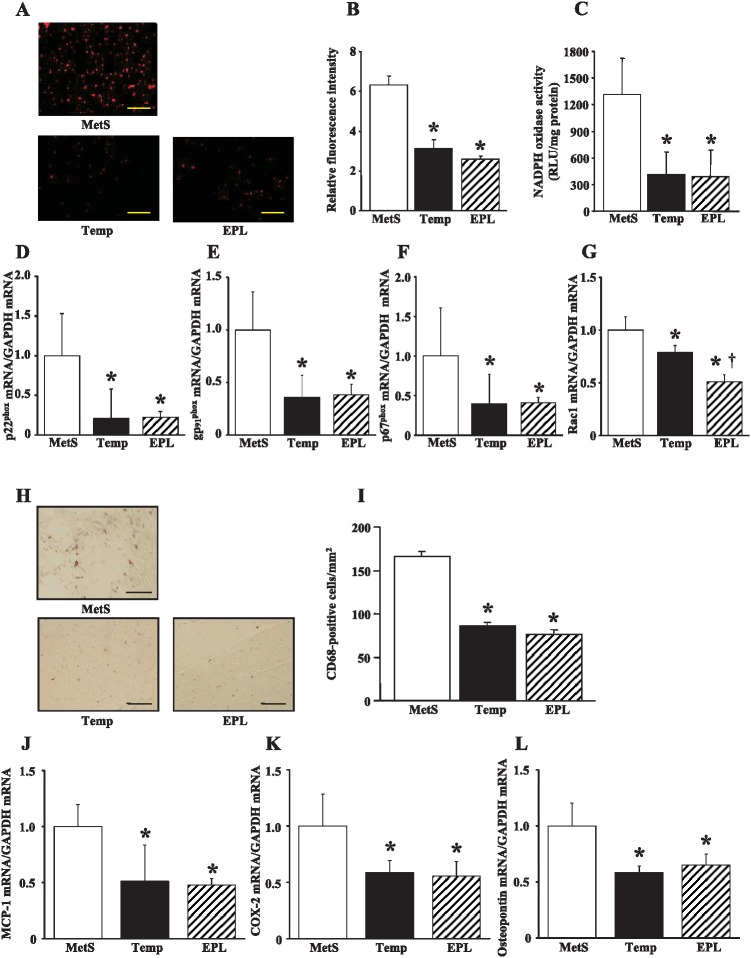
Superoxide production in myocardial tissue sections revealed by staining with dihydroethidium as well as the activity of NADPH oxidase in LV homogenates were both markedly decreased in the Temp and EPL groups compared with the MetS group (Figure 3A–C). The expression of genes for the p22phox and gp91phox membrane components and the p67phox cytoplasmic component of NADPH oxidase in the left ventricle was also down-regulated to a similar extent in the Temp and EPL groups (Figure 3D–F). The amount of mRNA for the NADPH oxidase subunit Rac1 in the left ventricle of DS/obese rats was decreased to a greater extent by eplerenone than by tempol (Figure 3G).
Fig. 3.
NADPH oxidase activity, macrophage infiltration, and inflammatory gene expression in the left ventricle of rats in the three experimental groups at 15 weeks of age. (A) Superoxide production as revealed by dihydroethidium staining in the LV myocardium. Scale bars, 100 µm. (B) Relative dihydroethidium fluorescence intensity determined from sections similar to those in (A). (C) NADPH-dependent superoxide production in LV homogenates. Results are expressed as relative light units (RLU) per milligram of protein. (D–G) Quantitative RT-PCR analysis of p22phox, gp91phox, p67phox, and Rac1 mRNAs, respectively. (H) Immunohistochemical analysis with antibodies to the monocyte-macrophage marker CD68. Scale bars, 50 µm. (I) Density of CD68-positive cells determined from sections similar to those in (H). (J–L) Quantitative RT-PCR analysis of MCP-1, COX-2, and osteopontin mRNAs, respectively. All quantitative data are means ± SEM (n = 8 rats per group). *P < 0.05 versus MetS group; †P < 0.05 versus Temp group.
Cardiac inflammation
Immunostaining for the monocyte-macrophage marker CD68 revealed that macrophage infiltration in the LV myocardium was decreased to a similar extent in the Temp and EPL groups compared with the MetS group (Figure 3H, I). The expression of MCP-1, COX-2, and osteopontin genes in the left ventricle of DS/obese rats was also decreased similarly in the Temp and EPL groups (Figure 3J-L).
Activity of RAAS
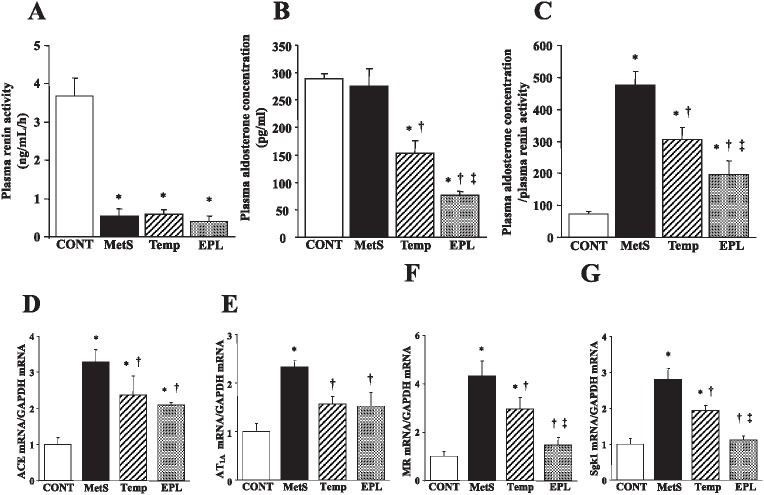
DS/obese rats showed a decrease in plasma renin activity compared with DS/lean rats, and there was no significant difference in plasma renin activity among the MetS, Temp, and EPL groups (Figure 4A). The plasma aldosterone concentration did not differ significantly between the MetS group and the CONT group, but it was decreased in the Temp group and, to a greater extent, in the EPL group (Figure 4B). The ratio of the plasma aldosterone concentration to plasma renin activity was significantly greater in the MetS group than in the CONT group, and this effect was also attenuated to a greater extent in the EPL group than in the Temp group (Figure 4C). The amounts of mRNAs for ACE and the AT1A receptor in the left ventricle were increased in the MetS group compared with the CONT group, and these effects were attenuated by both drugs (Figure 4D, E). Finally, expression of MR and Sgk1 genes in the left ventricle was increased in the MetS group compared with the CONT group, and these effects were attenuated to a greater extent in the EPL group than in the Temp group (Figure 4F, G).
Fig. 4.
Circulating levels of renin activity and aldosterone as well as expression of RAAS-related genes in the left ventricle of rats in the four experimental groups at 15 weeks of age. (A–C) Plasma renin activity, plasma aldosterone concentration, and the ratio of plasma aldosterone concentration to plasma renin activity, respectively. (D–G) Quantitative RT-PCR analysis of ACE, AT1A receptor, MR, and Sgk1 mRNAs, respectively. All quantitative data are means ± SEM (n = 8 rats per group). *P < 0.05 versus CONT group; †P < 0.05 versus MetS group; ‡P < 0.05 versus Temp group.
DISCUSSION
We have found that eplerenone, but not tempol, attenuated hypertension and LV hypertrophy in DS/obese rats, and that eplerenone ameliorated LV fibrosis and diastolic dysfunction more effectively than did tempol. Both tempol and eplerenone similarly reduced cardiac oxidative stress and inflammation in DS/obese rats. The superior cardioprotective action of eplerenone was associated with its greater attenuation of both the ratio of the plasma aldosterone concentration to plasma renin activity and MR signaling. Our results thus indicate that selective MR blockade with eplerenone ameliorated hypertension and cardiac damage more effectively than did the antioxidant agent tempol in DS/obese rats.
Obesity, especially when complicated with hypertension, is associated with changes in cardiac structure and function.34) Renal and cardiac injury in an experimental model of MetS were previously found to be strongly dependent on activation of the aldosterone-MR system.11-13) Aldosterone plays a key role in the pathogenesis of hypertension associated with diet-induced obesity by promoting glomerular hyperfiltration and sodium retention.35) Furthermore, patients with higher circulating aldosterone levels showed an increased risk of developing hypertension and MetS in the Framingham Offspring Study,36) and the development of these conditions was efficiently attenuated by MR blockade. DS/obese rats develop obesity and salt-sensitive hypertension as well as LV hypertrophy, fibrosis, and diastolic dysfunction, in the presence of normal serum aldosterone levels.17) In the present study, eplerenone, but not tempol, attenuated hypertension and LV hypertrophy in these animals. The dose of eplerenone administered (15 mg/kg per day) was sufficiently low that we did not expect it to reduce SBP. A previous study showed that a higher dose of eplerenone (40 mg/kg per day) did not reduce SBP in DS rats.37) The fact that a low dose of eplerenone was able to lower SBP in DS/obese rats is likely a result of the increased salt sensitivity of blood pressure in these animals. Recently, MR-associated hypertension with normal circulating aldosterone levels has been attracting more attention in relation to resistant hypertension.38) In such cases, MR antagonists may be indicated as add-on therapy to the inhibitors of renin-angiotensin system. Rac1 is shown to stimulate nuclear translocation of MRs, thus resulting in enhanced MR activity. Since DS/obese rats shows increased expression of Rac1 gene in the heart,17, 23) elevation of Rac1 levels may therefore be one mechanism of increased salt sensitivity of blood pressure in such rats.
LV hypertrophy was also attenuated in the EPL group compared with the MetS and Temp groups, probably reflecting in part the antihypertensive action of eplerenone. Cardiac fibrosis is a pathological feature associated with hypertension and is responsible for LV diastolic dysfunction, likely as a result of increased LV diastolic stiffness.18) DS/obese rats show increased levels of perivascular and interstitial fibrosis that were associated with impairment of LV relaxation as well as up-regulation of TGF-β1 and CTGF gene expression in the heart.17, 23) These changes in DS/obese rats were inhibited by both tempol and eplerenone, but the extent of inhibition was greater for eplerenone. These data are consistent with previous observations showing that TGF-β1 and CTGF contribute to the development of LV remodeling in a rat model of heart failure39) and in SHR/NDmcr-cp rats fed a high-salt diet.12) Furthermore, DS/obese rats showed LV diastolic dysfunction, as indicated by decreased E/A ratio, a prolonged DcT and increased LVEDP/LVDd ratio, and these changes were inhibited to a greater extent by eplerenone than by tempol. Aldosterone was shown to act on nonepithelial cells of the heart, vasculature, and kidney to promote tissue remodeling, inflammation, and fibrosis.40) Such nonclassical actions of aldosterone were markedly attenuated by MR blockade with eplerenone or spironolactone in association with improvement in the outcomes of patients with LV systolic dysfunction and heart failure.14, 41) Whereas eplerenone ameliorated LV fibrosis and diastolic dysfunction more effectively than did tempol in the present study, both drugs were found to attenuate LV diastolic dysfunction to a similar extent in SHR/NDmcr-cp rats.12) The superior cardioprotective action of eplerenone is likely attributable to its greater antihypertensive effect, which is likely related to its greater inhibition of aldosterone-MR signaling in the cardiovascular system. Our results are also consistent with previous observations showing that the addition of spironolactone to standard angiotensin II inhibition improved myocardial abnormalities and decreased fibrotic markers in MetS.42) However, our results do not warrant that the selective MR antagonist eplerenone is superior to the antioxidant tempol in its ability to inhibit MR, because they produced unequal antihypertensive potency in our experimental conditions.
Obesity is a proinflammatory state characterized by adipose tissue inflammation, including increased production of proinflammatory cytokines and MCP-1 by adipose tissue.43) This obesity-related change in adipose tissue is linked to the development of insulin resistance, type 2 diabetes mellitus, and cardiovascular injury.43) Activation of MR has been implicated in the inflammation apparent in vessels, the heart, or renal cortex of rodent models of diabetes mellitus or hypertension.44, 45) MR blockade also reduces markers of inflammation in patients with diabetes.15, 46) In our recent study, macrophage infiltration into the interstitial space of the LV myocardium was accompanied by increased expression of genes for proinflammatory proteins such as MCP-1, osteopontin, and COX-2 in the heart of DS/obese rats.23) These changes may contribute to the development of myocardial fibrosis in these animals.47) Both tempol and eplerenone treatment similarly prevented these inflammatory changes in DS/obese rats, whereas cardiac fibrosis was inhibited to a greater extent in the EPL group than in the Temp group. It is possible that eplerenone inhibited the up-regulation of TGF-β1 and CTGF gene expression to a greater extent than did tempol as a consequence of its direct inhibition of MR and the resultant marked antihypertensive effect.
MetS is associated with an increase in oxidative stress.9) MR activation can induce ROS overproduction, and selective MR blockade abolishes this effect.12) Furthermore, ROS are thought to contribute to MR activation.11, 48) We recently showed that NADPH-dependent superoxide generation and the expression of NADPH oxidase subunit genes were increased in the heart of DS/obese rats.17, 23) These effects in DS/obese rats were attenuated to a similar extent by both tempol and eplerenone. Excess ROS may contribute to impairment of LV diastolic function through inhibition of Ca2+-handing proteins.7) The decrease in the level of cardiac oxidative stress induced by tempol or eplerenone in DS/obese rats was accompanied by amelioration of cardiac inflammation. The antioxidant effect of eplerenone on the heart of DS/obese rats is thus similar to that of tempol. Our results suggest that some of the beneficial cardiovascular effects of eplerenone are mediated through changes in oxidative stress.12)
The RAAS has been implicated in the pathogenesis of MetS.10) In addition to the classical systemic RAAS, a local RAAS is thought to operate in the heart, vessels, and kidney.18) Our recent study demonstrated that the expression of genes for ACE, the AT1A receptor, MR, and the aldosterone effector kinase Sgk1 was up-regulated in the heart of DS/obese rats,17, 23) consistent with a causative role for RAAS activation in the development of cardiac injury associated with MetS.12) Our observations that cardiac RAAS gene expression was reduced by tempol and eplerenone are consistent with previous results showing that up-regulation of ACE and MR signaling in the cardiovascular system of SHR/NDmcr-cp rats was attenuated by these drugs.12) Notably, cardiac expression of the MR and Sgk1 genes was decreased to a greater extent by eplerenone than by tempol. In addition, we found that both the plasma aldosterone concentration and the ratio of the plasma aldosterone concentration to plasma renin activity were lower in the EPL group than in the Temp group. The classical systemic RAAS is modulated to increase renin and aldosterone levels by MR blockade with the feedback loop. Our results are not consistent with previous findings that plasma aldosterone levels were elevated by selective MR blockade with eplerenone in SHR/cp rats.12) Adipocyte-derived aldosterone-releasing factors (ARFs) stimulate aldosterone secretion by the adrenal gland, resulting in aldosterone excess in obese spontaneously hypertensive rats.13) It might be possible that eplerenone inhibited the secretion of these factors in DS/obese rats.
A previous in vitro study suggested that oxidative stress enhances MR sensitivity through desumoylation of MR protein.49) Eplerenone treatment attenuated LV fibrosis and failure in mice subjected to ascending aortic constriction, indirectly linking the involvement of aldosterone with chronic pressure overload.50) Since aldosterone-mediated LV hypertrophy and fibrosis was associated with increased oxidative stress and inflammation, it is possible that the beneficial effects of eplerenone may be attributable in part to reduced oxidative stress and its-associated decrease in MR sensitivity.51, 52) Our results also support the notion that MR activation by factors other than aldosterone, such as increased MR abundance, increased MR sensitivity, and overstimulation of MR by Rac1 may be involved in the pathogenesis of MR-associated hypertension with normal plasma aldosterone levels.38) In addition to genomic effects of aldosterone, rapid nongenomic effects of mineralocorticoids have been reported in various tissues, including in the heart, that are mediated by activation of a membrane receptor distinct from the classical MR.53, 54) It is thus not improbable that nongenomic effects of aldosterone play a role in the pathogenesis of cardiac injury in DS/obese rats.
In conclusion, we have shown that eplerenone, but not tempol, attenuated hypertension and LV hypertrophy in DS/obese rats and that eplerenone ameliorated LV fibrosis and diastolic dysfunction more effectively than did tempol. Cardiac oxidative stress and inflammation were similarly attenuated by both drugs. Our results suggest that abnormal activation of the aldosterone-MR system may play a key role in the development of salt-sensitive hypertension and cardiac damage in MetS. Selective MR blockade thus warrants further investigation for its ability to prevent hypertension as well as to protect the cardiovascular system in individuals with MetS.
ACKNOWLEDGEMENTS
We thank Masafumi Ohtake, Riyo Inagaki, Erina Suzuki, Miki Kato, Keigo Nashima, and Chieko Nakashima for technical assistance.
SOURCE OF FUNDING
This work was supported by unrestricted research grants from Mitsubishi Tanabe Pharma Corporation (Osaka, Japan), Kyowa Hakko Kirin Co. Ltd. (Tokyo, Japan), Mochida Pharmaceutical Co., Ltd. (Tokyo, Japan), Astellas Pharma Inc. (Tokyo, Japan), MSD K.K. (Tokyo, Japan), Pfizer Japan Inc. (Tokyo, Japan), Ajinomoto Pharmaceuticals Co., Ltd. (Tokyo, Japan), Takeda Pharmaceutical Company Limited (Osaka, Japan), Daiichi-Sankyo Company, Limited (Tokyo, Japan), Kowa Pharmaceutical Co. Ltd. (Tokyo, Japan) and Dr. Nagata (Nagoya University) as well as by Management Expenses Grants from the Japanese government to Nagoya University.
Conflict of Interest/Disclosure
None.
REFERENCES
- 1).Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation, 2005; 111: 1448–1454. [DOI] [PubMed]
- 2).Kannel WB, Brand N, Skinner JJ, Jr., Dawber TR, McNamara PM. The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med, 1967; 67: 48–59. [DOI] [PubMed]
- 3).Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA, 2002; 288: 2709–2716. [DOI] [PubMed]
- 4).Matsui H, Shimosawa T, Uetake Y, Wang H, Ogura S, Kaneko T, Liu J, Ando K, Fujita T. Protective effect of potassium against the hypertensive cardiac dysfunction: association with reactive oxygen species reduction. Hypertension, 2006; 48: 225–231. [DOI] [PubMed]
- 5).Masugata H, Senda S, Goda F, Yoshihara Y, Yoshikawa K, Fujita N, Daikuhara H, Nakamura H, Taoka T, Kohno M. Left ventricular diastolic dysfunction as assessed by echocardiography in metabolic syndrome. Hypertens Res, 2006; 29: 897–903. [DOI] [PubMed]
- 6).Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med, 2006; 355: 260–269. [DOI] [PubMed]
- 7).Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med, 2004; 10: 1200–1207. [DOI] [PubMed]
- 8).Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism, 2006; 55: 928–934. [DOI] [PubMed]
- 9).Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest, 2004; 114:1 752–1761. [DOI] [PMC free article] [PubMed]
- 10).Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol, 2004; 287: R943–R949. [DOI] [PubMed]
- 11).Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension, 2007; 50: 877–883. [DOI] [PubMed]
- 12).Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, Nagase M, Fujita T. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension, 2008; 52: 287–294. [DOI] [PubMed]
- 13).Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol, 2006; 17: 3438–3446. [DOI] [PubMed]
- 14).Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med, 2003; 348: 1309–1321. [DOI] [PubMed]
- 15).Matsumoto S, Takebayashi K, Aso Y. The effect of spironolactone on circulating adipocytokines in patients with type 2 diabetes mellitus complicated by diabetic nephropathy. Metabolism, 2006; 55: 1645–1652. [DOI] [PubMed]
- 16).Hattori T, Murase T, Ohtake M, Inoue T, Tsukamoto H, Takatsu M, Kato Y, Hashimoto K, Murohara T, Nagata K. Characterization of a new animal model of metabolic syndrome: the DahlS.Z-Leprfa/Leprfa rat. Nutr Diabetes, 2011; 1: e1. [DOI] [PMC free article] [PubMed]
- 17).Murase T, Hattori T, Ohtake M, Abe M, Amakusa Y, Takatsu M, Murohara T, Nagata K. Cardiac remodeling and diastolic dysfunction in DahlS.Z-Leprfa/Leprfa rats: a new animal model of metabolic syndrome. Hypertens Res, 2012; 35: 186–193. [DOI] [PubMed]
- 18).Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension, 2006; 47: 656–664. [DOI] [PubMed]
- 19).Takatsu M, Nakashima C, Takahashi K, Murase T, Hattori T, Ito H, Murohara T, Nagata K. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension, 2014; 62: 957–965. [DOI] [PubMed]
- 20).Nagata K, Somura F, Obata K, Odashima M, Izawa H, Ichihara S, Nagasaka T, Iwase M, Yamada Y, Nakashima N, Yokota M. AT1 receptor blockade reduces cardiac calcineurin activity in hypertensive rats. Hypertension, 2002; 40: 168–174. [DOI] [PubMed]
- 21).Hattori T, Murase T, Takatsu M, Nagasawa K, Matsuura N, Watanabe S, Murohara T, Nagata K. Dietary salt restriction improves cardiac and adipose tissue pathology independently of obesity in a rat model of metabolic syndrome. J Am Heart Assoc, 2014; 3(6): e001312. [DOI] [PMC free article] [PubMed]
- 22).Matsuura N, Asano C, Nagasawa K, Ito S, Sano Y, Minagawa Y, Yamada Y, Hattori T, Watanabe S, Murohara T, Nagata K. Effects of pioglitazone on cardiac and adipose tissue pathology in rats with metabolic syndrome. Int J Cardiol, 2015; 179: 360–369. [DOI] [PubMed]
- 23).Takahashi K, Takatsu M, Hattori T, Murase T, Ohura S, Takeshita Y, Watanabe A, Murohara T, Nagata K. Premature cardiac senescence in DahlS.Z-Leprfa/Leprfa rats as a new animal model of metabolic syndrome. Nagoya J Med Sci, 2014; 76: 35–49. [PMC free article] [PubMed]
- 24).Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation, 1978; 58: 1072–1083. [DOI] [PubMed]
- 25).Kato MF, Shibata R, Obata K, Miyachi M, Yazawa H, Tsuboi K, Yamada T, Nishizawa T, Noda A, Cheng XW, Murate T, Koike Y, Murohara T, Yokota M, Nagata K. Pioglitazone attenuates cardiac hypertrophy in rats with salt-sensitive hypertension: role of activation of AMP-activated protein kinase and inhibition of Akt. J Hypertens, 2008; 26: 1669–1676. [DOI] [PubMed]
- 26).Nagata K, Iwase M, Sobue T, Yokota M. Differential effects of dobutamine and a phosphodiesterase inhibitor on early diastolic filling in patients with congestive heart failure. J Am Coll Cardiol, 1995; 25: 295–304. [DOI] [PubMed]
- 27).Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985; 28: 412–419. [DOI] [PubMed]
- 28).Miyachi M, Yazawa H, Furukawa M, Tsuboi K, Ohtake M, Nishizawa T, Hashimoto K, Yokoi T, Kojima T, Murate T, Yokota M, Murohara T, Koike Y, Nagata K. Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension, 2009; 53: 701–707. [DOI] [PubMed]
- 29).Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol, 2005; 16: 2906–2912. [DOI] [PubMed]
- 30).Somura F, Izawa H, Iwase M, Takeichi Y, Ishiki R, Nishizawa T, Noda A, Nagata K, Yamada Y, Yokota M. Reduced myocardial sarcoplasmic reticulum Ca2+-ATPase mRNA expression and biphasic force-frequency relations in patients with hypertrophic cardiomyopathy. Circulation, 2001; 104: 658–663. [DOI] [PubMed]
- 31).Sakata Y, Yamamoto K, Mano T, Nishikawa N, Yoshida J, Hori M, Miwa T, Masuyama T. Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of Angiotensin-converting enzyme inhibitor. Circulation, 2004; 109: 2143–2149. [DOI] [PubMed]
- 32).Murase T, Hattori T, Ohtake M, Nakashima C, Takatsu M, Murohara T, Nagata K. Effects of estrogen on cardiovascular injury in ovariectomized female DahlS.Z-Leprfa/Leprfa rats as a new animal model of metabolic syndrome. Hypertension, 2012; 59: 694–704. [DOI] [PubMed]
- 33).Yamada T, Nagata K, Cheng XW, Obata K, Saka M, Miyachi M, Naruse K, Nishizawa T, Noda A, Izawa H, Kuzuya M, Okumura K, Murohara T, Yokota M. Long-term administration of nifedipine attenuates cardiac remodeling and diastolic heart failure in hypertensive rats. Eur J Pharmacol, 2009; 615: 163–170. [DOI] [PubMed]
- 34).Dhuper S, Abdullah RA, Weichbrod L, Mahdi E, Cohen HW. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity (Silver Spring), 2011; 19: 128–133. [DOI] [PubMed]
- 35).de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension, 2004; 43: 41–47. [DOI] [PubMed]
- 36).Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med, 2004; 351: 33–41. [DOI] [PubMed]
- 37).Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, Takeda Y, Yoshida J, Miwa T, Okamoto M, Masuyama T, Nonaka Y, Hori M. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: Beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol, 2007; 292: R946–R954. [DOI] [PubMed]
- 38).Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens, 2012; 25: 514–523. [DOI] [PubMed]
- 39).Ahmed MS, Oie E, Vinge LE, Yndestad A, Oystein Andersen G, Andersson Y, Attramadal T, Attramadal H. Connective tissue growth factor-a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol, 2004; 36: 393–404. [DOI] [PubMed]
- 40).Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med, 2007; 13: 189–197. [DOI] [PMC free article] [PubMed]
- 41).Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med, 1999; 341: 709–717. [DOI] [PubMed]
- 42).Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O’Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging, 2011; 4: 1239–1249. [DOI] [PubMed]
- 43).Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res, 2005; 96: 939–949. [DOI] [PubMed]
- 44).Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol, 2004; 217: 263–269. [DOI] [PubMed]
- 45).Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology, 2006; 147: 5363–5373. [DOI] [PubMed]
- 46).Takebayashi K, Matsumoto S, Aso Y, Inukai T. Aldosterone blockade attenuates urinary monocyte chemoattractant protein-1 and oxidative stress in patients with type 2 diabetes complicated by diabetic nephropathy. J Clin Endocrinol Metab, 2006; 91: 2214–2217. [DOI] [PubMed]
- 47).Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension, 2004; 43: 739–745. [DOI] [PubMed]
- 48).Young M, Funder JW. Eplerenone, but not steroid withdrawal, reverses cardiac fibrosis in deoxycorticosterone/salt-treated rats. Endocrinology, 2004; 145: 3153–3157. [DOI] [PubMed]
- 49).Yokota K, Shibata H, Kurihara I, Kobayashi S, Suda N, Murai-Takeda A, Saito I, Kitagawa H, Kato S, Saruta T, Itoh H. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem, 2007; 282: 1998–2010. [DOI] [PubMed]
- 50).Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation, 2005; 111: 420–427. [DOI] [PubMed]
- 51).Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol, 2001; 280: C53–C60. [DOI] [PubMed]
- 52).Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol, 2002; 161: 1773–1781. [DOI] [PMC free article] [PubMed]
- 53).Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science, 1987; 237: 268–275. [DOI] [PubMed]
- 54).Mihailidou AS, Mardini M, Funder JW. Rapid, nongenomic effects of aldosterone in the heart mediated by epsilon protein kinase C. Endocrinology, 2004; 145: 773–780. [DOI] [PubMed]