Abstract
To investigate the selective pressures acting on the protein-coding genes during the differentiation of indica and japonica, all of the possible orthologous genes between the Nipponbare and 93–11 genomes were identified and compared with each other. Among these genes, 8,530 pairs had identical sequences, and 27,384 pairs shared more than 90% sequence identity. Only 2,678 pairs of genes displaying a Ka/Ks ratio significantly greater than one were revealed, and most of these genes contained only nonsynonymous sites. The genes without synonymous site were further analyzed with the SNP data of 1529 O. sativa and O. rufipogon accessions, and 1068 genes were identified to be under positive selection during the differentiation of indica and temperate japonica. The positively selected genes (PSGs) are unevenly distributed on 12 chromosomes, and the proteins encoded by the PSGs are dominant with binding, transferase and hydrolase activities, and especially enriched in the plant responses to stimuli, biological regulations, and transport processes. Meanwhile, the most PSGs of the known function and/or expression were involved in the regulation of biotic/abiotic stresses. The evidence of pervasive positive selection suggested that many factors drove the differentiation of indica and japonica, which has already started in wild rice but is much lower than in cultivated rice. Lower differentiation and less PSGs revealed between the Or-It and Or-IIIt wild rice groups implied that artificial selection provides greater contribution on the differentiation than natural selection. In addition, the phylogenetic tree constructed with positively selected sites showed that the japonica varieties exhibited more diversity than indica on differentiation, and Or-III of O. rufipogon exhibited more than Or-I.
Introduction
Asian cultivated rice (O. sativa L.) is one of the oldest and most important crop species. It is the primary source of food and livelihood for more than a third of Asia’s population, accounting for 35–60% Asia’s and ~20% the world’s caloric intake respectively[1]. O. sativa has a broad geographic distribution across the world with a high phenotypic variability, an estimated 120,000 varieties [1]. Most varieties have been placed into two subspecies, O. sativa ssp. indica and O. sativa ssp. japonica, which differ in more than 40 characteristics, such as phenol reaction phenotype, KClO3 resistance, cold sensitivity, drought tolerance, germination, seed shedding, length-width ratio of spikelet, apiculus hair length, awn length, digestion of endosperm in KOH solution, hardening of endosperm and first internode [2]. Some of these characteristics have been used to distinguish the indica and japonica varieties [2, 3]. Further analyses with ecological traits, isozymes and/or DNA markers confirmed and developed the above classification [2–8]. Studying 950 accessions with 4.1 million SNPs, Huang et al further divided the japonica subspecies into two sub-groups, temperate japonica and tropical japonica, and the indica subspecies into indica and aus sub-group [9].
The immediate progenitor of O. sativa is O. rufipogon [10]. Previous studies mostly focused on the domestication of wild rice, indicating that O. sativa was domesticated from O. rufipogon approximately 8200–13,500 years ago [2, 11]. However, these studies have provided two hypotheses about the origin(s) of two subspecies. One proposed that the domesticated rice originated from a single common wild ancestor, and differentiation of indica and japonica occurred after the domestication of cultivated species, which is supported by the analyses of well-characterized domestication genes and SNPs from 630 gene fragments in wild and cultivated rice accessions [11–16]. The other hypothesis suggested that two major rice types were domesticated separately from different populations of wild rice, supported by phylogenetic analyses that showed distinct clades in O. sativa for indica and japonica with different O. rufipogon accessions associated with each clade [17–22], as well as the whole-genome SNPs analyses [10, 23]. The SNPs analyses further indicated that japonica was first domesticated from a specific population of O. rufipogon around the middle area of the Pearl River in southern China, and that indica was subsequently developed from crosses between japonica and local wild rice as the initial cultivars spread into South East and South Asia[10].
Incomplete observations with one or several isozymes or domestication-related genes have only dropped small hints about the domestication processes, whereas the application and development of molecular markers can provide considerable information for the understanding of rice evolution. However, analyses with molecular markers such as RFLPs, SSRs and SNPs, which are usually caused by mutation, need to specify whether the mutation is neutral or not. A neutral mutation cannot change the gene function, and has no effect on fitness. Thus, such mutations provide less useful information for evolutionary analyses, and may interfere with the prediction. An advantageous mutation would have a positive effect on phenotype, and increase the fitness of the organism. These mutations will be accumulated in the gene pool. Conversely, deleterious mutations would decrease the fitness of the organism, and get typically eliminated from the gene pool by selection. Thus, positively selected genes (PSGs) carry much more information that is relevant to the evolutionary history of a species than negatively selected genes. Furthermore, the PSGs are, or have been, functionally important, and identification will facilitate the understanding of genetic variation that contributes to phenotypic diversity, and help to annotate the functional genome. Therefore, the emphasis of the domestication and differentiation analyses should be placed on the PSGs.
Differentiation of indica and japonica was driven by both artificial and natural selection, which directly acted on many characteristics [2, 24–27]. Selection results in a difference in gene frequencies between populations. Various factors have been known to be as selective forces, e.g., temperature, light condition, day length, soil fertility, stress conditions like drought, submergence, salinity, pollution, and herbicide use [2]. Protein evolution is the outcome of interaction between mutational processes and selective forces; therefore, analyzing the coding region of a genome is fundamental to understand how selection influences evolution. As a model organism, O. sativa is a well-characterised species with a small genome (389 Mb). The indica variety 93–11 and the japonica variety Nipponbare have been fully sequenced [28], and more than 2000 accessions including wild rice have been partly sequenced (http://ricevarmap.ncpgr.cn/django/home/) [10]. These features afford unique opportunities to explore differentiation of indica and japonica via genomic approaches. In this study, the genes under positive selection were identified and analyzed systematically in order to provide information for further understanding of cultivated rice evolution.
Results
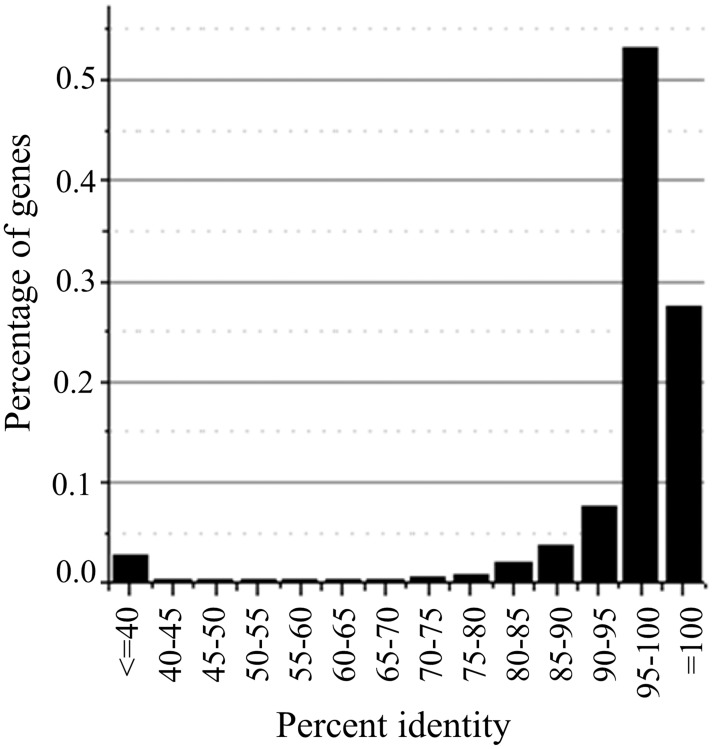
All of the gene annotations for Nipponbare and 93–11 were downloaded from online public databases. There were 40,354 and 67,393 annotations for Nipponbare from the Rice Annotation Project Database (RAP-DB) and Rice Genome Annotation Project (RGAP), respectively, and 40,745 annotations for 93–11 from Rice Information System (RISe). The annotations of RGAP contained more alternatively spliced genes, transposons and retrotransposon genes. The databases of Nipponbare protein sequences retrieved from RAP-DB and RGAP were queried with the 93–11 protein sequences to identify pairs of orthologs, respectively. Combining the two BLAST results, 30,995 pairs of orthologous genes were found. The identity values of these orthologs were re-calculated according to the ClustalW2 result, and the resulting distribution of percent identities was shown in Fig. 1A total of 8,530 gene pairs exhibited 100% identity, and 27,384 pairs had more than 90% identity. These pairs were used to evaluate positive selection between indica and japonica.
Fig 1. The distribution of the percent identity between the possible orthologs.
The most similar proteins between 93–11 and Nipponbare were selected with BLAST, and 30995 pairs of proteins were obtained; each pair was analyzed via ClustalW 2 to obtain the percent identity.
Positively selected genes between 93–11 and Nipponbare
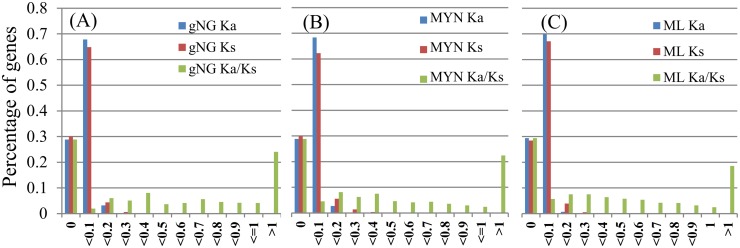
Positive selection is often evaluated by the ratio of nonsynonymous/synonymous substitution rates. This ratio, Ka/Ks, is expected to be greater than 1.0 in the case of positive selection [29]. The orthologous genes with high identity values (>90%) were used to detect instances of positive selection between 93–11 and Nipponbare by estimating their synonymous and nonsynonymous substitution rates. The Ka and Ks values of those genes were obtained with NG, gNG, YN, MYN, and maximum likelihood, respectively, and the results with maximum likelihood, gNG and MYN were shown in Fig. 2. The distributions of the Ka and Ks values were very narrow, with 99% of those genes displaying the Ka and Ks values below 0.3, and 18.5–24.5% of the gene pairs (more than 5000 pairs) showed Ka/Ks > 1 (Fig. 2). Fisher’s test was used to identify the Ka/Ks ratios that were significantly higher than one, suggesting more than 10% genes were positively selected during the differentiation of 93–11 and Nipponbare. In addition, the average percent identity of the PSGs was 99.29%.
Fig 2. The distribution of Ka, Ks and Ka/Ks.
(A) Using the gNG method. (B) Using the MYN method. (C) Using the Maximum Likelihood method. Note: Ka/Ks were specified as zero if both Ka and Ks were zero (5247 genes).
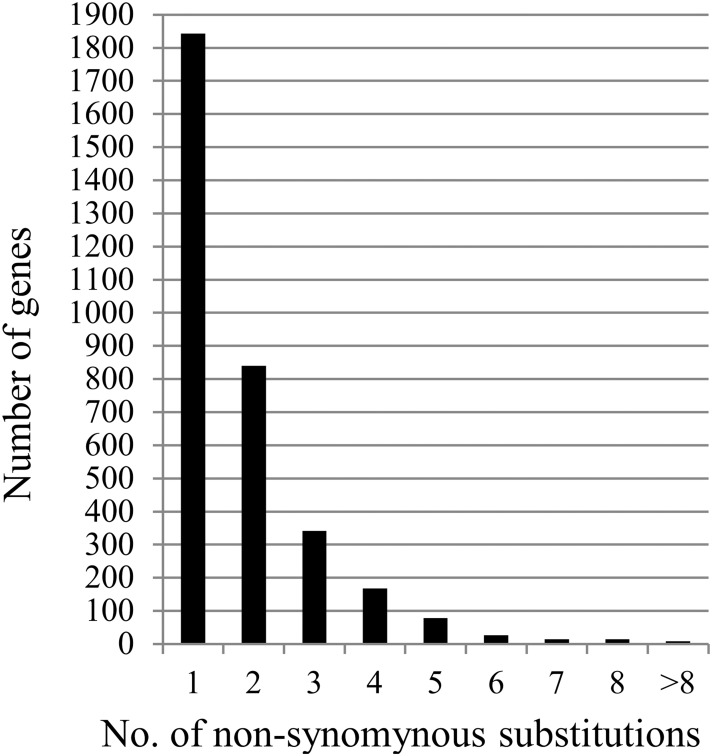
These PSGs were manually analyzed to remove annotation mistakes and ClustalW errors, followed by re-calculation. There were 2,977 PSGs detected with the gNG method and 2,799 PSGs with MYN. Among them, 2,664 PSGs were shared via both approaches. Interestingly, the synonymous substitution numbers of most these PSGs are zero (S1 Table). We denoted such type of genes as nonsynonymous substitution genes (NSSGs) including the genes whose Ka/Ks ratios were not significantly higher than one in Fisher’s test (S1 Table). Most of the NSSGs exhibited one or two substitutions (Fig. 3). Only seven PSGs with synonymous substitution sites were detected by at least one method (S2 Table).
Fig 3. The distribution of the numbers of non-synonymous substitutions in NSSGs.
To further investigate the selective pressure acting on protein-coding genes, all of the genes whose Ka/Ks ratios is not significantly higher than one were analyzed with an alternative approach to calculate the Ka/Ks ratios on sliding windows of fixed size. The alignment slicing procedure with sliding windows of 100 codons and a window shift of 34 codons generated 108,358 windows with less than 50% gap. Only 14 PSGs were identified by at least one of above methods (MYN, YN, NG and ML) after removing the annotation mistakes and ClustalW errors (S3 Table). Because it is difficult to do further analysis with the SNP data for these genes, we focused on NSSGs in the following study.
SNP data can be used to estimate the positively selected sites in NSSGs between indica and temperate japonica
To detect whether the above PSGs found between 93–11 and Nipponbare were also under selection among most of the other indica and japonica accessions, the data containing 520 indica (excluding aus rice) and 409 temperate japonica accessions with 4.1 million SNPs was downloaded [10] and analyzed, as 93–11 belonged to indica and Nipponbare to temperate japonica. The SNPs in the exons of all PSGs were exposed, and most of the positively selected sites (PSSs) in the PSGs were able to be found in these SNPs. In addition, some new sites that changed amino acids were also discovered. Taking chromosome 1 for example, we found 2847 SNPs in the exons of 497 NSSGs, which included 687 (77.6%) PSSs revealed between 93–11 and Nipponbare. There were 198 (22.4%) PSSs that did not contain in these SNPs, so we speculated that these PSSs were specific to the differences between 93–11 and Nipponbare or the SNP coverage was not enough.
We calculated the Fst value of each SNP site based on its frequency in indica and temperate japonica, which was thought to be a measure of population differentiation due to genetic structure [30–32]. Most of these SNP sites possess lower Fst values, with 70.6% less than 0.25 and 60.7% less than 0.1. These sites could less affect the early differentiation between indica and japonica, and thus we did not examine whether their alterations changed the amino acids. The frequencies of these SNPs were calculated in total, indicating that the minor base frequencies of 1687 (83.9%) SNP sites were less than 0.1. It showed that these SNPs merely affected a small amount of rice accessions when they changed the amino acids, explaining why so many SNPs could not be found in PSSs.
We found 115 new nonsynonymous sites and 80 synonymous sites in the SNP data (Table 1). The difference of 93–11 and Nipponbare could represent about 73.2% diversity among all the indica and temperate japonica accessions when the Fst values are not less than 0.25. This suggested that it was possible to find synonymous sites in NSSGs in the study of the differentiation of indica and temperate japonica. It is difficult to calculate the number of the synonymous sites in the unsequenced region, but the probability could be estimated in the sequenced region, and the two regions are supposed to have an identical probability. The average probability is about 11.0%, but the probability is much less for the NSSGs with higher Fst values (Table 1). These results inferred that SNPs data could be used to estimate the positively selected sites in NSSGs between indica and temperate japonica.
Table 1. Number of nonsynonymous sites and new synonymous sites of chromosome 1 between indica and temperate japonica.
| Fst | shared 1 | New Nonsyn. 2 | New syn. 3 | total | Probability 4 |
|---|---|---|---|---|---|
| > = 0.95–1 | 204 | 9 | 6 | 219 | 0.027 |
| 0.9–0.95 | 45 | 6 | 5 | 56 | 0.089 |
| 0.8–0.9 | 59 | 8 | 11 | 78 | 0.141 |
| 0.7–0.8 | 52 | 14 | 7 | 73 | 0.096 |
| 0.6–0.7 | 37 | 10 | 14 | 61 | 0.230 |
| 0.5–0.6 | 45 | 12 | 8 | 65 | 0.123 |
| 0.4–0.5 | 41 | 18 | 9 | 68 | 0.132 |
| 0.3–0.4 | 32 | 17 | 8 | 57 | 0.140 |
| 0.25–0.3 | 17 | 21 | 12 | 50 | 0.240 |
| < 0.25 | 155 |
1Number of nonsynonymous sites (PSSs) shared by sequence and SNP analyses.
2Number of new nonsynonymous sites found between indica and temperate japonica
3Number of new synonymous sites found between indica and temperate japonica
4The probability of synonymous site occurred in NSSGs.
Positively selected genes between indica and temperate japonica
We discovered 313 genes that only contain nonsynonymous sites (Fst > = 0.25) in chromosome 1 (S4 Table). To detect whether these SNP loci were the signatures of adaptation during the genetic differentiation between populations, Lositan, an Fst related statistic method, was employed to identify the outliers that were positively influenced by selection, acting on either the locus itself or the closely linked locus. We identified 105 (19.9%) outliers within 99% confidence interval and additional 56 (10.5%) within 95% confidence interval. Almost all of the sites whose Fst values are more than 0.95 were outliers. However, the sites with the Fst value of one were not able to be detected as outliers because one is the biggest Fst value, and the program cannot distinguish which is bigger between the simulation and the sample Fst. These sites should be under selection during the differentiation of indica and japonica, and have already been completely fixed. We also detected some outliers with lower Fst values, which would infer the recent selection occurred (Table 2).
Table 2. The distribution of NSSGs and outliers along Fst values between indica and temperate japonica, and the distribution of outliers along Fst values between Or-It and Or-IIIt.
| Fst values | No. of NSSGs between indica and temperate japonica 1 | No. of outliers between indica and temperate japonica | No. of outliers between Or-It and Or-IIIt 2 | ||
|---|---|---|---|---|---|
| Chr.1 | Chr.2–12 | Chr. 1 | Chr. 2–12 | ||
| 1 | 76 | 224 | 99 | 252 | 30 |
| 0.95-<1 | 68 | 310 | 93 | 429 | 66 |
| 0.9–0.95 | 19 | 162 | 34 | 240 | 64 |
| 0.8–0.9 | 34 | 68 | 13 | 85 | 22 |
| 0.7–0.8 | 34 | 4 | |||
| 0.6–0.7 | 21 | 7 | 1 | 3 | |
| 0.5–0.6 | 27 | 11 | 9 | 18 | 2 |
| 0.4–0.5 | 25 | 22 | 5 | 44 | 8 |
| 0.25–0.4 | 9 | 24 | 6 | 56 | 28 |
| Total | 313 | 832 | 161 | 1127 | 220 |
1Only the PSS with highest Fst was considered for some PSGs with more than one PSS.
2The number of the PSSs used to analyze O. rufipogon is 1354, including some of the PSSs from the PSGs with a synonymous site.
We detected 173 (55.3%) genes (including the genes with the site whose Fst is one) with positively selected outliers and only nonsynonymous sites (S4 Table). Natural and/or artificial selection could directly act on these genes. Some genes with more than one nonsynonymous site were measured again after combining the SNPs to get the haplotypes of the genes, but no additional genes were found to be under positive selection (data not shown).
We found some NSSGs with synonymous sites between indica and temperate japonica, in which some synonymous sites were detected as outliers, and these may be hitchhiking sites (S5 Table). The genes with higher Fst values nonsynonymous sites and lower synonymous sites were further analyzed, and those with nonsynonymous outliers should be under positive selection (S5 Table).
All NSSGs on the other chromosomes were analyzed between indica and temperate japonica with Lositan. The outlier sites corresponding to the amino residues and their positions on the proteins were shown in S6 Table. Additional 832 genes (1005 genes in total including chromosome 1) containing only nonsynonymous outlier sites were revealed (Table 2 and S6), and 83.6% of these genes have at least one high-Fst-value site (Fst > 0.9).
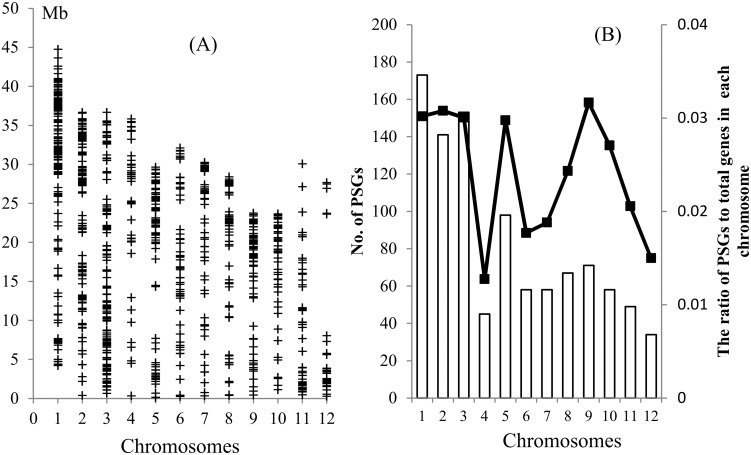
The PSGs were unevenly distributed among and along the chromosomes (Fig. 4). The numbers of the PSGs in the chromosome 1, 2, 3 are about 2 times more than those in the chromosome 4, 6, 7, 10, 11 and 12 (Fig. 4B). The gene density was usually higher near the ends of the chromosomes, but the distribution patterns on different chromosomes were distinct. For example, the PSGs were preferentially located near the ends of the short arms of chromosome 11 and 12, and the ends of the long arms of chromosomes 1, 2, 4, 7, 8, 9 and 10 (Fig. 4A). The ratios of the PSGs to total genes in each million base pairs were calculated, and the pattern of these ratios along the chromosomes was similar but not identical to that of the numbers of the PSGs (S1 Fig). In addition, the distribution of the ratios of PSGs to total genes in each chromosome was similar with that of the numbers of the PSGs except chromosome 9, in which the gene density is much higher with respect to the number of PSGs (Fig. 4). The result indicated that the uneven distribution was independent on the gene density in a chromosome.
Fig 4. The distribution of the PSGs along rice chromosomes.
(A) The distribution of the PSGs along the chromosomes. ‘+’ indicates the positions of the genes on the chromosomes. (B) The numbers of the PSGs (bars) and the ratios of PSGs (lines) to total genes in each chromosome.
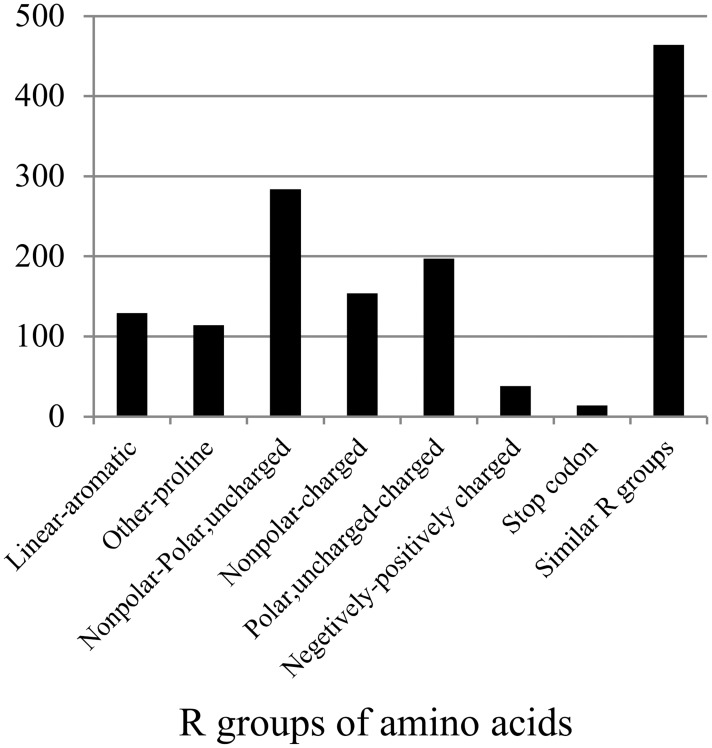
We found 1393 nonsynonymous outlier sites including one site whose change altered intron 3′ splice site and 14 sites whose changes resulted in stop codon. We found that 33.3% sites were replaced by the amino acids with similar R group (side chain), and the rest by the amino acids with different property (Fig. 5). Some substitutes were able to severely change the structures of the proteins, for example, the substitution of proline (Fig. 5).
Fig 5. The number of each type of substitutions in the proteins encoded by the PSGs.
We uncovered and selected additional 63 genes that contained only one synonymous site and some nonsynonymous outliers, in which the Fst value of the synonymous site was not higher than at least one nonsynonymous site (S7 Table). Some of these genes contained a nonsynonymous site, which altered the codon to stop codon, before the synonymous site on the gene. These genes were included in S7 Table as well, and could be under positive selection.
Functional classification of PSGs
Based on the gene annotations, we summarized the possible functions of the PSGs (S8 Table). However, the function annotations of at least 223 PSGs are hypothetical or unknown, and some other PSGs contained two or more domains or motifs. Many proteins encoded by PSGs possessed zinc finger domain, binding domain, F-box domain or ankyrin repeat etc., or belonged to the protein families of transcription factor, transferase, protein kinase, peptidase, synthase, transporter or hydrolase etc. (S8 Table).
Blast2GO, RAP-DB and MSU Rice Genome Annotation Project were used to reveal the GOs of these PSGs, and cellular component, molecular function and biological process annotations were found in only 492, 626 and 499 PSGs, respectively. These genes were involved in 218 molecular functions and 213 biological processes, respectively (S9 Table and S10 Table). More attentions should be paid for 402 (64.2%) proteins (including sequence-specific DNA binding transcription factor activity) encoded by the PSGs with binding activity, 116 (18.5%) with hydrolase activity, 93 (14.9%) with transferase activity. There were 118 (23.6%) PSGs involved in macromolecular metabolic processes, 75 (15.0%) in biological regulation, 64 (12.8%) in transport (transmembrane transport and vesicle-mediated transport) and 63 (12.6%) in response to stimulus (including cellular response to stimulus). Some proteins could contain more than one activity (462 proteins) or be involved in more than one process (316 proteins), or vice versa.
To reveal whether the PSGs contained some genes of known function, we searched NCBI, QTARO (http://qtaro.abr.affrc.go.jp/ogro/table) and Google Scholar with the IDs of RAP-DB and RGAP. We found 29 genes have been characterized, and 47 genes’ expression patterns have been revealed (Table 3 and S11). More than half of these genes are involved in the regulation of biotic and/or abiotic stresses, and seven of these genes regulate the germ cell development (Table 3), especially S5 gene (Os06t0213100 or LOC_Os06g11010), which regulates the hybrid sterility between indica and japonica variety [33] and was positively selected during differentiation of indica and japonica (S6 Table). The expressions of most genes can be induced or repressed by biotic and/or abiotic stresses (S11 Table). These results implied that many artificial/environmental factors could directly act on the genes and accelerated the variety differentiation. In particular, some known PSGs (Dpl2 and S5 genes) are involved in reproductive isolation (Table 3).
Table 3. The PSGs of known function.
| Locus ID | Gene | Isolation or expression | Characters | Functions | Ref* |
|---|---|---|---|---|---|
| Os01t0678600 | asl1 | Mutant | Albino seedling lethality | Chloroplast development. | [34] |
| Os01t0695900 | OsMYB4 | Overexpression | Chilling and freezing tolerance | Cold tolerance | [35, 36] |
| Os01t0756700 | OsKAT1 | Overexpression | Salinity tolerance | Salinity tolerance in protoplast. Maintenance of cytosolic cation homeostasis. | [37] |
| Os01t0816100 | OsNAC4 | Knockdown | Blast resistance | Blast resistance. HR cell death. | [38–40] |
| Os01t0831000 | lax | Mutant | Culm leaf, rachis-branches, lateral spikelet | Lateral organ development. Axillary meristem formation. | [41–44] |
| Os01t0867300 | Osabf1 | Mutant | Sensitive to drought and salinity treatment. | Drought and salinity tolerance. | [45] |
| Os01t0872800 | OsPdk1 | Mutant and overexpression | Overexpression of ospdk1 enhanced basal resistance against bacterial blight resistance and blast resistance | Ospdk1 participates in signal transduction through pathogen recognition | [46] |
| Os01t0929600 | rtS | Knockdown | Sterility | Pollen development. Anther development. | [47] |
| Os02t0664000 | OsGPX3 | Knockdown | Dwarf and shorter root. Accumulation of H2O2 | Root development. Dwarfism. H2O2 homeostasis. | [48] |
| Os02t0766700 | OsbZIP23 | Mutant and overexpression | Decreased sensitivity to ABA and tolerance to salinity and drought stress. | Drought and salinity tolerance. ABA sensitivity. | [49] |
| Os03t0119966 | rim1 | Mutant | Rice dwarf virus resistance. | Rice dwarf virus resistance. | [50] |
| Os03t0285800 | OsMAPK5 or OsMPK3 | Knockdown and Overexpression | Bacterial blight and blast resistance; cold, drought and salinity tolerance | Positively regulate response to biotic and abiotic stress, and JA pathway | [51–55] |
| Os03t0821300 | xb15 | Mutant | Bacterial blight resistance | Resistance to Xoo. Regulation of cell death. | [56] |
| Os05t0420300 | serf1 | Mutant | Sensitive to salt stress | Salinity tolerance. | [57] |
| Os06t0184100 | DPL2 | Natural variation | Sterility | Hybrid sterility. Pollen germination. Interaction with DPL1 | [58] |
| Os06t0213100 | S5 | Natural variation | Sterility | Single locus hybrid sterility. | [33] |
| Os06t0354700 | nyc3 | Mutant | Stay green | Leaf senescence. Chlorophyll degradation. | [59] |
| Os06t0665400 | apo1 or SCM2 | Mutant and natural variation | Grain number. Lodging resistance | Floral organ identity; panicle branching; culm strength | [60–63] |
| Os06t0712700 | spw1 or OsMADS16 | Mutant and overexpression | Alter floral organ | Floral organ formation. | [64, 65] |
| Os06t0724900 | ila1 | Mutant | Increase leaf angle | Abnormal vascular bundle formation and cell wall composition in the leaf lamina joint. | [66] |
| Os07t0687700 | rTGA2.1 | Knockdown | Bacterial blight resistance and reduced plant stature | Resistance to Xanthomonas oryzae pv. Oryzae. Growth retardation. | [67] |
| Os08t0139000 | OsDEG10 | Knockdown | Sensitive to high light and cold stresses | High-light and cold tolerance. | [68] |
| Os08t0522400 | OsAPx-R | Knockdown | Dwarf | Delay development and disturb steady state of the antioxidant | [69] |
| Os09t0439200 | OsJAZ8 | Overexpression | Bacterial blight resistance | JA induced resistance to Xanthomonas oryzae pv. Oryzae. | [70] |
| Os09t0441900 | DEP1 | Natural variation | Dense and erect panicle. | Enhance meristematic activity. Conferring cadmium tolerance | [71, 72] |
| Os09t0507200 | OsMADS8 | Knockdown | Panicle flower | Floral organ formation. | |
| Os09t0522000 | OsDREB1B | Overexpression | Cold, drought and salinity tolerance | Regulators of the abiotic stress | [73–76] |
| Os09t0537700 | OsRNS4 | Overexpression | Salinity tolerance | Salinity tolerance. Positive regulation in ABA response | [77] |
| Os12t0572800 | mel2 | Mutant | Developmental aberration of germline and nursery cells | Regulate the premeiotic G1/S-phase transition of male and female germ cells, | [78] |
*reference
Differentiation and positive selection among the O. rufipogon accessions
O. sativa have been classified into five groups—indica, aus, temperate japonica, tropical japonica and intermediate, while O rufipogon into three groups—Or-I, Or-II and Or-III [9, 10]. To investigate the population differentiation on indica and japonica, we constructed a neighbour-joining tree with 446 O. rufipogon accessions and 1,083 O. sativa varieties based on the PSSs revealed between indica and temperate japonica, in which some PSSs were deleted for lack of data in some groups. The results showed that indica and temperate japonica were separately located on the two sides of the phylogenetic tree with the largest difference as prediction. The temperate and tropical japonica varieties distributed over a large range on the tree comparing with indica and aus which were clustered together. Most indica including the aus accessions seemed to generate from one progenitor. O. rufipogon were located between indica and japonica, meanwhile a small number of the Or-I accessions were in indica or aus group. Most of the intermediate varieties were located between tropical japonica and Or-III, Or-II and Or-III, or Or-I and Or-II. The aromatic varieties in the collection were put together with some intermediate varieties (S2A Fig). We also constructed a Minimum Evolution tree and obtained a similar result (data not shown). To explore the phylogenetic relationships of the wild rice further, we constructed a tree only with the O. rufipogon accessions. Most of Or-I and Or-II were concentrated together, whereas Or-III distributed over a large range. Unexpectedly, some of Or-III seemed more close to the Or-II group (S2B Fig). We selected some O. rufipogon accessions into three new groups—Or-It, Or-IIt and Or-IIIt for the next analysis, which were concentrated on the tree separately. Almost all of the accessions in Or-IIIt were from China.
The phylogenetic tree exhibited that the degree of the indica-japonica differentiation of the wild rice was between indica and japonica, and Or-IIt was between Or-It and Or-IIIt. We then measured the Fst values according to the frequencies of the PSSs, as shown in Table 4. The biggest average Fst value was between indica and temperate japonica as we expected, followed by the one between temperate japonica and Or-It. However, the Fst value between indica and Or-IIIt was much lower than that between temperate japonica and Or-It. The indica-japonica differentiation of the wild rice is mainly between Or-It and Or-IIIt, which exhibited a relatively high level of population differentiation (Table 4).
Table 4. The Fst values between the rice groups.
| Or-It | Or-IIt | Or-IIIt | TeJ* | TrJ* | Indica | |
|---|---|---|---|---|---|---|
| Or-IIt | 0.192 | |||||
| Or-IIIt | 0.476 | 0.247 | ||||
| TeJ | 0.802 | 0.561 | 0.230 | |||
| TrJ | 0.658 | 0.452 | 0.198 | 0.076 | ||
| Indica | 0.064 | 0.299 | 0.592 | 0.919 | 0.771 | |
| aus | 0.040 | 0.196 | 0.443 | 0.750 | 0.610 | 0.102 |
*TeJ: temperate japonica; TrJ: tropical japonica
The PSSs were also revealed to check whether they were outliers during population differentiation in the wild rice. It showed that 23.2% PSSs were found to be under positive selection between Or-It and Or-IIIt, but 29.7% outliers were associated with the Fst values lower than 0.25 (S12 Table). The GOs of these PSGs with the Fst values over 0.25 were shown in S12 Table, and no significant difference was found with the GOs’ distribution of indica~temperate japonica. The distribution of the outliers along Fst values were shown in Table 2. Most of the outliers with a high Fst value between Or-It and Or-IIIt also exhibited a high Fst value between indica and temperate japonica (S13 Table). These results inferred that the differentiation of indica and japonica should have started before domestication, and the differentiation was becoming stronger during and after domestication.
Discussion
The basis for understanding the differentiation of indica and japonica
The indica and japonica types exist as natural varieties that differ in their adaptation to distinct climatic, ecogeographic and cultural conditions [79]. The rice cultivars in the temperate countries such as Japan, Korea and northern China are exclusively japonicas, and those grown in the tropical and subtropical regions such as Thailand, Burma, India and southern China are usually indicas. In addition, some japonicas are also distributed in high altitude areas of the tropics [2]. Both natural and artificial selection have affected the distribution of indica and japonica rice, and brought about many different morphological and physiological traits between the two groups. Our study discovered that these PSGs were involved in 213 biological processes, and had 218 molecular functions except the genes without functional annotation. Many of these proteins encoded by the PSGs had binding activity, and were involved in response to stimulus and in biological regulation (S9 Table and S10 Table). More than half of the PSGs with known function and/or expression were involved in the responses to biotic/abiotic stresses, and some of them (Dpl2 and S5 genes) are involved in reproductive isolation (Table 3 and S11). These results implied that selection played an important role in the differentiation of indica and japonica, and these PSGs might directly or indirectly regulate and control these different traits. Further studies on the functions of these genes are essential to reveal the mechanism underlying the differentiation between varieties. Our results provided the basis for a comprehensive and systematic understanding of the differentiation of indica and japonica, and would help explain some important inter-subspecies differences. In addition, each target of positive selection has a story to tell about the historical forces and events that have shaped the history of a population.
Whole-genome selection screening is necessary to study the differentiation of indica and japonica
By 4000 years ago, human societies worldwide had completed the domestication of all major crop species [80]. In the past ten or more years, researchers have identified the several specific genes that control some of the most important morphological changes associated with domestication. These genes include tb1 [81] and tga1 in maize [82]; qSH1 [83], sh4 [16], Prog1 [12, 13] and Rc [84] in rice; fw2.2 in tomato [85]; and the Q gene in wheat [86]. Although only a few domestication genes have been well documented, these analyses provided a great deal of information important to the understanding of how domestication modified plant development to produce today’s crops. Nevertheless, these data have not been sufficient to reveal the mechanism of domestication yet. Even fewer genes have been identified as involved in the differentiation of indica and japonica. Given this background, a whole-genome selection screen is a useful strategy for understanding the domestication and differentiation of indica and japonica. In this study, we revealed 1068 genes throughout the genome that underwent positive selection during differentiation, but found only 29 genes of known function. There were 15 genes involved in the regulation of biotic and/or abiotic responses; seven genes regulate the germ cell development. All of these genes except S5 and Dpl2 have not been reported to be involved in differentiation of indica and japonica in previous work. In addition, we found other 47 PSGs in response to various environment factors (Table 3 and S11). Our study laid the foundation for further research on evolution of cultivated rice.
The large differences between the Nipponbare and 93–11 proteomes due to the differences of gene annotations
When the genes in Nipponbare and 93–11 were compared, orthologs could not be found for more than 10,000 genes. This suggested a major difference between the Nipponbare and 93–11 proteomes. However, for most of these genes, highly similar sequences were found in the Nipponbare or 93–11 genome when used as queries to search the other genome. This result implied orthologs could not be found for these genes mainly due to the differences in the annotations of Nipponbare and 93–11. Further evidence to support this view came from the two different systems used to annotate the Nipponbare genes, RAP-DB and RGAP. We used the 93–11 protein sequences as queries to search the RAP-DB and RGAP databases, and obtained 21,884 and 25,538 orthologs, respectively. When these two sets of results were combined, 30,995 pairs of orthologs were discovered. In addition, previous study showed that approximately one-third of the automated annotations contained errors in the NBS-LRR encoding genes in Arabidopsis, and more than one-third in LRR-kinase genes in rice [85, 87]. The results suggested that inadequate gene annotation was the main impediment to finding the orthologous relationships between the Nipponbare and 93–11 genes. In this study, we selected a lower standard to reveal all of the possible orthologs, and then found the PSGs with a higher standard and manually corrected the annotation and ClustalW mistakes. These greatly reduced error rate and workload.
More genes than those detected were under positive selection during differentiation of indica and japonica
Several considerations make us to suppose that the actual number of the PSGs during differentiation of indica and japonica would be far more than that detected in this study. Firstly, some annotation errors brought about that no orthologs were found between 93–11 and Nipponbare. Secondly, some genes are pseudogenes in 93–11 or Nipponbare, but are functional genes in another. For example, the phr1 gene lost its function due to an 18 nucleotide deletion in the japonica lines, but it remained functional in the indica lines [25]. The Phr1 gene encoding a polyphenol oxidase controls the phenol reaction, which is an important trait for distinguishing indica and japonica. The grains of the indica cultivars turn brown after being soaked in phenol solution, whereas those of the japonica cultivars do not [2]. The genetic test revealed positive selection for the 18 bp deletion [25]. Unfortunately, our study failed to detect this selection on the Phr1 gene because the method used in this research was not suited for analyzing deletion. Thirdly, the method based on the average Ka/Ks ratio over all the sites in a sequence is low powerful to detect positive selection comparing with PAML-codeml because adaptive evolution occurs at only a few sites, as most amino acids in a protein are under structural and functional constraints [88, 89]. That is why only a few PSGs with synonymous sites were revealed and so many NSSGs were discovered in this study. However, the recent data are not fit to PAML-codeml. Fourthly, we adopted a relatively stringent condition, which required that the PSGs should be detected by both of methods at the same time. It led to the results that some supposed PSGs would be excluded because of the stringent condition.
Artificial selection accelerate indica-japonica differentiation
Our results indicated that the differentiation has already started in wild rice, but this differentiation is very low. Only 16.4% PSSs in indica~japonica were also detected in Or-It~ Or-IIIt, and the average Fst value between Or-It and Or-IIIt is 0.476 comparing with 0.919 between indica and temperate japonica. In addition, the populations of Or-It and Or-IIIt only constitute a small part of wild rice. If the differentiation in wild rice was supposed to be driven by natural selection, the indica-japonica differentiation in cultivated rice could be driven by natural and artificial selection. Moreover, artificial selection is much more powerful than natural selection on the differentiation.
Differentiation of indica and japonica is one of the most important evolution directions
We used the PSSs to reconstruct the phylogenetic tree, which showed that temperate japonica is far from all of wild rice, but indica and Or-I were almost clustered together (S2A Fig). This result looks like that in the principal component analysis (PCA) plot of 1529 accessions with ~8 million SNP sites in the Huang’s paper (Supplementary Figure 13), in which the japonica varieties clearly segregate from the other groups, and some Or-I accessions mixed with the indica varieties [10]. This implied that indica came from Or-I, whereas japonica maybe derived from another wild rice group that is similar to Or-III and not included in the collection. However, Huang et al suggested that japonica was first domesticated from Or-III in southern China, and was subsequently crossed to Or-I wild rice in South East Asia and South Asia, thus generating indica after many cross-differentiation-selection cycles according to the analysis of domestication loci [10]. That seemed inconsistent with the results from all the SNP data [10] and our tree.
The first component in the PCA plot separated indica and japonica, and the second component separated O. sativa and O. rufipogon [10]. The result implied that differentiations of indica and japonica, and wild and cultivated rice are two main evolution directions. The differentiation of indica and japonica started in wild rice. The accessions of japonica and Or-III distributed over a large range in our tree, whereas that of indica and Or-I concentrated together. This inferred that the japonica varieties exhibited more diversity than indica on differentiation, Or-III than Or-I (S2 Fig). Thus, the study on the origin and evolution of indica and japonica should consider the power that acted on the differentiation. However, Huang’s model neglected the indica-japonica evolution direction [10], and thus could not explain the evolution of indica and japonica well.
Materials and Methods
The sequences and SNP data used in the study
The annotations of the genes of Nipponbare (temperate japonica) were downloaded from RAP-DB (http://rapdb.dna.affrc.go.jp/download/irgsp1.html) and Rice Genome Annotation Project (ftp://ftp.plantbiology.msu.edu/pub/data/Eukaryotic_Projects/o_sativa/annotation_dbs/pseudomolecules/version_6.1/all.dir/), respectively. The gene annotations for 93–11 (indica) were downloaded from the RISe database (ftp://ftp.genomics.org.cn/pub/ricedb/rice_update_data/GLEAN_genes/Beijing_indica/GLEAN_genes/). The genome DNA sequences of Nipponbare and 93–11 were obtained from the IRGSP/RAP build 5 dataset (http://rapdblegacy.dna.affrc.go.jp/download/index.html) and ricedb/RGPVs9311/9311 (ftp://ftp.genomics.org.cn/pub/ricedb/rice_update_data/genome/9311/), respectively. All of the SNP data were download from Rice Haplotype Map Project Database (http://www.ncgr.ac.cn/RiceHap2/index.html).
Sequence alignment and the discovery of the orthologous genes between indica and japonica
The stand-alone BLAST programs (ncbi-blast-2.2.24+.exe; ftp://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/LATEST/) were used to search each database with default parameters. The BLAST results were parsed with the Bio::SearchIO module in perl (http://search.cpan.org/~cjfields/BioPerl-1.6.901/Bio/SearchIO.pm). The results were manually treated with EXCEL to find the most similar genes on the collinear regions of the 93–11 and Nipponbare chromosomes, which were considered orthologous. The pairs of orthologs were aligned with ClustalW2 [90] using the Bio::Tools::Run::Alignment::Clustalw module (http://search.cpan.org/~cjfields/BioPerl-Run-1.006900/lib/Bio/Tools/Run/Alignment/Clustalw.pm). The percent identity values were calculated according to the ClustalW2 results. To convert a multiple sequence alignment of proteins to a codon alignment of the corresponding DNA sequences, PAL2NAL[91] was implemented using the Bio::Tools::Run::Alignment::Pal2Nal module (http://search.cpan.org/~cjfields/BioPerl-Run-1.006900/lib/Bio/Tools/Run/Alignment/Pal2Nal.pm).
Analysis of selective pressure
Yang and Nielsen (YN) [92], maximum likelihood (ML) [93], Nei & Gojobori (NG) [94], MYN [95], gNG [96], and gMYN [96] methods were adopted to estimate the synonymous and nonsynonymous substitution rates in pairwise comparisons of protein-coding DNA sequences. The program PAML [97], Bio::Tools::Run::Phylo::PAML::Codeml (http://search.cpan.org/~cjfields/BioPerl-Run-1.006900/lib/Bio/Tools/Run/Phylo/PAML/Codeml.pm) and Bio::Tools::Run::Phylo::PAML::Yn00 modules (http://search.cpan.org/~cjfields/BioPerl-Run-1.006900/lib/Bio/Tools/Run/Phylo/PAML/Yn00.pm) were used to implement the YN, ML and NG methods; KaKs_calculator 2.0 [29] was used to execute the gNG, MYN and gMYN methods. The outcomes of these methods were compared, and only the results of gNG and MYN were further analyzed. The outputs were ordered by the Ka/Ks value and by the significance level of the associated Fisher’s test, which indicates whether the Ka/Ks ratio is significantly different from one.
The positions of the exons of the PSGs were revealed with Blast, and the SNPs in exons were selected, and their Fsts were calculated with EXCEL. All SNPs with more than 0.25 Fst values were manually checked to see if they changed the amino acids with the help of Sequencher (http://www.genecodes.com) and DNAsis Max Trial 1.0 (http://www.miraibio.com/download/).
Lositan, a Fst related statistic method, was employed to identify outliers for selection detection [98]. The phylogenetic tree was constructed with MEGA6 (http://megasoftware.net/).
GO analysis
The GO annotations of the PSGs were retrieved from Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/) and RAP-DB, or searched with Blast2GO using the default threshold [99, 100]. The GO annotations for biological processes, molecular functions and cellular component categories were classified into different groups with OBO-edit version 2.1.1 (http://sourceforge.net/projects/geneontology/files/). The chromosomal positions of the PSGs were recovered by searching the Nipponbare genome sequences, parsing the sequences with the Bio::SearchIO module and then mapping the resulting data with EXCEL.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(TIF)
(A) Neighbor-joining tree of 446 O. rufipogon accessions and 1,083 O. sativa varieties constructed with the PSSs. The five divergent groups, indica, aus, temperate japonica, tropical japonica and intermediate were indicated with different colors. The scale bar indicates the simple matching distance. (B) Neighbor-joining tree of 446 O. rufipogon accessions constructed with the PSSs.
(TIF)
Acknowledgments
We wish to thank Miss Lihua Zhang, a PhD student at the South Dakota University, for her assistance with Perl programming, and thank Dr. Bin Han’s group for their SNP data of 1529 O. sativa and O. rufipogon accessions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Khush GS. Origin, dispersal, cultivation and variation of rice. Plant molecular biology. 1997;35(1–2):25–34. Epub 1997/09/18 [PubMed] [Google Scholar]
- 2. Oka HI, editor. Origin of cultivated rice. Tokyo: Japan Scientific Societies Press; 1988. [Google Scholar]
- 3. Morishima H, Oka. H . Phylogenetic differentiation of cultivated rice. XXII. Numerical evaluation of the Indica—Japonica differentiation. Jpn J Breed. 1981;31:402–13. [Google Scholar]
- 4. Garris AJ, Tai TH, Coburn J, Kresovich S, McCouch S. Genetic structure and diversity in Oryza sativa L. Genetics. 2005;169(3):1631–8. Epub 2005/01/18 genetics.104.035642 [pii] 10.1534/genetics.104.035642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glaszmann JC. Isozymes and classification of Asian rice varieties. Theor Appl Genet. 1987;74:21–30. 10.1007/BF00290078 [DOI] [PubMed] [Google Scholar]
- 6. Second G. Evolutionary relationships in the sativa group of Oryza based on isozyme data. Genet Sel Evol. 1985;17:89–114. 10.1186/1297-9686-17-1-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang ZY, Tanksley SD. Restriction fragment length polymorphism in Oryza sativa L. Genome. 1989;32(6):1113–8. [Google Scholar]
- 8. Zhang P, Li J, Li X, Liu X, Zhao X, Lu Y. Population structure and genetic diversity in a rice core collection (Oryza sativa L.) investigated with SSR markers. PloS one. 2011;6(12):e27565 Epub 2011/12/14 10.1371/journal.pone.0027565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang X, Zhao Y, Wei X, Li C, Wang A, Zhao Q, et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nature genetics. 2012;44(1):32–9. 10.1038/ng.1018 [DOI] [PubMed] [Google Scholar]
- 10. Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, et al. A map of rice genome variation reveals the origin of cultivated rice. Nature. 2012;490(7421):497–501. 10.1038/nature11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molina J, Sikora M, Garud N, Flowers JM, Rubinstein S, Reynolds A, et al. Molecular evidence for a single evolutionary origin of domesticated rice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(20):8351–6. Epub 2011/05/04 1104686108 [pii] 10.1073/pnas.1104686108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan L, Li X, Liu F, Sun X, Li C, Zhu Z, et al. Control of a key transition from prostrate to erect growth in rice domestication. Nature genetics. 2008;40(11):1360–4. Epub 2008/09/30 ng.197 [pii] 10.1038/ng.197 [DOI] [PubMed] [Google Scholar]
- 13. Jin J, Huang W, Gao JP, Yang J, Shi M, Zhu MZ, et al. Genetic control of rice plant architecture under domestication. Nature genetics. 2008;40(11):1365–9. Epub 2008/09/30 ng.247 [pii] 10.1038/ng.247 [DOI] [PubMed] [Google Scholar]
- 14. Zhu BF, Si L, Wang Z, Zhou Y, Zhu J, Shangguan Y, et al. Genetic control of a transition from black to straw-white seed hull in rice domestication. Plant physiology. 2011;155(3):1301–11. 10.1104/pp.110.168500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang LB, Zhu Q, Wu ZQ, Ross-Ibarra J, Gaut BS, Ge S, et al. Selection on grain shattering genes and rates of rice domestication. The New phytologist. 2009;184(3):708–20. Epub 2009/08/14 NPH2984 [pii] 10.1111/j.1469-8137.2009.02984.x [DOI] [PubMed] [Google Scholar]
- 16. Li C, Zhou A, Sang T. Rice domestication by reducing shattering. Science. 2006;311(5769):1936–9. Epub 2006/03/11 1123604 [pii] 10.1126/science.1123604 [DOI] [PubMed] [Google Scholar]
- 17. Caicedo AL, Williamson SH, Hernandez RD, Boyko A, Fledel-Alon A, York TL, et al. Genome-wide patterns of nucleotide polymorphism in domesticated rice. PLoS genetics. 2007;3(9):1745–56. Epub 2007/10/03. 07-PLGE-RA-0120 [pii] 10.1371/journal.pgen.0030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng C, Motohashi R, Tsuchimoto S, Fukuta Y, Ohtsubo H, Ohtsubo E. Polyphyletic origin of cultivated rice: based on the interspersion pattern of SINEs. Mol Biol Evol. 2003;20(1):67–75. Epub 2003/01/10 [DOI] [PubMed] [Google Scholar]
- 19. Londo JP, Chiang YC, Hung KH, Chiang TY, Schaal BA. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(25):9578–83. Epub 2006/06/13. 0603152103 [pii] 10.1073/pnas.0603152103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rakshit S, Rakshit A, Matsumura H, Takahashi Y, Hasegawa Y, Ito A, et al. Large-scale DNA polymorphism study of Oryza sativa and O. rufipogon reveals the origin and divergence of Asian rice. Theor Appl Genet. 2007;114(4):731–43. Epub 2007/01/16 10.1007/s00122-006-0473-1 [DOI] [PubMed] [Google Scholar]
- 21. Tang T, Lu J, Huang J, He J, McCouch SR, Shen Y, et al. Genomic variation in rice: genesis of highly polymorphic linkage blocks during domestication. PLoS genetics. 2006;2(11):e199 Epub 2006/11/23 06-PLGE-RA-0344R2 [pii] 10.1371/journal.pgen.0020199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu Q, Ge S. Phylogenetic relationships among A-genome species of the genus Oryza revealed by intron sequences of four nuclear genes. The New phytologist. 2005;167(1):249–65. Epub 2005/06/14 NPH1406 [pii] 10.1111/j.1469-8137.2005.01406.x [DOI] [PubMed] [Google Scholar]
- 23. He Z, Zhai W, Wen H, Tang T, Wang Y, Lu X, et al. Two evolutionary histories in the genome of rice: the roles of domestication genes. PLoS genetics. 2011;7(6):e1002100 Epub 2011/06/23 10.1371/journal.pgen.1002100 PGENETICS-D-10–00553 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang CL, Hung CY, Chiang YC, Hwang CC, Hsu TW, Huang CC, et al. Footprints of natural and artificial selection for photoperiod pathway genes in Oryza. The Plant journal: for cell and molecular biology. 2012;70(5):769–82. 10.1111/j.1365-313X.2012.04915.x [DOI] [PubMed] [Google Scholar]
- 25. Yu Y, Tang T, Qian Q, Wang Y, Yan M, Zeng D, et al. Independent losses of function in a polyphenol oxidase in rice: differentiation in grain discoloration between subspecies and the role of positive selection under domestication. The Plant cell. 2008;20(11):2946–59. Epub 2008/11/27 tpc.108.060426 [pii] 10.1105/tpc.108.060426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olsen KM, Caicedo AL, Polato N, McClung A, McCouch S, Purugganan MD. Selection under domestication: evidence for a sweep in the rice waxy genomic region. Genetics. 2006;173(2):975–83. 10.1534/genetics.106.056473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takano-Kai N, Jiang H, Kubo T, Sweeney M, Matsumoto T, Kanamori H, et al. Evolutionary history of GS3, a gene conferring grain length in rice. Genetics. 2009;182(4):1323–34. 10.1534/genetics.109.103002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Project IRGS. The map-based sequence of the rice genome. Nature. 2005;436(7052):793–800. Epub 2005/08/16 10.1038/nature03895 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Z, Li J, Zhao XQ, Wang J, Wong GK, Yu J. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics Proteomics Bioinformatics. 2006;4(4):259–63. Epub 2007/05/29. S1672–0229(07)60007–2 [pii] 10.1016/S1672-0229(07)60007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–54. [DOI] [PubMed] [Google Scholar]
- 31. Wright S. The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution. 1965;19:395–420. [Google Scholar]
- 32. Wright S. Variability Within and Among Natural Populations: Univ. of Chicago Press, Chicago; 1978. [Google Scholar]
- 33. Chen J, Ding J, Ouyang Y, Du H, Yang J, Cheng K, et al. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(32):11436–41. 10.1073/pnas.0804761105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gong X, Jiang Q, Xu J, Zhang J, Teng S, Lin D, et al. Disruption of the rice plastid ribosomal protein s20 leads to chloroplast developmental defects and seedling lethality. G3. 2013;3(10):1769–77. 10.1534/g3.113.007856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park MR, Yun KY, Mohanty B, Herath V, Xu F, Wijaya E, et al. Supra-optimal expression of the cold-regulated OsMyb4 transcription factor in transgenic rice changes the complexity of transcriptional network with major effects on stress tolerance and panicle development. Plant, cell & environment. 2010;33(12):2209–30. 10.1111/j.1365-3040.2010.02221.x [DOI] [PubMed] [Google Scholar]
- 36. Vannini C, Locatelli F, Bracale M, Magnani E, Marsoni M, Osnato M, et al. Overexpression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. The Plant journal: for cell and molecular biology. 2004;37(1):115–27. [DOI] [PubMed] [Google Scholar]
- 37. Obata T, Kitamoto HK, Nakamura A, Fukuda A, Tanaka Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant physiology. 2007;144(4):1978–85. 10.1104/pp.107.101154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaneda T, Taga Y, Takai R, Iwano M, Matsui H, Takayama S, et al. The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. The EMBO journal. 2009;28(7):926–36. 10.1038/emboj.2009.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taga Y, Takai R, Kaneda T, Matsui H, Isogai A, Che FS. Role of OsHSP90 and IREN, Ca2+ dependent nuclease, in plant hypersensitive cell death induced by transcription factor OsNAC4. Plant signaling & behavior. 2009;4(8):740–2. 10.1038/emboj.2009.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kawahara Y, Oono Y, Kanamori H, Matsumoto T, Itoh T, Minami E. Simultaneous RNA-seq analysis of a mixed transcriptome of rice and blast fungus interaction. PloS one. 2012;7(11):e49423 10.1371/journal.pone.0049423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Komatsu M, Maekawa M, Shimamoto K, Kyozuka J. The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Developmental biology. 2001;231(2):364–73. 10.1006/dbio.2000.9988 [DOI] [PubMed] [Google Scholar]
- 42. Yang F, Wang Q, Schmitz G, Muller D, Theres K. The bHLH protein ROX acts in concert with RAX1 and LAS to modulate axillary meristem formation in Arabidopsis. The Plant journal: for cell and molecular biology. 2012;71(1):61–70. 10.1111/j.1365-313X.2012.04970.x [DOI] [PubMed] [Google Scholar]
- 43. Mach J. Rice axillary meristem formation requires directional movement of LAX PANICLE1 protein. The Plant cell. 2009;21(4):1027 10.1105/tpc.109.210410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oikawa T, Kyozuka J. Two-Step Regulation of LAX PANICLE1 Protein Accumulation in Axillary Meristem Formation in Rice. The Plant cell. 2009;21(4):1095–108. 10.1105/tpc.108.065425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amir Hossain M, Lee Y, Cho JI, Ahn CH, Lee SK, Jeon JS, et al. The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Plant molecular biology. 2010;72(4–5):557–66. 10.1007/s11103-009-9592-9 [DOI] [PubMed] [Google Scholar]
- 46. Matsui H, Miyao A, Takahashi A, Hirochika H. Pdk1 kinase regulates basal disease resistance through the OsOxi1-OsPti1a phosphorylation cascade in rice. Plant & cell physiology. 2010;51(12):2082–91. 10.1093/pcp/pcq167 [DOI] [PubMed] [Google Scholar]
- 47. Luo H, Lee JY, Hu Q, Nelson-Vasilchik K, Eitas TK, Lickwar C, et al. RTS, a rice anther-specific gene is required for male fertility and its promoter sequence directs tissue-specific gene expression in different plant species. Plant molecular biology. 2006;62(3):397–408. 10.1007/s11103-006-9031-0 [DOI] [PubMed] [Google Scholar]
- 48. Passaia G, Fonini LS, Caverzan A, Jardim-Messeder D, Christoff AP, Gaeta ML, et al. The mitochondrial glutathione peroxidase GPX3 is essential for H2O2 homeostasis and root and shoot development in rice. Plant science: an international journal of experimental plant biology. 2013;208:93–101. 10.1016/j.plantsci.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 49. Xiang Y, Tang N, Du H, Ye H, Xiong L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant physiology. 2008;148(4):1938–52. 10.1104/pp.108.128199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoshii M, Shimizu T, Yamazaki M, Higashi T, Miyao A, Hirochika H, et al. Disruption of a novel gene for a NAC-domain protein in rice confers resistance to Rice dwarf virus. The Plant journal: for cell and molecular biology. 2009;57(4):615–25. 10.1111/j.1365-313X.2008.03712.x [DOI] [PubMed] [Google Scholar]
- 51. Wang Q, Li J, Hu L, Zhang T, Zhang G, Lou Y. OsMPK3 positively regulates the JA signaling pathway and plant resistance to a chewing herbivore in rice. Plant cell reports. 2013;32(7):1075–84. 10.1007/s00299-013-1389-2 [DOI] [PubMed] [Google Scholar]
- 52. Xie G, Kato H, Sasaki K, Imai R. A cold-induced thioredoxin h of rice, OsTrx23, negatively regulates kinase activities of OsMPK3 and OsMPK6 in vitro. FEBS letters. 2009;583(17):2734–8. 10.1016/j.febslet.2009.07.057 [DOI] [PubMed] [Google Scholar]
- 53. Xie G, Kato H, Imai R. Biochemical identification of the OsMKK6-OsMPK3 signalling pathway for chilling stress tolerance in rice. The Biochemical journal. 2012;443(1):95–102. 10.1042/BJ20111792 [DOI] [PubMed] [Google Scholar]
- 54. Rao KP, Vani G, Kumar K, Wankhede DP, Misra M, Gupta M, et al. Arsenic stress activates MAP kinase in rice roots and leaves. Archives of biochemistry and biophysics. 2011;506(1):73–82. 10.1016/j.abb.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 55. Kishi-Kaboshi M, Okada K, Kurimoto L, Murakami S, Umezawa T, Shibuya N, et al. A rice fungal MAMP-responsive MAPK cascade regulates metabolic flow to antimicrobial metabolite synthesis. The Plant journal: for cell and molecular biology. 2010;63(4):599–612. 10.1111/j.1365-313X.2010.04264.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park CJ, Peng Y, Chen X, Dardick C, Ruan D, Bart R, et al. Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21-mediated innate immunity. PLoS biology. 2008;6(9):e231 10.1371/journal.pbio.0060231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schmidt R, Mieulet D, Hubberten HM, Obata T, Hoefgen R, Fernie AR, et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. The Plant cell. 2013;25(6):2115–31. 10.1105/tpc.113.113068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mizuta Y, Harushima Y, Kurata N. Rice pollen hybrid incompatibility caused by reciprocal gene loss of duplicated genes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20417–22. 10.1073/pnas.1003124107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morita R, Sato Y, Masuda Y, Nishimura M, Kusaba M. Defect in non-yellow coloring 3, an alpha/beta hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. The Plant journal: for cell and molecular biology. 2009;59(6):940–52. 10.1111/j.1365-313X.2009.03919.x [DOI] [PubMed] [Google Scholar]
- 60. Ookawa T, Hobo T, Yano M, Murata K, Ando T, Miura H, et al. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nature communications. 2010;1:132 10.1038/ncomms1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ikeda-Kawakatsu K, Yasuno N, Oikawa T, Iida S, Nagato Y, Maekawa M, et al. Expression level of ABERRANT PANICLE ORGANIZATION1 determines rice inflorescence form through control of cell proliferation in the meristem. Plant physiology. 2009;150(2):736–47. 10.1104/pp.109.136739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ikeda K, Nagasawa N, Nagato Y. ABERRANT PANICLE ORGANIZATION 1 temporally regulates meristem identity in rice. Developmental biology. 2005;282(2):349–60. 10.1016/j.ydbio.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 63. Ikeda K, Ito M, Nagasawa N, Kyozuka J, Nagato Y. Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. The Plant journal: for cell and molecular biology. 2007;51(6):1030–40. 10.1111/j.1365-313X.2007.03200.x [DOI] [PubMed] [Google Scholar]
- 64. Nagasawa N, Miyoshi M, Sano Y, Satoh H, Hirano H, Sakai H, et al. SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development. 2003;130(4):705–18. [DOI] [PubMed] [Google Scholar]
- 65. Lee S, Jeon JS, An K, Moon YH, Lee S, Chung YY, et al. Alteration of floral organ identity in rice through ectopic expression of OsMADS16. Planta. 2003;217(6):904–11. 10.1007/s00425-003-1066-8 [DOI] [PubMed] [Google Scholar]
- 66. Ning J, Zhang B, Wang N, Zhou Y, Xiong L. Increased leaf angle1, a Raf-like MAPKKK that interacts with a nuclear protein family, regulates mechanical tissue formation in the Lamina joint of rice. The Plant cell. 2011;23(12):4334–47. 10.1105/tpc.111.093419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fitzgerald HA, Canlas PE, Chern MS, Ronald PC. Alteration of TGA factor activity in rice results in enhanced tolerance to Xanthomonas oryzae pv. oryzae. The Plant journal: for cell and molecular biology. 2005;43(3):335–47. 10.1111/j.1365-313X.2005.02457.x [DOI] [PubMed] [Google Scholar]
- 68. Park HY, Kang IS, Han JS, Lee CH, An G, Moon YH. OsDEG10 encoding a small RNA-binding protein is involved in abiotic stress signaling. Biochemical and biophysical research communications. 2009;380(3):597–602. 10.1016/j.bbrc.2009.01.131 [DOI] [PubMed] [Google Scholar]
- 69. Lazzarotto F, Teixeira FK, Rosa SB, Dunand C, Fernandes CL, Fontenele Ade V, et al. Ascorbate peroxidase-related (APx-R) is a new heme-containing protein functionally associated with ascorbate peroxidase but evolutionarily divergent. The New phytologist. 2011;191(1):234–50. 10.1111/j.1469-8137.2011.03659.x [DOI] [PubMed] [Google Scholar]
- 70. Yamada S, Kano A, Tamaoki D, Miyamoto A, Shishido H, Miyoshi S, et al. Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant & cell physiology. 2012;53(12):2060–72. 10.1093/pcp/pcs145 [DOI] [PubMed] [Google Scholar]
- 71. Kunihiro S, Saito T, Matsuda T, Inoue M, Kuramata M, Taguchi-Shiobara F, et al. Rice DEP1, encoding a highly cysteine-rich G protein gamma subunit, confers cadmium tolerance on yeast cells and plants. Journal of experimental botany. 2013;64(14):4517–27. 10.1093/jxb/ert267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, et al. Natural variation at the DEP1 locus enhances grain yield in rice. Nature genetics. 2009;41(4):494–7. 10.1038/ng.352 [DOI] [PubMed] [Google Scholar]
- 73. Zhang T, Zhao X, Wang W, Pan Y, Huang L, Liu X, et al. Comparative transcriptome profiling of chilling stress responsiveness in two contrasting rice genotypes. PloS one. 2012;7(8):e43274 10.1371/journal.pone.0043274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, et al. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. The Plant journal: for cell and molecular biology. 2003;33(4):751–63. [DOI] [PubMed] [Google Scholar]
- 75. Figueiredo DD, Barros PM, Cordeiro AM, Serra TS, Lourenco T, Chander S, et al. Seven zinc-finger transcription factors are novel regulators of the stress responsive gene OsDREB1B. Journal of experimental botany. 2012;63(10):3643–56. 10.1093/jxb/ers035 [DOI] [PubMed] [Google Scholar]
- 76. Gutha LR, Reddy AR. Rice DREB1B promoter shows distinct stress-specific responses, and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. Plant molecular biology. 2008;68(6):533–55. 10.1007/s11103-008-9391-8 [DOI] [PubMed] [Google Scholar]
- 77. Zheng J, Wang Y, He Y, Zhou J, Li Y, Liu Q, et al. Overexpression of an S-like ribonuclease gene, OsRNS4, confers enhanced tolerance to high salinity and hyposensitivity to phytochrome-mediated light signals in rice. Plant science: an international journal of experimental plant biology. 2014;214:99–105. 10.1016/j.plantsci.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 78. Nonomura K, Eiguchi M, Nakano M, Takashima K, Komeda N, Fukuchi S, et al. A novel RNA-recognition-motif protein is required for premeiotic G1/S-phase transition in rice (Oryza sativa L.). PLoS genetics. 2011;7(1):e1001265 10.1371/journal.pgen.1001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chang T-T, editor. Origin, domestication, and diversification. New Jersey USA: J. Wiley and Sons, Inc.; 2003. [Google Scholar]
- 80. Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127(7):1309–21. Epub 2006/12/28. S0092-8674(06)01592-3 [pii] 10.1016/j.cell.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 81. Doebley J, Stec A, Hubbard L. The evolution of apical dominance in maize. Nature. 1997;386(6624):485–8. Epub 1997/04/03 10.1038/386485a0 [DOI] [PubMed] [Google Scholar]
- 82. Wang H, Nussbaum-Wagler T, Li B, Zhao Q, Vigouroux Y, Faller M, et al. The origin of the naked grains of maize. Nature. 2005;436(7051):714–9. Epub 2005/08/05 nature03863 [pii] 10.1038/nature03863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Konishi S, Izawa T, Lin SY, Ebana K, Fukuta Y, Sasaki T, et al. An SNP caused loss of seed shattering during rice domestication. Science. 2006;312(5778):1392–6. Epub 2006/04/15 1126410 [pii] 10.1126/science.1126410 [DOI] [PubMed] [Google Scholar]
- 84. Sweeney MT, Thomson MJ, Pfeil BE, McCouch S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. The Plant cell. 2006;18(2):283–94. Epub 2006/01/10 tpc.105.038430 [pii] 10.1105/tpc.105.038430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Frary A, Nesbitt TC, Grandillo S, Knaap E, Cong B, Liu J, et al. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science. 2000;289(5476):85–8. Epub 2000/07/07. 8651 [pii] [DOI] [PubMed] [Google Scholar]
- 86. Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai YS, Gill BS, et al. Molecular characterization of the major wheat domestication gene Q. Genetics. 2006;172(1):547–55. Epub 2005/09/21 genetics.105.044727 [pii] 10.1534/genetics.105.044727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sun X, Wang GL. Genome-wide identification, characterization and phylogenetic analysis of the rice LRR-kinases. PloS one. 2011;6(3):e16079 10.1371/journal.pone.0016079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Anisimova M, Bielawski JP, Yang Z. Accuracy and power of the likelihood ratio test in detecting adaptive molecular evolution. Mol Biol Evol. 2001;18(8):1585–92. [DOI] [PubMed] [Google Scholar]
- 89. Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13(5):555–6. Epub 1997/11/21 [DOI] [PubMed] [Google Scholar]
- 90. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. Epub 2007/09/12 btm404 [pii] 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 91. Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34(Web Server issue):W609–12. Epub 2006/07/18 34/suppl_2/W609 [pii] 10.1093/nar/gkl315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang Z, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol. 2000;17(1):32–43. Epub 2000/02/10 [DOI] [PubMed] [Google Scholar]
- 93. Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol. 1998;15(5):568–73. Epub 1998/05/15 [DOI] [PubMed] [Google Scholar]
- 94. Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3(5):418–26. Epub 1986/09/01 [DOI] [PubMed] [Google Scholar]
- 95. Zhang Z, Li J, Yu J. Computing Ka and Ks with a consideration of unequal transitional substitutions. BMC Evol Biol. 2006;6:44 Epub 2006/06/03. 1471–2148–6-44 [pii] 10.1186/1471-2148-6-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang DP, Wan HL, Zhang S, Yu J. Gamma-MYN: a new algorithm for estimating Ka and Ks with consideration of variable substitution rates. Biol Direct. 2009;4:20 Epub 2009/06/18. 1745–6150–4–20 [pii] 10.1186/1745-6150-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24(8):1586–91. Epub 2007/05/08 msm088 [pii] 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- 98. Antao T, Lopes A, Lopes RJ, Beja-Pereira A, Luikart G. LOSITAN: a workbench to detect molecular adaptation based on a Fst-outlier method. BMC Bioinformatics. 2008;9:323 10.1186/1471-2105-9-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. Epub 2005/08/06 bti610 [pii] 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- 100. Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–35. Epub 2008/05/01 gkn176 [pii] 10.1093/nar/gkn176 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(TIF)
(A) Neighbor-joining tree of 446 O. rufipogon accessions and 1,083 O. sativa varieties constructed with the PSSs. The five divergent groups, indica, aus, temperate japonica, tropical japonica and intermediate were indicated with different colors. The scale bar indicates the simple matching distance. (B) Neighbor-joining tree of 446 O. rufipogon accessions constructed with the PSSs.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.